Abstract
Objective
The development and patterns of spontaneous aging-related changes in the anterior cruciate ligament (ACL) and their relationship to articular cartilage degeneration are not well characterized. The aim of this study was to investigate the types and temporal sequence of aging-related ACL changes and establish the correlation with cartilage lesion patterns at all stages of OA development in human knee joints without prior joint trauma.
Methods
Human knee joints (n=120; 65 donors; age 23-92) were obtained at autopsy and ACL and cartilage were graded macroscopically and histologically. Inflammation surrounding the ACL was assessed separately.
Results
Histological ACL substance scores and ligament sheath inflammation scores increased with aging. Collagen fiber disorganization was the earliest and most prevalent change. The severity of mucoid degeneration and chondroid metaplasia in the ACL increased with development of cartilage lesions. A correlation between ACL and cartilage degeneration was observed, especially in the medial compartment of the knee joint.
Conclusion
ACL degeneration is highly prevalent in knees with cartilage defects, and may even precede cartilage changes. Hence, ACL deficiencies may not only be important in post-traumatic OA, but also a feature associated with knee OA pathogenesis in general.
Keywords: anterior cruciate ligament, aging, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) results from different risk factors that include genetic predisposition, malalignment, joint trauma, obesity and aging (1-4). The disease process may initiate in one particular joint structure but eventually manifests in all joint tissues (5-7). Among the different joint tissues, articular cartilage appears to be most susceptible to mechanical and aging-related damage (8). Traumatic lesions to menisci and ligaments are well-known risk factors for OA (9,10). The anterior cruciate ligament (ACL) is essential for knee kinematics especially in rotation and functions as anterior/posterior stabilizer (11,12). In the setting of ACL deficiency, the articular cartilage as well as the menisci in the medial tibio-femoral compartment are more susceptible to arthritic change than the lateral compartment (13).
Approximately 23% of painful knee OA patients have ACL deficiency and 48% of these patients do not have a prior history of ligament injury (6). A correlation between the radiologic OA grade and the histological grade of ACL degeneration has been reported in end-stage OA (14). MRI is an important tool in assessing articular cartilage, menisci and subchondral bone, but its application to ACL is limited to measuring ACL diameter, and detecting partial or complete ruptures and mucoid degeneration (6,15-17). Histology remains the most sensitive method to analyze ACL (18). Histological changes in ACL are highly prevalent in knees with severe OA and include cystic changes, disorientation of collagen fibers and mucoid degeneration (19).
Although there is an apparent correlation of ACL and cartilage degeneration in end-stage OA, intrinsic aging-related changes in the ACL and their relationship with changes in cartilage at earlier stages of the disease process have not been analyzed in human joints. It is also not known whether the ACL degeneration observed in end stage OA is a consequence of cartilage destruction or the result of a primary lesion within the ACL that causes articular cartilage degeneration.
The purpose of this study was to examine ACL in a large number of human knee joints across the entire adult age spectrum to determine the type, frequency and severity of changes in ACL. In addition, we characterized the phenotype of histological changes and examined the effect of aging and OA on inflammation in the ACL. A detailed analysis of the relationship of specific changes in the ACL with the development of cartilage lesions was performed. This is the first systematic study of macroscopic and histological changes in the human ACL during aging and at all stages of idiopathic OA.
MATERIALS AND METHODS
Tissue procurement
Human knee joints were obtained from three tissue banks with approval of the Scripps Human Subjects Committee. Joints were processed within 72 hours post-mortem. In this study, 120 human knee joints were analyzed. There were 30 male and 35 female donors, with a mean age of 66.1 years (range, 23-92 years) (Supplementary Table 1). From 55 of 65 donors we obtained both knees, while from 10 of 65 donors we obtained only one knee. The causes of death and co-morbidities are similar to the general population in the United States (20).
Macroscopic and histological analysis of ACL
ACL degeneration was assessed both macroscopically and histologically as described previously (14,18,19,21). The ACL was resected at the insertion sites on the femur and tibia. The macroscopic appearance was evaluated and classified in 3 stages: normal, abnormal (thinner than normal and sclerotic), or ruptured (complete disappearance of the ligament, or persistence of only a few fibers) (21).
For histology, the samples were immediately fixed in Z-Fix (Anatech, Battle Creek, MI) for 48-72 hours. After fixation, each specimen was cut transversely at the proximal one third of ligament and longitudinally through the center of the ligament from proximal one third of the ACL substance and femur attachment site where ACL tears frequently occur (22,23) (Supplementary Figure 1A).
After cutting, dehydration with alcohol, Pro-Par Clearant (Anatech) and infiltration with paraffin (Paraplast, McCormick) samples were embedded in paraffin. 4 μm sections were cut and stained with hematoxylin and eosin (H&E).
