Abstract
Purpose of review
Acute HIV-1 infection (AHI) is comprised of the eclipse phase, during which the transmitted virus struggles to avoid eradication and achieve amplification/spread; the expansion phase when virus disseminates and undergoes exponential replication associated with extensive CD4+ T cell destruction; and the containment phase when set-point levels of viremia and immune activation are established. The importance of interactions between HIV-1 and innate responses in determining events throughout AHI is increasingly recognised, and is reviewed here.
Recent findings
During the eclipse phase HIV-1 subverts dendritic cell functions to promote its replication at mucosal sites and employs multiple strategies to minimise control by type 1 interferons. Systemic virus dissemination is associated with widespread activation of innate responses, which fuels HIV-1 replication. To minimise the protective effects of innate responses HIV-1 resists control by natural killer cells and may impair innate regulation of adaptive responses. Innate responses remain chronically activated after HIV-1 containment, which is thought to drive HIV-1 pathogenesis.
Summary
Innate responses are pivotal determinants of events at all stages of AHI. Increased understanding of mechanisms involved in innate control of HIV-1 and pathways regulating innate activation during HIV-1 infection could facilitate development of novel approaches to combating this infection.
Keywords: Human immunodeficiency virus, innate immunity, type 1 interferon, dendritic cell, natural killer cell
Introduction
The acute phase of human immunodeficiency virus type 1 (HIV-1) infection is the most critical stage of this infection. Virus interactions with the immune system during acute HIV-1 infection (AHI) determine i. whether the transmitted virus is eliminated at mucosal sites or establishes expanding foci of infection and disseminates; ii. the magnitude of the ensuing acute burst of viral replication and extent of associated damage to the immune system; and iii. the efficiency of control of virus replication and establishment of set-point levels of viremia and immune activation, independent predictors of subsequent disease progression. Adaptive responses start to be induced during the acute viremic burst [1, 2] and contribute to containment of HIV-1 replication and establishment of the persisting virus load [3], but there is no evidence that they constrain HIV-1 replication during the initial stages of infection [4]. By contrast innate responses are activated very rapidly after virus exposure, and increasing evidence indicates that interactions between HIV-1 and the innate immune system are key determinants of events from the earliest stages of AHI onwards (Figure 1). This review covers recent advances in understanding of the activation of innate responses during AHI, roles played by innate responses in control of HIV-1 replication, and viral strategies for subverting and exploiting innate defences.
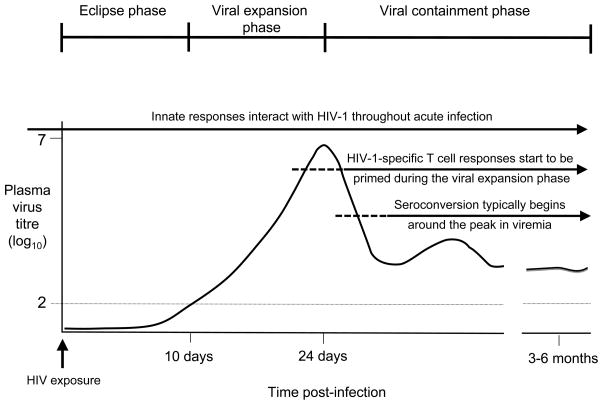
Figure 1. Diagram illustrating the different phases of acute HIV-1 infection and the host immune responses available to counteract virus replication at each.
Following mucosal HIV-1 transmission virus replication is initially confined to small foci of infection at the mucosal site. After local amplification, infection disseminates to the draining lymph node and blood; the eclipse phase ends when virus first reaches detectable levels in the plasma (the horizontal dotted line indicates the limit of sensitivity of standard HIV-1 RNA assays). An exponential burst of virus replication then ensues, associated with extensive depletion of CD4+ T cells, particularly from the gut-associated lymphoid tissues. Plasma virus titres often reach peaks of 1–100 million RNA copies/ml at the end of the viral expansion phase. HIV-1 replication is subsequently contained, and set-point levels of persisting viral replication and immune activation are established by 3–6 months post-infection. Interactions occur between HIV-1 and the innate immune system at all stages of acute infection. Adaptive responses start to be induced during the viral expansion phase and play an important role in containment of virus replication and establishment of the set-point persisting viral load.
