Abstract
(Pro)renin receptor (PRR) is expressed in renal vasculature, glomeruli and tubules. The physiologic regulation of this receptor is not well established. We hypothesized that sodium depletion increases PRR expression through cGMP-PKG signaling pathway. Renal PRR expressions were evaluated in Sprague-Dawley rats on normal sodium (NS) or low sodium (LS) diet and in cultured rat proximal tubular cells (PTCs) and mouse renal inner medullary collecting duct cells (IMCDs) exposed to LS concentration. LS augmented PRR expression in renal glomeruli, proximal tubules, distal tubules and collecting ducts. LS also increased cGMP production and protein kinase G (PKG) activity. In cells exposed to NS, cGMP analog increased PKG activity and upregulated PRR expression. In cells exposed to LS, blockade of guanylyl cyclase with ODQ decreased PKG activity and downregulated PRR expression. PKG inhibition decreased phosphatase PP2A activity, suppressed LS-mediated phosphorylation of Erk, JNK, c-Jun and NF-κB p65, and attenuated LS-mediated PRR upregulation. LS also enhanced DNA binding of cAMP response element binding protein 1(CREB-1) to cAMP response elements (CREs), NF-κB p65 to NF-κB elements and c-Jun to AP-1 elements in PRR promoter in PTCs. We conclude that sodium depletion upregulates renal PRR expression via cGMP-PKG signaling pathway by enhancing binding of CREB-1, NF-κB p65 and c-Jun to PRR promotor.
Keywords: (pro)renin receptor, sodium depletion, cyclic guanidine monophosphate, protein kinase G, kidney
INTRODUCTION
(Pro)renin receptor (PRR) is one of the newly discovered components of the reninangiotensin aldosterone system (RAAS) (1, 2) and is expressed in renal vasculature, glomeruli and tubules (1 - 4). PRR contributes to the conversion of angiotensinogen to angiotensin I (1). Recent studies also demonstrated involvement of PRR in the development of kidney diseases and inflammation (4 - 7). Overexpression of human PRR in transgenic rats resulted in an increase of aldosterone production and elevation of blood pressure (8). At present time, the physiologic regulation of PRR expression is unknown. The relationship between low sodium intake and increased activity of RAAS is well established. Low sodium intake is associated with increased production of renin (9, 10) and angiotensin II (10 - 12) and enhanced expression of the angiotensin receptor type-1 (13) and type-2 (14). Similarly, low sodium diet (LS) enhances renal production of cGMP (12, 15 - 18). Recent studies demonstrated upregulation of PRR in diabetic animals (3) and renal cells exposed to high glucose medium (4, 5). However, it is unknown whether PRR expression is regulated by sodium or cGMP. Defining the relationship between sodium and PRR could be the first step to elucidate the physiologic role of PRR in the kidney. This study was conducted to evaluate whether LS, cGMP or its messenger protein kinase G (PKG) influence PRR expression. We hypothesized that in the kidney, sodium depletion enhances PRR expression via cGMP-PKG mediated intracellular signaling pathway. We also identified the cellular signals, transcription factors and their functional binding sites in the promoter region of PRR that may influence PRR expression in response to sodium depletion.
MATERIALS AND METHODS
Animal Preparation, Salt Intake and Renal Expression of (Pro)renin Receptor
Study protocols were approved by the University of Virginia Animal Care and Use Committee. Sprague-Dawley rats (Charles River Laboratories; Wilmington MA) weighing 245 to 255 g were used in this study. The effects of low sodium intake on the renal PRR expression were studied by placing the animals for one week on normal-sodium (NS, 0.3% NaCl, Harlan Teklad, Madison WI) or LS (0.05% NaCl, Harlan Teklad, Madison WI) diet (n = 8 for each group). At the end of this period, animals were euthanized and the kidneys were harvested for protein and total RNA extraction, and parts of kidney were also fixed with Bounin's fixative. Renal PRR expression was evaluated with quantitative real time PCR, Western blotting and immunohistochemical staining.