The ACL sections were graded histologically using a modification of previous scoring systems (14). The following categories were examined and scored for each ligament: 1) inflammation in the ACL substance, 2) mucoid degeneration, 3) chondroid metaplasia, 4) cystic changes, 5) orientation of collagen fibers (Supplementary Figure 1B). Each category was scored for the severity of the degenerative change on both transverse and longitudinal sections and a single score was assigned for each ACL. The ACL histological changes were scored as follows: 0: no changes, 0.5: minimal changes, 1: mild changes, 2: moderate changes, 3: severe changes (Supplementary Figure 1C). The highest summed score of ligament degeneration (total ACL score) was 15 if all 5 histological categories were scored severe. Severity of ACL degeneration was graded as normal, mild, moderate, and severe after considering the total ACL substance score. Severity of degeneration of ACL was considered normal if the total ACL score was 0, mild if it was 0.5 to 5, moderate if it was 5.5 to 10 and severe if it was higher than 10. If orientation of collagen fibers, mucoid degeneration and/or cystic changes were scored 3, or ACL was completely degraded and only a few remnants were detectable macroscopically, it was considered severe.
The presence or absence of calcium deposition was also recorded. Selected sections with or without calcium deposition on H&E stains were stained with Von Kossa and Alizarin Red S. Although inflammation is considered in the total ACL score, a more detailed assessment was performed. Inflammation surrounding the ACL was analyzed separately as the extent of synovitis in the ligament sheath by using a histological grading system for synovitis (24). The following categories were examined and scored for inflammation in each ligament sheath: 1) hyperplasia / enlargement of synovial lining cell layer, 2) inflammatory cell infiltration, 3) activation of synovial stroma / pannus formation. The histological changes of ligament sheath were scored as follows: 0: absent, 1: slight, 2: moderate, 3: strong. The highest summed score of the ligament sheath (ligament sheath inflammation score) was 9 if all categories were scored as strong.
Morphologic analysis of articular cartilage
All cartilage surfaces (femoral condyles, trochlea and tibial plateaus) were graded macroscopically using a modified Outerbridge scoring system (25). We established a more detailed scoring method based on the International Cartilage Repair Society (ICRS) knee map (26) by dividing the cartilage into several compartments: 3 areas in the trochlea, and 9 areas each in each femoral condyle and tibial plateau. Each of these 39 areas was scored individually, and total compartment and total knee scores were calculated. For each area a score ranging from 1 – 4 was assigned as follows: 1: intact surface, 2: fibrillation, 3: fissuring, 4: erosion. The total knee cartilage scores range from 39 (normal) to 156 (maximum severity) (Supplementary Figure 2). The scoring system used in this study was reliable with 95% limits of agreement ranging from −4.548 to 4.615.
The total knee cartilage scores were translated into grades 0 – IV (grade 0: normal when total score was 39, grade I: minimal change when total score was 40 to 58, grade II: mild change when total score was 59 to 78, grade III: moderate change when total score was 79 to 97, and grade IV: severe change when total score was higher than 98.
Statistical analysis
Summary statistics are reported as mean ± SD (standard deviation). Differences among groups were determined by one-way analysis of variance (ANOVA) with continuous variables, Kruskal-Wallis (non-parametric one-way analysis of variance) procedures with ordinal variables [e.g., scales], and chi-square procedures with dichotomous variables. [Note that two-sample Kruskal-Wallis tests are equivalent to Mann-Whitney tests.] Pairwise group comparisons subsequent to the overall ANOVA tests were undertaken with Tukey’s studentized range procedure following parametric ANOVA, and with the Dwass-Steel-Critchlow-Fligner (DSCF) procedure following Kruskal-Wallis. Spearman’s nonparametric correlation coefficient ρ was computed when comparing continuous and ordinal variables, or two ordinal variables. 95% confidence intervals (CI) for the Spearman rank correlation coefficients were determined from percentiles of 1000 bootstrap samples. Calculations were undertaken in Systat 12 (Systat Inc., Chicago, IL) and SPSS 15 (SPSS Inc., Chicago, IL). P values of less than 0.05 were considered statistically significant.
RESULTS
Macroscopic assessment of ACL
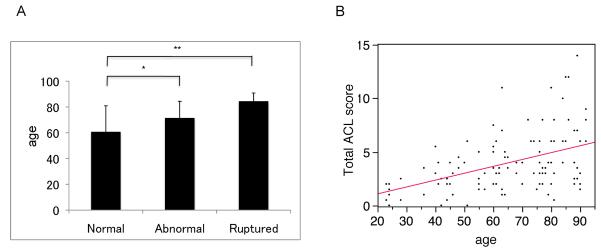
In this study, 120 knees from 65 individuals without joint trauma history were analyzed. Macroscopic assessment showed that ACL in 68 knees were normal, 40 were abnormal and 12 were ruptured. In 3 knees the ACL was completely degraded and only a few remnants were detectable. Overall, ages of donors differed significantly across the three categories of ACL status (F3,117 = 13.37, p<0.0001). In pairwise comparisons, the mean age of donors with normal ACL (59.7±20.4) was significantly less than that of donors with abnormal ACL (71.5±12.9; p=0.003) and that of donors with ruptured ACL (84.5±6.4; p<0.001). Differences in mean age of donors with abnormal ACL compared to ruptured ACL were of borderline significance (p=0.06). (Figure 1A).
Figure 1.
A. ACL macroscopic scores versus age. The mean age of donors with normal ACL was significantly less than that of donors with abnormal ACL and that of donors with ruptured ACL. Each ACL was treated as a separate unit for analysis. Values are the mean + SD. * = p< 0.05, ** = p<0.01. B. ACL microscopic scores versus age. The total ACL score, which is the sum of all histological changes within the ACL increased with donor age. Each data point represents one knee.