HIV-1 - innate interactions at mucosal infection sites during the initial stages of AHI
HIV-1 infection is generally acquired by sexual mucosal transmission. Studies in macaques infected intravaginally with simian immunodeficiency virus (SIV) show that virus replication is initially confined to the mucosal infection site. Small foci of infection are established locally, some of which expand and disseminate virus to the draining lymph node (DLN) and blood [5*]. The length of the eclipse period before widespread virus dissemination occurs ranges from 5–6 days to several weeks, with exposure to lower virus doses being associated with longer eclipse periods [6, 7*]. Mucosal HIV-1 transmission is inefficient: >100 exposures may be required before disseminated infection occurs, and infection is commonly initiated by a single founder virus [8]. This suggests that HIV-1 struggles to establish infection at mucosal sites and may frequently be eliminated during the eclipse period. Two factors hamper HIV-1 replication at the mucosa: relatively few CD4+ target cells are present, and local host defences need to be evaded. HIV-1’s ability to overcome these hurdles is critically dependent on interactions with the innate immune system.
HIV-1 exploits DCs to overcome the problem of limited target availability at mucosal infection sites
HIV-1 replication at mucosal transmission sites predominantly occurs in CD4+ T cells [5*]. CD4+ T cells are sparsely distributed at non-inflamed genital mucosae, so induction of an influx of additional target cells is crucial for virus propagation. In macaques infected intravaginally with SIV epithelial cells are rapidly stimulated to produce macrophage inflammatory protein (MIP)3α (CCL20), which recruits plasmacytoid dendritic cells (pDCs) to the endocervix. The virus then triggers pDCs to produce factors including type 1 interferons (IFN-1) (discussed below) and the chemokines MIP1α and β (CCL3 and CCL4), which recruit CD4+ T cells, amplifying the pool of locally-available target cells [9] (although they may also contribute to control of the replication of CCR5-utilising viruses).
HIV-1 also exploits conventional (c)DCs in the sub-epithelium to help it cope with the low density of CD4+ T cell targets. HIV-1 does not infect these dendritic cell-specific, intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN)+ cDCs efficiently, but by binding to DC-SIGN achieves multiple goals: DC-SIGN-bound virions are sequestered and efficiently transferred to CD4+ T cells with which the DC interacts [10], a process facilitated by signalling via DC-SIGN [11]; signalling via DC-SIGN also promotes production of pro-inflammatory cytokines that stimulate virus replication [12]; and signalling via both DC-SIGN and TLR8 can enable HIV-1 to replicate productively within the DC itself [13*]. cDCs also express other HIV-1 capture/transfer receptors [14]. The importance of DC-mediated HIV-1 transfer in virus spread during early AHI is suggested by the fact that stromal cell-derived factor-1 (SDF-1) (CXCL12) production by DCs impairs transfer of CXCR4-utilising viruses across the DC-T cell synapse, and CXCR4-utilising viruses are rarely transmitted [15, 16].
HIV-1 employs multiple strategies to evade control by IFN-1 early after transmission
Although local immune activation and cellular infiltration facilitate virus replication, HIV-1 must simultaneously avoid being controlled by innate antiviral defences activated at the mucosa, particularly IFN-1.
IFN-1 are pleiotropic cytokines that act by up-regulating transcription of hundreds of IFN-stimulated genes (ISGs), many of which have antiviral activity [17**]. The most intensively-studied of the ISGs that restrict the replication of HIV-1 and related viruses are tripartite motif (TRIM)5α apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC)3G and tetherin, the antiretroviral effects of which are emphasised by the fact that HIV-1 and related viruses have evolved strategies for counteracting them [18*]. The most recently-discovered of these restriction factors was tetherin, a cell-surface protein that “tethers” virions to virus-producing cells, preventing their release [19–21*] and is also incorporated into virions, reducing their infectivity [22*]. Tetherin is antagonised by HIV-1 Vpu, which reduces tetherin levels on infected cells by targeting it for degradation and sequestering it in a perinuclear compartment [23–25*]. Although tetherin restricts transfer of virions from an infected cell to neighbouring cells by cell-to-cell spread [26, 27*], increased tetherin expression on uninfected cells enhances their infection [27, 28*]. The IFN-induced increase in tetherin expression may thus benefit HIV-1 by enhancing cell-to-cell transmission.
