Abstract
Pain has both sensory-discriminative and emotional-affective dimensions. Previous studies demonstrate that electroacupuncture (EA) alleviates the sensory dimension but do not address the affective. An inflammatory pain rat model, produced by a complete Freund adjuvant (CFA) injection into the hind paw, was combined with a conditioned place avoidance (CPA) test to determine whether EA inhibits spontaneous pain-induced affective response and, if so, to study the possibility that rostral anterior cingulate cortex (rACC) opioids underlie this effect. Male Sprague-Dawley rats (250–275g, Harlan) were used. The rats showed place aversion (i.e. affective pain) by spending less time in a pain-paired compartment after conditioning than during a preconditioning test. Systemic non-analgesic morphine (0.5 and 1.0 mg/ kg, i.p.) inhibited the affective reaction, suggesting that the affective dimension is underpinned by mechanisms different from those of the sensory dimension of pain. Morphine at 0.5 and at 1 mg/kg did not induce reward. Rats given EA treatment before pain-paired conditioning at GB 30 showed no aversion to the pain-paired compartment, indicating that EA inhibited the affective dimension. EA treatment did not produce reward or aversive effect. Intra-rACC administration of D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr amide (CTOP), a selective mu opioid receptor antagonist, but not norbinaltorphimine (nor-BNI), a selective kappa opioid receptor antagonist, blocked EA inhibition of the affective dimension. These data demonstrate that EA activates opioid receptors in the rACC to inhibit pain-induced affective responses and that EA may be an effective therapy for both the sensory-discriminative and the affective dimensions of pain.
Keywords: Acupuncture, Anterior cingulate cortex, Opioid, Affective pain, CTOP
1. Introduction
For thousands of years, acupuncture, a traditional therapeutic modality, has been used in China and other Asian countries to treat a variety of diseases and symptoms, including pain (Cheng, 1999), which has both sensory-discriminative and emotional-affective dimensions. Recent studies, including our own (Lao et al., 2004), have shown that electroacupuncture (EA) increases noxious stimulation-induced paw withdrawal latency (PWL) in inflammation pain animal models (Zhang et al., 2002). These studies demonstrate that EA alleviates the sensory-discriminative dimension of pain. In a clinical trial, an acupuncture group experienced significantly greater improvement in pain and function scores and patient global assessment than did a sham group (Berman et al., 2004). Further, acupuncture on patients with fibromyalgia relieved both the sensation of pain and pain-related anxiety (Martin et al., 2006). In an uncontrolled observation, acupuncture improved the affective dimension of a variety of chronic pain conditions, including headache, facial, and spine-associated pain syndromes (Hammes et al., 2002). While these studies suggested that acupuncture may inhibit the affective dimension of pain, the underlying mechanisms were unknown and the effects of acupuncture/EA on the emotional-affective dimension of pain had not been investigated in preclinical studies.
Previous studies suggest that the anterior cingulate cortex (ACC) plays a role in affective pain. Early studies show that surgical ablation of the ACC and surrounding cortical tissues decreases affective responses (i.e. affective pain) to noxious stimuli but does not alter the ability of the human subject to judge the intensity or location of an unpleasant stimulus (Foltz and White, 1962; Hurt and Ballantine, 1974). Recent brain imaging studies on noxious stimulation and persistent pain conditions consistently report increased cerebral blood flow, indicating increased neuronal activity in the ACC (Casey, 1999; Wagner et al., 2007). In animals, bilateral ACC lesions decrease the development of place-avoidance behavior in a formalin-conditioned place avoidance (CPA) model (Johansen et al., 2001). ACC lesions in rats with nerve injury also significantly decrease escape/avoidance behavior but do not alter mechanical hypersensitivity (LaGraize et al., 2004). Moreover, intra-ACC microinjection of morphine produces a selective, naloxone-reversible reduction in affective pain, as evidenced by a decrease in the avoidance response induced by noxious cutaneous stimulation in a neuropathic pain rat model (LaGraize et al., 2006).
Since it has been well demonstrated that acupuncture induces release of endogenous endorphins (Mayer, 2000), we hypothesized that rostral ACC (rACC) opioids underpin acupuncture’s inhibition of affective pain. Affective pain has been studied by combining an inflammatory pain model with a CPA test (Hummel et al., 2008; Johansen et al., 2001). We used this combination to determine whether EA inhibits complete Freund adjuvant (CFA)-induced affective response and, if so, to study whether the opioids underlie this effect.
2. Methods
2.1 Experimental design
Male Sprague–Dawley rats weighing 250–275 g (Harlan) were kept under controlled environmental conditions (22 ± 0.5°, relative humidity 40–60%, 7 am to 7 pm alternate light–dark cycles, food and water ad libitum). The animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland School of Medicine. The ethical guidelines for the treatment of animals of the International Association for the Study of Pain were followed in these experiments.
Five experiments were carried out. The first two were to validate the affective pain animal model with morphine and the other three to investigate the effect and mechanisms of EA on affective pain.
In Experiment 1, rats were divided into three groups (n=6 / group): 1) naive, 2) saline-injected, and 3) CFA-injected. The saline or CFA (0.08 ml) was injected into one hind paw. The aim of this experiment was to determine whether the apparatus chambers produce neutral stimuli and whether CFA-induced spontaneous pain generated a negative affect that could be assessed with CPA. These three groups were not treated with EA or sham EA.
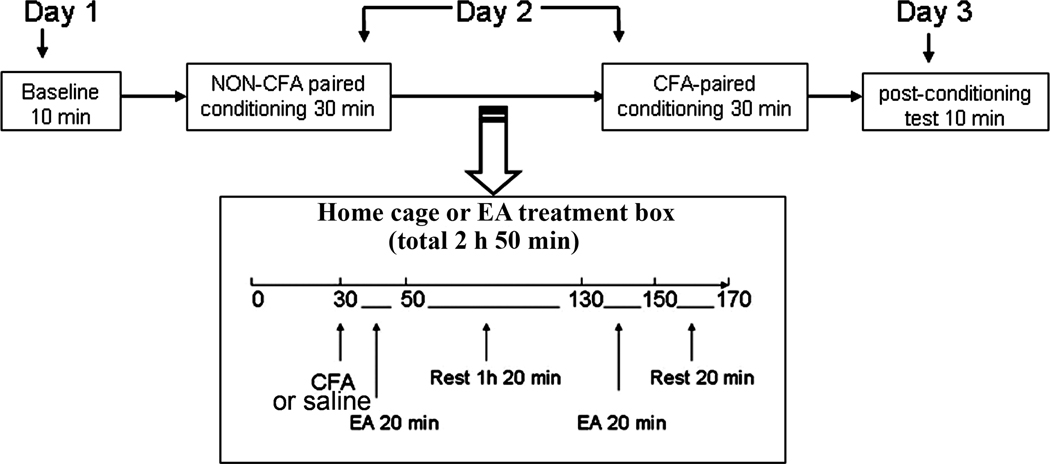
In Experiment 2, to investigate the effect of a systemic non-analgesic dosages (0.5 and 1 mg/kg) of morphine on CFA-induced negative affective response, three groups of rats were given intraperitoneal (i.p.) morphine or saline prior to pain-paired conditioning (n=6 /group): 1) saline (0.25 ml), 2) morphine at 0.5 mg/kg in 0.25 ml saline and 3) morphine at1 mg/kg. CFA was injected into a hind paw 30 min post non-CFA paired conditioning. Morphine was administered 30 min before pain-paired conditioning (i.e. 1 h 30 min post-CFA) on the second day. Saline (0.25 ml, i.p.) was injected at the same time points.
