Abstract
Endogenous estrogens mediate protective effects in the cardiovascular system, affecting both endothelium-dependent and -independent mechanisms. Previous studies have suggested that nonselective estrogen receptor agonists such as endogenous estrogens inhibit endothelium-dependent vasoconstriction; however, the role of estrogen receptors in this response has not yet been clarified. This study investigated whether the novel intracellular G protein-coupled estrogen receptor GPER regulates vascular reactivity in mice. Effects of chronic deficiency (using mice lacking the GPER gene) and acute inhibition (using the GPER-selective antagonist G15) on endothelium-dependent and -independent vascular reactivity, and the effects of GPER deficiency on vascular gene expression and structure were investigated. We found that chronic GPER deficiency is associated with increased endothelial prostanoid-mediated vasoconstriction, but had no effect on endothelial nitric oxide (NO) bioactivity, gene expression of endothelial NO synthase and thromboxane prostanoid (TP) receptor, or vascular structure. GPER deletion also increased TP receptor-mediated contraction. Acute GPER blockade enhanced endothelium-dependent contractions, and reduced endothelial NO bioactivity. Contractions to TP receptor activation were unaffected by G15. In conclusion, this study has identified GPER as the first estrogen receptor with inhibitory activity on endothelium-dependent contractility. These findings may be important for understanding and treating diseases associated with increased endothelial vasoconstrictor prostanoid activity such as hypertension and obesity.
Keywords: EDCF, Endothelium, Estrogen, GPER, GPR30, Nitric Oxide, Prostanoid
Introduction
Vascular tone is maintained by a balanced release of endothelial relaxing factors including nitric oxide (NO), and endothelium-derived contracting factors (EDCF) such as prostanoids and endothelin.1,2 NO is released under basal conditions, in response to shear stress and vasodilators such as acetylcholine.3 Acetylcholine and other agonists also stimulate the production of EDCF, including cyclooxygenase (COX)-derived prostanoids that activate thromboxane prostanoid (TP) receptors on vascular smooth muscle cells (VSMC).1,2,4 In arteries of patients with essential hypertension, endothelium-dependent NO generation is impaired, while activity of vasoconstrictor prostanoids is increased.1,2 These abnormalities in endothelial cell function are associated with an increased cardiovascular risk.5
Endogenous estrogens beneficially modulate the activity of endothelial factors by increasing the transcription of the endothelial NO synthase (eNOS) gene,6 and acutely stimulating NO production via eNOS.7 Effects of estrogens are mediated by multiple receptors including the classical estrogen receptors ERα and ERβ,8 with NO-dependent effects of estrogens requiring functional ERα.7,9–11 Estrogens also bind to the novel, 7-transmembrane spanning intracellular G protein-coupled estrogen receptor GPER (previously termed GPR30)8,12 cloned from human endothelial cells13 and expressed throughout the cardiovascular system.8 The development of the GPER-selective agonist G-114 has facilitated studies that demonstrate GPER activation induces acute vasodilation and lowers blood pressure in rodents.15–19 Moreover, G-1-dependent relaxation is absent in GPER-knockout (GPER0) mice, further corroborating the requirement of this receptor to mediate vascular responses.15 We18 and others17,19 have shown that acute GPER-mediated vasodilator effects are at least partly endothelium- and NO-dependent.
The role of GPER in endothelium-dependent vasoconstriction is unknown. Natural estrogens such as 17β-estradiol, a non-selective agonist of ERα, ERβ, and GPER,8 modulate vasoconstrictor prostanoid activity and the expression of TP receptors and prostanoid synthases.20–26 Furthermore, inhibitory effects of 17β-estradiol on COX-dependent responses to vasoconstrictors have suggested a role of estrogen receptors,27 although the specific estrogen receptor(s) involved in endothelium-dependent vasoconstriction have not been identified. Using GPER0 mice and the GPER-selective antagonist G15,28 the present study was therefore designed to test the hypothesis that GPER is involved in endothelium-dependent and -independent regulation of vasomotor tone, with a particular emphasis on endothelial vasoconstrictor prostanoids.
