Abstract
Naturally-occurring biomaterial scaffolds derived from extracellular matrix (ECM) have been the topic of recent investigation in the context of rotator cuff tendon repair. We previously reported a method to treat fascia ECM with high molecular weight tyramine substituted-hyaluronan (TS-HA) for use as a tendon augmentation scaffold. The presence of cross-linked TS-HA in fascia was associated with an increased macrophage and giant cell response compared to water treated controls after implantation in a rat abdominal wall model. The objective of this study was to determine the extent to which TS-HA treatment was associated with mechanical property changes of fascia after implantation in the rat model. Fascia samples in all groups demonstrated time-dependent decreases in mechanical properties. TS-HA treated fascia with cross-linking exhibited a lower toe modulus, a trend toward lower toe stiffness, and a higher transition strain than water treated controls not only after implantation, but also at time zero. TS-HA treatment, with or without cross-linking, had no significant effect on time-zero or post-implantation load relaxation ratio, load relaxation rate, linear-region stiffness, or linear-region modulus. Our findings demonstrated that the particular TS-HA treatment employed in this study decreased the low-load elastic mechanical properties of fascia ECM, in keeping with the heightened macrophage and giant cell host response seen previously. This work provides a starting point and guidance for investigating alternative HA treatment strategies.
Keywords: Extracellular matrix (ECM), Hyaluronan/hyaluronic acid, Mechanical properties, Biocompatibility, Tendon
Introduction
Tears of the rotator cuff tendons afflict more than 40% of our aging population.33, 40 Despite advances in surgical treatment options, however, the failure rate of rotator cuff repairs ranges from 20 to 90% depending on factors such as tear size and chronicity, muscle atrophy and degeneration, and tendon tissue quality.12, 22, 26, 37, 42, 44 Hence, there remains a critical need for improved repair strategies that provide effective mechanical reinforcement and stimulate tendon healing.
Over the past decade, scaffolds derived from extracellular matrix (ECM) have been investigated for the augmentation of rotator cuff repairs. The rationale for using such a device may include mechanical reinforcement and/or improving the rate and quality of biologic healing. Naturally-occurring ECM scaffolds are believed to provide a chemically and structurally instructive environment for infiltrating host cells via their natural three-dimensional structure and composition, intrinsic cytokines and growth factors, and/or remodeling byproducts.6, 24, 32, 36 Augmentation scaffolds derived from human fascia lata ECM may be particularly appropriate for tendon repair, because fascia has chemical, structural, and material properties similar to tendon.18
We propose to enrich fascia ECM with exogenous high molecular weight hyaluronan (HA), a molecule well-known for its anti-inflammatory and wound healing properties.8, 35, 39, 43 Conceptually, HA enriched fascia could provide an augmentation scaffold that has both robust time-zero mechanical properties and a biological milieu that minimizes chronic inflammation within the scaffold and enhances fibroblast infiltration. Decreased inflammation could limit resorption and allow engraftment of the scaffold. As a result, the mechanical properties of treated fascia could be maintained to an extent that prevents or limits re-tear over the course of soft tissue healing.
We use tyramine substituted-hyaluronan (TS-HA), which has tyramine adducts added to 5% of the HA chain.16 Neighboring tyramine adducts can cross-link to form dityramine bridges, resulting in an HA hydrogel that is not susceptible to hydrolysis and has a concentration-dependent resistance to hyaluronidase degradation. We previously reported a method to treat fascia ECM with TS-HA, which increased the amount of HA in fascia by an order of magnitude to approximately 1% tissue weight.10 The incorporated HA was distributed throughout the tissue and, upon cross-linking, was effectively immobilized within fascia ECM as a hydrogel network.
As well, we previously evaluated the host response to water and TS-HA treated fascia after one and three months implantation in a rat abdominal wall defect model.10 Although treated fascia was not evaluated at the site of clinical application, the rat abdominal wall model serves as an affordable, pre-clinical means to investigate in vivo material-tissue interactions and inflammation for comparative studies of biomaterials. This animal model has been previously employed by several other investigators to assess materials used for musculoskeletal repair.5, 15, 45 One and three months are appropriate time points for evaluating the chronic inflammatory phase of healing, and three months is sufficient for predicting either a constructive tissue remodeling outcome or a pro-inflammatory (destructive) tissue remodeling outcome.7
All grafts exhibited a chronic lymphocytic response that is suggestive of an immune response to the fascia xenograft. In contrast to our hypothesis, TS-HA treated fascia with cross-linking exhibited an increased macrophage and giant cell response and a lower density of fibroblast-like cells than water treated control. Macrophages and giant cells function to phagocytose and degrade implanted biomaterials and do so by secreting reactive oxygen intermediates, acid, and proteolytic enzymes such as matrix metalloproteinases (MMP).1, 47 MMP activity has been implicated in the degradation of ECM proteins and a subsequent decrease in matrix mechanical properties.2
Hence, the objective of this study was to determine changes in the mechanical properties of water and TS-HA treated fascia after implantation in a rat abdominal wall model. Specifically, load relaxation ratio, load relaxation rate, stiffness of the toe- and linear-regions, elastic modulus of the toe- and linear-regions, and transition strain were determined. Based on the heightened macrophage and giant cell response over water treated control observed in our previous histologic study,10 we hypothesized that TS-HA treated fascia with cross-linking would exhibit a lower load relaxation ratio, a higher load relaxation rate, lower toe- and linear-region stiffness, lower toe- and linear-region moduli, and higher transition strain compared to water treated controls and treated fascia without cross-linking.