Morphine induces reward and thus may confound the interpretation of CPA data. To determine whether low dosage morphine induces reward in the absence of pain, rats were divided into three groups (n=6 per group) and injected i.p. with 1) morphine (0.5 mg/kg), 2) morphine (1 mg/kg), or 3) saline only (0.25 ml) for a conditioned place preference (CPP) test. Like the CPA test described below (Fig. 1), the CPP test consisted of three phases: pretesting, conditioning, and testing. On day one, rats were allowed to freely explore the entire testing apparatus, two separate compartments, in a 10-min pretest. The amount of time spent in each compartment was recorded. On day two, the rats were injected with saline (0.25 ml, i.p.) and, 30 min later, were placed individually into one compartment for a 30-min conditioning session. Two h after its saline-paired conditioning, each rat in Group 1 or 2 was injected with its respective dosage of morphine and, 30 min later, was conditioned for 30 min in the second compartment. The saline-only group received nothing before conditioning in the second compartment in order to determine whether the injection and the conditioning schedule influences time spent in the apparatus. On day three, the rats were tested as in the pretest, but without morphine or saline, and the times spent in each compartment were again recorded.
Fig. 1.
Flow chart of the experimental design. On day one, time spent by the rats in each of two distinct compartments was recorded during a 10-min preconditioning period. On day two, each rat freely explored the first compartment for 30 min before a CFA injection and the second compartment for 30 min after CFA. On day three, the time spent in each compartment during a 10-min post-conditioning test was recorded again.
In Experiment 3, to investigate the effect of EA on pain-induced affective response, CFA-injected rats were divided into four groups (n=6–8 per group): 1) CFA + sham EA control, 2) CFA + EA treatment prior to pain-paired conditioning on day two to determine the effect of EA on CFA-induced affective response, 3) saline + sham EA control and 4) saline + EA treatment prior to pain-paired conditioning on day two to determine whether EA alone produces reward or aversion that may confound the data interpretation.
In Experiment 4, to investigate the involvement of opioid receptors in EA action, rats were prepared for bilateral intra-rACC cannulation and allowed to recover for five days prior to experiment. Four groups of saline- and CFA-injected rats were treated with EA or sham EA: 1) saline + sham EA, 2) CFA + sham EA, 3) saline + EA, and 4) CFA + EA. The rats in Group 4, CFA + EA, were randomly divided into three subgroups (n=8 per group) to reveal the role of endogenous opioids in the EA inhibition of affective response: 1) 6.25 nmol/0.25 µl/side of the mu opioid receptor antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr amide (CTOP) + EA, 2) 10 nmol/0.4 µl/side of the kappa opioid receptor antagonist norbinaltorphimine (nor-BNI) + EA, and 3) vehicle + EA. The antagonist dosages are based on our preliminary study. The antagonists, dissolved in saline, were administered five min before the CFA injection. Previous studies demonstrate that intracerebroventricular CTOP and nor-BNI both prevent acetaminophen-produced analgesia for two hours after administration (Bujalska, 2004), indicating that CTOP and Nor-BNI may block their respective receptors in the rACC for at least the two hours of the EA experiment.
Groups 1–3, saline + sham EA, CFA + sham EA and saline + EA, were similarly divided into three subgroups and tested.
In Experiment 5, to clarify whether EA inhibition of the affective dimension of pain depends on its inhibition of the nocifensive reflex, indicative of the sensory dimension, CFA-injected rats were divided into three groups (n=6/group): 1) intrathecal (i.t.) CTOP + EA prior to pain-paired conditioning, 2) saline (i.t.) + EA and 3) saline (i.t.) + sham EA. CTOP at 5 nmol, which blocked EA inhibition of nocifensive response in our previous study (Zhang et al. 2004), was given (i.t.) five min before the CFA injection, which was followed by EA treatment and pain-paired conditioning. After conditioning, PWL was measured to determine EA’s effect on the sensory component of pain.
2.2 EA treatment
EA treatment was performed according to the procedures previously developed in our laboratory (Lao et al., 2004). The animals were gently handled for 30 min each day for two or three days before the experiment. After cleaning a rat’s skin with alcohol swabs, one investigator held the animal while the second swiftly inserted disposable acupuncture needles (gauge #32, 0.5 in. in length), with electrodes soldered to their handles, bilaterally and approximately ½ in. deep, into each flank at the equivalent of human acupoint GB30. In humans, GB30, the 30th point on the Gallbladder Meridian, is located at the junction of the lateral third and medial two-thirds of the distance between the greater trochanter and the hiatus of the sacrum. The needles and electrodes were stabilized with adhesive tape. The procedure typically lasted less than 20 s and caused little distress to the animal.
The EA was delivered by an electrical stimulator via an isolator (A360D Stimulus Isolator, World Precision Instruments) that converts electrical voltage into constant electrical current. In order to minimize discomfort to the animal, current intensity was adjusted slowly over the period of approximately two min to the designated level of 3 mA, the maximum current the animal can tolerate without distress. Since the electrical stimulation was administered to the muscles, not directly to the peripheral nerve, mild muscle twitching was observed at this intensity.
EA treatment was given immediately after the CFA injection on day two. Twenty min of stimulation at10 Hz, 3 mA, 0.1 ms pulse width was applied bilaterally at GB30 once at the beginning and again at the end of a 2 h period (Lao et al., 2004). During EA treatment, each rat was placed on a glass surface under an inverted clear plastic chamber (approximately 5”× 8”×11”) without restraints or anesthetics. The animals remained awake and still during treatment, and no signs of distress were observed (Lao et al., 2004). For sham EA control, acupuncture needles were inserted bilaterally at acupoint GB30; a pair of electrodes from the stimulator was attached to the ends of the needles but no electrical current was delivered.
2.3 CPA test
Place conditioning was performed as described by Fenu et al. (Fenu et al., 2006), with modifications. The place conditioning apparatus is made of Plexiglas and consists of two equal, rectangular compartments (12” long × 8” high × 5” wide) positioned on top of mesh screens and separated by guillotine doors. One compartment is covered on three sides and the ceiling with horizontal yellow 0.5-inch stripes at 0.5-inch intervals; its bottom is #8×8 plain steel mesh (8 mm holes). The other compartment is covered on three sides and the ceiling with vertical orange stripes and has #3×3 plain steel mesh (3 mm holes) on its floor. The guillotine doors are covered with the colored stripes corresponding to their respective walls; they were inserted during conditioning sessions and removed during pre- and post-conditioning tests. The vertically striped compartment is laterally illuminated on the outside with a Coast Led Lenser mini-Tac Torch. The colored horizontal and vertical stripes and the light are the visual cues; the differing steel mesh is the tactile cue. The testing room has dim indirect lighting consisting of one 15-W bulb positioned about 1 m from the apparatus. The apparatus was cleaned with 75% ethanol after each test.