Methods
Vascular Function Experiments
Male C57Bl6 and GPER-deficient (GPER0) mice (3 months of age) were anesthetized by intraperitoneal injection of sodium pentobarbital and exsanguinated by cardiac puncture. All procedures were approved by and carried out in accordance with institutional policies and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The aorta was excised and prepared for measurements of isometric force in organ baths as described.15,18,29 To study acute effects of GPER inhibition on vascular function, selected rings were pretreated with the GPER-selective antagonist G15 (3 μmol/L)28 or vehicle (ethanol 0.27%, DMSO 0.03% vol/vol) for 20 min. Some rings were also pretreated with the non-selective COX inhibitor indomethacin (10 μmol/L), the TP receptor antagonist SQ 29,548 (1 μmol/L), or the NO synthase inhibitor L-NAME (300 μmol/L) for 30 min. Concentration-response curves to acetylcholine (0.1 nmol/L – 100 μmol/L), sodium nitroprusside (SNP, 0.01 nmol/L – 10 μmol/L), and U46619 (0.1 nmol/L – 10 μmol/L) were obtained. Basal NO bioactivity was determined as described.30 To exclude interference with NO-dependent effects of GPER,17–19 responses to SNP and U46619 were recorded in the presence of L-NAME.
Histological Analyses and Immunohistochemistry
Paraffin-embedded cross sections (2 μm) of WT and GPER0 aortas were stained with hematoxylin and eosin using a standard protocol. For immunohistochemical analyses, sections were stained as described31 using the following antibodies: α-smooth muscle actin (1:200), proliferating cell nuclear antigen (PCNA, 1:500), and Ki-67 (1:100).
Quantitative Real-time Polymerase Chain Reaction (qPCR)
RNA from thoracic aorta of WT and GPER0 mice was extracted and reverse transcribed. PCR was performed using TaqMan gene expression assays. Gene expression was calculated using the 2−ΔΔC(T) method.32
Statistical Analyses
Area under the curve (AUC) and EC50 values (as negative logarithm: pD2) of concentration-response curves were calculated by non-linear regression analysis.33 Data were analyzed using two-way repeated-measures ANOVA followed by Bonferroni post hoc analysis, the unpaired Student's t-test or the Mann-Whitney U test where appropriate. Values are shown as means±SEM. A P<0.05 value was considered statistically significant.
For expanded Materials and Methods please see the online supplement at http://hyper.ahajournals.org.
Results
Effect of GPER Deficiency on Endothelium-Dependent Vascular Reactivity
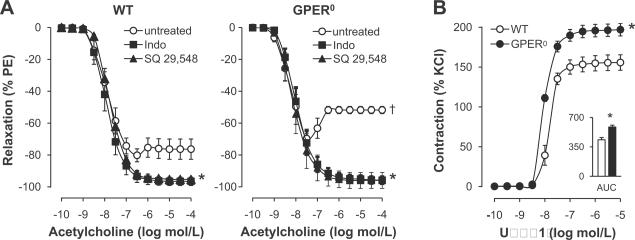
In order to assess the effect of endogenous GPER expression on endothelial vasoreactivity, we examined acetylcholine-induced endothelium-dependent relaxation and contraction34,35 in the aorta of wild type (WT) and GPER0 mice. Endothelium-dependent, receptor-stimulated NO release by acetylcholine induced similar extents of relaxation in phenylephrine-preconstricted rings from WT and GPER0 mice (Figure 1A). However, at acetylcholine concentrations ≥0.1 μmol/L, which stimulate the release of EDCF,21–23,34–36 a 2.3-fold increased contraction in GPER0 aorta was observed (44.1±2.3% vs. 19.6±6.4%, n=5, P<0.01 vs. WT, Figure 1A). Consistent with an EDCF-mediated response,1,2 the non-selective COX-inhibitor indomethacin and the TP receptor antagonist SQ 29,548 completely blocked contractions to acetylcholine (n=4–5, P<0.05 vs. untreated vessel rings, Figure 1A). To assess the role of GPER in EDCF-mediated activation of TP receptor signaling in VSMC,1,2 we measured concentration-dependent contraction to the selective TP receptor agonist U46619. Contractions to U46619 were increased in GPER0 aorta (maximal effect 197±8% vs. 156±10%; AUC 586±22 vs. 435±27 AU; pD2 8.01±0.03 vs. 7.82±0.12; n=5–6, P<0.01 vs. WT), consistent with an inhibitory effect on EDCF-mediated VSMC contractility by endogenous GPER in WT mice. TP receptor gene expression was unaffected by GPER deficiency (Table 1), which also had no effect on gene expression levels of prostanoid generating enzymes, including COX-1, prostacyclin synthase, and thromboxane A2 synthase (Table 1).
Figure 1.