Materials and Methods
The preparation and characterization of the water control and TS-HA treated fascia used in this study are described in detail as part of our previous work.10 Furthermore, we note that the fascia strips used for mechanical testing in this study were implanted into the same rats that were used for the histologic analysis of water and TS-HA treated fascia reported previously.10
Experimental Design
Patches of decellularized, lyophilized, sterile human fascia lata (5×5 cm) treated with water, TS-HA with cross-linking, or TS-HA without cross-linking were used for this study (n=8 patches per group). The fascia was derived from the iliotibial tract of donors aged 18–55 years and had been donated by the Musculoskeletal Transplant Foundation, Edison, NJ. TS-HA treated patches, both cross-linked and uncross-linked, had a concentration of 5–10 micrograms of TS-HA per milligram of dry weight tissue (μg/mg).10 Five mechanical test strips were cut from each fascia patch and were used for mechanical testing at time zero (n=3 per patch) and after one and three months implantation in a rat abdominal wall model (n=1 per patch per time point). A total of 48 rats were used for the in vivo implantation study (n=8 per treatment group per time point). Detailed methods are described below.
Rat Abdominal Wall Defect Model
All procedures were performed in accordance with the National Institutes of Health guidelines for care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Forty-eight adult, male Lewis rats were used (450–600 g, Harlan, Indianapolis, IN). Each rat was anesthetized with an intramuscular injection of ketamine, xylazine, and acepromazine (30/6/1 mg/kg). The abdomen was prepared for aseptic surgery. Via a ventral midline incision, a partial-thickness 0.4×4.5 cm defect was created in the anterior sheath adjacent to the linea alba. The anterior sheath was removed and the underlying rectus muscle, transversalis fascia, and peritoneum were left intact. One 0.4×4.5 cm fascia strip from each patch was wetted in saline for 10 min and secured into the defect using four corner sutures of 5-0 Prolene. As reported previously, on the contralateral side of the linea alba, a second defect (0.8×1 cm) was created and replaced with a wetted 0.8×1 cm fascia piece from the same patch for semi-quantitative histologic analysis.10 The skin incision was closed using 4-0 chromic gut suture, and the rat was allowed to recover from anesthesia under a heating lamp. For analgesia, each rat received 0.15 mg/kg buprenorphine post-operatively, 12 hours later, and thereafter as needed. Rats were housed individually for the duration of the study.
Euthanasia and Tissue Harvest
At one and three months, rats were sacrificed via carbon dioxide asphyxiation (n=8 per group per time point). Fascia grafts and the underlying muscle were harvested and frozen until ready for mechanical testing.
Mechanical Properties Testing
Three 0.4×4.5 cm strips cut from each water control and TS-HA treated fascia patch were used to quantify mechanical properties at time zero. Prior to testing, time-zero test strips were rehydrated for 4 hr in saline. Only a small volume was used (300 μl) to prevent leaching of HA from the uncross-linked TS-HA treated fascia. In preparation for mechanical testing, all explanted mechanical test strips were thawed and isolated from the underlying muscle by blunt dissection.
For mechanical testing, samples were gripped in custom clamps under 26 in-oz of torque with superglue and sandpaper, leaving a nominal gauge length of 28 mm. Thickness was determined at five points along the sample length with a linear variable displacement transducer probe under a constant pressure of ~0.001 MPa. Width was determined from optical analysis at three points along a longitudinal image captured with a Leica MZ6 stereomicroscope (Bannockburn, IL). Cross-sectional area was calculated as the product of average thickness and average width. Surface stain lines were placed 5 mm apart on the gage-section, for optical strain analysis and material property determination.18
All mechanical testing was conducted in 0.9% saline at 37°C on a MTS FlexTest SE electromechanical test system (Eden Prairie, MN) fitted with a 500-N load cell (Honeywell-Sensotec, Columbus, OH). Fascia strips were preconditioned from 0.2 to 2 N for five cycles, immediately elongated 1.2 mm (nominally 4% strain) at 3 mm/sec, and held at that position for 10 minutes to allow for relaxation. Samples were then returned to a slack position, allowed 5 minutes to recover,21 and pulled to failure at a rate of 10 mm/min.30
Data Analysis
The load-displacement data from each test was zeroed with 0.2 N load. From the relaxation portion of the test, the load-relaxation ratio was computed as the ratio of final to peak load. The load-relaxation rate, n, was calculated by fitting a power law relationship tn to the first ten seconds of the load-relaxation data.19
From the failure portion of the test, load and local (optical) displacement data were both plotted directly and normalized by cross-sectional area and initial gage length between the stain lines to generate load-displacement and stress–strain curves, respectively. The stress-strain data were analyzed by bilinear fitting of the data through 60% of the maximum load using the least squares method.4 Toe and linear modulus were calculated as the defined slopes of the two linear fits, respectively, and transition strain was defined as the strain corresponding to the intersection of the two linear fits. For a small portion of samples, the toe -region was deemed to be nonexistent based on the criterion that the toe modulus was greater than 70% of the linear modulus. In these cases, the linear modulus was recalculated as the slope of the entire stress-strain curve, and the transition strain was assigned a value of zero. For the few samples that exhibited decreasing strains at high loads (likely an artifact of surface strain mapping) these data points were removed and bilinear curve fitting was reapplied to the remaining data. Toe and linear stiffness were determined in a similar manner from the load-displacement curves. Data analysis was performed using a customized code in MATLAB software (Mathworks, Natick, MA).
