Abstract
Angiotensin converting enzyme (ACE) is composed of the N- and C-terminal catalytic domains. To study the role of the ACE domains in the inflammatory response, N-KO and C-KO mice, models lacking one of the two ACE domains, were analyzed during angiotensin II-induced hypertension. At 2 weeks, N-KO mice have systolic blood pressures that averaged 173 ± 4.6 mm Hg, which is more than 25 mm Hg higher than the blood pressures observed in wild-type or C-KO mice (146 ± 3.2 and 147 ± 4.2 mm Hg). After 3 weeks, blood pressure differences between N-KO, C-KO and WT were even more pronounced. Macrophages from N-KO mice have increased expression of tumor necrosis factor α after stimulation with either lipopolysaccharide (about 4-fold) or angiotensin II (about 2-fold), as compared to C-KO or wild-type mice. Inhibition of the enzyme prolyl oligopeptidase, responsible for the formation of acetyl SDKP and other peptides, eliminated the blood pressure difference and the difference in tumor necrosis factor α expression between angiotensin II treated N-KO and wild-type mice. However, this appears independent of acetyl SDKP. These data establish significant differences in the inflammatory response as a function of ACE N- or C-domain catalytic activity. They also indicate a novel role of prolyl oligopeptidase in the cytokine regulation and in the blood pressure response to experimental hypertension.
Keywords: Angiotensin converting enzyme, hypertension, angiotensin II, prolyl oligopeptidase, acetyl SDKP
Introduction
Angiotensin converting enzyme (ACE) is a zinc-dependent carboxy dipeptidase that is responsible for the conversion of angiotensin I to angiotensin II. Although ACE is a single polypeptide chain, it is composed of two homologous and independent catalytic domains, often termed the N- and C-terminal domains.1,2 Each domain binds a zinc molecule which is required for catalysis. Despite genetic divergence, the two catalytic domains retain significant structural homology, particularly in areas critical for catalysis. However, in vitro analysis has demonstrated that the two catalytic sites differ in affinity and effectiveness for cleaving individual peptide substrates. For example, the C-terminal catalytic site has a higher Kcat for angiotensin I than the N-terminal domain.3 In contrast, the four amino acid ACE substrate acetyl-SerAspLysPro (AcSDKP) is cleaved almost exclusively by the N-terminal catalytic domain.4 It is released from its precursor, the actin-sequestering protein thymosin β4, by the action of prolyl oligopeptidase (POP), a serine peptidase.5 AcSDKP is elevated in N-KO mice, and in patients on ACE inhibitors. However, AcSDKP is only one of several peptides produced or degraded by POP; other POP substrates include angiotensin I, angiotensin II, bradykinin, bradykinin potentiating protein, substance P, oxytoxin and vasopressin.6
There is growing recognition that inflammation plays an important role in the development of hypertension.7,8 Recent reports have demonstrated that immune cells significantly modulate blood pressure changes and target organ damage, particularly in angiotensin II induced hypertension.9,10 Moreover, mice deficient in certain pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα) and IL-6, have a blunted hypertensive response to chronic angiotensin II infusion.11,12
To understand the functional role of the N- and C-domains of ACE in the cytokine and the inflammatory response secondary to the induction of experimental hypertension, we chronically infused angiotensin II into two lines of genetically engineered mice harboring point mutations that specifically inactivate either the N- or the C-terminal catalytic domain of ACE; these mice are termed N-KO and C-KO, respectively.13,14 In these mouse models, the genetic mutations have no effect on the tissue distribution or levels of ACE expression. Under basal conditions, N-KO and C-KO mice have normal and equivalent blood pressures and plasma levels of angiotensin II. Thus, the N-KO and C-KO mice permit investigation of ACE domain-specific functions independent of secondary effects of basal blood pressure changes, a typical bias introduced with either ACE inhibitors or the genetic elimination of all ACE expression.
Materials and Methods
The systolic blood pressure of N-KO, C-KO and WT mice was repeatedly measured by tail cuff plethysmography for 2 or 3 weeks during the infusion of 980 ng/kg/min of angiotensin II by osmotic minipump. For the blood pressure analysis, at least 21 animals per group were studied. For in vivo experiments in which POP was inhibited, N-KO and WT mice received 2 weeks of angiotensin II (980 ng/kg/min) by minipump and received daily IP injections of 10 mg/kg of S-17092. Blood pressure was measured as described above. At least 9 mice per group were studied.
For in vitro production of cytokines, peritoneal macrophages from N-KO, C-KO and WT mice were collected after thioglycollate injection. After purification, cells were cultured overnight with 1 mg/ml LPS. Supernatant concentration of TNFα, IL-12 and IL-10 were measured by ELISA. To measure in vivo production of TNFα, N-KO, C-KO and WT mice were injected IP with 45 mg/kg of LPS and sera levels of TNFα were measured by ELISA. For all figures showing cytokine production, the number of mice studied is indicated by individual data points.