On day one, baseline time spent by the rats in each of the two distinct compartments during a 10-min preconditioning period was recorded (Fenu et al., 2006; Johansen et al., 2001). The animal was considered to be in a chamber when both its front paws were in it.
On day two, each rat was free to explore one of the conditioning compartments for 30 min, then removed from the conditioning compartment and returned to its home cage. Thirty min later, saline or CFA (Sigma, St Louis, MO; 0.08 ml, 40 µg Mycobacterium tuberculosis), suspended in an 1:1 oil/saline emulsion, was subcutaneously injected into the plantar surface of one hind paw using a 25-gauge hypodermic needle (Zhang et al., 2004). After the injection, each rat in experiments 1 and 2 was returned to its clear plastic home cage; each rat in experiments 3–5 received EA under a clear, inverted plastic chamber. Two h after the CFA injection, each rat was allowed to explore the second compartment for another 30 min. The pain-paired compartment was alternated so that half of the subjects were pain-conditioned in the orange chamber, the other half in the yellow.
During the10-min post-conditioning test on day three, the time spent by the rats in each compartment was recorded again (Fig. 1). CPA score magnitudes, used as an indicator of affective response, were determined by subtracting time spent in the pain-paired compartment during the preconditioning test from time spent in that compartment during the post-conditioning test: the less post-conditioning time spent in the compartment, the greater the affective response. The investigator who performed the CPA test was blinded to the group assignment.
2.4 Intra-Bilateral Cingulate Cortex Cannulation
Animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and held in a stereotaxic frame (Stoelting, Wood Dale IL). An incision was made on the midline of the head and a small hole was drilled. A double, bilateral 26-gauge stainless steel guide cannula (Plastic One, Roanoke, VA) was implanted toward the ACC, 2.6 mm rostrally, 0.6 mm laterally, and 2.4 mm ventrally to the bregma according to the Paxinos and Watson flat skull coordinate system. This double guide cannula was secured with dental cement and two small screws. A double, bilateral dummy cannula, cut to extend 0.5 mm beyond the guide cannula and covered with a dust cap, remained in the guide except during drug infusion. Following cannulation, animals were housed singly and allowed to recover for five days prior to the experiment.
For drug infusion, a 0.6 cm length of PE-50 tubing was connected to each end of a 15-cm length of PE-10 tubing. During infusion, the dummy cannula was replaced by an injector that was inserted 0.5 mm beyond the guide cannula to target the ACC. One end of the tubing was connected to the injector and the other to a 50-µl Hamilton syringe. The solution was infused at 0.1µl/min for a total of 0.25 µl on each side of the rACC with a pump (KD Scientific, Model 780210). After infusion, the injector was left in the cannula for another 2 min to allow the chemicals to spread at the injected area.
2.5 Intrathecal drug delivery
Lumbar punctures were performed as previously described (Li et al., 2011). A PE10 polyethylene tube (Clay Adams) was submerged in 70°C water, stretched to about 150% of its original length to reduce the diameter, and used as an injection catheter. With a 29-gauge needle, another 10-cm PE10 tube was connected to one end of the catheter and then to a 50-µl glass Hamilton syringe with a PE50 tube. The injection catheter was pre-filled with 10 µl of drug or vehicle and 5 µl of saline separated by a small air bubble. Under isoflurane anesthesia, the dorsal pelvic area was shaved and swabbed with 70% alcohol. A 21-gauge sterile needle with the plastic hub removed was inserted between lumbar vertebrae L5 and L6. The catheter was inserted into the guide needle and rostrally advanced 4 cm from the tip of the needle into the lumber enlargement, where its arrival was confirmed by a tail-flick. The drug CTOP, or vehicle, was injected and followed by a saline flush. Five min after injection, the catheter was withdrawn and the needle was removed from the inter-vertebral space.
2.6 Paw withdrawal latency
Hyperalgesia was determined by a decrease in PWL to a noxious thermal stimulus. PWL was tested with Hargreaves’s method (Hargreaves et al., 1988; Lao et al., 2004). Each rat was placed under an inverted clear plastic chamber on the glass surface of the Paw Thermal Stimulator System (UCSD, San Diego) and allowed to acclimatize for 30 min before the test. A radiant heat stimulus was applied to the plantar surface of each hind paw from underneath the glass floor with a projector lamp bulb (CXL/CXR, 8 V, 50 W). PWL to the nearest 0.1 sec was automatically recorded when the rat withdrew its paw from the stimulus. Stimulus intensity was adjusted to derive a baseline PWL of approximately 10.0 sec in naïve animals. Paws were alternated randomly to preclude order effects; a 20-sec cut-off was used to prevent tissue damage. Four tests were conducted for each hind paw, with a 5-min interval between each test. Mean PWL was established by averaging the tests.
2.7 Histology
After the experiment, the infusion site was verified by histology. The animals were perfused with saline and 10% formalin under analgesia with sodium pentobarbital. The brains were removed and immersed in 10% formalin for 2 h and transferred to 30 % sucrose. The tissue at the cannula site was cut at 40-µm thick coronal sections which were stained and examined under a microscope to determine the location of the cannula according to Paxinos and Watson’s atlas.
2.8 Statistical analysis
Raw data or CPA score magnitudes (Figs. 3A, 4 and 6A) were presented. For the magnitudes, preconditioning time spent in the pain-paired compartment by each rat was subtracted from the post-conditioning time it spent there, averaged for each group of rats. The data were analyzed with one- or two-way ANOVA to reveal whether CFA-induced pain produced an affective response, whether EA inhibited such a response, and whether opioid receptors were involved in the EA effect. Bonferroni post-tests were conducted to reveal differences among groups of rats at each time point (GraphPad Prism 5.0). P<0.05 was considered significant.
Fig. 3.