Statistical Analysis
Two-way ANOVA was performed to examine the effects of treatment and time on cross-sectional area, load relaxation ratio and rate, toe- and linear-region stiffness, toe- and linear-region modulus, and transition strain. When appropriate, multiple comparisons were performed with a Tukey HSD post-hoc test. A p value of ≤ 0.05 was considered significant, and a p value of ≤0.10 was indicative of a trend. Results are expressed as mean ± standard deviation.
Results
Gross Observations
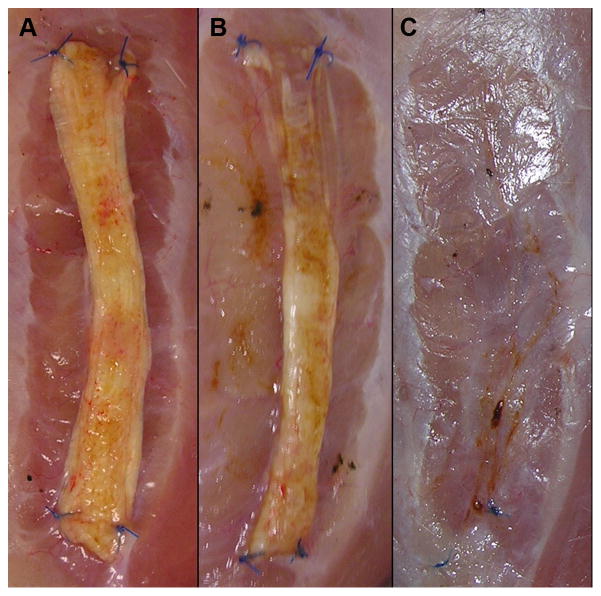
At one month, grafts of all groups appeared to have experienced a minimal degree of resorption or remodeling, independent of treatment. However, at three months, grafts of all groups experienced a variable degree of resorption or remodeling. TS-HA with cross-linking test strips occasionally demonstrated extensive or even complete resorption/remodeling (Figure 1). In addition, two of the 48 implanted mechanical test strips (one TS-HA with cross-linking at one month and one TS-HA with cross-linking at three months) could not be mechanically tested due to a loss of structural integrity. For two other test strips (both TS-HA with cross-linking at three months), the posterior and anterior ends were extensively resorbed or remodeled and, therefore, were mechanically tested using a shorter grip-to-grip gage length of 15 mm. Because the mechanical integrity of three month fascia strips from all groups was uncertain, three month samples were exempted from viscoelastic testing in order to avoid sub-failure damage prior to the collection of elastic mechanical properties.
Figure 1.
Representative images of TS-HA with cross-linking treated fascia strips at explantation at three months. Grafts appeared to have experienced a variable degree of resorption or remodeling, ranging from minimal (A) to extensive (B) to complete (C).
Cross-sectional Area
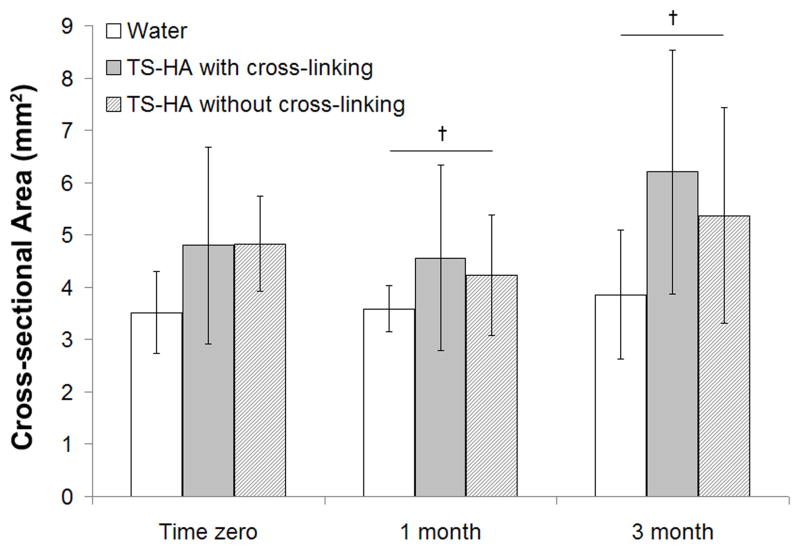
ANOVA indicated that only group had a significant effect on cross-sectional area (Figure 2, p=0.002), and there was a trend for time (p=0.07). No significant group-time interaction was found. The cross-sectional area of TS-HA treated fascia pieces, with or without cross-linking, was significantly greater than water control. Fascia grafts at three months trended towards a greater cross-sectional area than at one month.