A, Endothelium-dependent responses to acetylcholine in the aorta of WT and GPER0 mice. Responses in the absence and presence of the COX-inhibitor indomethacin (Indo) or the TP receptor antagonist SQ 29,548 are shown. Values are means±SEM; n=4–5. *P<0.05 vs. untreated rings; †P<0.01 vs. WT. B, Concentration-dependent contraction and area under the curve (AUC) in response to the TP receptor agonist U46619 in WT and GPER0 aorta in the presence of L-NAME. Values are means±SEM; n=4–6. *P<0.01 vs. WT.
Table 1.
Vascular gene expression levels (calculated using the 2−ΔΔC(T) method32 and expressed as arbitrary units) in WT and GPER0 mice.
| Gene | WT | GPER0 | P-Value |
|---|---|---|---|
| Cyclooxygenase 1 | 28.98±3.07 | 21.82±2.70 | 0.13 |
| Prostacyclin Synthase | 2.427±0.313 | 1.830±0.213 | 0.15 |
| Thromboxane A2 Synthase | 517.4±71.0 | 428.8±10.7 | 0.25 |
| TP Receptor | 158.5±15.2 | 168.9±10.7 | 0.59 |
| Endothelial NO Synthase | 120.9±33.6 | 121.9±30.9 | 0.98 |
| sGC (α-subunit) | 187.4±42.1 | 171.7±19.6 | 0.75 |
| sGC (β-subunit) | 170.1±32.5 | 140.6±7.2 | 0.41 |
Values are means±SEM; n=4–5. TP receptor, thromboxane prostanoid receptor; sGC, soluble guanylate cyclase; NO, nitric oxide.
Chronic and Acute Effects of GPER Inhibition on Endothelial NO and EDCF Activity
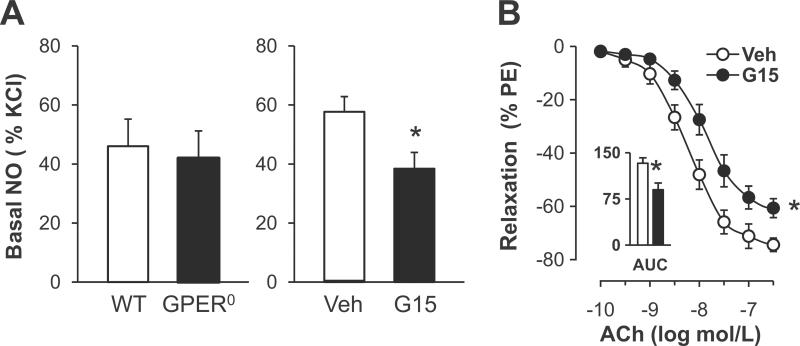
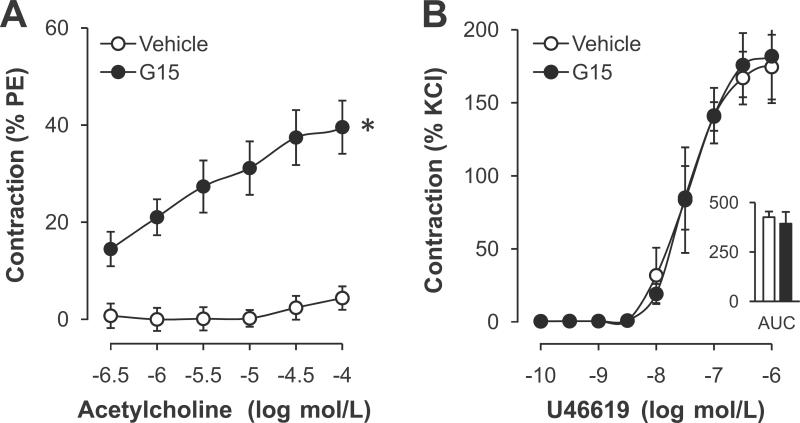
Based on previous findings that the GPER-selective agonist G-1 induces endothelium- and NO-dependent relaxation,17–19 we next investigated the role of GPER in basal and stimulated bioactivity of NO. GPER deficiency had no effect on receptor-stimulated, NO-mediated relaxation (Figure 1A), on basal NO bioactivity (41.9±9.3% vs. 46.0±9.2%, n=6–9, P=n.s. vs. WT, Figure 2A), or on eNOS gene expression (Table 1). In contrast, acute inhibition of GPER with the selective antagonist G15 reduced basal NO bioactivity in WT mice (38.3±5.7% vs. 57.7±5.2%, n=4–6, P<0.05 vs. vehicle, Figure 2A). Moreover, receptor-stimulated endothelial NO release in response to acetylcholine in WT mice was attenuated by G15 (maximal effect - 60.7±3.5% vs. −74.4±2.5%; AUC 90±11 vs. 133±9 AU; n=5–6, P<0.05 vs. vehicle, Figure 2B). At higher concentrations of acetylcholine, blockade of GPER by G15 yielded an increase in contraction to acetylcholine21–23,34–36 (9-fold at 0.1 mmol/L, n=5–6, P<0.05 vs. vehicle, Figure 3A). In contrast, contraction to TP receptor activation by U46619 remained unaffected by G15 (Figure 3B) compatible with the notion that acute GPER inhibition augments endothelial EDCF activity and/or release but not VSMC TP receptor signaling. In addition, G15 alone had no effect on vascular tone in quiescent aortic rings (Meyer MR and Prossnitz ER, unpublished data, 2011).