To examine the role of AcSDKP in blood pressure control, WT or N-KO mice received 980 ng/kg/min of angiotensin II for 2 weeks. Groups of 10 WT mice received 0, 0.5, 1.5 or 3.0 mg/kg/day AcSDKP beginning 1 week before the start of angiotensin II infusion and continuing throughout the experiment. Blood pressure was repeatedly measured as described above. In a separate experiment, all N-KO mice received daily IP injections of 10 mg/kg of S-17092 in addition to angiotensin II. Five N-KO mice also received 1.5 mg/kg/day of AcSDKP by osmotic minipumps. Blood pressure was repeatedly measured. A full description of the Materials, Methods and Statistics is found in the online Data Supplement, see http://hyper.ahajournals.org.
Results
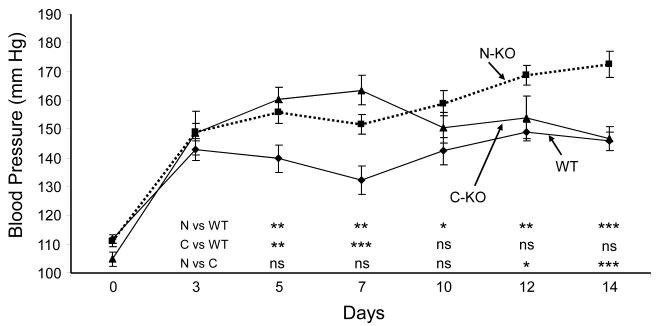
Under basal conditions, the systolic blood pressures of the N-KO, C-KO and WT mice averaged 111 ± 2 mm Hg, 105 ± 2 mm Hg, and 112 ± 1 mm Hg. The mice were implanted with osmotic minipumps that delivered 980 ng/kg/min of angiotensin II for 2 weeks. The blood pressure was determined 3,5,7,10,12 and 14 days after the start of angiotensin II infusion (Fig. 1).15 At day 5, the blood pressures of both N-KO and C-KO mice were significantly higher than WT mice. The blood pressures of the C-KO mice reached a peak at day 7 (164 ± 5 mm Hg) and then decreased so that by day 14 the pressures of these mice were virtually identical to WT. In contrast, the blood pressures of the N-KO mice remained significantly above the WT animals from day 5 onwards, and at day 14 averaged 173 ± 5 mm Hg (p<0.001 for N-KO vs. WT or C-KO). Thus, after a 2 week infusion of angiotensin II, N-KO mice had systolic blood pressures that averaged 26 and 27 mm Hg greater than C-KO or WT mice.
Figure 1.
Blood pressure response to angiotensin II infusion. On day 0, blood pressure was determined and the mice were implanted with an osmotic minipump delivering angiotensin II. Blood pressure was determined on the indicated days. n=30, 21 and 30 for N-KO, C-KO and WT, respectively. The significance of the data was examined by one-way ANOVA with Tukey correction and is indicated beginning on day 5 for N-KO vs WT (N vs WT), C-KO vs WT (C vs WT), and N-KO vs C-KO (N vs C). * p = 0.05 to 0.01, ** p = 0.01 to 0.001, and *** p < 0.001.
To investigate if the pressure differences between N-KO mice, C-KO and WT mice were sustained, smaller numbers of mice were infused with angiotensin II for 21 days. On days 19 and 21, the N-KO mice averaged 200 ± 5 and 190 ± 4 mm Hg (n=5) while WT averaged 147 ± 5 and 147 ± 4 mm Hg (n=4, p≤0.001). C-KO mice averaged 142 ± 8 and 127 ± 10 mm Hg (n=3, p<0.001). Thus, the sustained elevation of blood pressure in the N-KO group was maintained in the 3rd week of angiotensin II infusion.
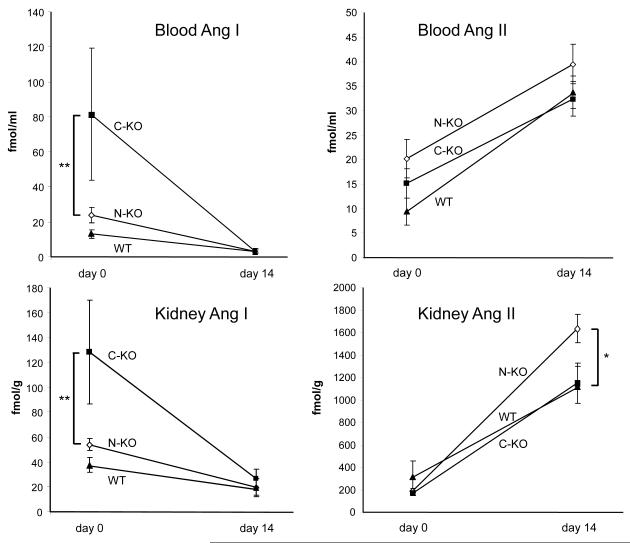
To understand the difference in blood pressure response between the N-KO, C-KO and WT mice, we measured blood and kidney angiotensin peptides under basal conditions and after 14 days of angiotensin II infusion (Fig. 2). Under basal conditions, the three groups had similar levels of angiotensin II in the blood; kidney levels were also equivalent between the three groups. Infusion of angiotensin II significantly elevated blood angiotensin II in all mice, but between the groups, there were no significant differences. At 14 days, kidney angiotensin II levels were also markedly higher than basal levels. However, whereas levels in C-KO and WT mice were virtually identical, renal levels of angiotensin II in the N-KO mice were 1.5-fold those of the other two groups (n≥14 per group; p<0.015).