Figure 2.
Cross-sectional area of water treated control, TS-HA with cross-linking, and TS-HA without cross-linking treated fascia (n=7–8 per group per time point). The cross-sectional area of TS-HA treated fascia with and without cross-linking was significantly greater than water control at all time points (p=0.002), which is not denoted with symbols because of the manner in which the data is graphically presented here. †Fascia grafts at three months trended towards a greater cross-sectional area than at one month (p=0.07).
Mechanical Properties Testing
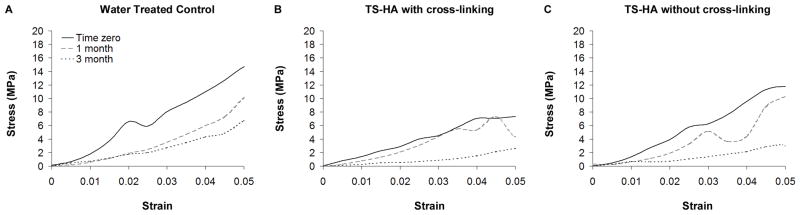
The average stress versus local strain curves for water control, TS-HA with cross-linking, and TS-HA without cross-linking treated fascia at all time points are shown in Figure 3. Failure is under-represented because all test strips failed at the grips.
Figure 3.
Average stress versus local strain curves for (A) water control, (B) TS-HA with cross-linking, and (C) TS-HA without cross-linking test strips at all time points (n= 7–8 per group per time point). The wavy nature of the curves at higher strains is a consequence of including fewer samples in the average as samples fail. Failure is under-represented because all test strips failed at the grips.
Viscoelastic Properties
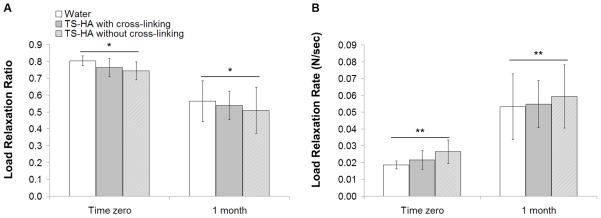
ANOVA indicated that only time had a significant effect on both load relaxation ratio (Figure 4A, p<0.0001) and load relaxation rate (Figure 4B, p<0.0001), and there was no significant group-time interaction. Load relaxation ratio significantly decreased and load relaxation rate significantly increased from time zero to one month.
Figure 4.
(A) Load relaxation ratio and (B) load relaxation rate of water control, TS-HA with cross-linking, and TS-HA without cross-linking treated fascia at time zero and one month (n=7–8 per group per time point). *Load relaxation ratio significantly decreased with time (p<0.0001). **Load relaxation rate significantly increased with time (p<0.0001).
Elastic Properties
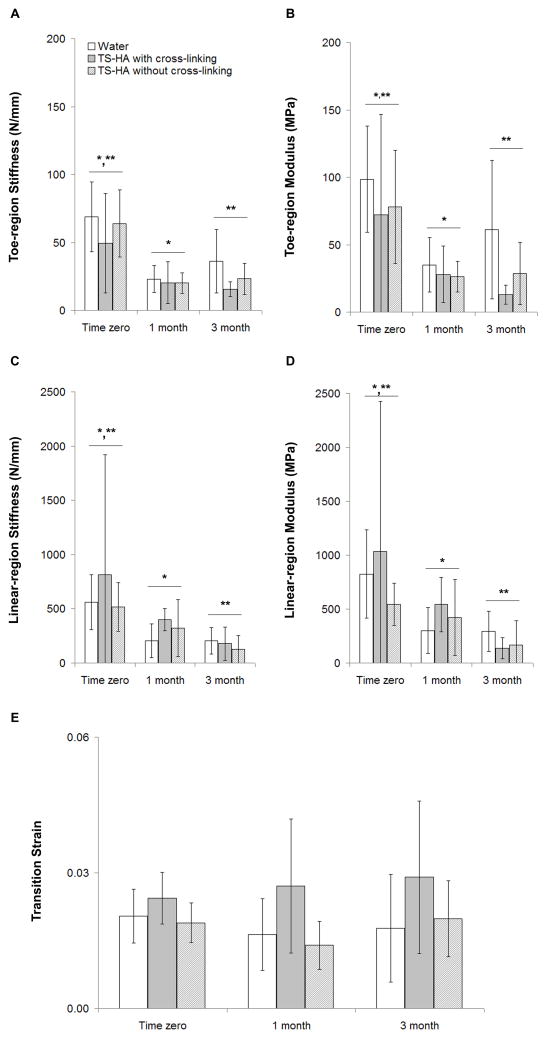
ANOVA indicated that there was no significant group-time interaction for any of the elastic mechanical properties. Only time had a significant effect on toe-region stiffness, which was greater at time zero than at one and three months (Figure 5A, p<0.001). Post-hoc multiple comparison testing indicated that treated fascia with cross-linking trended toward a lower toe-region stiffness than water treated controls (p=0.06).
