Abstract
Bovine Adeno-Associated Virus (BAAV) can enter a cell either through a transcytosis or transduction pathway. We previously demonstrated that particles entering via the transcytosis pathway can be redirected to transduce the cell by blocking particle exocytosis with tannic acid (TA). To investigate whether this approach is useful in lung gene therapy applications, we tested the effect of TA on BAAV transduction in cystic fibrosis airway epithelia in vitro, and mouse lung in vivo. Our findings suggest that BAAV transcytosis can occur in vivo and that treatment with TA reduces transcytosis and increases lung transduction. TA treatment did not impair the sorting and the activity of the BAAV expressed CFTR membrane protein.
In AAV transcytosis studies we used tannic acid (TA) to inhibit vector exocytosis from the basolateral side of polarized epithelial barrier models1. As a consequence of viral transcytosis inhibition, the vector was redirected to the nucleus, resulting in a dramatic increase in transduction. In order to better understand the response of primary epithelial cells to TA treatment, as well as maximize transduction at the lowest concentration of TA, we performed a series of experiments varying TA concentrations and incubation times. Since TA treatment might result in disruption or damage epithelial integrity, thus affecting BAAV transcytosis, we monitored the trans-epithelial resistance (TER) as a parameter of epithelia integrity. No loss of TER was noted at the TA concentrations reported. We first incubated primary culture of human airway epithelia (HAE) with BAAV-GFP at different concentrations of TA for 3 hrs. Forty-eight hrs post incubations, GFP positive cells were observed by fluorescent microscopy and