Abstract
The intercellular propagation of action potential is a necessary prerequisite of cardiac function. It is widely held that this is achieved by direct coupling between myocytes mediated by gap junctions – aggregates of cell-to-cell connexon channels. Connexons in the plasma membrane of cardiomyocytes not docked within gap junction aggregates are thought tightly regulated, though there is evidence that so-called connexon hemichannels may open in certain pathological conditions. It is presently not known whether undocked connexons are concentrated in specialized domains of membrane. Recently, we reported that interaction between the MAGUK scaffolding protein Zonula Occludens-1 (ZO-1) and the gap junction protein connexin 43 (Cx43) is concentrated in a region of the plasma membrane surrounding the gap junction plaque called the perinexus. It was found that ZO-1-Cx43 interaction governs a balance between undocked connexons in the perinexus and connexons docked in functional intercellular channels in the gap junction. In ongoing work it has been determined that the perinexus of cardiomyocyte gap junctions likely does contain high concentrations of undocked connexons composed of Cx43. This connexon-enriched zone of membrane appears to be a specialized nidus for integration of channel, junctional, and signal transduction molecules. Further insight into the function of the perinexus could provide new therapeutic avenues for the treatment of arrhythmia and other cardiac diseases.
Keywords: Connexin43, ZO-1, Gap Junction, Hemichannel, Perinexus
A Controversy of Connexins
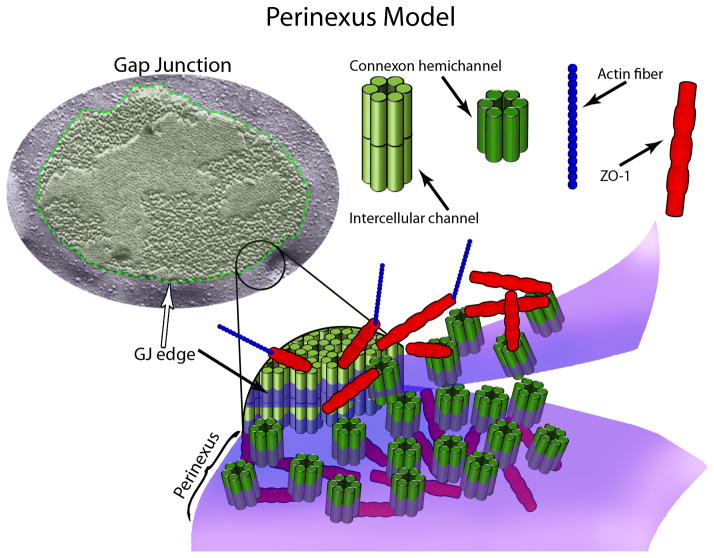
One of the more interesting debates going on in gap junction (GJ) biology is whether connexin hemichannels function in vivo or not? For those who accept the evidence for such structures, the hemichannel is envisaged as a gated pore in the plasma membrane formed by a hexamer of connexins (also known as a connexon) that is capable of undergoing regulated opening and closure. For those who do not, the concept that a connexon could be functionally operational as a membrane channel in living tissues prior to docking with another connexon in a GJ is viewed as highly improbable. Figure 1 provides a model illustrating conceptions of connexon hemichannels, intercellular channels and GJs.
Figure 1.
Cx43 oligomerizes to form hexameric half-channels called connexons or hemichannels, which interact with connexons on adjacent cells to create intercellular channels (top right). Intercellular channels aggregate in structures called gap junctions (GJs), which have a defined border in the Caspar and Makowski model (top left). The MAGUK scaffolding protein ZO-1 and actin have recently been demonstrated to complex with GJ-associated Cx43 (top left and bottom). Importantly, Cx43/ZO-1 interaction (sans actin) was shown to extend well beyond the edge of the GJ into a novel region called the perinexus (bottom). This region appears to function in regulating the transition of connexons from a non-junctional to a junctional configuration, and may be a hub for integration of channel-related, signaling, and cytoskeletal molecules.