Figure 2.
Angiotensin peptide levels. Angiotensin I and angiotensin II were measured in the blood and kidney of N-KO, C-KO and WT mice before and after infusion of angiotensin II for 14 days. Blood and kidney angiotensin I levels were significantly elevated in C-KO mice on day 0 (** p<0.01, n=12, 6 and 12 for N-KO, C-KO and WT). In contrast, there was no significant difference in blood or kidney angiotensin II levels between the 3 groups of mice. After the 14 day infusion, blood angiotensin II levels were increased, but there was no significant difference between groups. However on day 14, there was a significant increase of kidney levels of angiotensin II in N-KO mice (* p<0.015, n≥14 per group).
Previously, we reported that basal blood angiotensin I levels were elevated in C-KO mice.14 This is also true for renal angiotensin I levels and reflects the elevated renin found in this model (Fig. 2).
Angiotensin 1-7 is reported as an N-terminal specific substrate of ACE.16 Thus, we wondered if the level of this peptide would be elevated in the N-KO mice, similar to the elevated levels of the N terminal substrate AcSDKP. However, under basal conditions, no significant differences in blood or renal angiotensin 1-7 were found in WT, N-KO or C-KO mice (Supplemental Fig. 1). With the infusion of angiotensin II, there was a rise in angiotensin 1-7 levels, with the slope of the rise being greatest for the N-KO group. However, by 2-way ANOVA, taking into account time and genotype, there was no group significance. Finally, at 14 days, there were no significant differences by 1-way ANOVA. Bradykinin showed no significant differences between the groups (Supplemental Fig. 1).
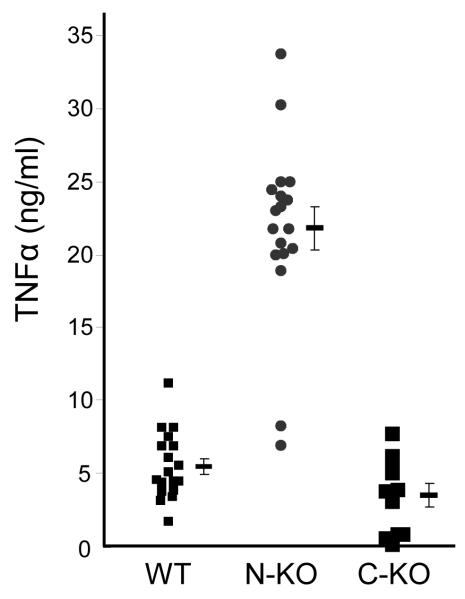
To understand the role of inflammation in the blood pressure response of the N-KO mice, we first studied a simple model of inflammatory cytokine release in response to LPS. In this experiment, there was no angiotensin II infusion. Four days after intraperitoneal (IP) injection of thioglycolate, peritoneal macrophages from N-KO, C-KO and WT mice were collected and cultured overnight with LPS (a model activator of macrophages). TNFα levels in the supernatants were then measured (Fig. 3a). To our surprise, there was a marked difference in cytokine profile; macrophages derived from N-KO mice produced far more TNFα than either C-KO or WT mice (N-KO vs. C-KO or WT, p<0.001). Study of the time course of TNFα protein expression showed that as early as 4 hrs after LPS addition, there were differences between N-KO and WT cells; by 8 hrs, the differences were pronounced (Supplemental Figure 2).
Figure 3.
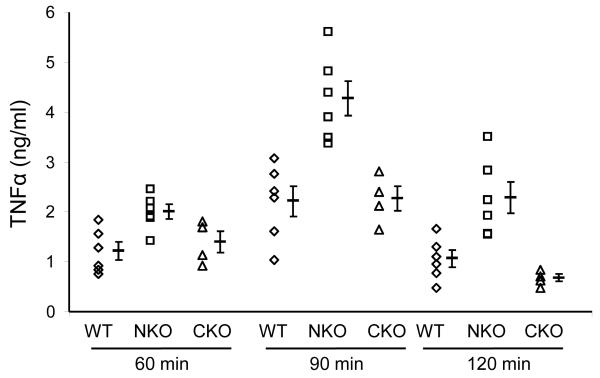
Macrophage TNFα production in response to LPS. A, Peritoneal macrophages from N-KO, C-KO and WT mice were collected after thioglycollate injection. After purification, the macrophages were cultured with LPS for 18 hrs and TNFα was measured. Each dot represents data from one mouse. Group means and SEM are also shown (p<0.001 for N-KO vs either C-KO or WT). Cells from N-KO mice produced far more TNFα than the equivalent cells from the other groups. B, N-KO, C-KO and WT mice received a single IP injection of LPS. After 60, 90 and 120 min, plasma concentrations of TNFα were determined. Each dot is data from a single mouse. Group means and SEM are also shown. At 90 min, levels of TNFα are significantly higher in N-KO mice compared to the two other groups (p<0.005).