Many other ISGs also inhibit HIV-1 replication, including protein kinase R (PKR), Mx, ISG15, TRIM22 and interferon-induced transmembrane proteins (IFITMs)1–3 [17, 29–31*]. There are also additional as-yet-unidentified factors that restrict HIV-1 replication, e.g. IFNα induces a post-entry block to HIV-1 replication in macrophages [32, 33] and HIV-1 replication in monocyte-derived DCs (MDDCs) is blocked by a restriction factor antagonised by SIV Vpx [34**]. The relative importance of these different antiviral pathways in constraining HIV-1 replication in vivo remains to be determined. Correlations have been reported between adjuvant/vaccine-induced APOBEC3G expression and virus replication following mucosal SIV challenge in macaques [35, 36], but although this suggests a role for ISGs in virus control it is unclear whether the effects observed were mediated by APOBEC3G or other ISGs up-regulated in parallel.
The importance of IFN-1 in HIV-1 control is underlined by the fact that HIV-1 employs multiple strategies to block IFN-1 production in infected cells. HIV-1 genomic RNA is recognised by the cytoplasmic RNA sensor retinoic acid-inducible gene I (RIG-I), but in HIV-1-infected cells the viral protease sequesters RIG-I and targets it to lysosomes to block IFN-I induction [37**]. Likewise HIV-1 DNA would be recognised by an as-yet-unidentified nucleic acid sensor in infected cells, but this is prevented by the cytosolic nuclease 3′ repair exonuclease 1 (TREX1), which binds to and digests excess cytoplasmic HIV-1 DNA [38**]. In MDDCs HIV-1 infection can also be sensed by a pathway involving interaction of newly-synthesised capsids with cyclophilin A and subsequent IRF3 activation [34] that HIV-1 does not appear to evade, perhaps because it does not normally replicate efficiently in cDCs. HIV-1 also prevents IRF3-mediated triggering of IFN-1 production: in T cells and macrophages Vpr and Vif target IRF3 for degradation [39], whilst in MDDCs Vpr blocks IRF3 activation without inducing its degradation [40*].
Although HIV-1 avoids triggering IFN-1 production in infected cells, IFN-1 are nonetheless produced by pDCs at the mucosal transmission site and subsequently in LNs [9, 41–43]. HIV-1 is endocytosed by pDCs following binding to CD4 and chemokine co-receptors, and interaction of viral RNA with TLR7 in endosomes triggers IRF7 activation and IFN-1 induction [44]. pDCs can also recognise HIV-1-infected cells by both endosomal (IRF7-dependent) and cytoplasmic (IRF3-dependent) pathways [45*]. Notably, HIV-1-stimulated pDCs can be repeatedly triggered to produce IFN-1, which is associated with virion trafficking to early endosomes and induction of a partially-matured, persistently IFN-1-secreting phenotype [46**]. That HIV-1 activates potent secretion of IFN-1 and other cytokines/chemokines by pDCs but suppresses IFN-1 production by infected cells likely reflects a balance between its need to drive inflammation and attract CD4+ cells to enhance replication, whilst simultaneously minimising local up-regulation of antiviral ISGs. Table 1 summarises HIV-1-host pattern-recognition receptor interactions that are subverted/exploited during AHI.
Table 1.