quantified by determining the mean fluorescence intensity (Fig. 1A and B). TA mediated BAAV transduction was observed to be dose dependent with extensive transduction at a TA concentration of 0.125% w/v, a concentration 4 fold lower than that (0.5%) previously reported1.
FIG. 1.
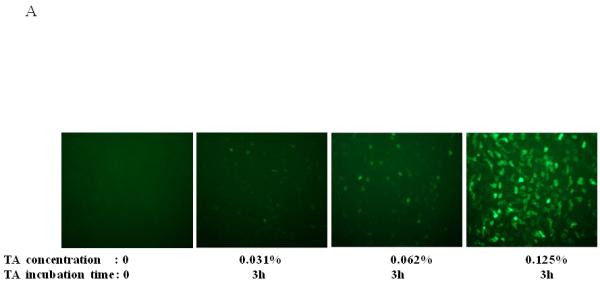
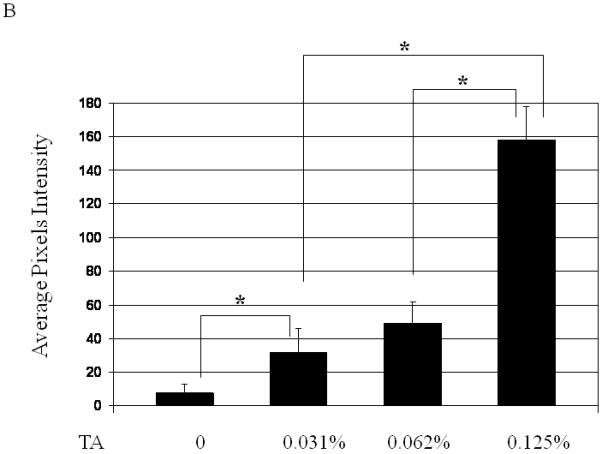
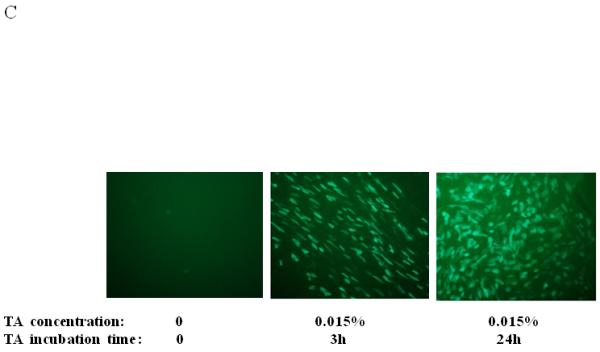
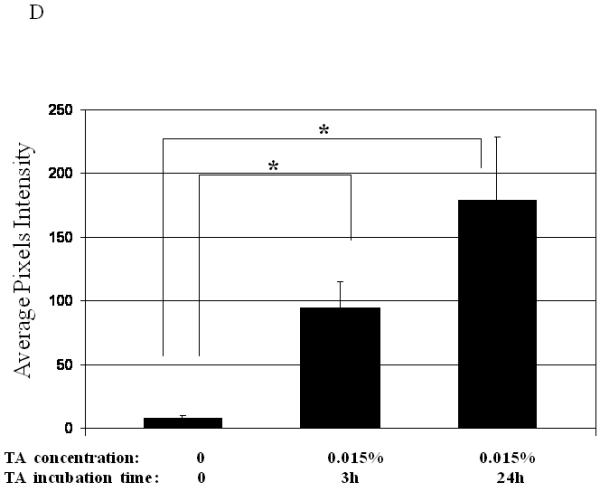
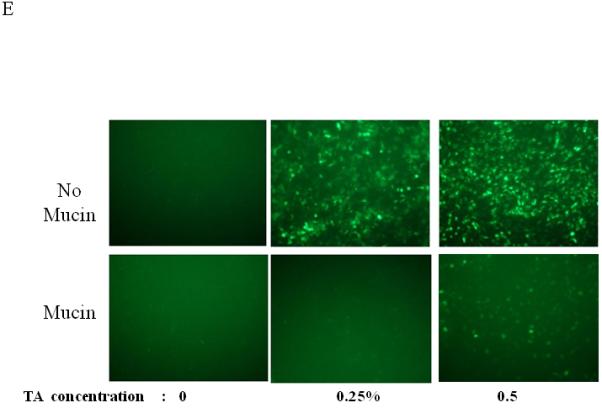
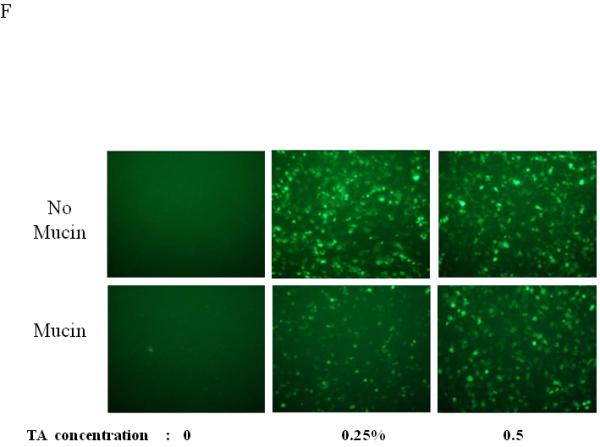
Dose, time and mucins effects on primary HAE BAAV TA mediated transduction. (A and B) Monolayer of differentiated HAE, plated in 6mm transwell filters, were incubated apically with 108 DNAse resistant particles (DRP) of BAAV-GFP and treated on the basolateral surface with 0, 0.031, 0.062 or 0.125, % w/v of TA for 3 hrs. 48 hrs post incubation, GFP positive cells were observed by fluorescent microscopy and fluorescence quantified using the ImageJ software. (C and D) As above, cells were incubated apically with BAAV-GFP but treated with 0.015% w/v of TA for 3 or 24 h respectively. 96 hrs later GFP positive cells were observed and quantified. (E, F and G) HAE cultures secreting mucins were extensively washed with cell medium or left untreated. BAAV was applied apically and cell treated on the basolateral surface with 0.25 or 0.5% w/v TA for 3 hrs. 48 (E) and 96 (F) hrs later positive cell were observed and quantified. N=2 in duplicate. Student’s t-test * (P value <0.05). Positive cells of 4 random fields in experiment A were also counted and similar fold changes compared with control were measured (data not shown).
Cells treated with TA and no BAAV did not differ from those incubated with BAAV without TA (data not shown).