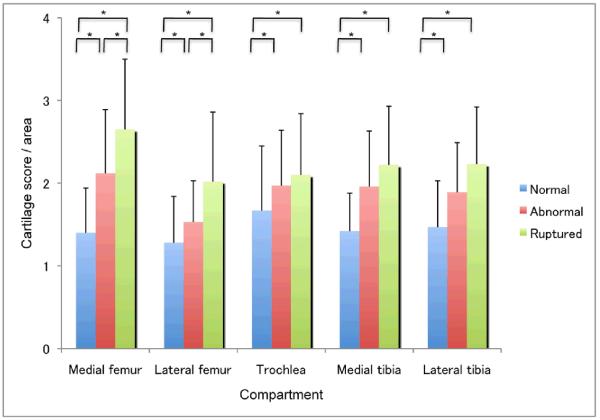
Overall, total knee cartilage scores differed significantly across the three categories of ACL status (X22 = 39.7, p<0.0001). Mean scores ± SD per group were: knees with normal ACL, 54.8±18.9; knees with abnormal ACL, 73.3±18.6; knees with ruptured ACL, 89.6±24.4. All pairwise comparisons were significant, via DSCF, at p<0.001. ACL rupture was present in 7 of 29 (24.1%) of knees with moderate and severe cartilage lesions and this was significantly less common (5 of 91, 5.5%) in knees with normal, minimal and mild cartilage lesions (P<0.01 by chi-square analysis) (Supplementary Table 2). Macroscopic ACL lesions correlated with cartilage degeneration, especially in the medial femoral condyle (Figure 2).
Figure 2. Macroscopic changes in ACL versus cartilage changes.
Macroscopic changes in ACL correlated with cartilage degeneration, especially in the medial femoral condyle, compared to the other compartments of the knee joint. Within each compartment, all pairwise comparisons of normalized cartilage scores among the three ACL categories were statistically significant [at p<0.05 from the nonparametric Dwass-Steel-Critchlow-Fligner procedure], with the exceptions of abnormal vs. ruptured, in the trochlea, medial tibia, and lateral tibia. Values are the mean + SD. * = p<0.05.
These results from macroscopic analysis show a high prevalence of ACL lesions, which increased with donor age. Interestingly, ACL lesions (abnormal and ruptured) were also observed in 13 of 60 joints (16.7%) with normal or only minimally degenerated cartilage.
ACL histology and cartilage scores
The total ACL score, which is the sum of all histological changes within the ACL increased with donor age (Spearman’s ρ =0.42, 95% CI 0.25 to 0.57) (Figure 1B). Histological abnormalities in the ACL (mild, moderate and severe) were detected in 65 of 68 (95.6%) of macroscopically normal ACL.
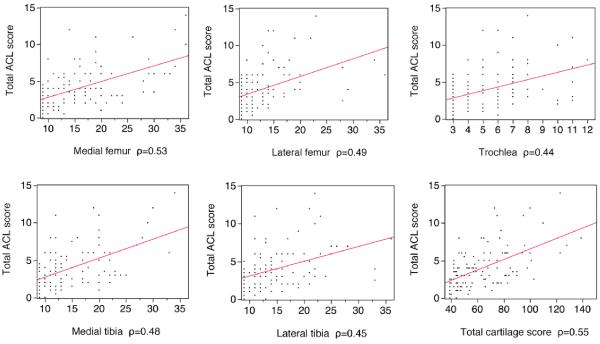
We investigated the correlation between histological changes in ACL and cartilage lesion patterns. The total ACL score correlated significantly with the total knee cartilage scores, most strongly with the medial compartment cartilage scores (Figure 3).
Figure 3. Histological changes in ACL versus cartilage changes.
The relationship of total ACL score versus cartilage scores in knee compartments was analyzed. ACL degeneration correlated with cartilage degeneration, especially in the medial compartments, compared to the lateral compartments of the knee joint.
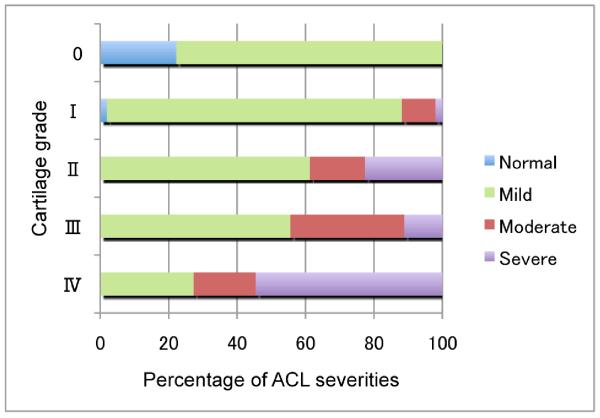
To test if histological ACL changes can occur before cartilage degeneration, we investigated the correlations between each histological ACL grade and each cartilage grade (Figure 4). There were no normal ACL in knees with Grade II, III or IV cartilage. This suggests that ACL degeneration is involved already at early stages of cartilage degeneration. Interestingly, there were also degenerated ACL in Grade 0 and I (normal, minimal cartilage degeneration).
Figure 4. Severity of ACL changes versus cartilage degeneration.