Examples of interactions between HIV-1 and host pattern-recognition receptors that are subverted during acute infection
| Host pattern-recognition receptor | Expressed by | Cellular location | HIV-1 component(s) recognised | “Intended” outcome(s) of the interaction | Is this subverted by HIV-1? |
|---|---|---|---|---|---|
| Langerin | Langerhans cells (LC) | Plasma membrane | Envelope glycoprotein | Virion capture, degradation in Birbeck granules and antigen presentation | Yes if LC are activated: virions are then not degraded, but are transferred to CD4+ T cells. |
| DC-SIGN | Conventional (c)DCs in tissues | Plasma membrane | Envelope glycoprotein | Virion capture for antigen processing and presentation | Yes. HIV-1 is captured and transferred to CD4+ T cells. Signalling via DC-SIGN also impairs DC functions and (with TLR8 signalling) promotes HIV-1 replication in cDCs. |
| DCIR | Conventional (c)DCs | Plasma membrane | Envelope glycoprotein | Virion capture for antigen processing and presentation | Yes. HIV-1 is captured and transferred to CD4+ T cells. Signalling via DCIR may also impair DC functions. |
| TLR7 | Plasmacytoid (p)DCs and monocytes | Endocytic vesicles | Viral RNA | Triggering of type 1 IFN and pro-inflammatory cytokine/chemokine production | Yes. HIV-1 is able to repeatedly stimulate pDCs to produce high levels of cytokines/chemokines via this pathway. This promotes HIV-1 replication and spread. |
| TLR8 | Conventional (c)DCs and monocytes | Endocytic vesicles | Viral RNA | Triggering of pro-inflammatory cytokine/chemokine production | Yes. Interaction of HIV-1 with both DC-SIGN and TLR8 promotes virus replication in cDCs. |
| RIG-I | Most cells | Cytoplasm | Viral RNA | IRF3 activation and triggering of type 1 IFN production | Yes. The HIV-1 protease sequesters RIG-I so that type 1 IFN is not triggered via this pathway. |
| Unknown DNA sensor | Most cells | Cytoplasm | Viral DNA | IRF3 activation and triggering of type 1 IFN production | Yes. TREX1 digests excess cytoplasmic HIV-1 DNA so that this pathway is not activated. Vpr and Vif also target IRF3 for degradation. |
| Unknown mechanism dependent on cyclophilin A | Conventional (c)DCs | Cytoplasm | Viral capsid | IRF3 activation and triggering of type 1 IFN production | Not efficiently, perhaps because HIV-1 does not normally replicate efficiently in cDCs. Vpr blocks IRF3 without inducing its degradation. |
Activation and subversion of systemic innate responses during the viral expansion phase of AHI
After amplification at the transmission site HIV-1 spreads to the DLN and rapidly undergoes systemic dissemination [5*]. An exponential increase in viral replication ensues, associated with extensive depletion of CD4+ T cells, particularly from the gut-associated lymphoid tissues (GALT). During this phase of AHI there is widespread activation of innate responses.
Systemic activation of innate responses during the viral expansion phase
The earliest systemic perturbations in innate factors detected in AHI are elevations in acute-phase proteins (APPs) including the acute form of serum amyloid A (A-SAA), plasma concentrations of which increase transiently during the eclipse phase then increase again during the acute viremic phase [47**]. The second increase in APP levels is coincident with systemic elevations in pro-inflammatory cytokines including interleukin (IL)-1β and IL-6 [43], which are known to trigger APP production by the liver. No perturbations are detected in plasma cytokines during the eclipse phase, but the initial burst of APP production may reflect viral dissemination to the GALT and local production of pro-inflammatory cytokines that reach the liver via the portal venous system. APPs including A-SAA and α1-antitrypsin plus proteolytic fragments of the latter inhibit HIV-1 replication in vitro [47**], hence may mediate direct antiviral activity during AHI; and A-SAA and other APPs have immunomodulatory effects [48, 49] so may also help to control HIV-1 replication indirectly. The increase in viremia during AHI is associated with widespread activation of DCs, coupled with elevations in circulating levels of innate cytokines/chemokines including IFN-1, IL-15, IL-18, tumor necrosis factor (TNF)α and IFNγ-induced protein (IP)-10 (CXCL10) [43]. Circulating frequencies of pDCs and cDCs are dramatically reduced prior to the peak in viremia [50*], and although they recover somewhat as viremia is contained they remain reduced throughout infection. The decline in circulating DC frequencies during AHI likely reflects a combination of activation-associated recruitment into LNs and apoptotic death. In SIV and HIV-1 infections pDCs accumulate in LNs where they produce IFNα and undergo apoptosis [42, 51–53] and high levels of cDC apoptosis are also observed [54, 55*]. The DCs that remain in the blood during AHI retain functional capacity and are in a heightened activation state where they exhibit hyper-responsiveness to stimulation with TLR7/8 ligands [50*].