Cell Culture, Sodium Depletion and Inhibition of cGMP-PKG signaling cascade
Mouse renal inner medullary collecting duct epithelial cells (IMCD) were obtained from the American Type Culture Collection (ATCC, Manassas VA) and cultured according to ATCC recommended protocols. Proximal tubular epithelial cells from Wistar Kyoto rats (PTCs) were kindly provided by Dr. John J. Gildea at the University of Virginia. Cells were grown to confluence in Dulbecco's Modified Eagle Medium / Nutrient Mixture F12 Medium (DMEM / F12) (Invitrogen, Carlsbad CA) supplemented with 10% fetal calf serum and antibiotics. Serum starvation was conducted with Opti-MEM I medium (Invitrogen, Carlsbad CA) for 12 hrs.
NS and LS media were prepared according to Yang's methods (19). LS medium was prepared by Opti-MEM I medium in a 1:1 mixture with 300 mM D-mannitol (to reduce Na+ concentration to 57.03 ± 0.37 mM). In control groups, cells were exposed to Opti-MEM I medium in a 1:1 mixture with isotonic saline (final Na+ concentration about 132.00 ± 0.32 mM). In time-response course studies, cells were serum-starved for 12 hrs prior and then exposed to NS or LS medium for 1, 3, 6, 12 and 24 hrs, respectively. At the end of experiments, cells were harvested for total RNA and protein extraction. For different treatments experiments, each drug was added to serum free medium 30 min prior to the end of serum starvation. After 30 min pretreatment, cells were refreshed with NS or LS medium with or without treatment which included one of the following: cGMP analogue (8-bromo-GMP, Calbiochem, La Jolla CA), 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-one (ODQ) (Sigma-Aldrich, St. Luis MO) or PKG inhibitor (Calbiochem, La Jolla CA).
Assessing Gene Expression and Protein Phosphorylation
Determination of gene expression and protein phosphorylation was conducted with real time PCR and Western blotting assay. The details are as described in Online Supplement (please see http://hyper.ahajournals.org).
Measurement of Cell Viability, cGMP Production, PKG Activity and Phosphatase PP2A Activity
The measurement of cell viability, cGMP production, PKG and phosphatase PP2A activities was carried out as described in Online Supplement (please see http://hyper.ahajournals.org).
Real-time mapping and in vitro binding activities of CRE, NF-κB and AP-1 regulatory elements in PRR promotor
The protocol for real time mapping of transcription factors cAMP response element binding protein 1(CREB-1), NF-κB p65 and c-Jun to cAMP response element (CRE), NF-κB and activator protein 1 (AP-1) elements and their in vitro binding activity is detailed in Online Supplement (please see http://hyper.ahajournals.org).
Statistical analysis
The data analysis was carried out using STATISTICA version 5.0 (StatSoft, Tulsa OK). Results are expressed as mean ± SE. Comparisons among different treatment groups were evaluated by analysis of variance (ANOVA) with repeated measures, and the Bonferroni correction method as a post-hoc test. A p-value of < 0.05 was defined as statistically significant.
RESULTS
Sodium depletion increased PRR expression in the kidneys of rats
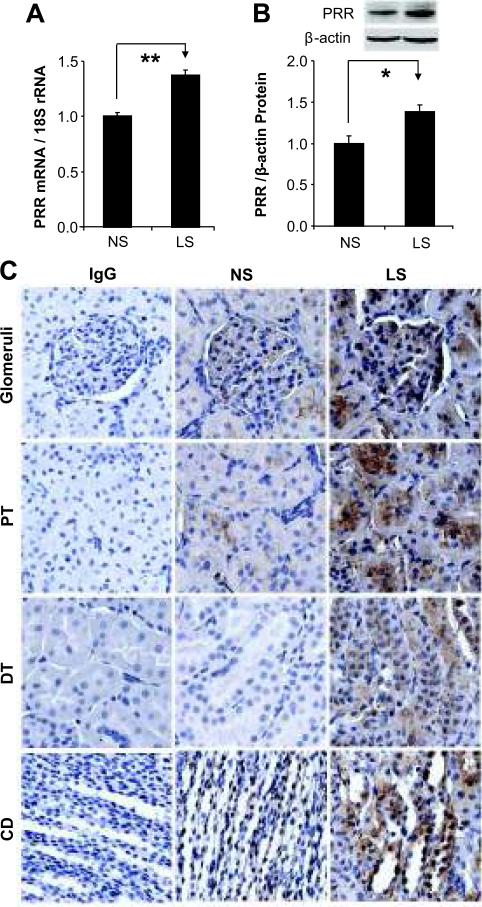
Compared to NS treated rats, PRR mRNA and protein expression were upregulated in the kidney of LS treated rats (Figure 1A-B). Similarly, immunostaining of PRR was increased in the glomeruli, proximal and distal tubules, and collecting ducts in LS group (Figure 1C).