There were no normal ACL in knees with Grade II, III and IV cartilage (mild, moderate and severe cartilage degeneration), while there were also degenerated ACL in Grade 0 and I (normal and minimal cartilage degeneration).
Characterization of specific histological ACL abnormalities
Scores for the individual histological parameters are shown in Table 1.
Table 1.
Scores for specific ACL histological parameters.
| 0 | 0.5 | 1 | 2 | 3 | |
|---|---|---|---|---|---|
| Inflammation | 61(52.1) | 1(0.9) | 25(21.4) | 19(16.2) | 11(9.4) |
| Mucoid degeneration | 44(37.6) | 12(10.3) | 38(32.5) | 16(13.7) | 7(6.0) |
| chondrid metaplasia | 76(64.9) | 2(1.7) | 17(14.5) | 18(15.4) | 3(2.6) |
| Cystic changes | 74(63.2) | 3(2.6) | 20(17.1) | 16(13.7) | 4(3.4) |
| Orientation of collagen fibers | 12(10.3) | 22(18.8) | 36(30.7) | 40(34.1) | 7(6.0) |
| Values represent the number of cases and (%). | |||||
ACL inflammation
ACL inflammation manifests as leukocyte infiltration and neovascularization in the ligament sheath and within the ACL substance (Supplementary Figure 3). There was no significant correlation between ACL substance inflammation score and donor age (Spearman’s ρ =0.16, 95% CI −0.04 to 0.34). There was a positive correlation between ligament sheath inflammation score and donor age (Spearman’s ρ =0.41, 95% CI 0.24 to 0.54). There was a positive correlation between ligament sheath inflammation score and ACL substance inflammation score (Spearman’s ρ =0.53, 95% CI 0.38 to 0.66). In 52 of 78 (74.3%) ACL with abnormal ligament sheath, inflammation within the ACL substance was observed. Conversely only 4 of 39 (10.3%) ACL without inflammation of the ligament sheath, had inflammation within the ACL substance. This relationship between inflammation in the ACL substance and in ligament sheath suggests that the ligament sheath is more predisposed to the inflammation than the ACL substance and the inflammatory processes in ACL substance may follow inflammation of the ligament sheath. In a small subset of individuals, there was leukocyte infiltration in the ligament substance without inflammation of the ligament sheath.
Relationship between ACL inflammation and ACL degeneration
There was a positive correlation between ACL substance inflammation score and total ACL score (Spearman’s ρ =0.58, 95% CI 0.46 to 0.69). In addition, there was also a positive correlation between ligament sheath inflammation score and total ACL score (Spearman’s ρ =0.50, 95% CI 0.36 to 0.64).
Relationship between ACL inflammation and cartilage degeneration
There was a weak correlation between ACL substance inflammation score and total knee cartilage score (Spearman’s ρ =0.29, 95% CI 0.11 to 0.45). There was a positive correlation between ACL sheath inflammation scores and total knee cartilage scores (Spearman’s ρ =0.51, 95% CI 0.35 to 0.65).
Thus, changes in the ligament sheath correlate more strongly with cartilage damage than changes within the ligament.
Mucoid degeneration
Mucoid degeneration reflects degradation of collagen and deposition of new glycosaminoglycans. Mucoid degeneration was observed in 73 of 117 ACL (62.3%) (Supplementary Figure 4). There was no significant correlation between mucoid degeneration score and donor age (Spearman’s ρ =0.18, 95% CI −0.01 to 0.34). Even in 4 of 9 ACL from young (<45 years old) donors without cartilage degeneration there was mucoid degeneration. There was a positive correlation between mucoid degeneration scores and total ACL scores (Spearman’s ρ =0.59, 95% CI 0.44 to 0.72). There was no significant correlation between mucoid degeneration and inflammation scores of ACL substance (Spearman’s ρ =0.12, 95% CI −0.07 to 0.30). There was also a weak correlation between mucoid degeneration scores and ligament sheath inflammation scores (Spearman’s ρ =0.20, 95% CI 0.02 to 0.39). There was a weak correlation between mucoid degeneration scores and total knee cartilage scores (Spearman’s ρ =0.22, 95% CI 0.22 to 0.39).
Thus, mucoid degeneration is frequent, occurs during the aging process and even in relatively young donors.
Chondroid metaplasia
Chondroid metaplasia, which represents a shift in ligament cell phenotype towards a more chondrocytic cell morphology, was observed in 40 of 117 ACL (34.2%) (Supplementary Figure 5). There was a positive correlation between chondroid metaplasia scores and total ACL scores (Spearman’s ρ =0.56, 95% CI 0.41 to 0.68). There was also a positive correlation between chondroid metaplasia scores and mucoid degeneration scores (Spearman’s ρ =0.62, 95% CI 0.47 to 0.73). There was no significant correlation between chondroid metaplasia scores and total knee cartilage scores (Spearman’s ρ =0.19, 95% CI −0.02 to 0.37).
There was no significant correlation between chondroid metaplasia scores and inflammation scores of ACL substance or ligament sheath scores (Spearman’s ρ =0.09, 95% CI −0.09 to 0.27 for the former, Spearman’s ρ =0.10, 95% CI −0.09 to 0.29 for the latter).