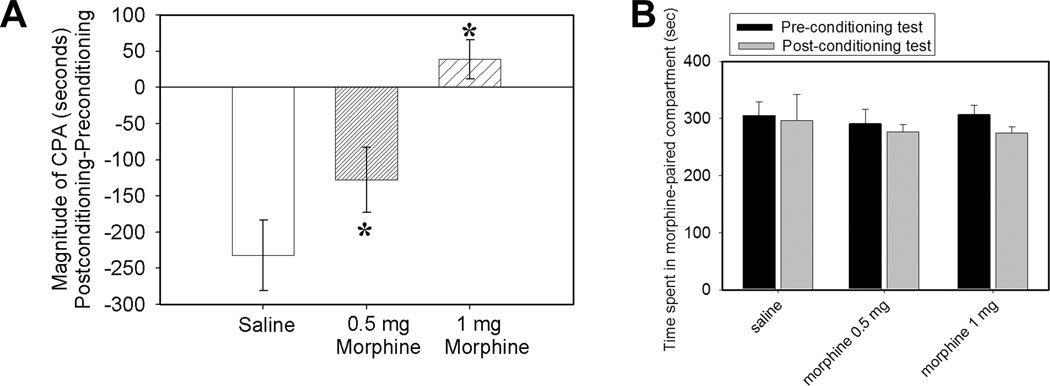
Fig. 3A. Effects of systemic non-analgesic dosages (0.5 and 1 mg/kg, i.p.) of morphine on CFA-induced affective pain (n=6/group). Compared to saline control, these dosages significantly blocked CFA-induced aversive response. *P<0.05 vs saline control. Fig. 3B. Conditioned place preference score in morphine-treated rats (0.5 and 1 mg/kg; n=6 per group). Times spent in a morphine-paired chamber during pre- and post-conditioning tests are presented as mean ± se. Neither saline- nor morphine-paired compartments produced preference.
Fig. 4.
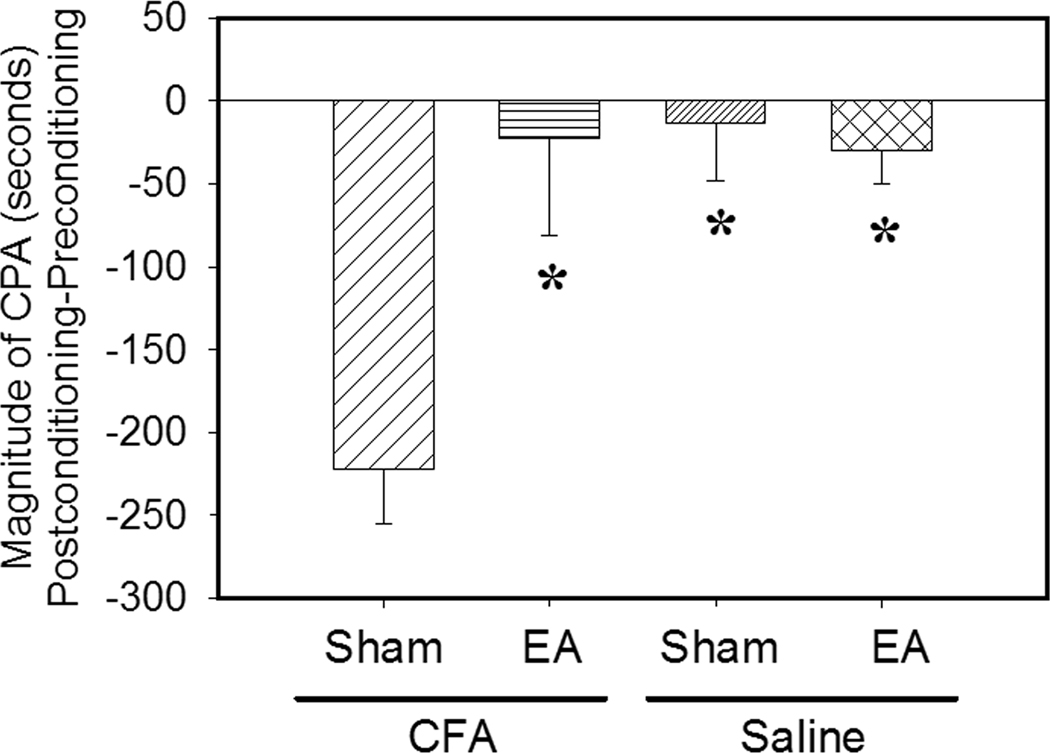
Columns 1 & 2: EA inhibition of CFA-induced affective response (n=8 per group). EA was given on day two before pain-paired conditioning. CFA-injected rats given sham EA showed avoidance to a pain-paired compartment. EA treatment blocked the avoidance response. *P<0.05 compared to sham control. Columns 3 & 4: Conditioned place preference score in saline-injected rats with EA or sham EA (n=6 per group). Times spent in an EA- or sham-paired chamber during pre- and post-conditioning tests are presented as mean ± se. Neither paired conditioning produced preference or avoidance of the respectively paired compartment. *P<0.05 compared to sham control in CFA-injected rats.
Fig. 6.
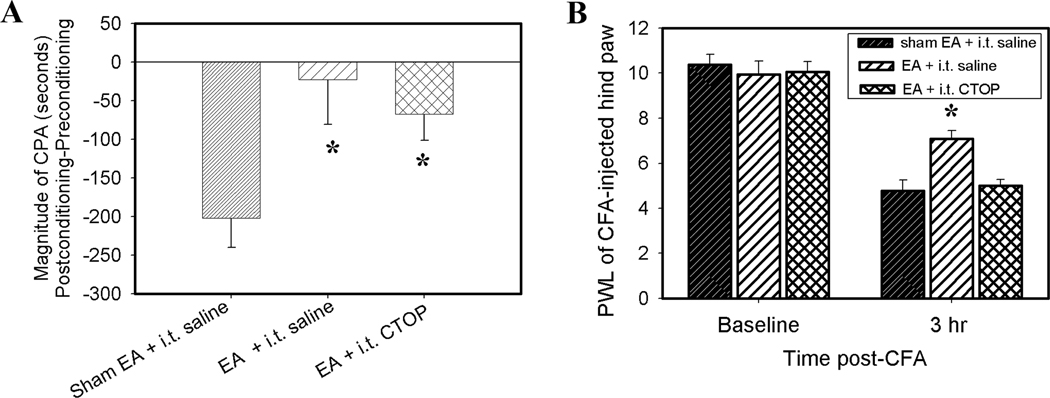
Fig. 6A. Effects of CTOP (5 nmol, i.t.), a mu-selective opioid receptor antagonist, on EA inhibition of CFA-induced affective response (n=6/group). EA plus saline (i.t.) or CTOP significantly inhibited CFA-induced affective response compared to sham EA plus saline (i.t.). This indicates that spinal CTOP did not block EA inhibition of the affective response. *P<0.05 vs sham EA + saline control. Fig. 6B. Effects of CTOP on EA inhibition of the nocifensive reflex (n=6/group). EA plus saline (i.t.) significantly increased PWL of the CFA-injected hind paw compared to sham EA plus saline (i.t.), suggesting EA inhibition of the nocifensive reflex. However, EA plus CTOP (i.t.) did not increase PWL of the CFA-injected hind paw compared to sham EA plus saline (i.t.), suggesting that spinal CTOP blocked EA inhibition of the nocifensive reflex.