Figure 2.
A, Basal NO release in aorta from WT and GPER0 mice (left panel), and acute effects of the GPER-selective antagonist G15 (right panel). Basal NO release was calculated as the increase in contraction to phenylephrine (300 nmol/L) in rings treated with L-NAME compared to untreated rings. Values are means±SEM; n=4–9. *P<0.05 vs. vehicle. B, Acute effect of G15 on vascular reactivity to receptor-stimulated NO release by acetylcholine (ACh). AUC, Area under the curve. Values are means±SEM; n=5–6. *P<0.05 vs. vehicle (Veh).
Figure 3.
A, Acute effects of the GPER-selective antagonist G15 on endothelium-dependent contraction to acetylcholine. Values are means±SEM; n=5–6. *P<0.05 vs. vehicle. B, Concentration-dependent contraction and area under the curve (AUC) to U46619 after acute G15 incubation in the presence of L-NAME. Values are means±SEM; n=4–5.
Acute and Chronic Effects of GPER Inhibition on Endothelium-Independent Relaxation
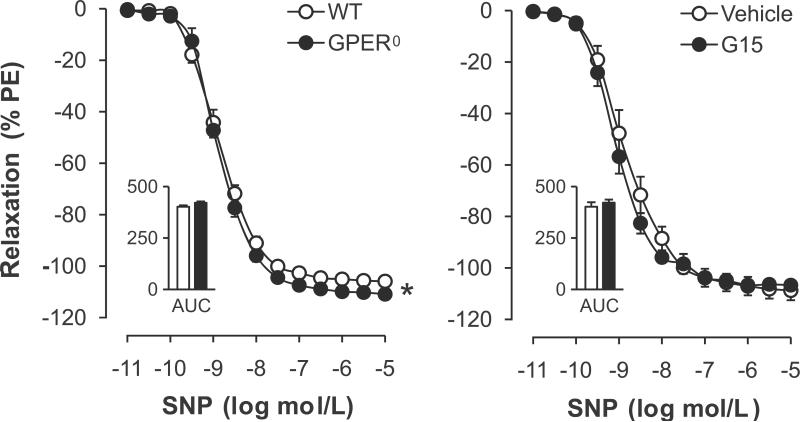
Endothelium-independent, NO-mediated relaxation in response to SNP was slightly but significantly enhanced in GPER0 mice (maximal effect −111±1% vs. −106±1%, P<0.01 vs. WT; AUC 421±7 vs. 402±7 AU, P=n.s.; pD2 8.84±0.05 vs. 8.82±0.08, P=n.s.; n=5–6, Figure 4A). GPER deficiency had no effect on gene expression levels of the α- or β-subunits of soluble guanylate cyclase, the downstream target of NO in VSMC3 (Table 1). Moreover, G15 did not acutely affect endothelium-independent relaxation (Figure 4B), suggesting that only the chronic lack of GPER to a very small extent affects smooth muscle sensitivity to NO.
Figure 4.
Endothelium-independent relaxation to the NO donor sodium nitroprusside (SNP) in aorta from WT and GPER0 mice (left panel), and role of acute inhibition with the GPER-selective antagonist G15 (right panel). Responses were recorded in the presence of L-NAME. AUC, Area under the curve. Values are means±SEM; n=5–6. *P<0.01 vs. WT.