To investigate whether differences in TNFα expression also exist in vivo, groups of N-KO and WT mice were injected IP with LPS. Maximum blood levels of TNFα were found 90 min after the LPS injection (Fig. 3b). At this point, TNFα levels in the N-KO mice were nearly double those of the WT mice (p<0.005). In contrast, the same experiment performed with C-KO mice showed a rise of TNFα virtually identical to WT. Thus, both in vitro and in vivo data show that, in the absence of ACE N-terminal catalytic activity, TNFα expression was increased.
Thioglycollate-elicited macrophages were also examined for LPS-stimulated expression of other cytokines. IL-12, a pro-inflammatory cytokine, showed a pattern similar to TNFα, namely after culture for 18 hr with LPS, N-KO derived macrophages made 3-fold the cytokine levels of WT cells (p<0.02) and 8-fold the cytokine levels of C-KO cells (p<0.005) (Supplemental Fig. 3a). In contrast, expression of the anti-inflammatory cytokine IL-10 by N-KO cells was only about half that produced by either WT or C-KO macrophages (Supplemental Fig. 3b). In summary, the inflammatory response of macrophages from N-KO mice was substantially different from that of either WT or C-KO derived cells; in response to LPS, N-KO cells produced cytokines typical of a strong pro-inflammatory response. In contrast, C-KO derived cells resembled WT.
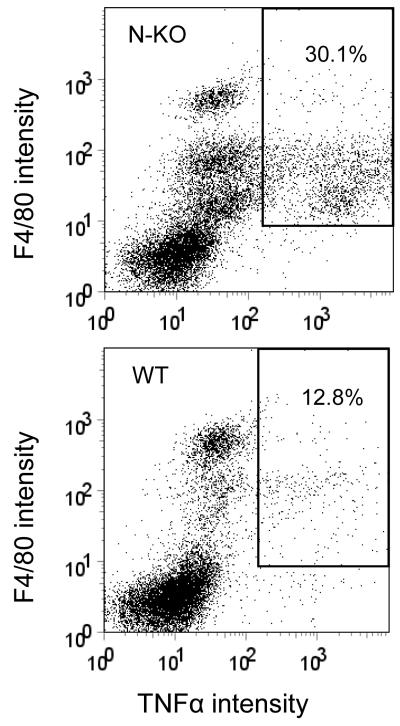
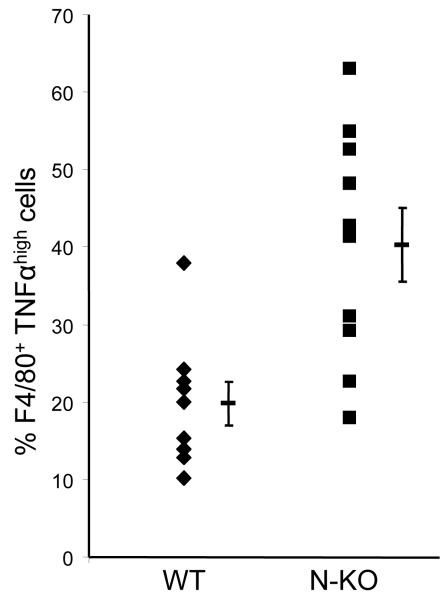
The above data examined N-KO mice for TNFα expression after LPS. To study the effects of angiotensin II, N-KO and WT mice were infused with the peptide for 14 days. At sacrifice, peritoneal cells were collected, treated with brefeldin A to inhibit protein secretion, then stained for surface expression of F4/80 (a macrophage marker), fixed, permeabilized, and stained for cytoplasmic TNFα, which was analyzed by flow cytometry. Representative data are shown in Fig. 4a where the top panel is data from an N-KO mouse, while a WT is shown on the bottom. The percentage of F4/80+/TNFαhigh cells vs. total F4/80+ cells is shown in the box. Combining data from at least 9 mice per group, N-KO mice averaged 40.3 ± 4.7% F4/80+/TNFαhigh, while WT averaged 19.8 ± 2.8% (p<0.002)(Fig. 4b). Cumulatively, our data establish a compelling case for differences in cytokine expression; to our knowledge, no other group has ever identified differences in the inflammatory response as a function of ACE N- or C-domain catalytic activity.
Figure 4.