Figure 1.
Effects of low sodium on the expression of PRR in rat kidney. (A) PRR mRNA, n = 8; (B) PRR protein, n = 8; (C) PRR immunohistochemical staining. NS: normal sodium; LS: low sodium; IgG control: IgG control negative staining; PT: proximal tubules; DT: distal tubules; CD: collecting ducts. *p<0.01; **p<0.001.
Sodium depletion upregulated PRR expression in time-dependent manner in PTCs and IMCD
Compared to NS, PRR mRNA and protein were upregulated after 6 hrs of LS exposure and reached the peak after 12 hrs in PTCs and IMCD as demonstrated in Supplement Figure S1 (please see http://hyper.ahajournals.org).
Sodium depletion increased cGMP production and relative PKG activity in PTCs and IMCD
cGMP production was significantly increased in culture supernatants and cells of both PTCs and IMCD cells after 12 hrs exposure to LS (Supplement Figure S2, please see http://hyper.ahajournals.org). cGMP analogue, 8-bromo-cGMP, significantly enhanced relative PKG activity in PTC and IMCD cells exposed to NS (Supplement Figure S2, please see http://hyper.ahajournals.org). LS significantly increased relative PKG activity. Blockade of soluble guanylyl cyclase (sGC) with ODQ inhibited LS-induced increase of relative PKG activities in both PTCs and IMCD cells (Supplement Figure S2, please see http://hyper.ahajournals.org).
Effect of cGMP stimulation and sGC blockade on PRR expression in PTCs
8-bromo-cGMP treatment upregulated PRR mRNA and protein expression in PTCs cells exposed to NS (Figure 2A-B). There was no dose dependent effect of 8-bromo-cGMP on PRR mRNA and protein expression at the utilized doses.
Figure 2.
Effects of cGMP stimulation and guanylyl cyclase inhibition on PRR expression in PTCs. NS: normal sodium; LS: low sodium; 8-bromo-cGMP: 8-bromocGMP; ODQ: guanylyl cyclase inhibitor; Mean is the average of three independent experiments. *p<0.01; **p<0.001.
sGC blockade with ODQ did not influence PRR expression in cells exposed to NS. In contrast, ODQ at the concentration of 100 nM significantly attenuated PRR upregulation in PTCs exposed to LS (Figure 2C-D).
Effect of PKG inhibition on PRR expression, PP2A activities and protein phosphorylation of signaling molecules in PTCs
PKG inhibition did not influence PRR expression in PTCs exposed to NS but attenuated PRR expression in a dose-dependent manner in LS treated PTCs cells (Figure 3A-B). Relative phosphatase PP2A activities were decreased in LS, which this process was reversed by PKG inhibition (Figure 3C).
Figure 3.
Effects of low sodium and PKG inhibition on PRR expression, relative PP2A activity and protein phosphorylation in PTCs. NS: normal sodium; LS: low sodium; PKGi: PKG inhibitor; Mean is the average of three independent experiments. *p<0.05; **p<0.01; †p<0.001.