Vascular Histology and Immunohistochemistry
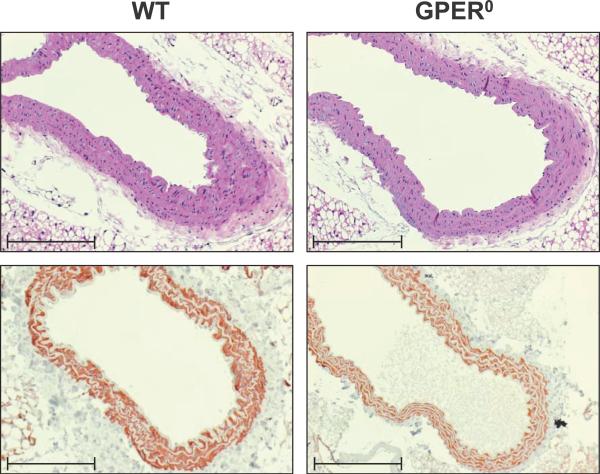
To determine whether the increased vasoreactivity in GPER0 animals might be associated with structural changes in the vasculature, histological analyses of the aorta were performed. Hematoxylin and eosin staining of aorta cross sections revealed structurally normal vascular walls in WT and GPER0 animals, in particular no changes in thickness of the smooth muscle layer (Figure 5). There was also no evidence of changes in VSMC differentiation and proliferation as assessed by staining for α-smooth muscle actin (Figure 5), PCNA, and Ki-67 (Amann K and Prossnitz ER, unpublished data, 2011).
Figure 5.
Histological sections of thoracic aorta of WT and GPER0 mice. Staining with hematoxylin and eosin (top) and immunohistochemical staining for α-smooth muscle actin (bottom) are shown revealing normal vascular structure. Scale bar, 100 μm.
Discussion
The present study provides evidence that endogenous GPER regulates vascular activity of prostanoid-dependent endothelial vasoconstriction. Chronic deficiency of the GPER gene has no effect on NO bioactivity but is associated with enhanced VSMC contraction to endothelium-dependent, COX-derived vasoconstrictor prostanoids and responses to TP receptor activation. In addition, histological and immunohistochemical analyses revealed structurally normal arteries in GPER0 mice. By contrast, acute blockade of GPER decreases both basal and stimulated endothelial NO bioactivity and increases EDCF-mediated responses while contractions to TP receptor activation remain unchanged. These data identify GPER as a novel, inhibitory regulator of endothelial vasoconstrictor prostanoids, and as the first estrogen receptor specifically associated with the regulation of endothelial vasoconstriction.
Acetylcholine induces the release of two counter-acting endothelial mediators in the mouse aorta: NO-mediated relaxation is induced at low concentrations of acetylcholine whereas a moderate EDCF-mediated contraction is observed at higher concentrations of acetylcholine.34 In the present study, we demonstrate that GPER deficiency augments acetylcholine-mediated contraction, which is completely abolished by either inhibition of COX or TP receptors, consistent with an EDCF-based mechanism.1,2 Moreover, direct activation of the EDCF-responsive element in VSMC, the TP receptor, reproduced the enhanced constrictor response in the absence of GPER with TP receptor gene expression levels being unaffected. Similar to the present findings, sensitivity to the prostanoid PGH2 is increased in spontaneously hypertensive rats (SHR) compared to normotensive animals, with no change in TP receptor expression levels in SHR.36,37 This suggests that a hyper-responsiveness of VMSC may contribute to endothelium-dependent contractions in both GPER0 mice and the SHR,2,36 possibly mediated by either increased calcium influx following TP receptor activation or enhanced sensitivity of contractile proteins.38 Consistent with this possibility, we observed that the calcium sensitivity of VSMC to endothelin-1-mediated constrictions is enhanced in GPER0 arteries.39 The present study also shows that endothelium-independent NO-mediated relaxation was slightly increased in GPER0 vessels with no change in guanylate cyclase gene expression, suggesting the possibility that chronic enhanced sensitivity to endothelium-derived constrictors might be compensated for by enhanced smooth muscle sensitivity to NO.
The enhanced EDCF response in GPER0 mice is likely unrelated to reduced NO release,1,2 since GPER deficiency had no effect on basal or stimulated NO bioactivity, or eNOS gene expression. Moreover, GPER deficiency had no effect on the gene expression levels of enzymes involved in prostanoid synthesis, including COX-1, which mediates endothelium-dependent contractions in large murine arteries,35 as well as synthases for thromboxane A2 and prostacyclin, molecules that function as EDCF under certain conditions.1,2 It is possible that an increased prostanoid release alone or in conjunction with an exaggerated production of reactive oxygen species (ROS), which stimulate formation of vasoconstrictor prostanoids,1,2 contributes to the enhanced EDCF response in GPER0 animals.