Macrophage production of TNFα in response to angiotensin II. After mice received angiotensin II for 14 days, peritoneal cells were collected by lavage. The cells were then stained with fluorescent tagged antibody to F4/80 and TNFα. Fluorescence intensity was measured by FACS. A, Typical results from an N-KO (top) and WT mouse (bottom). The percentage of F4/80+/TNFαhigh cells vs. total F4/80+ cells is shown in the box. B, This percentage is shown for 9 WT and 10 N-KO mice. The group mean and SEM are also indicated (p<0.002).
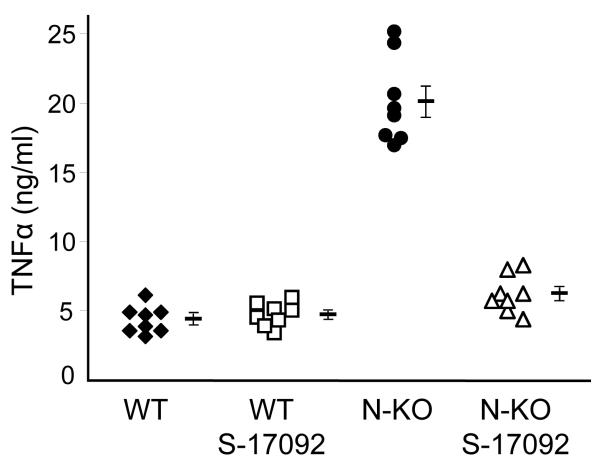
Part of the reason why N-KO mice have higher blood pressures in angiotensin II-induced hypertension may be the differences in the inflammatory response discussed above. Further, we wondered if these differences might be a consequence of the known increased AcSDKP in the N-KO model, as AcSDKP can influence the inflammatory response. To test this, we blocked formation of AcSDKP in N-KO and WT mice by injection of the prolyl oligopeptidase (POP) inhibitor S-17092 for 4 days before the collection of thioglycollate-induced peritoneal macrophages.17,18 Macrophages were then cultured overnight with LPS, and TNFα levels in the supernatants were measured (Fig. 5a). These data showed that POP inhibition reduced N-KO macrophage production of TNFα to levels equivalent to those of cells from WT mice. Further, the effect of S-17092 was limited to the N-KO mice. Thus, these data indicate that POP activity affects in vitro macrophage production of TNFα.
Figure 5.
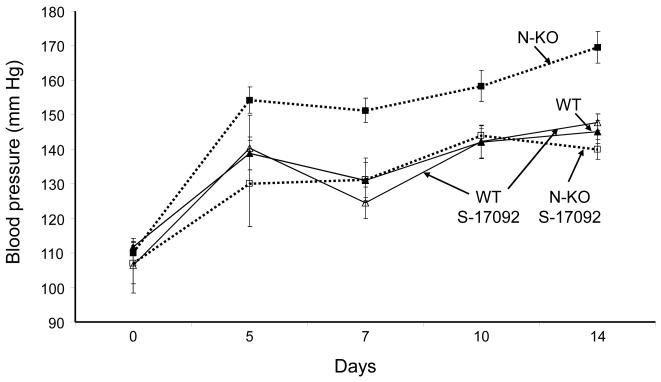
Effect of POP inhibition on cytokine production and blood pressure. A, N-KO and WT macrophages were collected 4 days after IP thioglycollate. Some animals received a daily IP injection of S-17092 during this period. After culture with LPS for 18 h, the concentration of TNFα was measured. The figure shows results from individual mice, as well as group means and SEM. Treatment with S-17092 markedly reduced TNFα levels in cells from N-KO mice (p<0.001). B, N-KO and WT mice were treated for 14 days with angiotensin II. Groups of these mice were pretreated with daily IP injection of S-17092 for 4 days before minipump implantation, and continued to receive daily S-17092 during the length of the experiment. Blood pressure was measured on days 5, 7 10 and 14 after minipump implantation. The figure shows the group means and SEM for each time point. Treatment of N-KO mice with S-17092 reduced the blood pressure elevation to that of WT mice. n=10, 10, 9, 10 for WT, N-KO, WT/S-17092 and N-KO/S-17092, respectively.
To test the effects of POP in vivo, and specifically whether this contributes to the increased blood pressure response to angiotensin II, groups of N-KO and WT mice were treated with the POP inhibitor S-17092 by daily IP injection beginning three days before implantation of the minipumps delivering angiotensin II (Fig. 5b). Blood pressure was then measured during the 14 day angiotensin II infusion. S-17092 had little effect on WT mice. However, N-KO mice treated with S-17092 increased blood pressure in response to angiotensin II significantly less than N-KO mice without S-17092 (p<0.001 on day 14). Or, put differently, the 25 mm Hg average difference in blood pressure between N-KO and WT mice treated with angiotensin II was completely eliminated by blocking POP.
While AcSDKP is produced by POP, it is only one of several peptide end-products of this peptidase. To directly investigate the effect of AcSDKP on blood pressure, we infused this peptide by minipump in WT mice. The mice were treated with one of three doses of AcSDKP (0.5, 1.5 or 3.0 mg/kg/day) beginning 1 week before the start of angiotensin II infusion, and AcSDKP infusion was continued through the 14 day angiotensin II infusion period. Blood pressure was measured at several time points throughout the experiment. At no time did the mice receiving AcSDKP show a significant difference in blood pressure from that observed in WT mice treated with angiotensin II in the absence of AcSDKP (Supplemental Fig. 4).