Figure 5.
Elastic mechanical properties of water control, TS-HA with cross-linking, and TS-HA without cross-linking treated fascia (n=7–8 per group per time point). (A) *,** Toe-region stiffness was significantly greater at time zero than at one and three months (p<0.001). Treated fascia with cross-linking trended toward a lower toe-region stiffness than water treated controls (p=0.06), which is not denoted with symbols because of the manner in which the data are graphically presented here. (B) For toe-region elastic modulus, group (p<0.001) and time (p=0.05) had significant effects. *,** Toe-region modulus was significantly greater at time zero than at one and three months. TS-HA treated fascia with cross-linking exhibited a significantly lower toe-region elastic modulus than water treated controls, which is not denoted with symbols because of the manner in which the data are graphically presented here. (C) *,** Linear-region stiffness was significantly higher at time zero than at one and three months (p=0.001). (D) *,** Linear-region modulus was significantly higher at time zero than at one and three months (p=0.001). (E) TS-HA treated fascia with cross-linking had a significantly greater transition strain than treated fascia without cross-linking and water treated controls (p=0.003). Neither result is denoted with symbols because of the manner in which the data is graphically presented here.
For toe-region elastic modulus, group (Figure 5B, p<0.001) and time (p=0.05) had significant effects. Toe-region modulus was significantly greater at time zero than at one and three months. TS-HA treated fascia with cross-linking exhibited a significantly lower toe-region elastic modulus than water treated controls.
Only time had a significant effect on linear-region stiffness (Figure 5C, p=0.001) and elastic modulus (Figure 5D, p=0.001), both of which were greater at time zero than at one and three months.
Only group had a significant effect on transition strain (Figure 5E, p=0.003). TS-HA treated fascia with cross-linking had a significantly greater transition strain than treated fascia without cross-linking and water treated controls.
Discussion
The objective of this study was to determine changes in the mechanical properties of water and TS-HA treated fascia after implantation in a rat abdominal wall model. Fascia samples in all groups demonstrated time-dependent decreases in mechanical properties. TS-HA treated fascia with cross-linking exhibited a lower toe-region modulus, a trend toward lower toe stiffness, and a higher transition strain than water treated controls not only after implantation, as hypothesized, but also at time zero. However, in contrast to our hypothesis, TS-HA treatment, with or without cross-linking, had no significant effect on time-zero or post-implantation load relaxation ratio, load relaxation rate, linear-region stiffness, or linear-region modulus. Hence, the TS-HA treatment employed in this study decreased only the low-load elastic mechanical properties of fascia ECM. Identifying which mechanical properties, and consequently which ECM components, are affected by the TS-HA treatment allows us to consider possible mechanisms for these mechanical property decreases. Additionally, we will discuss the results in light of the host cell response to these materials.10
The elastic properties of water treated fascia – as defined by toe stiffness, toe modulus, linear stiffness, and linear modulus – decreased after implantation. The viscoelastic properties also changed after implantation; load relaxation ratio decreased, while load relaxation rate increased. Previously, we reported that water treated fascia was infiltrated by a moderate degree of macrophages and a mild degree of giant cells,10 both of which are known to secrete the degradative enzymes matrix metalloproteinase (MMP)-1, -2, -9, -12, and -1413, 31, 47 Because elastin and other non-collagenous ECM proteins are the main contributors to the mechanical behavior of fascia at low strains,20 it is possible that the decreased toe modulus following implantation is a result of elastase, MMP-12, and/or broad spectrum MMP-14 activity. Additionally, the interaction between collagen and other ECM proteins could be physically disrupted by tissue swelling or edema, thus interrupting force transmission through the ground substance and lowering the toe modulus. Swelling and edema could also contribute to the increased stress relaxation of implanted fascia.3
The mechanical behavior of fascia at higher strains is attributed to collagen fibers that are un-crimped and load-bearing.23, 27, 49 Hence, the observed decrease in linear modulus in water controls after implantation is possibly a result of collagenase (MMP-1, -2, and -9) activity. In support of these proposed mechanisms, it has been well documented that MMP activity is a contributing factor in the loss of mechanical properties of ECM grafts post implantation.38, 50
Similar to water controls, both groups of TS-HA treated fascia demonstrated time-dependent decreases in mechanical properties. In addition, TS-HA treated fascia with cross-linking demonstrated a lower toe modulus, a trend toward lower toe stiffness, and a higher transition strain (i.e., longer toe-region) than water controls at both time zero and post implantation. The decreased low-load mechanical properties of cross-linked treated fascia at time zero may be attributed to the disruption of the interaction between collagen and other ECM proteins, possibly as a result of increased water uptake 14 during treatment (TS-HA treated fascia with cross-linking was uniquely subjected to a 24 hour rinse in 125 ml of water following the cross-linking reaction to remove any uncross-linked material). As well, the hydrogen peroxide that was used to initiate the cross-linking reaction could have activated endogenous tissue peroxidases, which may have disrupted or degraded ECM proteins involved in low-load mechanical behavior. Both hypothesized mechanisms are supported by the observation that uncross-linked treated fascia, which was not subjected to the 24 hour rinse or the cross-linking reaction, exhibited a similar toe stiffness, toe modulus, and transition strain as water controls. As part of our ongoing work, we intend to determine the mechanisms by which TS-HA treatment decreases the time-zero mechanical properties of fascia and subsequently modify the treatment protocol to limit or prevent their effects.