LS significantly increased the phosphorylation of Erk (T185Y187), JNK1/2 (T183Y185), c-Jun (S63), CREB-1 (S133) and NF-κB p65 (S276). These protein phosphorylations were attenuated by PKG inhibition (Figure 3C).
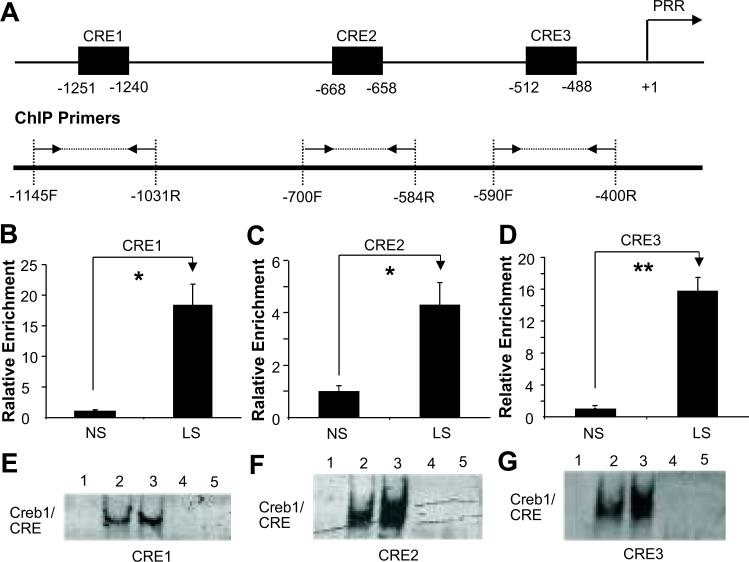
Sodium depletion increased CREB-1 binding to CRE elements in PRR promoter
Three CREs were predicted in rat PRR promoter (Figure 4A). ChIP results (Figure 4B-D) demonstrated that LS significantly increased CREB-1 binding to all three CRE elements. Compared to NS, EMSA results showed increased CREB1/CRE complex formation and band shift in LS. CREB-1 antibody completely inhibited the formation of CREB-1/CRE complexes (Figure 4E-G).
Figure 4.
Effects of low sodium on CREB1 binding to CRE elements in PRR promoter in PTCs. (A) Illustrated diagram of positions of CRE elements and ChIP primer pairs in predicted PRR promoter. Δ Fold: relative enrichment; (B)-(D): ChIP; (E)-(G): EMSA. NS: normal sodium; LS: low sodium; 1: Negative control; 2 & 4: NS; 3 & 5: LS; 4 & 5: CREB-1 antibody competitive assay. Mean is the average of three independent experiments. *p<0.05; **p<0.01.
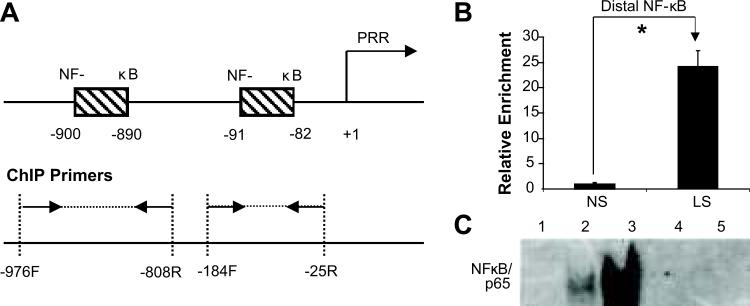
Sodium depletion increased NF-κB p65 binding to NF-κB elements in PRR promoter
Two NF-κB binding elements were predicted in rat PRR promoter (Figure 5A). Results from ChIP and EMSA demonstrated that LS significantly increased NF-κB p65 binding to distal NF-κB element (Figure 5B), and NF-κB p65/NF-κB complex formation and band shift (Figure 5C). NF-κB p65 antibody completely inhibited the formation of NF-κB p65/NF-κB element complexes (Figure 5C).
Figure 5.