To determine if TA mediated BAAV transduction was also time-dependent, we incubated cells with 0.015% w/v TA for 3 or 24 hrs, then measured transduction at 48 or 96 hrs post incubation. At 48 hrs post incubation, we observed only a few positive cells (data not shown). However, by 96 hrs post incubation, a majority of the cells were positive (Fig. 1C and D).
Taken together these experiments demonstrate the effect of TA is both dose and time dependent and can increase transduction at concentrations much lower than previously reported1.
For a number of pulmonary gene therapy applications, delivery via the apical surface of the lung may be the preferred route of vector delivery. As part of the innate immune system, however, a number of factors are secreted from the apical surface to prevent infection or colonization. Mucins have previously been shown to inhibit the transduction of some sialic acid binding AAV serotypes such as AAV4 2. To test the effect of mucin on TA mediated BAAV transduction of HAE, primary cultures were prepared and either extensively washed to remove cell surface mucins, or left untreated prior to the addition of TA to the basolateral surface and BAAV vector encoding GFP to the apical surface. Transduction was quantified at 48 and 96 hrs post vector addition (Fig. 1E, F and G). At 48 hrs post incubation, transduction was reduced in the cultures containing mucin compared with the washed cultures (Fig. 1E and G); however, this reduction was less evident by 96 hrs (Fig. 1F and G). No transduction was detected in cells incubated with BAAV without TA. This experiment suggests that mucins slow TA mediated BAAV transduction, but like AAV5, TA mediated BAAV transduction is insensitive to mucins. In vitro data on differentiated HAE suggests that utilizing the transcytosis entry pathway of BAAV can achieve significant transduction of HAE. For this to occur, BAAV particle trafficking must be altered by treatment with the transcytosis inhibitor TA. Although the exact mechanism of action of TA is not clear, it is reported to inhibit the membrane distribution of proteins that traffic from the basal side to the apical side3, 4. This effect could alter the trafficking of the recombinant proteins delivered by the BAAV vector. In order to test for an effect on genes delivered by BAAV vectors, we studied the biodistribution and activity of the cystic fibrosis transmembrane regulator (CFTR) gene delivered to cystic fibrosis (CF) HAE epithelia via BAAV vectors, following treatment of cultures with TA. We have previously demonstrated that a truncated form of this gene (CFTRΔR) can be delivered to CF HAE with an AAV5 vector, and that transduction resulted in an increase in cAMP-stimulated bumetanide-sensitive current 5. Immunocytochemical staining of the tannic acid treated and BAAV-CFTRΔR transduced CF HAE suggested apical distribution of the CFTRΔR protein (Fig. 2A, B and C), and was similar in distribution to that of CFTRΔR protein expressed by an adenovirus vector used as positive control, (Fig. 2D, E and F) applied to the basolateral surface of the cells 6, 7. To verify that the expressed CFTRΔR could correct the CF chloride transport defect, we applied BAAV-CFTRΔR for 4 hrs to the apical surface of differentiated primary cultures of CF HAE in the presence of TA. Seven days post incubation, we measured transepithelial chloride current and detected a cAMP-stimulated bumetanide-sensitive change in current in the TA BAAV-CFTRΔR transduced cells (Fig. 2G and H). The correction in the chloride current was dose dependent (1 to 8.5 Δμams/cm2) and approached chloride current levels of our previous data for non-CF airways (8 to 15 Δμams/cm2) 5. A similar degree of current correction was also obtained with an adenovirus vector (Fig. 2H).
FIG. 2.