Following implantation, the lower toe-modulus of TS-HA treated fascia with cross-linking may simply be a reflection of the decrease in this property at time zero. As well, TS-HA treated fascia with cross-linking was shown to elicit a heightened macrophage and giant cell response compared to water controls.10 Hence the potential for increased MMP activity is another possible mechanism for the lower toe-region mechanical properties of cross-linked treated fascia following implantation. However, no difference was seen in high-load (linear region) mechanical properties between TS-HA treated fascia with cross-linking and water controls, suggesting that any increase in MMP activity largely targeted non-collagenous ECM proteins and not the collagen fibers. It is also possible that the TS-HA hydrogel network that surrounds the large fascicle bundles sequesters infiltrating inflammatory cells and mediators17 or physically shields the collagen fascicles from degradative enzymes.
TS-HA treatment, with or without cross-linking, increased the cross-sectional area of fascia compared to water controls at time zero. This finding is consistent with our previous results10 and suggests that inter-fascicular spaces expand to accommodate the added TS-HA. Further, the cross-sectional area of all groups tended to increase from one to three months, presumably as a result of tissue swelling, edema, or cellular infiltration. This result may seem to contradict our previous work, in which we reported a decrease in cross-sectional area of fascia grafts from one to three months.10 The apparent discrepancy can be explained by noting that the cross-sectional area of mechanical test strips was measured transversely in this study, while the cross-sectional area of histologic grafts was measured longitudinally in our previous study. Hence, remodeling or resorption of the implanted grafts occurred primarily from the ends, which is supported by the gross observation of the explanted fascia strips.
The time-zero elastic modulus of water treated fascia quantified in this study (825 ± 410 MPa) is slightly higher than the modulus of fascia previously reported by our laboratory (532 ± 106 MPa).18 This discrepancy may be explained by the bilinear curve fitting methodology employed in this study. Here, elastic modulus was defined as the maximum slope of the second linear region of the curve, while in our previous study, as the slope from a defined strain range of the curve. Furthermore, whereas the elastic modulus of fascia following three months implantation in a rabbit vagina model was reported to decrease by 96%,46 we report a 64% drop here. One explanation for these disparate results may be related to the very different in vivo implantation environments between the two studies. In addition, sample geometry and test methods were different between the two studies (fascia strips in the Walter study had an aspect ratio of 4:1, and the elastic modulus was computed using grip-to-grip strain), so direct comparison of data is difficult. To our knowledge, there are no other reports on the post-implantation mechanical properties of fascia at similar time points to which to compare our work.
The current study is not without limitations. The stress-relaxation test employed in this study involved a single step to 4% strain, which is beyond the toe-region of these materials. Hence viscoelastic differences between groups at low-strains could not be evaluated. Second, all fascia strips explanted at three months were exempted from viscoelastic testing, due to their unknown mechanical integrity. Third, a small number of cross-linked TS-HA treated fascia samples, which experienced extensive remodeling or resorption, could not be mechanically tested. Thus, the mechanical properties of TS-HA treated fascia with cross-linking reported here are likely overestimated, because the mechanically inferior samples were not included. Fourth, fascia samples were frozen and subsequently thawed prior to mechanical testing for operator convenience. Although test strips could have been tested immediately after harvest, previous studies have demonstrated no effect of freeze/thaw on the mechanical properties of tendon or fascia tissue.11, 25, 29, 41, 48 Fifth, all specimens failed at the grips during mechanical testing, most likely as a result of stress concentrations.9, 34 Although other methods of gripping could have been investigated to prevent or limit grip failure, we did not thoroughly explore these alternatives, because we were interested in comparing material and structural properties, not failure outcomes. Lastly, we can only hypothesize that the mechanisms responsible for the mechanical property decreases reported herein involve the degradation of collagen or other ECM proteins, as changes to the ECM components were not quantified.
This work provides a starting point and guidance for the ongoing development of HA treated fascia ECM. Future work will investigate modifications to the treatment protocol to prevent or limit decreases in time-zero and post-implantation mechanical properties. Specifically, we will explore the effects of a lower concentration of hydrogen peroxide for cross-linking, different TS-HA concentrations which may influence host response as well as the swelling potential of the ECM, and lower molecular weight TS-HA which may allow for a more uniform HA distribution and potentially limit the accumulation of macrophages and giant cells which were previously observed around islands of hydrogel. Lastly, in the setting of rotator cuff repair, the host response to a scaffold may be uniquely influenced by exposure to synovial fluid and/or mechanical load. Hence, investigating host response in a site-specific animal model may be appropriate and instructive.28
Conclusion
This study is novel in that it characterizes the time-zero and post-implantation mechanical properties of decellularized fascia ECM and TS-HA treated fascia ECM with or without cross-linking. TS-HA treated fascia with cross-linking exhibited a lower toe-region modulus, a trend toward lower toe stiffness, and a higher transition strain than water treated controls at time zero and post implantation. This study evaluates the post-implantation mechanical properties of TS-HA treated fascia in light of previously reported histologic outcomes.10 Together, the results suggest that this particular preparation of TS-HA treatment (at the concentration, molecular weight, and tyramine substitution rate used here) adversely affects the host response and elastic mechanical properties of fascia ECM. Hence, the preparation of TS-HA treated fascia described in this study would likely not be beneficial as an augmentation device for the repair of rotator cuff tendon or other soft tissues. However, this study demonstrates that augmentation of an ECM with HA can alter both the host response and mechanical properties of the ECM. Although this particular preparation of TS-HA treated fascia was not effective in modulating inflammation or maintaining the mechanical properties of fascia, we will use these findings to further investigate TS-HA treatment with the intent to modulate inflammation, enhance fibroblast infiltration, and consequently promote the regeneration of mechanically functional host tissue.