The position that connexin hemichannel-associated phenomena do not occur, except as an experimental artifact, draws support from a number of propositions. First, a good deal of the data on connexin hemichannels comes from studies of cultured cells, heterologous expression systems and related experimental models1. Accounts of hemichannel function in vivo are less frequent, and often associated with pathology1. Second, with few exceptions, the connexin subunits of connexons form channels of large conductance1. The unregulated opening of even just a few of such large pores in the plasma membrane is generally thought to be inconsistent with cell survival. In a related third proposition, there is as yet little evidence for a specialized mechanism, cellular machinery and/or domain that could serve in the tight regulation that would be surely required for such prospectively dangerous channels.
In this perspective, data will be reviewed from recent work that could have bearing on the third proposition outlined above. In particular, the perinexus – a novel region surrounding GJ channel aggregates composed of connexin 43 (Cx43) that contains undocked connexons bound to a ZO-1 scaffold – will be discussed. The perinexus represents a specialized domain of plasma membrane that is enriched for connexons and patterns of protein-protein interaction that may provide opportunity for regulation of hemichannel activity.
Established Models of Gap Junction Structure
The origin of present understandings of the structure of GJs comes largely from the electron microscope (EM). Tangential sections through lanthanum-traced GJs in the EM gave the first hints of the channel array2. Freeze fracture EM provided higher spatial resolution views of GJs3. Aggregates of particles within GJs thought to represent individual connexons were revealed by freeze fracture to cluster in variably sized plaques in the membrane of nearly every cell type examined.
In a landmark study by Caspar and Makowski, low-dose EM was employed to resolve the micro-organization of the GJ and its connexon sub-units from negatively stained preparations of isolated plaques, giving us a structural model that persists almost unchanged in its primary features to this day4 (e.g. Figure 1). Ongoing work on GJ sub-structure has largely focused using methods such as cryoEM, X-ray diffraction, NMR, and atomic force microscopy to resolve the deployment of connexin proteins within channels at increasingly impressive levels of molecular resolution5. That being said, over the last decade other new insights into GJ organization have emerged. It turns out that not all parts of the plaque are the same. Of most pertinence to this essay is the periphery of the channel aggregate. It is now widely accepted that the edge of the GJ is of particular importance to its assembly. In key papers by Gaietta et al and Lauf et al undertaken in the early 2000s it was shown that GJ aggregates accumulate new channels at the periphery of the junction3.
Subsequent work from our laboratory provided evidence for a mechanism in which connexon accretion could be regulated at the GJ periphery6, 7. Zonula occludens-1 (ZO-1), which had previously been shown to interact with the Cx43, was shown to colocalize preferentially with the edge of GJ plaques, and regulate GJ size through its interaction with Cx43. As we will describe, it was via this work on the regulation of connexon recruitment that the significance of the perinexal domain of plasma membrane proximal to Cx43 GJs came into focus.
ZO-1 at the Edge
Before discussing our recent work in which the perinexus was described, a brief primer on ZO-1: Originally reported from studies of the tight junction, ZO-1 is a member of the membrane-associated guanylate kinase (MAGUK) family of multifunctional adaptor proteins that have roles in channel clustering, trafficking, signaling, and cell polarity. MAGUKs, such as ZO-1, synaptic protein PSD95 and the Drosophila tumor suppressor discs large (dlg) are characterized by amino-terminal protein-protein binding motifs, including one or more PDZ domains. A four amino acid PDZ-binding ligand at the extreme carboxyl terminus (CT) of Cx43 has been shown to be necessary for interaction with the second of three PDZ domains on ZO-18, though ongoing work has indicated that stable ZO-1-binding includes the last 19 amino acids of Cx43 CT and likely involves interaction with a ZO-1 homodimer9, 10.