These findings suggest that chondroid metaplasia represents an ACL intrinsic process that is associated with mucoid degeneration and not with inflammation.
Cystic changes
Cystic changes, which represent areas within fascicles that are devoid of extracellular matrix were observed in 43 of 117 ACL (36.7%) (Supplementary Figure 6). There was a weak correlation between cystic change scores and donor age (Spearman’s ρ =0.28, 95% CI 0.09 to 0.44). There was a positive correlation between cystic change scores and total ACL scores (Spearman’s ρ =0.45, 95% CI 0.29 to 0.58). There was a weak correlation between cystic change scores and total knee cartilage scores (Spearman’s ρ =0.26, 95% CI 0.07 to 0.43). There was no significant correlation between cystic change scores and inflammation scores of ACL substance (Spearman’s ρ =0.07, 95% CI −0.12 to 0.26). There was no significant correlation between cystic change scores and ligament sheath scores (Spearman’s ρ =0.18, 95% CI −0.02 to 0.36). These findings suggest that cystic changes are a relatively late event in ACL degeneration and are independent of inflammation.
Calcium deposition
Calcium deposition was observed in 11 of 117 ACL (9.4%) (Supplementary Figure 7). All of these ACL were from older (>70 years old) donors that also had degenerated cartilage. Calcium deposition was observed in 3 of 7 ACL (27.3%) from knees with calcium deposition in cartilage. However, calcium deposition was observed in 8 of 110 ACL (7.3%) from knees without calcium deposition in cartilage (P<0.01 by chi-square analysis). ACL with calcium deposition showed higher total knee cartilage scores (83.5±16.4) than those without calcium deposition (61.8±21.6; X21 = 10.82, p=0.001). Differences between ACL substance inflammation scores with calcium deposition (0.8±1.1) and without calcium deposition (0.8±1.0) were not statistically significant (X21 = 0.005, p=0.94).
These results suggest that calcium deposition in ACL is the latest event in ACL degeneration. Of interest is the observation, that in some cases ACL calcification can occur without cartilage calcification.
Collagen fiber organization
Disorganization of collagen fibers was observed in 105 of 117 ACL (89.7%) (Supplementary Figure 8). There were positive correlations between the collagen fiber orientation scores and donor age (Spearman’s ρ =0.52, 95% CI 0.36 to 0.66), total ACL scores (Spearman’s ρ =0.57, 95% CI 0.42 to 0.70) and total knee cartilage scores (Spearman’s ρ =0.54, 95% CI 0.37 to 0.69). There were weak correlations between the collagen fiber orientation scores and inflammation scores of ACL substance (Spearman’s ρ =0.21, 95% CI 0.02 to 0.38) and ligament sheath (Spearman’s ρ =0.32, 95% CI 0.14 to 0.49).
These observations indicate that collagen fiber disorganization is highly prevalent and can occur without other histological changes.
Earliest detectable aging-related changes in the ACL
To detect the earliest change in ACL, we investigated 9 ACL from young (<45 years old) donors with normal grade 0 cartilage (Supplementary Figure 9). Disorientation of collagen fibers was observed in 6 of these 9 ACL. Mucoid degeneration was observed in 4 of these 9 ACL. Inflammation of ACL substance, inflammation of ligament sheath and cystic changes were observed in 1 of 9 ACL, respectively. Chondroid metaplasia was not observed in ACL from young normal knees.
These results suggest that the disorientation of collagen fibers and mucoid degeneration are the earliest detectable aging-related changes in the ACL and can occur in the absence of cartilage degeneration.
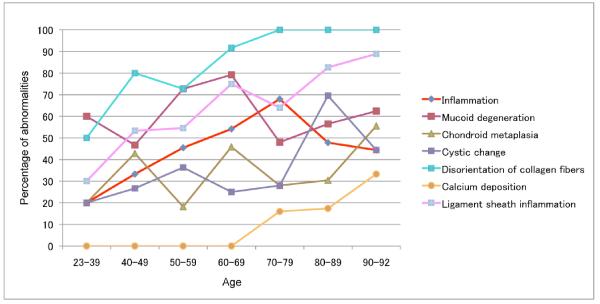
Furthermore, we analyzed the prevalence of abnormalities in each histopathological category versus age to reveal a temporal sequence of changes (Figure 5). The earliest and most prevalent change is collagen fiber disorganization. Calcification is the latest and least prevalent change. A relatively high percentage of young donors already have mucoid degeneration, which does not increase with age. Inflammation, chondroid metaplasia and cystic changes increase with age.
Figure 5. Aging-related prevalence of histopathological abnormalities in ACL.
For each histological abnormality, the percentage per age decade of ACL that had scores indicating pathologic change was calculated. The earliest change and most prevalent change is collagen fiber disorganization. Calcium deposition is the latest and least prevalent change. A relatively high percentage of young donors already have mucoid degeneration, which does not increase with age. Inflammation, chondroid metaplasia, cystic changes and ligament sheath inflammation also increase with age.
DISCUSSION
ACL degeneration: prevalence, correlation with age and cartilage degeneration
The present study is a comprehensive analysis of all major histological parameters of ACL pathology, using 120 ACL from 65 human knee donors across the adult age spectrum. The donor population was screened for history of joint injury. While major injuries were excluded it is possible that some donors had minor injuries that contributed to the observed changes in the ACL.