Acute GPER inhibition by G15 reduced endothelium-dependent, receptor-stimulated and basal NO bioactivity. This is consistent with previous studies demonstrating that stimulation of GPER using the selective agonist G-1 causes acute relaxation that is endothelium- and NO-dependent,17–19 an effect blocked by G15.19 In the present study, we also report that at higher concentrations capable of EDCF stimulation,21–23,34–36 acetylcholine causes a pronounced endothelium-dependent contraction in the presence of G15. This may be in part due to the observed G15-dependent reduction of NO bioactivity, which potentiates EDCF-mediated contractions.1,2 Moreover, the response to VSMC TP receptor activation was unaffected by G15, suggesting no increase in VSMC sensitivity to prostanoids in contrast to the observation in GPER0 mice.
The activity of endothelium-derived vasoconstrictor prostanoids increases with aging and diseases such as hypertension and obesity, conditions associated with structural changes in the vascular wall.1,2,40,41 In the present study, we used healthy adult mice and found no effect of GPER deficiency on media thickness, differentiation, or proliferation in the smooth muscle layer of the aorta consistent with normal vascular structure. Our results confirm previous findings in adult female GPER0 mice showing normal structure of the aortic media.42 In these animals, media thickness of non-pressure-fixed mesenteric arteries was also unaffected by GPER deficiency, whereas the vessel diameter was slightly decreased in nine months old GPER0 compared to WT mice.42 However, mean arterial blood pressure (MAP) in anesthetized GPER0 animals did not increase with age, although the MAP of GPER0 mice at nine months was higher than WT mice of the same age.42 Whether and to what extent the increased endothelium-dependent vasoreactivity observed in GPER0 in the present study contributes to blood pressure or structural vascular changes associated with the physiological aging process40 remains to be determined.
GPER is expressed and functional in the cardiovascular system of both males and females.15,17,42,43 Similar to the findings of the present study in male GPER0 mice, the absence of ERα or ERβ adversely affects endothelial-dependent vasoreactivity and blood pressure in male animals as well as in men.9,44–46 Thus, estrogen receptors are involved in the regulation of endothelium-derived factors independent of sex.9 Interestingly, many G protein-coupled receptors display a basal intrinsic activity even in the absence of their ligand that might be sufficient to mediate GPER-dependent vascular effects.47 Furthermore, the activity of the enzyme aromatase within the vascular wall (where it is localized intracellularly, like GPER, to the endoplasmic reticulum12,48), mediates local conversion of androgen precursors into the non-selective estrogen receptor agonist 17β-estradiol, which could yield local concentrations high enough to activate estrogen receptors.49 In fact, male mice lacking the enzyme aromatase display impaired endothelium-dependent relaxation.50 These findings are supported by human studies in men treated with an aromatase inhibitor, which impairs endothelium-dependent, flow-mediated dilation.51 Furthermore, there is evidence that 17β-estradiol inhibits endothelium-dependent contractions in salt-sensitive hypertension: in the renal artery, ovary-intact females exhibit much lower EDCF-mediated contractions than males, and only females are sensitive to inhibition of these responses by 17β-estradiol.23 As it is unclear to what degree GPER may have mediated these estrogen-dependent effects, further studies are required to clarify any sex-dependent roles of GPER or other ERs for EDCF or NO bioactivity under healthy conditions and in the presence of disease.
Perspectives
Conditions such as aging, hypertension, and obesity accelerate vascular disease development and are associated with exacerbated endothelium-dependent contractions and impaired NO bioactivity.1,2,41 In this study, we have identified GPER as the first estrogen receptor to specifically regulate endothelial protanoid vasoconstrictor activity. The ability of endogenous GPER to mitigate endothelium-dependent vasoconstriction and to enhance basal and stimulated endothelial NO bioactivity suggests the therapeutic potential of GPER-selective agonists such as G-114 to improve endothelium-dependent vascular dysfunction in conditions associated with increased cardiovascular risk.
Supplementary Material
Acknowledgements
We thank Daniel F. Cimino for expert technical assistance.