3. Results
3.1 CFA injection into one hind paw induced affective response
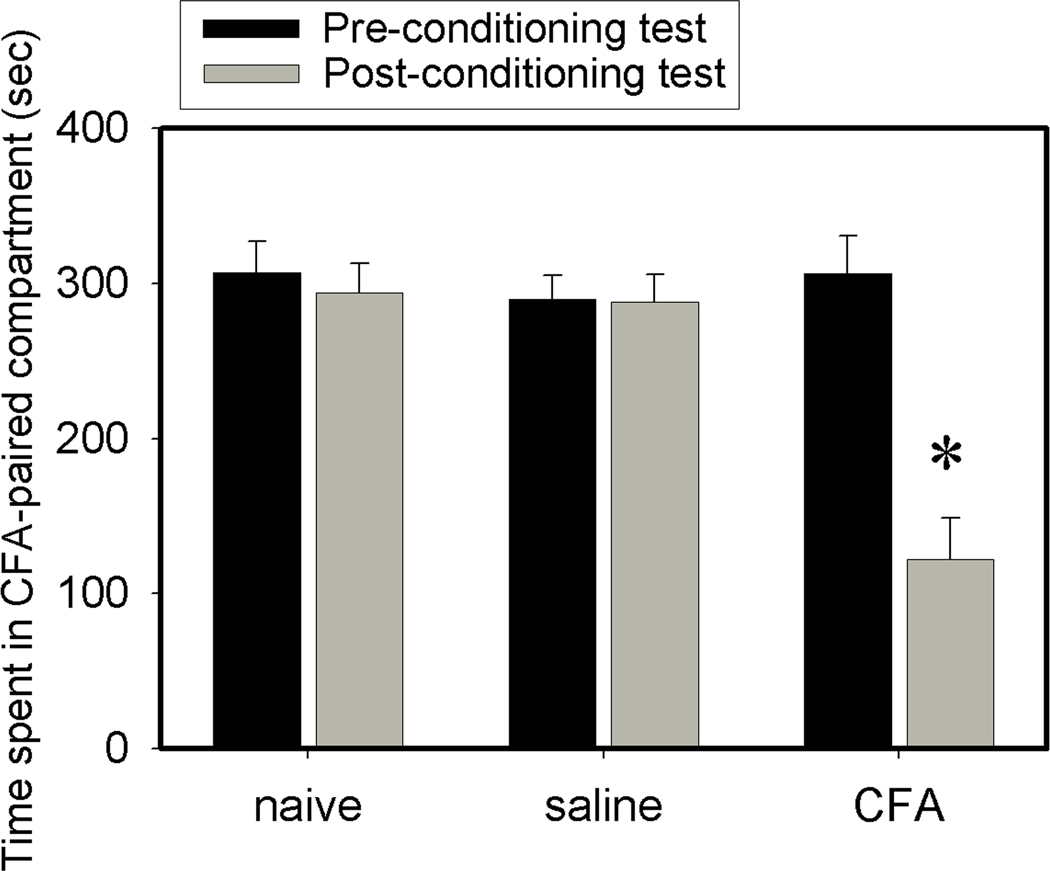
During the 10-min preconditioning test, the three groups of rats, naive, saline-, and CFA-injected, spent similar amounts of time in the two compartments, indicating no preference for either. But when a hind-paw CFA injection was paired with a particular compartment, rats spent less time in this compartment during the post-conditioning test (122 ±26 sec) than during the preconditioning test (306 ± 24 sec), demonstrating place aversion to this compartment. By contrast, saline-injected rats showed no aversion to the saline-paired compartment, spending 288 ± 18 sec during the post-conditioning test vs 290 ± 15 sec during the preconditioning test. Naive rats spent similar time in both compartments during the post-conditioning (294 vs 306 sec) and the preconditioning tests (307 vs 293 sec). These results indicate that the apparatus chambers produced neutral stimuli and demonstrated that a CFA injection induced pain affect compared to saline-injected and naive controls (Fig. 2).
Fig. 2.
CFA injection into one hind paw induced conditioned place avoidance (n=6 per group). Naive and saline-injection controls showed no preference or aversion to either chamber. CFA-injected rats showed aversive responses to the CFA-paired chamber during the post-conditioning test. Data are presented as mean ± se. *P<0.05 vs preconditioning test.
3.2 Systemic non-analgesic morphine inhibited CFA-induced affective response
As shown in Fig. 3A, a systemic non-analgesic dosage of morphine (0.5 or 1 mg /Kg) inhibited emotional-affective dimensions. Morphine at 2.5 mg/kg did not reverse decreased PWL (i.e. hyperalgesia) in the CFA-injected pain model in our previous studies (Zhang et al., 2004). The data demonstrate that the affective response is underpinned by mechanisms different from those of hyperalgesia. Morphine at 0.5 and at 1 mg/kg did not induce conditioned place preference (Fig. 3B).
3.3 EA treatment inhibited CFA-induced affective response
During the preconditioning test, the rats spent similar amounts of time in the two compartments, indicating no aversion to either. When a hind paw CFA injection was paired with a particular compartment, rats receiving sham EA spent less time in that compartment on the post-conditioning test day than on the preconditioning test day, demonstrating place aversion to that compartment (Fig. 4, column 1). By contrast, the EA-treated rats showed no aversion to the pain-paired compartment (Fig. 4, column 2). These results suggest that EA treatment inhibited the CFA-produced affective response. Saline-injected rats showed no preference or aversion to EA- or sham EA-paired chambers, suggesting that EA treatment did not produce reward or aversive effect in this single-trial place preference test (Fig. 4, columns 3 and 4). Both EA- and sham EA-treated rats explored and moved normally during conditioning; EA rats were not abnormally quiet.