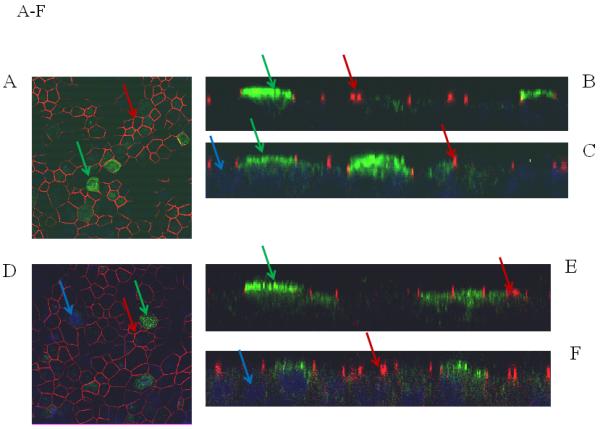
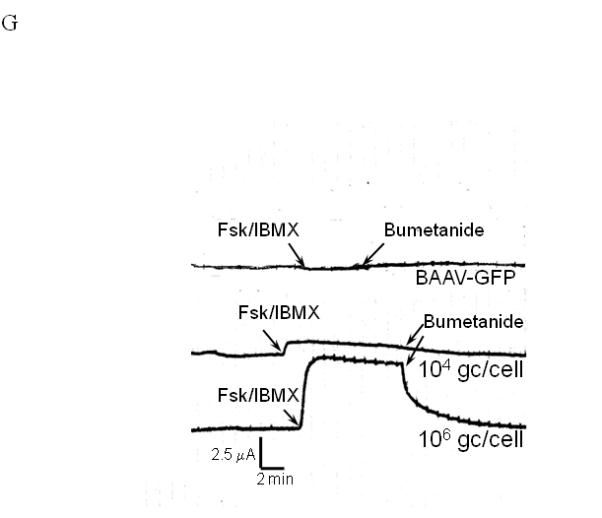
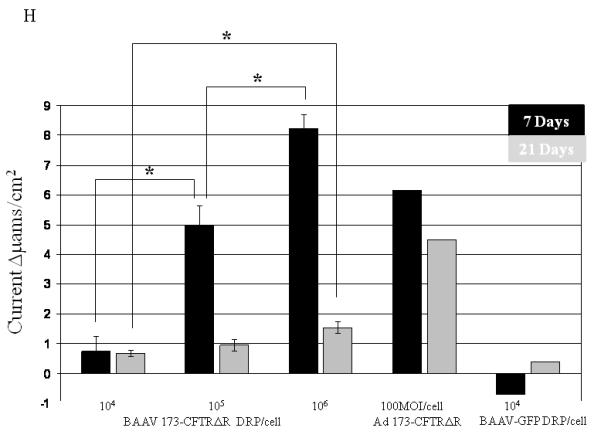
Immunostaining and current tracing of TA BAAV-CFTRΔR transduced CF HAE. Immunostaining of differentiated airway epithelia expressing BAAV-CFTRΔR (10×106 genomes (gc)/cell) (panels A-C) or ad5-CFTRΔR (100MOI) (D-F). Data are X-Y confocal images (B-C and E-F) or en face images (A and D). CFTR is shown in green, tight junction protein zonula occludens-1 (ZO-1) in red and indicated by arrows of the same color respectively. DAPI nuclear stain is in blue. (G) Current tracings of CF airway epithelia transduced with the indicated amounts of BAAV-173CMV-CFTRΔR (106 or 104 gc/cell). Millicells were treated sequentially with, amiloride (10−4M), forskolin (10−4M)/IBMX (10−5M), and bumetanide (10−4M) as indicated. Asterisk indicates a change in the recorder scale. (H) Bumetanide-sensitive forskolin-stimulated current (in μamps/cm2) in well-differentiated CF epithelia expressing the indicated amount of BAAV-CFTRΔR at 7 or 21 days post-transduction. N=3 for each AAV viral titer; N=1 for ad5-GFP control at 7 and 21 day time points. One-way ANOVA with Bonferroni post test * (P value <0.05).
As cystic fibrosis is a chronic condition, we next studied the persistence of CFTRΔR expression following BAAV TA mediated transduction. Long-term observation of BAAV-CFTRΔR TA transduced cultures suggested a decrease in transduction activity over time. As shown in Fig. 2H, by 21 days post transduction, the average current had decreased 4-fold compared with the level of chloride current correction measured on day 7. Thus, activity may decline over time due to decreased protein expression, although TA mediated BAAV transduction does not alter the distribution of CFTRΔR or protein function.