Initial work on Cx43/ZO-1 interaction suggested that, like other MAGUK-membrane protein interactions, ZO-1 stabilized Cx43 at the cell surface11. Subsequent work has indicated an interesting interplay between ZO-1 and activated c-Src competing for mutually exclusive binding of the Cx43 CT9, 12. At the outset, a simple mechanism in which c-Src displacement of ZO-1 from the Cx43 CT resulted in reduced cell-surface Cx43 and GJIC was favored12. However, recent elegant microscopic work by Baker et al, and Gilleron et al has demonstrated that the c-Src-mediated GJ internalization mechanism requires both Cx43/ZO-1 and Cx43/c-Src interaction in an asymmetric mode, with c-Src on the outside and ZO-1 on the inside of annular junctions 13, 14. Importantly, these data corroborated earlier work in which we observed an increase in annular junctions and Cx43 colocalization with ZO-1 in primary cardiomyocytes post-isolation15.
In the early and mid 2000s, our lab developed evidence for two further aspects of ZO-1 structure and function with respect to GJs composed of Cx43. First, we observed an accumulation of ZO-1 at the GJ perimeter. Second, it was determined that Cx43/ZO-1 interaction was controlling the rate at which Cx43 moved between non-junctional (detergent-soluble) and junctional (detergent-insoluble) pools of the protein on the basis of experimental strategies involving mutant constructs and competitive inhibitors6. However, whether ZO-1 interactions at the GJ, or elsewhere in the cell, controlled the transition between non-junctional and junctional pools of Cx43 was not clear until recently.
The Perinexus is a Novel GJ Associated Domain of Membrane
The rationale underpinning our recently published work on where and how ZO-1 governs the transition of Cx43 into GJs were driven by two questions. First, it was of interest to determine the molecular mechanism by which ZO-1 controlled Cx43 GJ size. Second, we were curious to find whether ZO-1 modulated Cx43 functions via its effects on GJ size.
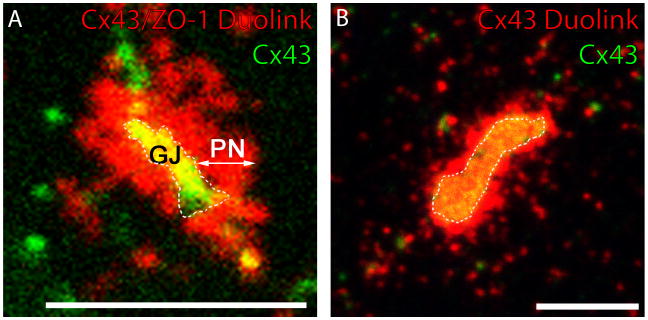
In addressing the first of these questions, the most intensive work has focused on localizing Cx43/ZO-1 interaction at increased sub-cellular resolution. An important technical aid to this effort has been an ability to amplify patterns of Cx43/ZO-1 association with improved sensitivity using the Duolink assay. In addition to confirming that ZO-1 was concentrated at the periphery of the GJ, this novel assay also resolved an extended domain of Cx43/ZO-1 interaction in the plasma membrane proximal to the GJ edge i.e., the perinexus (Fig. 2a). Using Duolink in combination with competitive inhibition approaches similar to those employed previously6, it was determined that the perinexus likely represented the primary site where ZO-1 controls the rate at which connexons composed of Cx43 transition to GJs7.
Figure 2.
Cardiomyocytes possess a perinexus concentrated in Cx43 connexons and Cx43/ZO-1 interaction. (A) Rat epicardial cells expressing GFP-tagged Cx43 in addition to endogenous Cx43 (Green), and Duolink labeling of Cx43/ZO-1 interaction (Red). (B) Cardiomyocytes labeled for Cx43 (Green) and Duolink Cx43/Cx43 interaction (Red). Scale bars = 5μM.