Effects of low sodium on NF-κB p65 binding to NF-κB elements in PRR promoter in PTCs. (A) Illustrated diagram of positions of NF-κB elements and ChIP primer pairs in predicted PRR promoter. Δ Fold: relative enrichment; (B), (D) and (F): ChIP; (C), (E) and (G): EMSA. NS: normal sodium; LS: low sodium; 1: Negative control; 2 & 4: NS; 3 & 5: LS; 4 & 5: NF-κB p65 antibody competitive assay. Mean is the average of three independent experiments. *p<0.001.
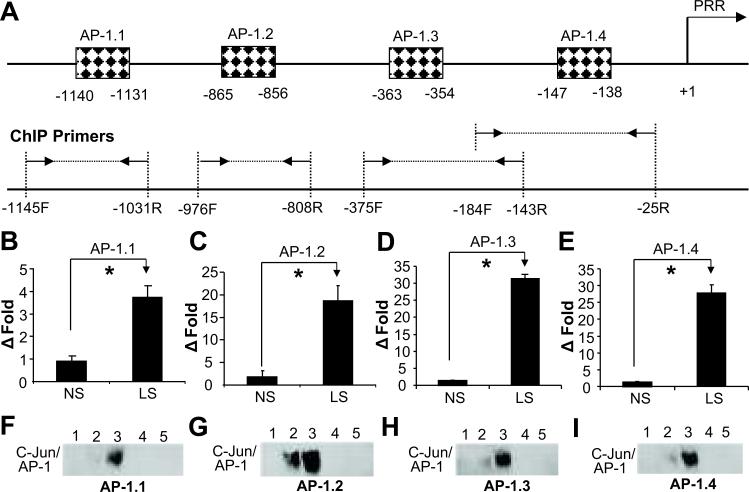
Sodium depletion increase c-Jun binding to AP-1 elements in PRR promoter
Four AP-1 binding elements were predicted in rat PRR promoter (Figure 6A). ChIP results demonstrated that LS significantly increased c-Jun binding to AP-1 elements (Figure 6B-E). In contrast to NS, EMSA results showed increased c-Jun/AP-1 complex formation with LS (Figure 6F-I). The c-Jun antibody completely inhibited the formation of c-Jun/AP-1 complexes (Figure 6F-I).
Figure 6.
Effects of low sodium on c-Jun binding to activator protein 1 (AP-1) elements in PRR promoter in PTCs. (A) Illustrated diagram of positions of AP-1 elements and ChIP primer pairs in predicted PRR promoter. Δ Fold: relative enrichment; (B)-(E): ChIP; (F)-(I): EMSA. NS: normal sodium; LS: low sodium; 1: Negative control; 2 & 4: NS; 3 & 5: LS; 4 & 5: c-Jun antibody competitive assay. Mean is the average of three independent experiments. *p<0.001.
DISCUSSION
In the present study, our first finding demonstrated that LS intake significantly upregulated PRR mRNA and protein expression in rat kidney. Although upregulation of PRR was observed in whole kidney, it was more pronounced in proximal tubules and collecting ducts. Similarly, in cultured proximal tubular and innermedullary collecting duct epithelial cells, LS exposure also upregulated PRR expression. These results implied that PRR might play a physiological role in proximal tubules and collecting ducts during sodium depletion.
Our in vivo studies were designed to evaluate renal expression of PRR after one week of LS diet. However, PRR expression in cultured renal cells was significantly upregulated after 6 hrs of LS exposure and reached a peak after 12 hrs. These results suggested a rapid change in renal PRR expression in response to changes in sodium depletion. Due to lack of availability of renal specific PRR knockout animal model or specific antagonists, we were not able to determine the exact role of this receptor in the regulation of renal functions. Our previous studies demonstrated that PRR is functional since it enhances the intracellular signaling protein phosphorylation (4, 5) and contributes to renal production of inflammatory factors (4 - 7). A possible function of PRR, though not evaluated in present study, is the regulation of renal sodium handling. A candidate for the link between PRR and sodium reabsorption is Erk. Previous studies demonstrated enhanced Erk phosphorylation by PRR (4, 5). Erk was shown to regulate the expression/activity of Na+, K+-ATPase (20), Na+/H+ exchanger (21) and Na+ channel (ENaC) (22). Future studies should evaluate the link between PRR and sodium reabsorption.