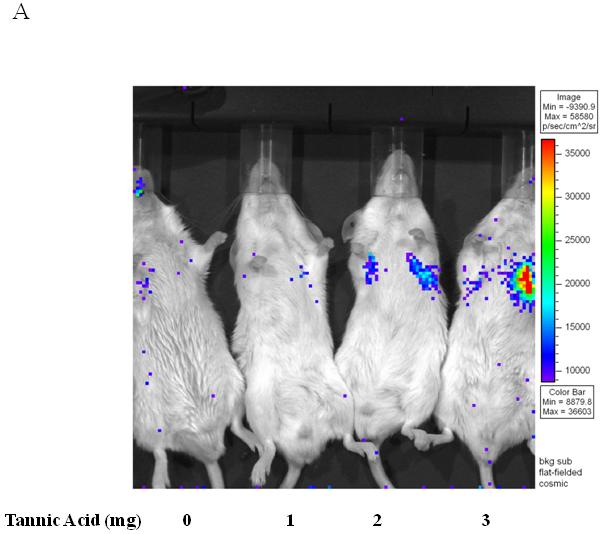
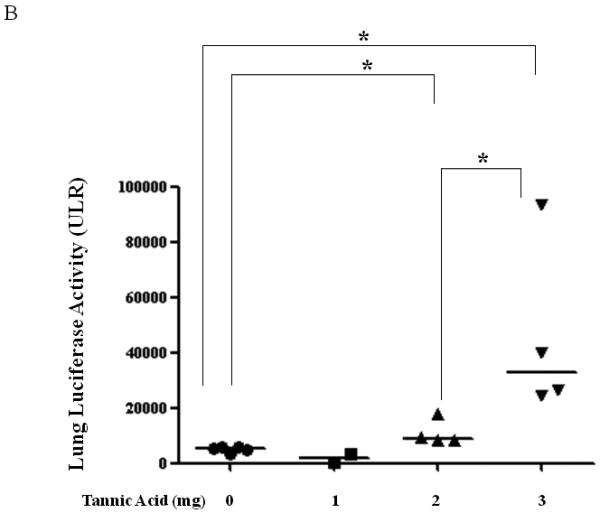
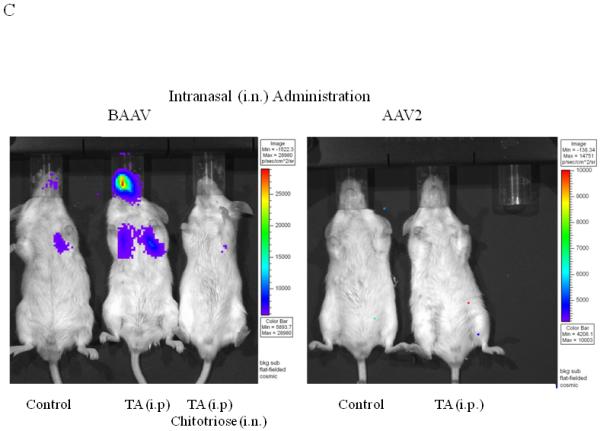
To determine if TA mediated BAAV transduction could occur in vivo, TA was injected into mice by intraperitoneal (i.p.) injection, followed by delivery of BAAV vector encoding luciferase (Luc) to the lungs via intranasal administration (i.n.). The level of transduction was observed and quantified by Xenogen camera imaging (Fig. 3A and B). 24 to 72hrs post administration, little luminescence signal was detected in the lung of control or mice treated with 1mg TA. However, pretreatment of mice with increasing doses of TA (2 and 3 mg) enhanced BAAV transduction of the lungs 2 and 8-fold respectively, compared with controls (Fig. 3A and B) N=4. In contrast, pretreatment of mice with tannic acid had no effect on AAV2 transduction, which is not reported to have transcytosis activity in HAE (Fig. 3C).
FIG. 3.
Lung TA mediated BAAV-Luc transduction in vivo.