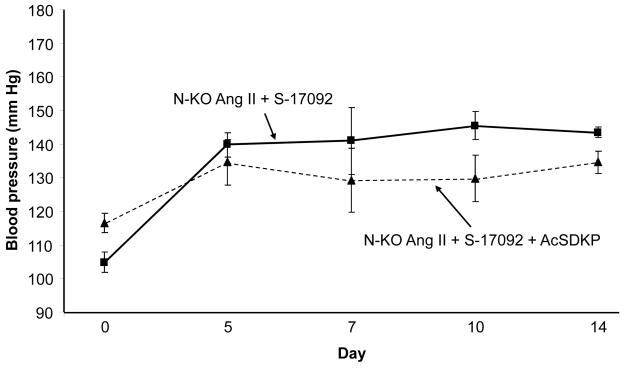
The lack of blood pressure effects observed with AcSDKP infusion may be due to the large amount of ACE in WT mice, and the ability of this enzyme (which has N-terminal activity) to degrade AcSDKP. To address this, we studied N-KO mice treated with angiotensin II in which POP activity was blocked by daily S-17092 injection, but in which AcSDKP levels were increased by minipump infusion of 1.5 mg/kg/day of peptide, a dose consistent with previous studies.19 These mice were compared to equivalent N-KO mice treated with angiotensin II and S-17092 but without AcSDKP supplementation (Fig. 6). No significant elevation of blood pressure was found with AcSDKP infusion. Further, FACS analysis of TNFα production by peritoneal macrophages showed no effect with AcSDKP infusion, as compared to mice treated with angiotensin II and S-17092 but without AcSDKP supplementation (Supplemental Fig. 5). Thus, these data do not indicate a role of AcSDKP in the elevated blood pressure or inflammation of N-KO mice treated with angiotensin II.
Figure 6.
Effects of AcSDKP on blood pressure and inflammation. N-KO mice were all treated with angiotensin II and S-17092. One group also received 1.5 mg/kg/day AcSDKP by minipump. n=5 per group. By Student’s t-test, p>0.05 for all days.
Discussion
ACE inhibitors are widely used to treat hypertension and heart disease. The goal of such therapy is blocking all ACE activity and these inhibitors appropriately block both ACE domains. However, the clinical emphasis on the role of ACE in cardiovascular disease often obscures the implications of an enzyme that has maintained two independent catalytic sites throughout millions of years of evolution. These domains can be individually studied in the N-KO and C-KO mice; perhaps the most important conclusion from these mice is that they establish beyond doubt that the two ACE catalytic domains are different. In vivo, it is the ACE C-terminal domain that normally produces the substantial majority of angiotensin II.14 In the C-KO mouse, typical concentrations of blood angiotensin II are only maintained through marked compensatory elevations of renin and angiotensin I levels. In contrast, N-KO mice have blood renin and angiotensin I levels that are essentially equivalent to those of WT animals.13
Further evidence for the asymmetry of ACE domain activity is the behavior of N-KO and C-KO mice when each strain was infused for two weeks with angiotensin II. C-KO mice demonstrated a very marked elevation of blood pressure during the first week of peptide infusion. This peaked at 7 days and then diminished slowly during the second week to levels equivalent to those of WT mice. While C-KO mice have a basal elevation of angiotensin I and renin, we do not know whether this contributes to the exaggerated blood pressure 7 days after the start of angiotensin II infusion.
In contrast to the C-KO model, the N-KO mouse shows an initial elevation of blood pressure that is greater than that of WT, and which continues to increase and to maintain the pressure differential with WT mice for at least 21 days. At the end of the infusion period, N-KO mice have blood pressures that averaged approximately 25 mm Hg higher than those seen in C-KO or WT mice. Our studies present two possible reasons for this difference in blood pressure response to angiotensin II. One possibility reflects the asymmetric accumulation of angiotensin II within the kidneys of the N-KO mice, as compared to WT or C-KO animals. The increased renal angiotensin II in the N-KO model may partly explain the elevated blood pressure, since previous work has demonstrated that elevated renal levels of angiotensin II are critical for the hypertensive response to angiotensin II.20,21 Both previous study of N-KO mice, and the data presented here, show no significant difference in blood levels of angiotensin II in N-KO vs WT mice.13 Thus, some other mechanism must be responsible for enhanced renal accumulation of angiotensin II in a low renin environment. One possibility, suggested by the literature, is differences in intratubular generation of angiotensin II.22,23
In addition to the contribution of renal angiotensin II, we believe an additional influence is differences in the inflammatory milieu present in N-KO vs C-KO or WT mice. N-KO mice show both in vitro and in vivo evidence for elevated production of TNFα and other cytokines generally associated with an increased inflammatory response. This was seen in response to in vitro stimulation with LPS, and also in peritoneal macrophages collected after the two week infusion of angiotensin II. There is now substantial evidence that a significant part of blood pressure elevation seen in response to angiotensin II infusion is due to immune activation.9 Thus, the enhanced macrophage pro-inflammatory cytokine production in N-KO mice exposed to angiotensin II is a second physiologic explanation for why these mice have an exaggerated blood pressure response.