3.4 Effects of injection of opioid receptor antagonists into the rACC on EA inhibition of CFA-induced affective responses
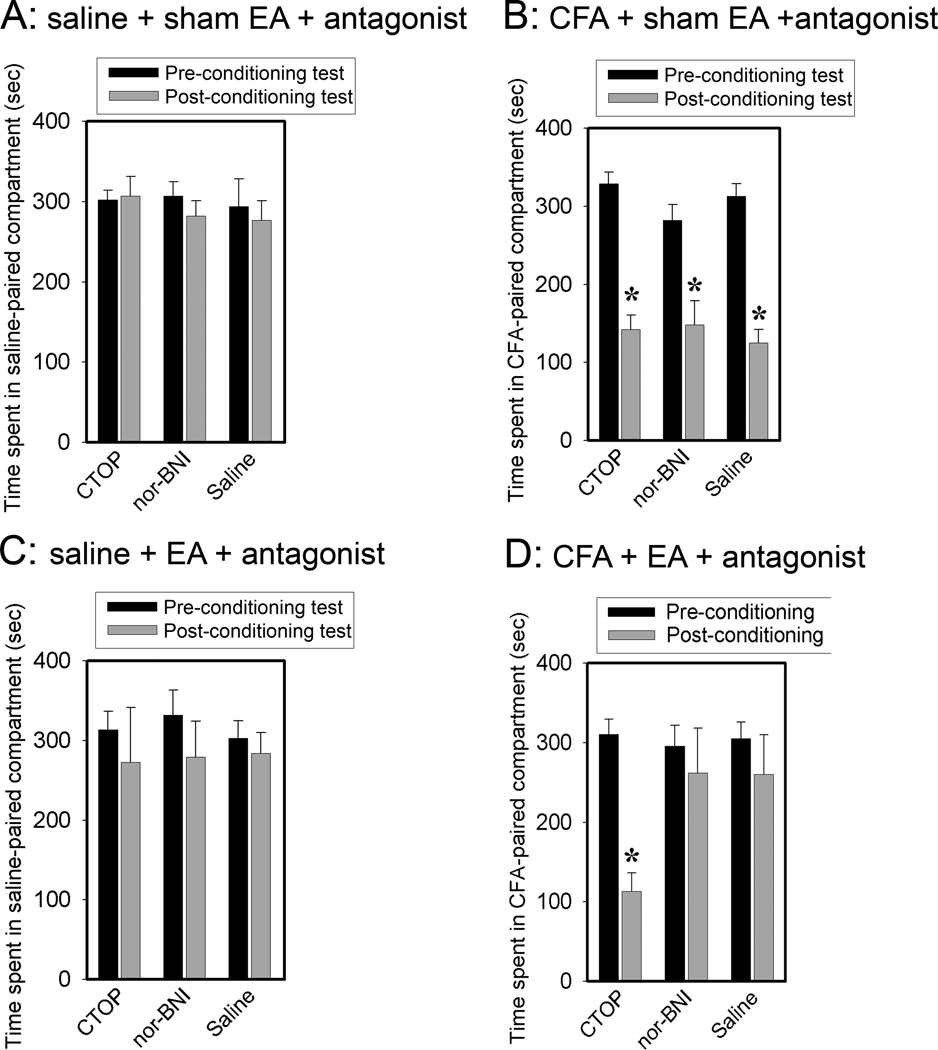
We injected CTOP, a mu-selective opioid receptor antagonist, and nor-BNI, a kappa-selective opioid receptor antagonist, into the rACC. As shown in Fig. 5A, saline-injected rats given sham EA and CTOP or nor-BNI showed no place avoidance or preference. Since hind paw saline-injection plus sham EA controls (Fig. 4) per se produced no preference or aversive effect, the data indicate that the antagonists did not produce preference or aversion in the absence of pain. In contrast, CFA-injected rats given sham EA and CTOP or nor-BNI showed significant aversion to a CFA-paired chamber (Fig. 5B). Since the extent of CPA was similar in the CFA + sham + antagonist and the CFA + sham + vehicle groups (Fig. 5B), the data suggest that the CFA injection induced little activation of endogenous opioid receptors in the rACC. Saline-injected rats given EA and CTOP or nor-BNI also showed no CPA behavior (Fig. 5C). This is consistent with the finding that saline plus EA (Fig. 4) and an antagonist (Fig. 5A) did not produce CPA.
Fig. 5.
Effects of μ and κ opioid receptors on EA action. Data are presented as mean ± SE (n=8 per group). 5A: Antagonists, CTOP for μ and nor-BNI for κ, were infused into the rACC in saline-injected, sham EA-treated rats. The rats showed no CPA. 5b. Antagonists were infused in CFA-injected, sham EA-treated rats. The rats showed significant CPA. *P<0.05 vs pre-conditioning test results. 5C. Antagonists were infused in saline-injected, EA-treated rats. The rats showed no CPA. 5D: Opioid receptor antagonist effects on EA inhibition of a CFA-induced affective response. EA was given on day two before pain-paired conditioning. CTOP or nor-BNI was given prior to EA treatment. CTOP blocked EA inhibition of the affective response, but nor-BNI did not. *P<0.05 compared to saline control.
As shown in Fig. 5D, CFA-injected rats receiving EA and CTOP showed significant affective response compared to those given saline, indicating that the CTOP blocked EA inhibition of the CFA-induced affective response. CFA-injected rats receiving EA and Nor-BNI showed no significant affective response compared to saline rats, indicating that the nor-BNI did not block EA inhibition of the affective response to CFA. These results suggest that mu but not kappa opioid receptors in rACC are involved in the EA-produced inhibition of affective pain.
3.5 EA inhibition of affective response did not depend on its inhibition of nocifensive reflex
As shown in Fig. 6A, rats given sham EA and intrathecal saline showed place aversion to the pain-paired compartment, while rats given EA and intrathecal saline or CTOP showed no place aversion. This indicates that intrathecal CTOP did not influence the EA inhibition of affective response. However, rats given EA and intrathecal saline showed a significant increase of PWL compared to rats given sham EA and intrathecal saline. Rats given EA and intrathecal CTOP did not show increased PWL (Fig. 6B). This indicates that CTOP blocked the EA inhibition of nocifensive reflex.
3.6 Histology
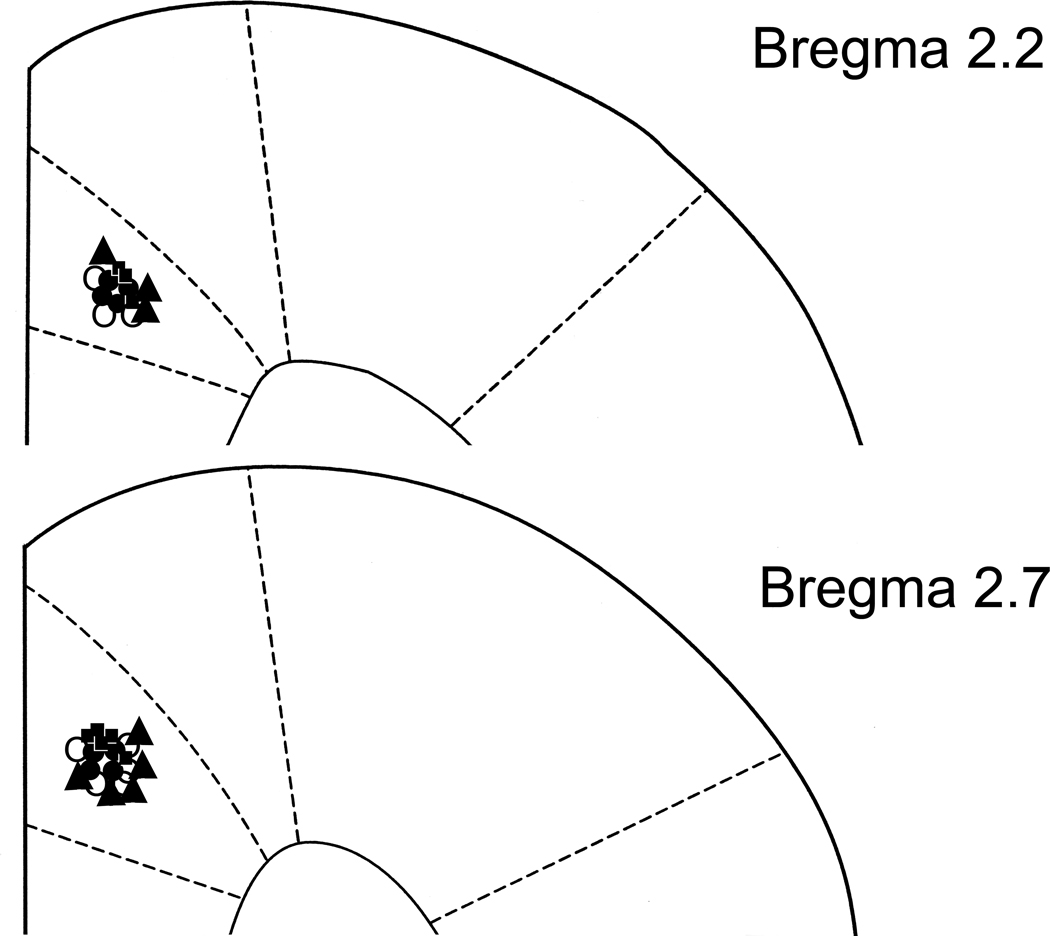
Cannula and injection sites were located in the rACC as shown in Fig. 7.