Sources of Funding
Supported by National Institutes of Health (NIH) grant CA127731 (to E.R.P.), Swiss National Science Foundation (SNSF) grants PBZHP3-135874 (to M.R.M.), 3200-108528/1 and K-33KO-122504/1 (to M.B.), the Interdisciplinary Centre for Clinical Research (IZKF) Erlangen, project A11 (to K.A.), and NIH grants HL82799 (to N.L.K.) and HL078914 (to M.K.W.).1
Footnotes
Disclosures: None
E.R.P. holds a US patent on G-1 and G15.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoutte PM. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension. 2011;57:526–531. doi: 10.1161/HYPERTENSIONAHA.110.165100. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3:31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- 4.Barton M. The discovery of endothelium-dependent contraction: the legacy of Paul M. Vanhoutte. Pharmacol Res. 2011;63:455–462. doi: 10.1016/j.phrs.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 6.Kleinert H, Wallerath T, Euchenhofer C, Ihrig-Biedert I, Li H, Förstermann U. Estrogens increase transcription of the human endothelial NO synthase gene: analysis of the transcription factors involved. Hypertension. 1998;31:582–588. doi: 10.1161/01.hyp.31.2.582. [DOI] [PubMed] [Google Scholar]
- 7.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 8.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99:2429–2437. doi: 10.1172/JCI119426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darblade B, Pendaries C, Krust A, Dupont S, Fouque MJ, Rami J, Chambon P, Bayard F, Arnal JF. Estradiol alters nitric oxide production in the mouse aorta through the alpha-, but not beta-, estrogen receptor. Circ Res. 2002;90:413–419. doi: 10.1161/hh0402.105096. [DOI] [PubMed] [Google Scholar]
- 11.Traupe T, Stettler CD, Li H, Haas E, Bhattacharya I, Minotti R, Barton M. Distinct roles of estrogen receptors alpha and beta mediating acute vasodilation of epicardial coronary arteries. Hypertension. 2007;49:1364–1370. doi: 10.1161/HYPERTENSIONAHA.106.081554. [DOI] [PubMed] [Google Scholar]
- 12.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 13.Takada Y, Kato C, Kondo S, Korenaga R, Ando J. Cloning of cDNAs encoding G protein-coupled receptor expressed in human endothelial cells exposed to fluid shear stress. Biochem Biophys Res Commun. 1997;240:737–741. doi: 10.1006/bbrc.1997.7734. [DOI] [PubMed] [Google Scholar]
- 14.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 15.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009;150:3753–3758. doi: 10.1210/en.2008-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broughton BR, Miller AA, Sobey CG. Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries. Am J Physiol Heart Circ Physiol. 2010;298:H1055–1061. doi: 10.1152/ajpheart.00878.2009. [DOI] [PubMed] [Google Scholar]
- 18.Meyer MR, Baretella O, Prossnitz ER, Barton M. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology. 2010;86:58–64. doi: 10.1159/000315497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsey SH, Carver KA, Prossnitz ER, Chappell MC. Vasodilation in response to the GPR30 agonist G-1 is not different from estradiol in the mRen2.Lewis female rat. J Cardiovasc Pharmacol. 2011;57:598–603. doi: 10.1097/FJC.0b013e3182135f1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller VM, Vanhoutte PM. 17 beta-Estradiol augments endothelium-dependent contractions to arachidonic acid in rabbit aorta. Am J Physiol. 1990;258:R1502–1507. doi: 10.1152/ajpregu.1990.258.6.R1502. [DOI] [PubMed] [Google Scholar]
- 21.Kauser K, Rubanyi GM. Gender difference in endothelial dysfunction in the aorta of spontaneously hypertensive rats. Hypertension. 1995;25:517–523. doi: 10.1161/01.hyp.25.4.517. [DOI] [PubMed] [Google Scholar]
- 22.Dantas AP, Scivoletto R, Fortes ZB, Nigro D, Carvalho MH. Influence of female sex hormones on endothelium-derived vasoconstrictor prostanoid generation in microvessels of spontaneously hypertensive rats. Hypertension. 1999;34:914–919. doi: 10.1161/01.hyp.34.4.914. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Kosaka H. Sex-specific acute effect of estrogen on endothelium-derived contracting factor in the renal artery of hypertensive Dahl rats. J Hypertens. 2002;20:237–246. doi: 10.1097/00004872-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Stallone JN. Estrogen potentiates vasopressin-induced contraction of female rat aorta by enhancing cyclooxygenase-2 and thromboxane function. Am J Physiol Heart Circ Physiol. 2005;289:H1542–1550. doi: 10.1152/ajpheart.01024.2004. [DOI] [PubMed] [Google Scholar]