(A and B) BAAV-Luc (5.109 DRP) was administered to the lungs of 2-5 mice as indicated for each TA concentration tested, by nasal instillation. Transduction was imaged (A) 24 hrs later using a Xenogen camera 15 min following administration of luciferin substrate. (B) Luciferase activity was quantified 24 to72 hrs post BAAV administration, by Living Image (R), version 2.60.1. Medians represented by horizontal lines. Student’s t-test * P value <0.05. (C) BAAV or AAV2-Luc (5.109 DRP) was delivered to the lungs of mice via an intranasal route with or without chitotriose (20mg/ml). In the indicated mice, prior to vector delivery, mice were treated with TA as described above. Transduction was imaged 24 hrs later using a Xenogen camera 15 min following injection of luciferin.
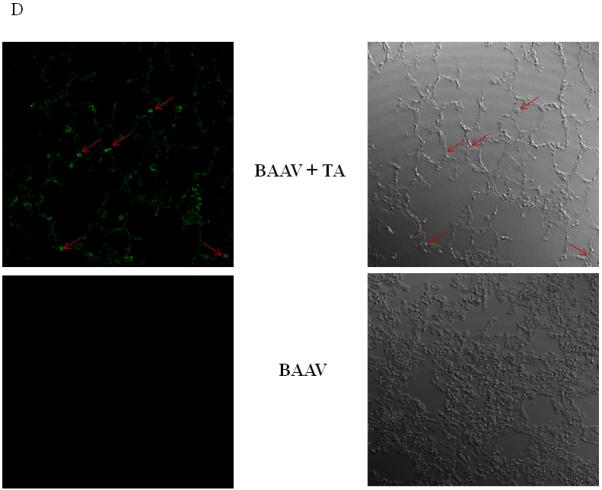
(D) Lungs of mice were removed 4 days post-transduction, inflated with agarose prior to imbedding in paraffin. Luciferase positive cells were detected by immunostaining and pseudocolored in green (left panel). The bright field image is shown on the right. Original magnification 63X.
Recent work has demonstrated that chitotriose is an important attachment molecule in BAAV transcytosis8. To test if this carbohydrate is also important in vivo in TA mediated transduction, BAAV vector was incubated with chitotriose prior to lung instillation in TA treated mice. As previously reported in vitro, preincubation of BAAV vector with chitotriose blocked TA mediated transduction in vivo (Fig. 3C, TA versus TA + chitotriose). Immunofluorescent staining of sections from the TA treated BAAV-infected mice showed transduction of alveolar cells (Fig. 3D).
To determine if TA mediated BAAV transduction persisted in vivo, mice were treated with or without TA i.p. followed by i.n. delivery of BAAV encoding luciferase and expression was measured 24 hrs or 8 weeks post delivery (N=4/group). In isolated organs at 24 hrs post delivery, mice treated with BAAV alone showed some transduction in the lung and stomach (Fig. 4A, B and C). BAAV+TA treated mice showed an increase in luciferase activity in the lung, whereas that of the stomach decreased compared to the BAAV alone treated mice (Fig. 4A and C). At 8 weeks post delivery, the level of luciferase in BAAV+TA treated animals was similar to the BAAV alone treated mice (Fig. 4B). The stomachs of the BAAV+TA treated mice still maintained a lower level of activity compared with BAAV alone treated mice; however the decrease was not statistically significant (Fig. 4C). Some signal was also detected in the liver 8 weeks post transduction, but the intensity was only 20% of that of the lung (data not shown). No luciferase activity was detected in the heart or sternum (data not shown).
FIG. 4.