Fig. 7.
Coronal brain section reconstructions showing microinjection sites and cannula placement in the rACC. Each saline + sham EA + antagonist rectangle (□) represents three rats. Each saline + EA + antagonist circle (○) represents three rats. Each CFA + sham EA + antagonist triangle (▲) represents three rats. Each CFA+EA+ antagonist black dot (●) represents three rats.
4. Discussion
4.1 CFA-injection into the hind paw induces affective pain
In the present study, a CFA injection induced affective pain compared to saline injection and naive control, as reflected by an avoidance response to a pain-paired compartment. Previous study showed that suprathreshold mechanical stimulation of the paw ipsilateral to carrageenan-caused tissue injury induces affective pain, as shown by significant aversion to a pain-paired environment (Hummel et al., 2008). These data demonstrate that nociceptive stimuli evoke negative affective responses. During pain-paired conditioning, the rats in our experiments experienced spontaneous pain. The data indicate that CFA injection-induced spontaneous pain produces an affective response.
4.2 A non-analgesic dose of morphine inhibits the affective response
A systemic non-analgesic dose of morphine inhibited the affective response. This indicates that the affective and sensory components of pain are underpinned by different mechanisms. Previous studies demonstrated that low dosage aspirin attenuates escape/avoidance behavior but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain (LaBuda and Fuchs, 2001). Moreover, low dosage celecoxib, diclofenac, and duloxetine significantly inhibited the avoidance response associated with stimulation of an injured paw, whereas higher dosages of these drugs were needed to attenuate mechanical allodynia assessed with von Frey filaments (Boyce-Rustay et al., 2010). In another study, microinjection of morphine into the ACC inhibited affective response but did not change the sensory threshold in a nerve-injured animal model (LaGraize et al., 2006). Collectively, these data suggest that the mechanisms underlying pain-related affective responses are dissociated from nocifensive reflexes. In other words, affective response is underpinned by mechanisms different from those of noxious stimuli-evoked paw withdrawal.
It is well known that morphine induces reward, thus possibly confounding interpretation of the data. Morphine-induced reward is usually assessed with a CPP test. Rats are commonly conditioned with high dosage morphine (4 mg/kg body weight) for multiple, usually four, days to establish CPP (Chen et al., 2005). Previous study has demonstrated that morphine at 4 mg/kg does not induce rewarding effects in carrageenan-injected rats although it does induce rewarding effects in twice-conditioned saline-injected rats (Suzuki et al., 1996). Once-conditioned carrageenan-injected rats showed preference to a higher dosage (10 mg/kg) morphine-paired compartment (van der Kam et al., 2008). These data suggest that the functioning of the reward circuitry is impaired in an inflammatory condition. Indeed, the extracellular level of dopamine in the nucleus accumbens, the major neural substrate for the rewarding effect produced by opioids, was markedly increased by 8 mg/kg, i.p. morphine in vehicle- but not formalin-injected rats (Narita et al., 2005). In our experiment, rats were conditioned once with low dosage (0.5 or 1 mg/kg) morphine and showed no preference to the morphine-paired compartment. Consequently, morphine inhibition of spontaneous pain-related negative affect is not due to morphine reward in our experiments.
In the CPA paradigm, animals associate spontaneous pain with the environment of a compartment. Opioids play an important role in learning and memory (Izquierdo et al., 1980). There is a possibility that morphine interfered with the acquisition of this relationship, thus confounding data interpretation. However, it should be noted that morphine impairment of memory requires chronic, high dosage (10– 40 mg/kg) morphine (Alaei et al., 2006). It has been reported that even morphine at 5.6mg/kg did not impair acquisition and performance in a nose-poke repeated acquisition and performance test that permits the assessment of drug effects on learning (Pitts et al., 2006). Therefore, the lower dosage (0.5 or 1 mg/kg) morphine used in our studies may not interfere with acquisition, but inhibit the spontaneous pain-related negative affect.
4.3 EA inhibits the affective response
The study further demonstrates that EA inhibited affective response. Since EA induces release of endogenous opioids (Han, 2003), it is possible that EA per se may results in reward which mask the spontaneous pain-induced avoidance response. Previous study showed that EA-paired conditioning once daily for four days produced preference to an EA-paired compartment, an EA reward effect (Chen et al., 2005). In our experiment, a single EA-paired conditioning did not produce reward effect. Moreover, EA treatments showed no effect on spatial learning but did promote memory (Chen et al., 2005). Taken together, these studies provide evidence that EA inhibition of affective pain is not a result of a potential reward effect or of deterioration in learning or memory. Further, as previously mentioned, acupuncture improved the affective dimension in patients with various chronic pain conditions in an uncontrolled observation (Hammes et al., 2002). These studies indicate that acupuncture may be useful for treating affective pain.
Previous studies demonstrated that low dosage celecoxib, diclofenac, and duloxetine significantly inhibit the affective dimension of pain but not the sensory dimension (Boyce-Rustay et al., 2010). These studies indicate that the affective and sensory components of pain are underpinned by different mechanisms. In our studies, intrathecal CTOP blocked EA inhibition of the sensory dimension of pain but did not influence EA inhibition of the affective dimension. This suggests that EA may act on these two components of pain through different mechanisms. Spinal opioids are involved in EA inhibition of the sensory component (Kim et al., 2009; Zhang et al., 2004), while rACC opioids are involved in EA inhibition of the affective component as discussed below. Further, since EA dramatically eliminates the affective response but only partially alleviates the sensory component of pain in the CFA-inflammatory pain rat model (Lao et al., 2004), it seems that EA, like drug treatment, acts more easily on the affective component (Boyce-Rustay et al., 2010).