One obvious difference between N-KO and WT mice is that N-KO mice have chronically elevated AcSDKP levels.13 AcSDKP is one of four peptides reported to be specific substrates of the ACE N-domain (AcSDKP, angiotensin 1-7, LH-RH, amyloid β1-42).24,25 AcSDKP is produced by the enzyme POP, which can be inhibited by the pharmaceutical S-17092, a very specific POP inhibitor.6 We treated N-KO mice with S-17092 using conditions previously shown to reduce plasma AcSDKP levels to WT levels.19 With POP inhibition, N-KO macrophage production of TNFα in response to LPS was restored to WT levels. Further, POP inhibition reduced the angiotensin II-mediated elevation of blood pressure in N-KO mice to the levels seen in WT mice. However, we were unable to establish that AcSDKP was the cause of the N-KO phenotype. Specifically, when N-KO mice were treated with angiotensin II, S-17092 and AcSDKP, no increase in the blood pressure response was observed as compared to mice treated with angiotensin II and S-17092, but without AcSDKP. Further, AcSDKP supplementation did not affect peritoneal macrophage production of TNFα. Previous published work showed that the short term administration of AcSDKP was associated with a reduced fibrotic response, and not with any type of pro-inflammatory response.26 Thus, our data and previous published findings are consistent in indicating that the elevated AcSDKP levels in N-KO mice are not the primary cause of the N-KO phenotype. Of the other known ACE N-terminal specific substrates, none appear to be good candidates to explain the N-KO data. Angiotensin 1-7 is generally considered a vasodilator. LH-RH and β amyloid are not suspected of influencing blood pressure effects.
POP is a 710 residue serine endopeptidase that is widely distributed in tissues.18 The renal cortex is particularly rich in this enzyme. While POP was discovered in human uterus through its ability to degrade oxytocin, the enzyme is thought to be active on several peptides including angiotensin I and angiotensin II (releasing angiotensin 1-7), bradykinin, and vasopressin. Several studies have implicated POP in central nervous system function, including learning and memory, and this has led to the development of several specific and high affinity inhibitors.6 For example, S-17092 was shown to have a Ki for POP of 1.3 nM in the rat and to not inhibit several other peptidases, including aminopeptidase M, dipeptidylpeptidase IV, neprilysin, endopeptidases 3.4.24.15 and 3.4.24.16, calpains and ACE. While there has been some speculation about a role of POP in blood pressure control, there has been little experimentation addressing this topic.27 POP knockout mice have been created, but their blood pressure was not reported.28 Our data are notable for the difference in effect between N-KO and WT mice treated with a POP inhibitor; the blood pressure lowering effect is far more pronounced in the N-KO. To us, this implies an important balance between the actions of POP and ACE. One well recognized product of POP is AcSDKP, which is destroyed by ACE. However, this does not appear to be the cause of the N-KO phenotype. We conclude that other POP products must also interact with the ACE N-terminus.
Perspectives
The lack of ACE N-terminal activity is associated with an exaggerated blood pressure response to angiotensin II, and an increased pro-inflammatory immune response to LPS or angiotensin II, as compared to WT or C-KO mice. These data underline important physiologic differences in the function of the two catalytic domains of ACE, a novel finding. All commonly used ACE inhibitors block catalytic activity of both ACE domains; once we understand the actions of the ACE N- and C-terminal domains, there may be clinical implications of ACE site specific inhibition which may ultimately translate into new treatment strategies using site specific ACE inhibitors.
Supplementary Material
Acknowledgments
We thank Dr. Denis Guedin, Institut de Recherche Servier, France, for his generous gift of S-17092. Ellen E. Bernstein, Tea Janjulia and Brian L. Taylor provided technical assistance. F.S.O. thanks Dr. Jane Z. Kuo for technical and statistical assistance.
Source of Funding
This work was supported by National Institutes of Health grant T32 DK007770 (FSO, WLBB), F32 HL105036 (FSO), K99 HL088000 (SF), K99 DK083455 (RAGV), R01 DK039777 and R01 HL110353 (KEB). Duncan Campbell is recipient of a Senior Research Fellowships from the National Health and Medical Research Council of Australia (Grant ID 395508).