Acknowledgments
This study was financially supported by the National Institutes of Health (NIAMS Grant # R01AR056633, F31AR057305, T32AR50959) and the Ohio Third Frontier (Grant # BRTT05-30). The authors would like to acknowledge the Musculoskeletal Transplant Foundation for their fascia donation and Lifecore Biomedical for their TS-HA donation.
Footnotes
1. The author, or one or more of the authors, has received or will receive remuneration or other prequisites for personal or professional use from a commercial or industrial agent in direct or indirect relationship to their authorship
References
- 1.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763–769. doi: 10.1177/0363546506296043. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson TS, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- 4.Aurora A, Mesiha M, Tan CD, Walker E, Sahoo S, Iannotti JP, McCarron JA, Derwin KA. Mechanical characterization and biocompatibility of a novel reinforced fascia patch for rotator cuff repair. J Biomed Mater Res A. 2011;99A:221–230. doi: 10.1002/jbm.a.33179. [DOI] [PubMed] [Google Scholar]
- 5.Badylak S, Kokini K, Tullius B, Simmons-Byrd A, Morff R. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190–202. doi: 10.1006/jsre.2001.6349. [DOI] [PubMed] [Google Scholar]
- 6.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 7.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantor JO, Nadkarni PP. Hyaluronan: the Jekyll and Hyde molecule. Inflamm Allergy Drug Targets. 2006;5:257–260. doi: 10.2174/187152806779010936. [DOI] [PubMed] [Google Scholar]
- 9.Cheung JT, Zhang M. A serrated jaw clamp for tendon gripping. Med Eng Phys. 2011;28:379–382. doi: 10.1016/j.medengphy.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Chin L, Calabro A, Rodriguez ER, Tan CD, Walker E, Derwin KA. Characterization of and host response to tyramine substituted-hyaluronan enriched fascia extracellular matrix. J Mater Sci Mater Med. 2011;22:1465–1477. doi: 10.1007/s10856-011-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clavert P, Kempf JF, Bonnomet F, Boutemy P, Marcelin L, Kahn JL. Effects of freezing/thawing on the biomechanical properties of human tendons. Surg Radiol Anat. 2001;23:259–262. doi: 10.1007/s00276-001-0259-8. [DOI] [PubMed] [Google Scholar]
- 12.Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears. A prospective long-term study. J Bone Jt Surg [Am] 2001;83-A:71–77. doi: 10.2106/00004623-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Colombat M, Caudroy S, Lagonotte E, Mal H, Danel C, Stern M, Fournier M, Birembaut P. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J. 2008;32:1399–1403. doi: 10.1183/09031936.00132007. [DOI] [PubMed] [Google Scholar]
- 14.Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- 15.Cook JL, Fox DB, Kuroki K, Jayo M, De Deyne PG. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–156. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 16.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20:33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 17.Day AJ, De La Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–643. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Derwin KA, Baker AR, Spragg RK, Leigh DR, Farhat W, Iannotti JP. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. J Biomed Mater Res A. 2008;84:500–507. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 19.Duenwald SE, Vanderby R, Jr, Lakes RS. Viscoelastic relaxation and recovery of tendon. Ann Biomed Eng. 2009;37:1131–1140. doi: 10.1007/s10439-009-9687-0. [DOI] [PubMed] [Google Scholar]
- 20.Eshel H, Lanir Y. Effects of strain level and proteoglycan depletion on preconditioning and viscoelastic responses of rat dorsal skin. Ann Biomed Eng. 2001;29:164–172. doi: 10.1114/1.1349697. [DOI] [PubMed] [Google Scholar]
- 21.Flatow EL, Nasser P, Lee L, Schaffler MB, Jepsen KL. Overestimation of the Degradation State in Fatigue Loaded Tendon due to Transient Effects. 2002. [Google Scholar]
- 22.Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg. 2003;12:550–554. doi: 10.1016/s1058-2746(03)00211-8. [DOI] [PubMed] [Google Scholar]