In this study, the rate of ACL rupture was 10.0%. It was smaller than previously described in the literature (6,27). However, in knees with moderate and severe cartilage, the rate of ACL rupture in this study was 24.1%. This rate was consistent with previous studies that examined the rate of ACL rupture from symptomatic OA patients (6,27).
Histologically, aging-related changes in ACL and their relationship with changes in cartilage at earlier stages of the disease process have not been analyzed in human joints. In the present study, we found degenerated ACL in knees without cartilage degeneration. Also, we observed knees with minimal cartilage degeneration that have moderate to severe ACL degeneration. These findings suggest that ACL degeneration can be initiated before or progresses more rapidly than cartilage degeneration, at least in a subpopulation of individuals.
A close correlation was observed between the presence of ACL degeneration at all stages of cartilage destruction. This may suggest that ligament extrinsic changes trigger or accelerate ACL degeneration. For example, femoral intercondylar notch stenosis and notch osteophytes can lead to ACL destruction. A smaller notch width has been found in ACL deficient OA knees (28,29) and causes impingement of the ACL (30,31). The strongest correlation between with ACL and cartilage degeneration was found at the medial femoral condyle. Taken together, medial femoral OA with osteophyte growth in the notch area might contribute to the progression of ACL degeneration.
Inflammation
We investigated inflammation as a potential mechanism of ACL degeneration. ACL substance inflammation scores from knees with cartilage damage were significantly higher than those from knees with normal cartilage. However, there was only a weak correlation between ACL substance inflammation score and total knee cartilage score. Furthermore, in a subset of individuals, inflammation in the ACL substance was observed in the absence of cartilage degradation.
In normal ACL, inflammatory cells were found mainly in perivascular areas in the ligament sheath. There were few inflammatory cells between collagen fibers within ACL substance. In contrast, ACL with inflammation showed increased inflammatory cell infiltrates not only in perivascular regions but also between collagen fibers within ACL substance, regardless of the presence of cartilage degeneration. These results indicate a process that is primarily driven by ACL intrinsic mechanisms.
Interestingly, we observed only a modest correlation between ACL inflammation and total ACL scores. These results suggest that mechanisms that are independent of ACL inflammation contribute to ACL degeneration and ACL inflammation is not an initial trigger of ACL degeneration. Once inflammation is initiated, its interplay with mechanical forces may accelerate ACL degeneration (10).
ECM changes
The earliest and most prevalent change in ACL extracellular matrix was disorganization of collagen fibers. In this study, there was a positive correlation between collagen fiber orientation scores and donor age (Spearman’s ρ =0.52, 95% CI 0.36 to 0.66). Also, disorientation of collagen fibers was observed in ACL from young donor without cartilage degeneration or inflammation. These results suggest that disorganization of collagen fibers might be induced by repetitive mechanical stress. An aging-related decrease in the diameter of the collagen fibrils and a corresponding increase in the concentration of small fibrils have been described for the human ACL (32).
Mucoid degeneration reflects disorganized collagen fibers, and the presence of stainable mucoid matrix (33-35) and is rare, with a reported prevalence of 1 – 5% in studies using MRI (16,17,36). However, we observed a high prevalence (62.2%) of mucoid degeneration. The severity of mucoid degeneration increased with the development of cartilage degeneration although mucoid degeneration was also observed in ACL from young donors with normal cartilage.
Cystic changes represent areas within fascicles that are devoid of extracellular matrix and indicate ACL degeneration (14,19,37). We noted cystic changes in ACL from knees with severe cartilage degeneration. There was no correlation between cystic change score and inflammation score of ACL substance. These findings suggest that cystic changes are a relatively late event in ACL degeneration and independent from inflammation. Calcium deposition within ACL is diagnosed in the presence of slightly basophilic material, compatible with calcium pyrophosphate dihydrate (CPPD) crystals (38). CPPD crystal deposition occurs with a frequency of 0.1% in adults and increases with age. Higher frequency of crystal deposition is found in locations that are in contact with synovial fluid such as the menisci and hyaline cartilage (38). In this study, calcium deposition was observed in 11 of 117 ACL (9.4%) and all of these ACL were from older (>70 years old) donors that also had degenerated cartilage.
ACL cellular changes
Chondroid metaplasia is a well-known feature of degenerated ACL (14,19). In normal ACL, fusiform and ovoid cells are located in the proximal one quarter of the ACL, close to the attachment site on the femur, and a spheroid cell zone occupies the distal three quarters of the ACL (39,40). In this study, chondroid metaplasia was observed in knees with cartilage degeneration but not in knees with normal cartilage. Chondroid metaplasia represents an ACL intrinsic process that is associated with mucoid degeneration and independent of inflammation. Mechanisms for the change in ligament cell phenotype may include mechanical and biochemical stimuli. Compression of tendons over bone can increase GAG content and the proportion of round cells (41). Biochemical mediators, such as cytokines, growth and differentiation factors, that are part of the abnormal synovial fluid composition in arthritic joints may also lead to the alteration in ligament cell phenotype.