Rhett et al also provided information on the partitioning mechanism of Cx43 between detergent-soluble (non-junctional) and detergent-insoluble (junctional) pools of the protein7. Using immunolabeling and co-immunoprecipitation we determined that Cx43/ZO-1 complexes in the perinexus have less avid interactions with actin than those at or within the GJ. Detergent solubility thus appears to be determined by whether Cx43 is linked to the actin cytoskeleton or not. In a more recent use of the Duolink assay, we amplified Cx43-Cx43 interactions within connexon channels. The rationale was to resolve Cx43 in the GJ and surrounding perinexus simultaneously. The distance that Cx43/Cx43 interaction extends from the GJ edge was identical to that resolved for Cx43/ZO-1 (Fig. 2b). These data indicated that the perinexus was indeed a site of Cx43 connexon enrichment in the plasma membrane adjacent to the GJ proper, as well as a site of interaction with ZO-1.
Taken together, our results indicate a new aspect of structure that might be considered for addition to Caspar and Makowski’s classical model (Fig. 1), at least for GJs composed of Cx43. In this revised model the Cx43 GJ plaque does not stand alone, but is surrounded by a perinexal scaffold - a sub-membrane network containing ZO-1 that extends beyond the edge of the GJ proper. This network appears to act as a meshwork that concentrates undocked connexons and regulates the rate at which connexons transition from the surrounding plasma membrane into the gap junction (Fig. 1).
The Functional Significance of The Perinexus
The question of whether ZO-1 regulation of the connexon transition has functional correlates was also addressed in our recent report. Based on the structural and biochemical data discussed above, it was anticipated that disruption of Cx43/ZO-1 interaction should increase GJ intercellular communication (GJIC) and reduce connexon hemichannel function. In other words, disruption of Cx43/ZO-1 interaction should increase GJ size (i.e. intercellular channels) at the expense of perinexal hemichannels - resulting in an increase in functional intercellular channels and a concomitant decrease in hemichannels available to act as pores between the cytoplasm and extracellular space.
The changes observed in Cx43 function observed experimentally were in line with these expectations7. Using multiple methodologies to reduce Cx43/ZO-1 interaction, GJIC was assayed by scrape loading and fluorescence recovery after photobleaching (FRAP), and hemichannel activity was assessed by ethidium bromide (EtdBr) uptake. It was found that when Cx43/ZO-1 was inhibited, HeLa cells expressing Cx43 (Cx43-HeLa) showed greater dye spread in a scrape-loaded monolayer and increased rates of bleached-cell fluorescence recovery (i.e., FRAP) through GJ connections to neighboring unbleached cells7. Conversely, hemichannel-mediated membrane permeability was reduced by blockade of Cx43/ZO-1 interaction7. Specifically, disrupting Cx43/ZO-1 interaction reduced the rate and magnitude of EtdBr uptake from the extracellular medium in monolayers of Cx43-HeLas. Interestingly, we could show that the observed changes were not likely to be due to changes in gating of connexon hemichannels, as cells not in contact with other cells did not display changes in dye uptake when Cx43/ZO-1 was reduced. Together, the results indicate that by controlling the conversion of perinexal connexons to GJ intercellular channels, ZO-1 not only regulates GJ organization, but also may govern a balance between levels of intercellular communication and hemichannel-mediated membrane permeability.
Is there a physiological counterpart to ZO-1 regulation of Cx43 function? In the heart it is well established that GJs are disrupted in a variety of cardiac pathologies in a process called remodeling. A feature of remodeling is redistribution of Cx43 from intercalated disks to the lateral borders of myocytes. Given the redistribution of connexons observed when Cx43/ZO-1 interaction is perturbed, it is difficult not to draw parallels to GJ remodeling processes occurring during cardiac disease states. Consistent with this, work from the Severs lab has demonstrated a negative correlation between ZO-1 and Cx43 immunofluorescence levels in the intercalated disc of working ventricular myocytes from the transplanted hearts of patients suffering from dilated and ischemic cardiomyopathies16. It was further shown in this study that Cx43 had greater co-immunoprecipitation with ZO-1 in the disease samples.