Despite being exposed to different intra-tubular sodium concentrations, PRR expression in proximal tubules and inner-medullary collecting ducts is similar in response to low sodium intake. It is likely that the sensing mechanism of sodium level that influences renal PRR expression may not be directly related to intra-tubular fluid sodium concentration. Both PTCs and IMCD cells increased cGMP production and PKG activity in response to LS exposure. These results suggest that these cells mount similar PRR signaling pathways in response to sodium depletion.
Our second finding was that the increment of cGMP production by sodium depletion contributes to renal PRR expression. Our previous studies showed that LS intake increased cGMP production in the kidneys (12, 15 - 18). In this study, we also demonstrated that LS increased cGMP production in PTCs and IMCD cells. Previous studies (12, 15 - 18) demonstrated that cGMP is a primary messenger to activate downstream target molecules to initiate secondary response. In this study, PTCs treated with 8-bromo-cGMP in the presence of NS upregulated PRR mRNA and protein expression. In contrast, inhibition of sGC attenuated PRR expression in cells exposed to LS. These results confirm that PRR is regulated by cGMP.
Similarly, we found that PKG activity was increased in cells exposed to LS and to 8-bromo-cGMP during NS exposure. PKG activity was attenuated by GC inhibition. These results confirm the existence of LS-cGMP-PKG pathway. The involvement of PKG in regulation of PRR expression was confirmed by PKG inhibition. PKG inhibition, in a dose dependent manner, attenuated the observed increase in PRR mRNA and protein expression in cells exposed to LS. These results demonstrated that activated PKG participate in PRR transcriptional regulation in the kidney exposed to LS via cGMP-PKG signaling pathway.
Multiple CRE, NF-κB and AP-1 binding elements were found in PRR promoter. In the present study, we confirmed the involvement of transcription factors CREB-1, NF-κB p65 and c-Jun in LS-stimulated PRR expression. We demonstrated that these regulatory elements were actively functional in PRR promoter in renal cells exposed to LS to enhance this receptor expression. Both ChIP and EMSA results showed that LS comprehensively enhanced dynamic binding of transcriptional factors CREB-1, NF-κB p65 and c-Jun to CRE, NF-κB and AP-1 elements in vivo and in vitro, respectively. Previously we reported that NF-κB p65 and c-Jun positively enhance transcriptional regulation of PRR expression in renal mesangial cells exposed to high glucose concentration (5). In this study we further confirmed that CREB-1, NF-κB p65 and c-Jun enhance transcriptional regulation of PRR and their binding to PRR promoter were significantly amplified in renal proximal tubular cells exposed to LS.
Previous studies demonstrated the involvement of cGMP-PKG signaling pathway in enhancing the effect of CRE (23, 24), NF-κB (25) and AP-1 (26, 27) in different tissues or cells. In present study, kinase phosphorylation of Erk1/2, JNK, CREB-1, p65 and c-Jun was observed in renal cells exposed to LS. The phosphorylation of these kinases was attenuated by PKG inhibition. These results suggested that PKG mediated the activation of CREB-1, NF-κB p65 and c-Jun and further influenced their binding to regulatory elements of PRR promoter in renal cells.
Several studies demonstrated that phosphatase PP2A negatively regulates PKC activity in many experimental models (28, 29). In this study, LS suppressed phosphatase PP2A activity. This effect was reversed by PKG. Thus LS seems to decrease phosphatase PP2A and increase PKC activities. These findings are in agreement with our previous study demonstrating the involvement of PKCs as regulators of MAPKs and PRR expression in renal mesangial cells (5). Taken together, PKG regulates PRR expression by activating CREB-1, p65 and c-Jun via PKG-PP2A-PKC signaling pathways.