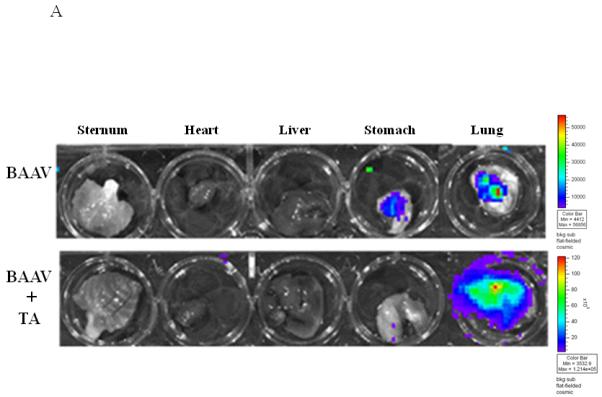
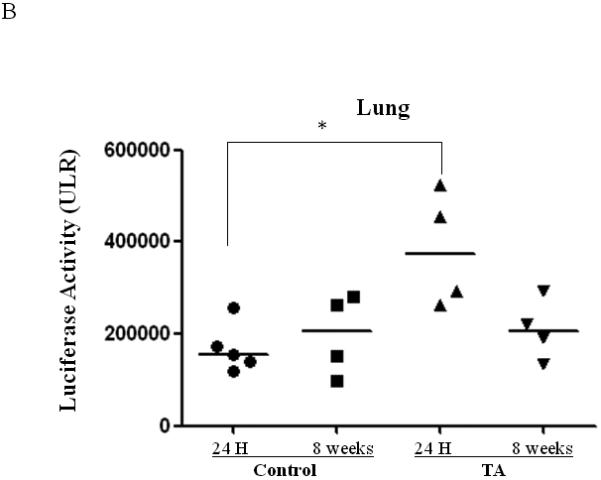
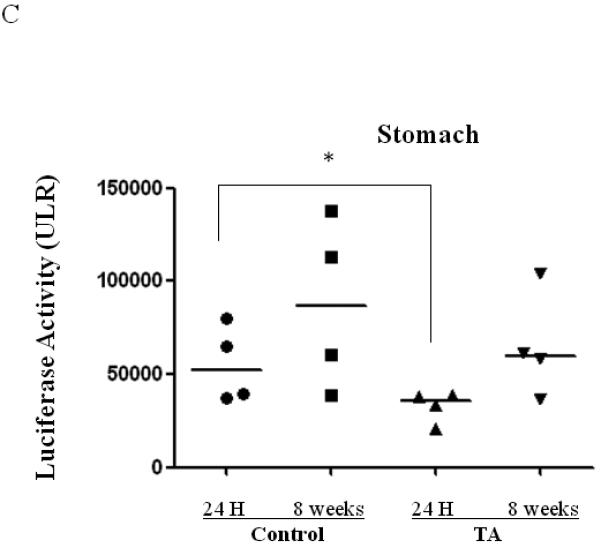
Biodistribution of i.n. administered BAAV–Luc. Four mice per group were injected with 200ul of PBS containing none or 3mg of TA prior to i.n. delivery of 5.109 DRP of BAAV-Luc. 24 hrs or 8 weeks post treatment respectively mice received luciferin substrate then were sacrificed and expression quantified. (A) Luciferase activity of representative mice receiving BAAV alone or BAAV+TA 24 hrs post transduction. (B and C) Average luciferase activity of whole lung or stomach respectively of mice receiving BAAV alone (control) or BAAV+TA (TA), 24 hrs or 8 weeks post treatment respectively. Medians represented by horizontal lines. Student’s t-test * P value <0.05.
Just as the use of alternative serotypes of vectors has enhanced gene transfer to the lung, the addition of chemical modulators of vector trafficking could also enhance transduction. TA induces re-trafficking of BAAV vectors, which can dramatically increase BAAV transduction activity without affecting localization or activity of the therapeutic proteins encoded by the vector. Although TA treatment does not alter the overall long-term transduction of the lung by BAAV in vivo, it does increase the kinetics of transduction. Following i.n. delivery of BAAV vector, transduction could be detected in other tissue, such as the stomach and liver. This finding supports our previous in vitro observations of transcytosis activity with BAAV vectors. Transcytosis of vector can be inhibited by treatment with TA in vivo, which then results in enhanced transduction at the site of delivery and consequent limited biodistribution.