It is tempting to speculate that changes observed in Cx43/ZO-1 interaction in some diseases of heart may occur preferentially in the perinexus. Cx43 redistribution during cardiac remodeling and/or lateralization may also represent a pathological shift in connexon hemichannel location from constraint in the perinexus and GJ, to free mobility in the plasma membrane. Unregulated opening of hemichannels would slow action potential velocity by decreasing space constant and also increase the chance of spontaneous depolarizations by altering membrane potential. In addition, hemichannels in isolated myocytes have been reported to prompt release of ATP and promote cell death under pathologic conditions17.
This model of GJ lateralization is complicated by other studies demonstrating a reduction in Cx43/ZO-1 interaction following an ischemic injury. In one recent example, NMR, yeast 2-hybrid, and surface plasmon resonance studies demonstrated that c-Src can bind ZO-1 with greater affinity than Cx4318. It was additionally demonstrated that this interaction between Src and ZO-1 increased in the injury border zone following coronary occlusion in a dog model of myocardial infarction. GJ lateralization and reduction of Cx43 levels were observed, and concomitantly, Cx43/ZO-1 interaction was reduced. Given the works of Gilleron et al, Baker et al, and Barker et al discussed earlier in this viewpoint essay, it is possible that these data represent GJ internalization through the mechanism of asymmetric ZO-1 and c-Src binding to opposite faces of the plaque13–15. The differences in accounts of the role of Cx43/ZO-1 interaction in cardiac pathology may be explained by considering that a variety of species, models and pathologies have been examined in these studies. Detailed molecular studies will be needed to resolve the various mechanisms that may be involved in pathological GJ remodeling and the role that the perinexus plays.
Are there additional physiological roles for the perinexus other than as a staging post for connexons waiting to dock in the GJ? Mori et al may provide the context for such a function. Using a 3D electrodiffusion model of cardiac conduction, these authors found that within a small range of cleft widths (i.e., the span of extracellular space in the intercalated disc between the sarcolemma of two myocytes) sodium channel-mediated conduction between cells can theoretically take place without recourse to GJs19.
The perinexus is physically located at the cleft between the membranes of adjacent cells where these membranes come together to form a GJ. The extracellular width at the perinexus thus has the potential to be extremely narrow. Additionally, it is well established that a population of Nav1.5 channels are concentrated at the intercalated disk and that some of these channels co-localize at, or in the vicinity of, GJs20. These data, together with our demonstration that the myocyte perinexus contains high concentrations of connexons, suggests the possibility that one function of the Cx43 perinexus may be to provide the framework for an ephapse between cardiomyocytes. Interestingly, a related unconventional ephaptic role for connexin hemichannels in inhibitory signaling between horizontal cells and cones in the vertebrate retina has recently been identified by Klaaseen and colleagues21.
Concluding Comments
The identification of the perinexus opens up a number of new opportunities in further understanding GJ biology and cardiac physiology. First, the perinexus likely has a biochemistry that is distinct from the GJ. This would include characteristic dynamic patterns of interaction between Cx43 and known partnering proteins, as well as unique protein-protein interactions. Uncovering the partitioning of Cx43 binding partners between the GJ and perinexus should assist in elucidating the unique functions of these two distinct domains. Second, novel aspects of hemichannel biology could be uncovered by studies specifically targeted to the perinexus. For example, it would be most interesting to determine whether hemichannel gating differs between the perinexus and other non-junctional regions of plasma membrane.
Finally, the study of cardiac GJ biology in the context of the perinexus could have clinical implications. Indeed, work from our laboratory has already shown that competitive inhibition of Cx43/ZO-1 interaction may contribute to reduced arrhythmogenic potential in injured mouse hearts22. Strategies for targeting the transition of connexons between the perinexus and the GJ, as well as the inhibiting potential loss of connexons from the perinexal domain, may hold promise in therapeutic strategies for treating heart disease.
Acknowledgments
Financial Support: This work was supported in part by grants from the National Institutes of Health (RO1 HL56728-10A2 to RGG, RO11DE019355-1 RGG subcontract, F30 HL095320-01 RGG mentor, and 5P20RR016434-07 RGG mentor), and an AHA Grant-in-Aid (RGG).