Our previous studies demonstrated that sodium depletion increases renal cGMP concentration by enhancing nitric oxide production by angiotensin type II and bradykinin B2 receptors (15, 16). Combined with the data in this study, we conclude that sodium depletion upregulates renal PRR expression via cGMP-PKG signaling pathway and enhancing the activities of transcription factors CREB-1, NF-κB p65 and c-Jun.
PERSPECTIVES
The present study confirms that sodium depletion significantly upregulates renal expression of (pro)renin receptor via cGMP-protein kinase G signaling pathways. These findings may help in identifying new mechanisms related to the regulation of this receptor expression under physiologic conditions. In addition, this study suggests the possibility of involvement of PRR in regulation of renal function. Elucidation of this effect could lead to better understanding of the importance of PRR in health and disease.
Supplementary Material
ACKNOELEDGEMENT
The authors would like to thank Dr. John J. Gildea for providing proximal tubular epithelial cell line and Mr. William Pitkin for technical assistance.
SOURCES OF FUNDING
This study was supported by grant DK-078757 and HL091535 from the National Institutes of Health to Helmy M. Siragy, M.D.
Footnotes
DISCLOSURE
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int. 1996;50:1897–1903. doi: 10.1038/ki.1996.511. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siragy HM, Huang J. Renal (pro)renin receptor upregulation in diabetic rats through enhanced angiotensin AT1 receptor and NADPH oxidase activity. Exp Physiol. 2008;93:709–714. doi: 10.1113/expphysiol.2007.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Siragy HM. Glucose promotes the production of interleukine-1beta and cyclooxygenase-2 in mesangial cells via enhanced (Pro)renin receptor expression. Endocrinology. 2009;150:5557–5565. doi: 10.1210/en.2009-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Siragy HM. Regulation of (pro)renin receptor expression by glucose-induced mitogen-activated protein kinase, nuclear factor-kappaB, and activator protein-1 signaling pathways. Endocrinology. 2010;151:3317–3325. doi: 10.1210/en.2009-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matavelli LC, Huang J, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol Physiol. 2010;37:277–282. doi: 10.1111/j.1440-1681.2009.05292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang J, Matavelli LC, Siragy HM. Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-beta1 - connective tissue growth factor signalling cascade. Clin Exp Pharmacol Physiol. 2011;38:215–221. doi: 10.1111/j.1440-1681.2011.05486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension. 2006;47:552–556. doi: 10.1161/01.HYP.0000199912.47657.04. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S, Kato J, Kuwasako K, Kitamura K. Plasma and tissue levels of proangiotensin-12 and components of the renin-angiotensin system (RAS) following low- or high-salt feeding in rats. Peptides. 2010;31:889–892. doi: 10.1016/j.peptides.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Effects of dietary salt changes on renal renin-angiotensin system in rats. Am J Physiol Renal Physiol. 2002;283:F995–F1002. doi: 10.1152/ajprenal.00321.2001. [DOI] [PubMed] [Google Scholar]
- 11.Siragy HM, Howell NL, Ragsdale NV, Carey RM. Renal interstitial fluid angiotensin. Modulation by anesthesia, epinephrine, sodium depletion, and renin inhibition. Hypertension. 1995;25:1021–1024. doi: 10.1161/01.hyp.25.5.1021. [DOI] [PubMed] [Google Scholar]
- 12.Siragy HM, Inagami T, Carey RM. NO and cGMP mediate angiotensin AT2 receptor-induced renal renin inhibition in young rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1461–R1467. doi: 10.1152/ajpregu.00014.2007. [DOI] [PubMed] [Google Scholar]