4.4 Opioids in the rACC are involved in EA inhibition of affective response
Previous studies provide evidence that acupuncture treatment increases opioid levels in brain and cerebral spinal fluid (Cao, 2002; Mayer, 2000). Regarding pain-related affective response, human and animal studies suggest that the ACC plays a role in affective behavior related to pain. Bilateral ACC lesions decrease pain-induced escape/avoidance behavior in rats (Johansen et al., 2001; LaGraize et al., 2004). Study shows that microinjection of morphine into the ACC produces a selective naloxone-reversible decrease in the aversion to noxious mechanical stimulation of the hind paw in nerve-injured rats (LaGraize et al., 2006), demonstrating that ACC opioids are involved in pain-induced affective response. In our study, CTOP pretreatment blocked EA inhibition of CPA, indicating that rACC mu opioid receptors are involved in EA inhibition of affective response. Since a microinjection of morphine into the ACC inhibited affective response but did not change the threshold of sensory pain (LaGraize et al., 2006), our data suggest that EA induces the rACC release of endorphins to block affective response and that this effect is not a consequence of its inhibition of the sensory dimension of pain.
It is known that opioid systems interact with glutamatergic transmission. For example, the mu-opioid receptor agonist [D-Ala2-N-Me-Phe4, Gly-ol5]-enkephalin (DAMGO) significantly decreases both N-Methyl-D-aspartic acid (NMDA)- and non-NMDA-EPSP (excitatory postsynaptic potential) amplitudes in nucleus accumbens neurons, which are reversed by CTOP (Martin et al., 1997). Previous study also demonstrated that intra-ACC microinjection of 2-amino-5-phosphonovalerate (AP5), an NMDAR antagonist, inhibits formalin-induced affective pain (Lei et al., 2004), indicating that NMDA receptors in the ACC is involved in affective responses. Taken together, rACC mu opioid receptors may be involved in EA action through modulating NMDA receptor activities. This warrants further investigation.
In conclusion, the present study provides direct evidence that EA inhibits spontaneous pain-induced affective response by activating mu opioid receptors in the rACC. Thus, EA inhibits both the sensory and the affective dimension of pain. The results of this study provide a promising therapeutic approach, i.e. low frequency EA, for the treatment of both sensory-discriminative and emotional-affective dimensions of pain.
Acknowledgement
This publication was made possible by grant number R21AT005474-01 and P01AT002605 from the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health. We would like to thank Dr. Lyn Lowry for her editorial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaei H, Borjeian L, Azizi M, Orian S, Pourshanazari A, Hanninen O. Treadmill running reverses retention deficit induced by morphine. Eur J Pharmacol. 2006;536:138–141. doi: 10.1016/j.ejphar.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Berman BM, Lao L, Langenberg P, Lee WL, Gilpin AM, Hochberg MC. Effectiveness of acupuncture as adjunctive therapy in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2004;141:901–910. doi: 10.7326/0003-4819-141-12-200412210-00006. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, Decker MW, Honore P. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology. 2010;58:537–543. doi: 10.1016/j.neuropharm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Bujalska M. Effect of nonselective and selective opioid receptors antagonists on antinociceptive action of acetaminophen [part III] Pol J Pharmacol. 2004;56:539–545. [PubMed] [Google Scholar]
- Cao X. Scientific bases of acupuncture analgesia. Acupunct Electrother Res. 2002;27:1–14. doi: 10.3727/036012902816026103. [DOI] [PubMed] [Google Scholar]
- Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96:7668–7674. doi: 10.1073/pnas.96.14.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-H, Liang J, Wang G-b, Han J-s, Cui C-l. Repeated 2 Hz peripheral electrical stimulations suppress morphine-induced CPP and improve spatial memory ability in rats. Exp Neurol. 2005;194:550–556. doi: 10.1016/j.expneurol.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Cheng X. Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1999. [Google Scholar]
- Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology. 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- Foltz EL, White LEJ. Pain "relief" by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Hammes MG, Flatau B, Bäcker M, Ehinger S, Conrad B, Tölle TR. Investigations on the effect of acupuncture on affective and sensory components of pain in patients with different stages of chronic pain. Schmerz. 2002;16:103–113. doi: 10.1007/s00482-002-0147-0. [DOI] [PubMed] [Google Scholar]
- Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hummel M, Lu P, Cummons TA, Whiteside GT. The persistence of a long-term negative affective state following the induction of either acute or chronic pain. Pain. 2008;140:436–445. doi: 10.1016/j.pain.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Hurt RW, Ballantine HTJ. Stereotactic anterior cingulate lesions for persistent pain: a report on 68 cases. Clin Neurosurg. 1974;21:334–351. doi: 10.1093/neurosurgery/21.cn_suppl_1.334. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Dias RD, Souza DO, Carrasco MA, Elisabetsky E, Perry ML. The role of opioid peptides in memory and learning. Behav Brain Res. 1980;1:451–468. doi: 10.1016/0166-4328(80)90001-7. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain. 2009;145:332–340. doi: 10.1016/j.pain.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBuda CJ, Fuchs PN. Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain. Neurosci Lett. 2001;304:137–140. doi: 10.1016/s0304-3940(01)01787-6. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol. 2006;197:22–30. doi: 10.1016/j.expneurol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN, LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp Neurol. 2004;188:139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Lei L-G, Sun S, Gao Y-J, Zhao Z-Q, Zhang Y-Q. NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp Neurol. 2004;189:413–421. doi: 10.1016/j.expneurol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang Y, Lao L, Xin J, Ren K, Berman BM, Zhang R-X. Serotonin Receptor 2A/C is Involved in Electroacupuncture Inhibition of Pain in an Osteoarthritis Rat Model. eCAM. 2011 doi: 10.1093/ecam/neq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Sletten CD, Williams BA, Berger IH. Improvement in fibromyalgia symptoms with acupuncture: results of a randomized controlled trial. Mayo Clinic Proc. 2006;81:749–757. doi: 10.4065/81.6.749. [DOI] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J Neurosci. 1997;17:11–22. doi: 10.1523/JNEUROSCI.17-01-00011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res. 2000;122:457–477. doi: 10.1016/s0079-6123(08)62157-3. [DOI] [PubMed] [Google Scholar]
- Narita M, Kishimoto Y, Ise Y, Yajima Y, Misawa K, Suzuki T. Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2005;30:111–118. doi: 10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- Pitts RC, Buda DR, Keith JR, Cerutti DT, Galizio M. Chlordiazepoxide and dizocilpine, but not morphine, selectively impair acquisition under a novel repeated-acquisition and performance task in rats. Psychopharmacology. 2006;189:135–143. doi: 10.1007/s00213-006-0538-5. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kishimoto Y, Misawa M. Formalin- and carrageenan-induced inflammation attenuates place preferences produced by morphine, methamphetamine and cocaine. Life Sci. 1996;59:1667–1674. doi: 10.1016/0024-3205(96)00498-5. [DOI] [PubMed] [Google Scholar]
- van der Kam EL, Vry JD, Schiene K, Tzschentke TM. Differential effects of morphine on the affective and the sensory component of carrageenan-induced nociception in the rat. Pain. 2008;136:373–379. doi: 10.1016/j.pain.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Wagner KJ, Sprenger T, Kochs EF, Tolle TR, Valet M, Willoch F. Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology. 2007;106:548–556. doi: 10.1097/00000542-200703000-00020. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang Y-Q, Ji G-C, Wu G-C, Zhao Z-Q. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]