Abbreviations
- Cx43
Connexin43
- ZO-1
Zonula Occludens-1
- GJ
Gap Junction
- EM
Electron Microscope
- MAGUK
Membrane Associated GUanylate Kinase
- dlg
discs large
- CT
carboxyl terminus
- GJIC
Gap Junction Intercellular Communication
- EtdBr
Ethidium Bromide
- FRAP
Fluorescence Recovery After Photobleaching
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003 Oct;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 2.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967 Jun;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palatinus JA, Rhett JM, Gourdie RG. The Connexin43 Carboxyl Terminus and Cardiac Gap Junction Organization. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011 Aug 9; doi: 10.1016/j.bbamem.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspar DL, Goodenough DA, Makowski L, Phillips WC. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol. 1977 Aug;74:605–628. doi: 10.1083/jcb.74.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Arce FT, Ramachandran S, Lal R. Nanomechanics of hemichannel conformations: connexin flexibility underlying channel opening and closing. The Journal of biological chemistry. 2006 Aug 11;281:23207–23217. doi: 10.1074/jbc.M605048200. [DOI] [PubMed] [Google Scholar]
- 6.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005 Dec;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Molecular Biology of the Cell. 2011 May;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Current Biology. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 9.Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem. 2004 Dec 24;279:54695–54701. doi: 10.1074/jbc.M409552200. [DOI] [PubMed] [Google Scholar]
- 10.Xiao F, Weng J, Fan K, Wang W. Detailed Regulatory Mechanism of the Interaction between ZO-1 PDZ2 and Connexin43 Revealed by MD Simulations. PLoS One. 2011;6:e21527. doi: 10.1371/journal.pone.0021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998 May 22;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 12.Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001 Jan 19;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- 13.Baker SM, Kim N, Gumpert AM, Segretain D, Falk MM. Acute internalization of gap junctions in vascular endothelial cells in response to inflammatory mediator-induced G-protein coupled receptor activation. FEBS Lett. 2008 Dec 10;582:4039–4046. doi: 10.1016/j.febslet.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilleron J, Fiorini C, Carette D, et al. Molecular reorganization of Cx43, ZO-1 and Src complexes during the endocytosis of gap junction plaques in response to a non-genomic carcinogen. J Cell Sci. 2008 Nov 25; doi: 10.1242/jcs.033373. [DOI] [PubMed] [Google Scholar]
- 15.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002 Feb 22;90:317–324. doi: 10.1161/hh0302.104471. [DOI] [PubMed] [Google Scholar]
- 16.Bruce AF, Rothery S, Dupont E, Severs NJ. Gap junction remodelling in human heart failure is associated with increased interaction of connexin43 with ZO-1. Cardiovasc Res. 2008 Mar 1;77:757–765. doi: 10.1093/cvr/cvm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shintani-Ishida K, Uemura K, Yoshida K. Hemichannels in cardiomyocytes open transiently during ischemia and contribute to reperfusion injury following brief ischemia. Am J Physiol Heart Circ Physiol. 2007 Sep;293:H1714–1720. doi: 10.1152/ajpheart.00022.2007. [DOI] [PubMed] [Google Scholar]
- 18.Kieken F, Mutsaers N, Dolmatova E, et al. Structural and Molecular Mechanisms of Gap Junction Remodeling in Epicardial Border Zone Myocytes following Myocardial Infarction. Circ Res. 2009 Apr 2; doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A. 2008 Apr 29;105:6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petitprez S, Zmoos AF, Ogrodnik J, et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res. 2011 Feb 4;108:294–304. doi: 10.1161/CIRCRESAHA.110.228312. [DOI] [PubMed] [Google Scholar]
- 21.Klaassen LJ, Sun Z, Steijaert MN, et al. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011 Jul;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A Peptide Mimetic of the Connexin43 Carboxyl Terminus Reduces Gap Junction Remodeling and Induced Arrhythmia Following Ventricular Injury. Circ Res. 2011 Jan 27; doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]