- 13.Schmid C, Castrop H, Reitbauer J, Della Bruna R, Kurtz A. Dietary salt intake modulates angiotensin II type 1 receptor gene expression. Hypertension. 1997;29:923–929. doi: 10.1161/01.hyp.29.4.923. [DOI] [PubMed] [Google Scholar]
- 14.Ozono R, Wang ZQ, Moore AF, Inagami T, Siragy HM, Carey RM. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–1246. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- 15.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptors mediates renal production of nitric oxide in conscious rats. J Clin Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 17.Millatt LJ, Siragy HM. Renal cyclic 3',5'-guanosine monophosphate and sodium excretion in Dahl salt-resistant and Dahl salt-sensitive rats: comparison of the roles of bradykinin and nitric oxide. J Hypertens. 2000;18:1491–1496. doi: 10.1097/00004872-200018100-00018. [DOI] [PubMed] [Google Scholar]
- 18.Millatt LJ, Siragy HM. Age-related changes in renal cyclic nucleotides and eicosanoids in response to sodium intake. Hypertension. 2000;35:643–647. doi: 10.1161/01.hyp.35.2.643. [DOI] [PubMed] [Google Scholar]
- 19.Yang T, Park JM, Arend L, Huang Y, Topaloglu R, Pasumarthy A, Praetorius H, Spring K, Briggs JP, Schnermann J. Low chloride stimulation of prostaglandin E2 release and cyclooxygenase-2 expression in a mouse macula densa cell line. J Biol Chem. 2000;275:37922–37929. doi: 10.1074/jbc.M006218200. [DOI] [PubMed] [Google Scholar]
- 20.Khundmiri SJ, Ameen M, Delamere NA, Lederer ED. PTH-mediated regulation of Na+-K+-ATPase requires Src kinase-dependent ERK phosphorylation. Am J Physiol Renal Physiol. 2008;295:F426–F437. doi: 10.1152/ajprenal.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts BA, 3rd, George T, Good DW. Aldosterone inhibits apical NHE3 and HCO3- absorption via a nongenomic ERK-dependent pathway in medullary thick ascending limb. Am J Physiol Renal Physiol. 2006;291:F1005–F1013. doi: 10.1152/ajprenal.00507.2005. [DOI] [PubMed] [Google Scholar]
- 22.Falin R, Veizis IE, Cotton CU. A role for ERK1/2 in EGF- and ATP-dependent regulation of amiloride-sensitive sodium absorption. Am J Physiol Cell Physiol. 2005;288:C1003–C1011. doi: 10.1152/ajpcell.00213.2004. [DOI] [PubMed] [Google Scholar]
- 23.Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol. 2002;88:1270–1278. doi: 10.1152/jn.2002.88.3.1270. [DOI] [PubMed] [Google Scholar]
- 24.Yang SN, Yang CH, Huang LT, Wu YT, Wang CL. Enhancement of CREBSerine-133 phosphorylation through nitric oxide-mediated signaling induced by bacterial lipopolysaccharide in vascular smooth muscle cells from rats. Chin J Physiol. 2002;45:69–74. [PubMed] [Google Scholar]
- 25.He B, Weber GF. Phosphorylation of NF-kappaB proteins by cyclic GMP-dependent kinase. A noncanonical pathway to NF-kappaB activation. Eur J Biochem. 2003;270:2174–2185. doi: 10.1046/j.1432-1033.2003.03574.x. [DOI] [PubMed] [Google Scholar]
- 26.Soh JW, Mao Y, Liu L, Thompson WJ, Pamukcu R, Weinstein IB. Protein kinase G activates the JNK1 pathway via phosphorylation of MEKK1. J Biol Chem. 2001;276:16406–16410. doi: 10.1074/jbc.C100079200. [DOI] [PubMed] [Google Scholar]
- 27.Andoh T, Chiueh CC, Chock PB. Cyclic GMP-dependent protein kinase regulates the expression of thioredoxin and thioredoxin peroxidase-1 during hormesis in response to oxidative stress-induced apoptosis. J Biol Chem. 2003;278:885–890. doi: 10.1074/jbc.M209914200. [DOI] [PubMed] [Google Scholar]
- 28.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsao CC, Nica AF, Kurinna SM, Jiffar T, Mumby M, Ruvolo PP. Mitochondrial protein phosphatase 2A regulates cell death induced by simulated ischemia in kidney NRK-52E cells. Cell Cycle. 2007;6:2377–2385. doi: 10.4161/cc.6.19.4737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.