An interesting observation in our study is the rapid kinetics of BAAV transduction induced by TA treatment compared with other permissive AAVs in the lung9. Previous research has demonstrated that other factors, such as addition of a helper virus, can dramatically increase the time course of transduction. While the complete mechanism for helper virus assisted transduction is not clear, several steps such as trafficking to the nucleus, and second strand synthesis are likely affected10, 11. Although there is no research addressing TA involvement in cellular DNA second strand synthesis or nuclear transport, our research suggests these pathways are likely affected by TA.
Tannic acid is a complex mixture of compounds contained in many plants that are consumed by humans. In addition it has been used medically to treat burns and some of its biological properties are known at the molecular level12, 13. As it is currently available, at high concentrations TA will affect transepithelial resistance of cells grown in transwells, limiting its use. However, fractionation may allow the isolation of the active compounds affecting BAAV transcytosis and increase TA’s therapeutic window for in vivo use.
In this manuscript we have established a proof of concept that blocking the transcytosis pathway of BAAV dramatically increases both the kinetics and overall transduction activity.
Acknowledgments
We thank Michael Welsh for his helpful suggestions.
Footnotes
The authors declare no conflict of interest.
References
- 1.Di Pasquale G, Chiorini JA. AAV transcytosis through barrier epithelia and endothelium. Mol Ther. 2006;13(3):506–16. doi: 10.1016/j.ymthe.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Walters RW, Pilewski JM, Chiorini JA, Zabner J. Secreted and transmembrane mucins inhibit gene transfer with AAV4 more efficiently than AAV5. J Biol Chem. 2002;277(26):23709–13. doi: 10.1074/jbc.M200292200. [DOI] [PubMed] [Google Scholar]
- 3.Polishchuk R, Di Pentima A, Lippincott-Schwartz J. Delivery of raft-associated, GPI-anchored proteins to the apical surface of polarized MDCK cells by a transcytotic pathway. Nat Cell Biol. 2004;6(4):297–307. doi: 10.1038/ncb1109. [DOI] [PubMed] [Google Scholar]
- 4.Yui N, Okutsu R, Sohara E, Rai T, Ohta A, Noda Y, et al. FAPP2 is required for aquaporin-2 apical sorting at trans-Golgi network in polarized MDCK cells. Am J Physiol Cell Physiol. 2009;297(6):C1389–96. doi: 10.1152/ajpcell.00098.2009. [DOI] [PubMed] [Google Scholar]
- 5.Ostedgaard LS, Rokhlina T, Karp PH, Lashmit P, Afione S, Schmidt M, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102(8):2952–7. doi: 10.1073/pnas.0409845102. Epub 2005 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostedgaard LS, Zabner J, Vermeer DW, Rokhlina T, Karp PH, Stecenko AA, et al. CFTR with a partially deleted R domain corrects the cystic fibrosis chloride transport defect in human airway epithelia in vitro and in mouse nasal mucosa in vivo. Proc Natl Acad Sci U S A. 2002;99(5):3093–8. doi: 10.1073/pnas.261714599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274(15):10219–26. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 8.Di Pasquale G, Kaludov N, Agbandje-McKenna M, Chiorini JA. BAAV transcytosis requires an interaction with β-1-4 linked- glucosamine and gp96. PloS ONE. 2010;5:e9336. doi: 10.1371/journal.pone.0009336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostedgaard LS, Rokhlina T, Karp PH, Lashmit P, Afione S, Schmidt M, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102(8):2952–7. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao W, Warrington KH, Jr., Hearing P, Hughes J, Muzyczka N. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J Virol. 2002;76(22):11505–17. doi: 10.1128/JVI.76.22.11505-11517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70(5):3227–34. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung KT, Wong TY, Wei CI, Huang YW, Lin Y. Tannins and human health: a review. Crit Rev Food Sci Nutr. 1998;38(6):421–64. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 13.Yang EB, Wei L, Zhang K, Chen YZ, Chen WN. Tannic acid, a potent inhibitor of epidermal growth factor receptor tyrosine kinase. J Biochem. 2006;139(3):495–502. doi: 10.1093/jb/mvj050. [DOI] [PubMed] [Google Scholar]