- 25.Hermenegildo C, Oviedo PJ, Cano A. Cyclooxygenases regulation by estradiol on endothelium. Curr Pharm Des. 2006;12:205–215. doi: 10.2174/138161206775193136. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Kuo L, Stallone JN. Estrogen potentiates constrictor prostanoid function in female rat aorta by upregulation of cyclooxygenase-2 and thromboxane pathway expression. Am J Physiol Heart Circ Physiol. 2008;294:H2444–2455. doi: 10.1152/ajpheart.01121.2007. [DOI] [PubMed] [Google Scholar]
- 27.Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am J Physiol. 1997;272:H2264–2270. doi: 10.1152/ajpheart.1997.272.5.H2264. [DOI] [PubMed] [Google Scholar]
- 28.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–427. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton M, Haudenschild CC, d'Uscio LV, Shaw S, Munter K, Luscher TF. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 1998;95:14367–14372. doi: 10.1073/pnas.95.24.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton CA, Brosnan MJ, McIntyre M, Graham D, Dominiczak AF. Superoxide excess in hypertension and aging: a common cause of endothelial dysfunction. Hypertension. 2001;37:529–534. doi: 10.1161/01.hyp.37.2.529. [DOI] [PubMed] [Google Scholar]
- 31.Haas CS, Amann K, Schittny J, Blaser B, Muller U, Hartner A. Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol. 2003;14:2288–2296. doi: 10.1097/01.asn.0000082999.46030.fe. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- 35.Tang EH, Ku DD, Tipoe GL, Feletou M, Man RY, Vanhoutte PM. Endothelium-dependent contractions occur in the aorta of wild-type and COX2−/− knockout but not COX1−/− knockout mice. J Cardiovasc Pharmacol. 2005;46:761–765. doi: 10.1097/01.fjc.0000187174.67661.67. [DOI] [PubMed] [Google Scholar]
- 36.Ge T, Hughes H, Junquero DC, Wu KK, Vanhoutte PM, Boulanger CM. Endothelium-dependent contractions are associated with both augmented expression of prostaglandin H synthase-1 and hypersensitivity to prostaglandin H2 in the SHR aorta. Circ Res. 1995;76:1003–1010. doi: 10.1161/01.res.76.6.1003. [DOI] [PubMed] [Google Scholar]
- 37.Tang EH, Vanhoutte PM. Gene expression changes of prostanoid synthases in endothelial cells and prostanoid receptors in vascular smooth muscle cells caused by aging and hypertension. Physiol Genomics. 2008;32:409–418. doi: 10.1152/physiolgenomics.00136.2007. [DOI] [PubMed] [Google Scholar]
- 38.Huang JS, Ramamurthy SK, Lin X, Le Breton GC. Cell signalling through thromboxane A2 receptors. Cell Signal. 2004;16:521–533. doi: 10.1016/j.cellsig.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Meyer MR, Field AS, Kanagy NL, Barton M, Prossnitz ER. Novel role of G protein-coupled estrogen receptor GPER/GPR30 as a regulator of intracellular calcium concentration during endothelin-1-induced arterial vasoconstriction. Abstract book, 12th International Conference on Endothelin, Cambridge, UK; September 11 – 14, 2011; p. 67. Abstract LBP011. [Google Scholar]
- 40.Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiol Rev. 1993;73:725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]
- 41.Barton M, Baretella O, Meyer MR. Obesity and risk of vascular disease: importance of endothelium-derived vasoconstriction. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01472.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 43.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sudhir K, Chou TM, Messina LM, Hutchison SJ, Korach KS, Chatterjee K, Rubanyi GM. Endothelial dysfunction in a man with disruptive mutation in oestrogen-receptor gene. Lancet. 1997;349:1146–1147. doi: 10.1016/S0140-6736(05)63022-X. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]
- 46.Corbacho AM, Eiserich JP, Zuniga LA, Valacchi G, Villablanca AC. Compromised aortic vasoreactivity in male estrogen receptor-alpha-deficient mice during acute lipopolysaccharide-induced inflammation. Endocrinology. 2007;148:1403–1411. doi: 10.1210/en.2006-0399. [DOI] [PubMed] [Google Scholar]
- 47.Gilchrist A, Blackmer T. G-protein-coupled receptor pharmacology: examining the edges between theory and proof. Curr Opin Drug Discov Devel. 2007;10:446–451. [PubMed] [Google Scholar]
- 48.Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457:219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simpson ER. Sources of estrogen and their importance. J Steroid Biochem Mol Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 50.Kimura M, Sudhir K, Jones M, Simpson E, Jefferis AM, Chin-Dusting JP. Impaired acetylcholine-induced release of nitric oxide in the aorta of male aromatase-knockout mice: regulation of nitric oxide production by endogenous sex hormones in males. Circ Res. 2003;93:1267–1271. doi: 10.1161/01.RES.0000103172.98986.25. [DOI] [PubMed] [Google Scholar]
- 51.Lew R, Komesaroff P, Williams M, Dawood T, Sudhir K. Endogenous estrogens influence endothelial function in young men. Circ Res. 2003;93:1127–1133. doi: 10.1161/01.RES.0000103633.57225.BC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.