- 23.Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Engin. 2002;124:72–77. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- 24.Hodde J. Extracellular matrix as a bioactive material for soft tissue reconstruction. ANZ J Surg. 2006;76:1096–1100. doi: 10.1111/j.1445-2197.2006.03948.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang H, Zhang J, Sun K, Zhang X, Tian S. Effects of repetitive multiple freeze-thaw cycles on the biomechanical properties of human flexor digitorum superficialis and flexor pollicis longus tendons. Clin Biomech (Bristol , Avon ) 2011;26:419–423. doi: 10.1016/j.clinbiomech.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Iannotti JP. Full Thickness Rotator Cuff Tears: Factors Affecting Surgical Outcome. J Am Acad Orthop Surg. 1994;2:87–95. doi: 10.5435/00124635-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leigh DR, Baker AR, Mesiha M, Rodriguez ER, Tan CD, Walker E, Derwin KA. Effect of implantation site and injury condition on host response to human-derived fascia lata ECM in a rat model. J Orthop Res. 2011:10. doi: 10.1002/jor.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitschuh PH, Doherty TJ, Taylor DC, Brooks DE, Ryan JB. Effects of postmortem freezing on tensile failure properties of rabbit extensor digitorum longus muscle tendon complex. J Orthop Res. 1996;14:830–833. doi: 10.1002/jor.1100140522. [DOI] [PubMed] [Google Scholar]
- 30.Lin TW, Cardenas L, Soslowsky LJ. Tendon properties in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2005;38:99–105. doi: 10.1016/j.jbiomech.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 31.MacLauchlan S, Skokos EA, Meznarich N, Zhu DH, Raoof S, Shipley JM, Senior RM, Bornstein P, Kyriakides TR. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol. 2009;85:617–626. doi: 10.1189/jlb.1008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67:637–640. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 33.Milgrom C, Schaffler MB, Van Holsbeeck Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg [Br ] 1995;77:296–298. [PubMed] [Google Scholar]
- 34.Ng BH, Chou SM, Krishna V. The influence of gripping techniques on the tensile properties of tendons. Proc Inst Mech Eng H. 2005;219:349–354. doi: 10.1243/095441105X34239. [DOI] [PubMed] [Google Scholar]
- 35.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 36.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 37.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts JM, Goldstrohm GL, Brown TD, Mears DC. Comparison of unrepaired, primarily repaired, and polyglactin mesh-reinforced Achilles tendon lacerations in rabbits. Clin Orthop Relat Res. 1983:244–249. [PubMed] [Google Scholar]
- 39.Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem. 2002;277:4581–4584. doi: 10.1074/jbc.R100037200. [DOI] [PubMed] [Google Scholar]
- 40.Tempelhof S. Age-related prevalance of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi: 10.1016/s1058-2746(99)90148-9. [DOI] [PubMed] [Google Scholar]
- 41.THOMAS ED, GRESHAM RB. COMPARATIVE TENSILE STRENGTH STUDY OF FRESH, FROZEN, AND FREEZE-DRIED HUMAN FASCIA LATA. Surg Form. 1963;14:442–443. [PubMed] [Google Scholar]
- 42.Thomopoulos S, Soslowsky LJ, Flanagan CL, Tun S, Keefer CC, Mastaw J, Carpenter JE. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239–247. doi: 10.1067/mse.2002.122228. [DOI] [PubMed] [Google Scholar]
- 43.Toole BP, Gross J. The extracellular matrix of the regenerating newt limb: synthesis and removal of hyaluronate prior to differentiation. Dev Biol. 1971;25:57–77. doi: 10.1016/0012-1606(71)90019-4. [DOI] [PubMed] [Google Scholar]
- 44.Uhthoff HK, Trudel G, Himori K. Relevance of pathology and basic research to the surgeon treating rotator cuff disease. J Orthop Sci. 2003;8:449–456. doi: 10.1007/s10776-002-0624-5. [DOI] [PubMed] [Google Scholar]
- 45.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg [Am ] 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 46.Walter AJ, Morse AN, Leslie KO, Zobitz ME, Hentz JG, Cornella JL. Changes in tensile strength of cadaveric human fascia lata after implantation in a rabbit vagina model. J Urol. 2003;169:1907–1910. doi: 10.1097/01.ju.0000061182.21353.a5. [DOI] [PubMed] [Google Scholar]
- 47.Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80:1052–1066. doi: 10.1189/jlb.0306152. [DOI] [PubMed] [Google Scholar]
- 48.Woo SL, Orlando CA, Camp JF, Akeson WH. Effects of postmortem storage by freezing on ligament tensile behavior. J Biomech. 1986;19:399–404. doi: 10.1016/0021-9290(86)90016-3. [DOI] [PubMed] [Google Scholar]
- 49.Woo SLY, An KN, Frank CB, Livesay GA, Ma CB, Zeminski J, Wayne JS, Myers BS. Anatomy, Biology, and Biomechanics of Tendon and Ligament. In: Buckwalter J, Einhorn T, Simon S, editors. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2000. pp. 581–616. [Google Scholar]
- 50.Yildirim Y, Kara H, Cabukoglu C, Esemenli T. Suture holding capacity of the Achilles tendon during the healing period: an in vivo experimental study in rabbits. Foot Ankle Int. 2006;27:121–124. doi: 10.1177/107110070602700209. [DOI] [PubMed] [Google Scholar]