Footnotes
Conflict of Interest: None
References
- 1.Soubrier F, Alhenc-Gelas F, Hubert C, Allegrini J, John M, Tregear G, Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci USA. 1988;85:9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein KE, Martin BM, Edwards AS, Bernstein EA. Mouse angiotensin I-converting enzyme is a protein composed of two homologous domains. J Biol Chem. 1989;264:11945–11951. [PubMed] [Google Scholar]
- 3.Wei L, Alhenc-Gelas F, Corvol P, Clauser E. The two homologous domains of human angiotensin I-converting enzyme are both catalytically active. J Biol Chem. 1991;266:9002–9008. [PubMed] [Google Scholar]
- 4.Rousseau A, Michaud A, Chauvet MT, Lenfant M, Corvol P. The hemoregulatory peptide N-acetyl-Ser-Asp-Lys-Pro is a natural and specific substrate of the N-terminal active site of human angiotensin-converting enzyme. J Biol Chem. 1995;270:3656–3661. doi: 10.1074/jbc.270.8.3656. [DOI] [PubMed] [Google Scholar]
- 5.Sosne G, Qiu P, Goldstein AL, Wheater M. Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB J. 2010;24:2144–2151. doi: 10.1096/fj.09-142307. [DOI] [PubMed] [Google Scholar]
- 6.Lawandi J, Gerber-Lemaire S, Juillerat-Jeanneret L, Moitessier N. Inhibitors of prolyl oligopeptidases for the therapy of human diseases: defining diseases and inhibitors. J Med Chem. 2010;53:3423–3438. doi: 10.1021/jm901104g. [DOI] [PubMed] [Google Scholar]
- 7.Harrison DG, Vinh A, Lob H, Madhur MS. Role of the adaptive immune system in hypertension. Curr Opin Pharmacol. 2010;10:203–207. doi: 10.1016/j.coph.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs S, Xiao HD, Adams JW, Frenzel K, Keshelava G, Capecchi MR, Corvol P, Bernstein KE. Role of the N-terminal catalytic domain of ACE investigated by targeted inactivation in mice. J Biol Chem. 2004;279:15946–15953. doi: 10.1074/jbc.M400149200. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs S, Xiao HD, Michaud A, Campbell DJ, Hubert C, Capecchi MR, Corvol P, Bernstein KE. The ACE C-terminal catalytic domain is the main site of angiotensin I cleavage in vivo. Hypertension. 2008;51:267–274. doi: 10.1161/HYPERTENSIONAHA.107.097865. [DOI] [PubMed] [Google Scholar]
- 15.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13:2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 16.Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdös EG. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension. 1998;31:912–917. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 17.Barelli H, Petit A, Hirsch E, Wilk S, De Nanteuil G, Morain P, Checler F. S 17092-1, a highly potent, specific and cell permeant inhibitor of human proline endopeptidase. Biochem Biophys Res Commun. 1999;257:657–661. doi: 10.1006/bbrc.1999.0366. [DOI] [PubMed] [Google Scholar]
- 18.Szeltner Z, Polgár L. Structure, function and biological relevance of prolyl oligopeptidase. Curr Protein Pept Sci. 2008;9:96–107. doi: 10.2174/138920308783565723. [DOI] [PubMed] [Google Scholar]
- 19.Li P, Xiao HD, Xu J, Ong FS, Kwon M, Roman J, Gal A, Bernstein KE, Fuchs S. Angiotensin-converting enzyme N-terminal inactivation alleviates bleomycin-induced lung injury. Am J Pathol. 2010;177:1113–1121. doi: 10.2353/ajpath.2010.081127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Villalobos RA, Satou R, Seth DM, Semprun-Prieto LC, Katsurada A, Kobori H, Navar LG. Angiotensin-converting enzyme-derived angiotensin II formation during angiotensin II-induced hypertension. Hypertension. 2009;53:351–355. doi: 10.1161/HYPERTENSIONAHA.108.124511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol. 2011;22:449–459. doi: 10.1681/ASN.2010060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–362. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya KR, Sturrock ED, Riordan JF, Ehlers MR. ACE revisited: a new target for structure-based drug design. Nat Rev Drug Discov. 2003;2:891–902. doi: 10.1038/nrd1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou K, Maeda T, Watanabe A, Liu J, Liu S, Oba R, Satoh Y, Komano H, Michikawa M. Abeta42-to-Abeta40- and angiotensin-converting activities in different domains of angiotensin-converting enzyme. J Biol Chem. 2009;284:31914–31920. doi: 10.1074/jbc.M109.011437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CX, Rhaleb NE, Yang XP, Liao TD, D’Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goossens F, De Meester I, Vanhoof G, Scharpé S. Distribution of prolyl oligopeptidase in human peripheral tissues and body fluids. Eur J Clin Chem Clin Biochem. 1996;34:17–22. doi: 10.1515/cclm.1996.34.1.17. [DOI] [PubMed] [Google Scholar]
- 28.Di Daniel E, Glover CP, Grot E, Chan MK, Sanderson TH, White JH, Ellis CL, Gallagher KT, Uney J, Thomas J, Maycox PR, Mudge AW. Prolyl oligopeptidase binds to GAP-43 and functions without its peptidase activity. Mol Cell Neurosci. 2009;41:373–382. doi: 10.1016/j.mcn.2009.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.