The present study provides a detailed analysis of changes in ACL cells and extracellular matrix. Based on all parameters examined on the present ACL sample set, we propose a temporal sequence of histopathological changes. The earliest changes are collagen fiber disorganization and mucoid degeneration. Inflammation, chondroid metaplasia and cystic changes increase with the presence of cartilage degradation. Calcium deposition is a late and rare event. The pathogenic process involves a marked phenotypic shift in ligament cells with an abnormal chondroid differentiation. It will be of interest to identify stimuli that may be generated in ACL itself or originate from cells in synovium, synovial fluid or cartilage. Notably, abnormal cellular differentiation is also observed in OA-affected menisci and cartilage. If these changes in the different tissues were driven by similar mechanisms and molecules, this would represent a powerful therapeutic target.
CONCLUSION
The relatively large sample set available in this study for histological analysis of ACL indicates a general association between ACL degeneration and aging and between degeneration of ACL and cartilage. However, the present results also suggest subpopulations where ACL changes may precede or initiate cartilage damage, whereas in other subsets ACL changes occur simultaneous or subsequent to cartilage lesions. Subsets can also be differentiated based on the degree of inflammation and on the type of cellular change within the ACL.
Supplementary Material
ACKNOWLEDGMENTS
Lilo Creighton, Margaret Chadwell and Anita San Soucie are gratefully acknowledged for their support for the histology of the specimens.
The study was supported by NIH grant AG007996, the Sam and Rose Stein Endowment Fund and by Postdoctoral Fellowships from the Arthritis Foundation (SO) and the Arthritis National Research Foundation (SM).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the manuscript critically for important content, and all authors approved the final version. Dr. Lotz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Hasegawa, Otsuki, Kinoshita, Koziol, D’Lima, Lotz
Acquisition of data. Hasegawa, Otsuki, Pauli, Patil, Steklov
Analysis and interpretation of data. Hasegawa, Otsuki, Pauli, Miyaki, Kinoshita, Koziol, D’Lima, Lotz.
REFERENCES
- 1.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;17(133):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheumatol. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol. 2005;32:2192–9. [PubMed] [Google Scholar]
- 4.Cooper C, Snow S, McAlindon TE, Kellingray S, Stuart B, Coggon D. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Bennett LD, Buckland-Wright JC. Meniscal and articular cartilage changes in knee osteoarthritis: a cross-sectional double-contrast macroradiographic study. Rheumatology (Oxford) 2002;41:917–23. doi: 10.1093/rheumatology/41.8.917. [DOI] [PubMed] [Google Scholar]
- 6.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52:794–9. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 7.Bergin D, Keogh C, O’Connell M, Rowe D, Shah B, Zoga A. Atraumatic medial collateral ligament oedema in medial compartment knee osteoarthritis. Skeletal Radiol. 2002;31:14–8. doi: 10.1007/s002560100418. [DOI] [PubMed] [Google Scholar]
- 8.Nichols EH, Richardson FL. Arthritis deformans. J Med Res. 1909;21:149–U54. [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 10.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament Injury, Reconstruction and Osteoarthritis. Curr Opin Orthop. 2005;16:354–62. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo SL, Livesay GA, Engle C. Biomechanics of the human anterior cruciate ligament. Muscle stabilization and ACL reconstruction. Orthop Rev. 1992;21:935–41. [PubMed] [Google Scholar]
- 12.Fleming BC. Biomechanics of the anterior cruciate ligament. J Orthop Sports Phys Ther. 2003;33:A13–5. [PubMed] [Google Scholar]
- 13.Louboutin H, Debarge R, Richou J, Selmi TA, Donell ST, Neyret P. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee. 2009;16:239–44. doi: 10.1016/j.knee.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Mullaji AB, Marawar SV, Simha M, Jindal G. Cruciate ligaments in arthritic knees: a histologic study with radiologic correlation. J Arthroplasty. 2008;23:567–72. doi: 10.1016/j.arth.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior cruciate ligament-injured subjects have smaller anterior cruciate ligaments than matched controls: a magnetic resonance imaging study. Am J Sports Med. 2009;37:1282–7. doi: 10.1177/0363546509332256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergin D, Morrison WB, Carrino JA, Nallamshetty SN, Bartolozzi AR. Anterior cruciate ligament ganglia and mucoid degeneration: coexistence and clinical correlation. AJR Am J Roentgenol. 2004;182:1283–7. doi: 10.2214/ajr.182.5.1821283. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes JL, Viana SL, Mendonca JL, Freitas FM, Bezerra AS, Lima GA. Mucoid degeneration of the anterior cruciate ligament: magnetic resonance imaging findings of an underdiagnosed entity. Acta Radiol. 2008;49:75–9. doi: 10.1080/02841850701660497. [DOI] [PubMed] [Google Scholar]
- 18.Trompeter AJ, Gill K, Appleton MA, Palmer SH. Predicting anterior cruciate ligament integrity in patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2009;17:595–9. doi: 10.1007/s00167-008-0701-0. [DOI] [PubMed] [Google Scholar]
- 19.Cushner FD, La Rosa DF, igorita VJ, Scuderi GR, Scott WN, Insall JN. A quantitative histologic comparison: ACL degeneration in the osteoarthritic knee. J Arthroplasty. 2003;18:687–92. doi: 10.1016/s0883-5403(03)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Minino A, Xu J, Kochanek K. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010:1–72. [PubMed] [Google Scholar]
- 21.Allain J, Goutallier D, Voisin MC. Macroscopic and histological assessments of the cruciate ligaments in arthrosis of the knee. Acta Orthop Scand. 2001;72:266–9. doi: 10.1080/00016470152846592. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior cruciate ligament. As determined by clinical and morphological studies. J Bone Joint Surg Am. 1974;56:223–35. [PubMed] [Google Scholar]
- 23.Wang IE, Mitroo S, Chen FH, Lu HH, Doty SB. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745–55. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 24.Krenn V, Morawietz L, Haupl T, Neidel J, Petersen I, Konig A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198:317–25. doi: 10.1078/0344-0338-5710261. [DOI] [PubMed] [Google Scholar]
- 25.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–7. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 26.ICRS Cartilage Evaluation Package. 2000 http://www.cartilage.org/Evaluation_Package/ICRS_Evaluation.pdf.
- 27.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226:373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 28.Hernigou P, Garabedian JM. Intercondylar notch width and the risk for anterior cruciate ligament rupture in the osteoarthritic knee: evaluation by plain radiography and CT scan. Knee. 2002;9:313–6. doi: 10.1016/s0968-0160(02)00053-4. [DOI] [PubMed] [Google Scholar]
- 29.Leon HO, Blanco CE, Guthrie TB, Martinez OJ. Intercondylar notch stenosis in degenerative arthritis of the knee. Arthroscopy. 2005;21:294–302. doi: 10.1016/j.arthro.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Lee GC, Cushner FD, Vigoritta V, Scuderi GR, Insall JN, Scott WN. Evaluation of the anterior cruciate ligament integrity and degenerative arthritic patterns in patients undergoing total knee arthroplasty. J Arthroplasty. 2005;20:59–65. doi: 10.1016/j.arth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Wada M, Tatsuo H, Baba H, Asamoto K, Nojyo Y. Femoral intercondylar notch measurements in osteoarthritic knees. Rheumatology (Oxford) 1999;38:554–8. doi: 10.1093/rheumatology/38.6.554. [DOI] [PubMed] [Google Scholar]
- 32.Strocchi R, De Pasquale V, Facchini A, Raspanti M, Zaffagnini S, Marcacci M. Age-related changes in human anterior cruciate ligament (ACL) collagen fibrils. Ital J Anat Embryol. 1996;101:213–20. [PubMed] [Google Scholar]
- 33.Wright T, Yoon C, Schmit BP. Shoulder MRI refinements: differentiation of rotator cuff tear from artifacts and tendonosis, and reassessment of normal findings. Semin Ultrasound CT MR. 2001;22:383–95. doi: 10.1016/s0887-2171(01)90028-9. [DOI] [PubMed] [Google Scholar]
- 34.Buck FM, Grehn H, Hilbe M, Pfirrmann CW, Manzanell S, Hodler J. Degeneration of the long biceps tendon: comparison of MRI with gross anatomy and histology. AJR Am J Roentgenol. 2009;193:1367–75. doi: 10.2214/AJR.09.2738. [DOI] [PubMed] [Google Scholar]
- 35.Nelissen RG, Hogendoorn PC. Retain or sacrifice the posterior cruciate ligament in total knee arthroplasty? A histopathological study of the cruciate ligament in osteoarthritic and rheumatoid disease. J Clin Pathol. 2001;54:381–4. doi: 10.1136/jcp.54.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salvati F, Rossi F, Limbucci N, Pistoia ML, Barile A, Masciocchi C. Mucoid metaplastic-degeneration of anterior cruciate ligament. J Sports Med Phys Fitness. 2008;48:483–7. [PubMed] [Google Scholar]
- 37.Stubbs G, Dahlstrom J, Papantoniou P, Cherian M. Correlation between macroscopic changes of arthrosis and the posterior cruciate ligament histology in the osteoarthritic knee. ANZ J Surg. 2005;75:1036–40. doi: 10.1111/j.1445-2197.2005.03610.x. [DOI] [PubMed] [Google Scholar]
- 38.Abreu M, Johnson K, Chung CB, De Lima JE, Jr., Trudell D, Terkeltaub R. Calcification in calcium pyrophosphate dihydrate (CPPD) crystalline deposits in the knee: anatomic, radiographic, MR imaging, and histologic study in cadavers. Skeletal Radiol. 2004;33:392–8. doi: 10.1007/s00256-004-0767-9. [DOI] [PubMed] [Google Scholar]
- 39.Murray MM, Spector M. Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: the presence of alpha-smooth muscle actin-positive cells. J Orthop Res. 1999;17:18–27. doi: 10.1002/jor.1100170105. [DOI] [PubMed] [Google Scholar]
- 40.Murray MM, Weiler A, Spindler KP. Interspecies variation in the fibroblast distribution of the anterior cruciate ligament. Am J Sports Med. 2004;32:1484–91. doi: 10.1177/0363546504263700. [DOI] [PubMed] [Google Scholar]
- 41.Gillard GC, Reilly HC, Bell-Booth PG, Flint MH. The influence of mechanical forces on the glycosaminoglycan content of the rabbit flexor digitorum profundus tendon. Connect Tissue Res. 1979;7:37–46. doi: 10.3109/03008207909152351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.