Natural killer (NK) cells also become highly activated during the viral expansion phase and increase in frequency in the circulation [56]. NK cell activation is stimulated by innate cytokines including IFN-1, IL-15 and IL-18 and is also regulated by receptor-ligand interactions. There is a specific increase in the frequency of NK cells expressing killer immunoglobulin receptor (KIR)s 3DS1 and 3DL1 in subjects expressing human leukocyte antigen (HLA)-Bw4 alleles with an isoleucine residue at position 80 (HLA-Bw480I), the putative HLA class I ligand for KIR3DL1/3DS1 [57], which suggests an important role for KIR-HLA interactions in regulating NK cell expansion and/or survival during AHI.
The dramatic systemic activation of innate responses in AHI (Figure 2) contrasts with the much more muted immune activation during acute infection with hepatitis B and C viruses [43], which adopt a “stealth” approach to minimise control by innate defences. Triggering of widespread immune activation favours HIV-1 replication and spread – but HIV-1 then has to avoid innate control and impede the induction of adaptive responses by innate responses.
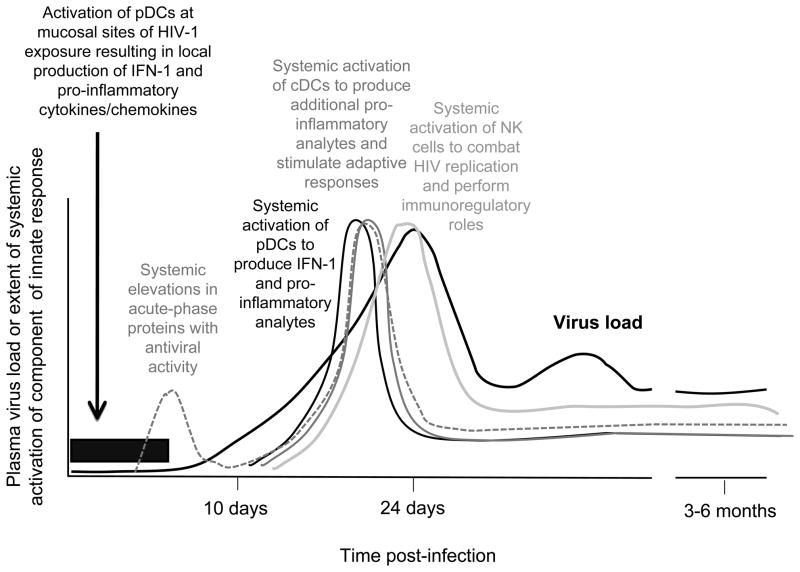
Figure 2. Diagram illustrating the dynamics with which innate responses are activated during AHI.
pDCs are recruited to mucosal sites of HIV-1 infection within hours of viral exposure, and produce pro-inflammatory cytokines and chemokines that attract CD4+ T cells to the transmission site hence facilitating viral replication, plus IFN-1 that mediate local antiviral activity. Systemic activation of innate responses is triggered as widespread virus dissemination occurs. There is rapid activation of pDCs and cDCs associated with elevations in plasma levels of multiple innate cytokines and chemokines, followed by activation and expansion of NK cells. Although innate effector mechanisms contribute to control of virus replication, the intense systemic immune activation simultaneously promotes viral replication. The initial intense activation of innate responses is not sustained after the acute viral burst, but a heightened state of activation continues to be maintained after viremia is contained. This chronic up-regulation of immune activation is thought to be an important factor in HIV-1 pathogenesis.
NK cell-mediated control of HIV-1 replication and viral strategies for its evasion
NK cells combat HIV-1 replication by killing infected cells and producing antiviral factors including IFNγ, TNFα and β-chemokines. Triggering of NK effector functions following contact with HIV-1-infected cells involves a reduction in signalling through inhibitory receptors including KIRs that interact with HLA-A/B molecules (surface expression of which is down-modulated to reduce CD8+ T cell recognition [58]), together with enhanced stimulation through activating/co-stimulatory/adhesion receptors including activating KIRs [59], NKG2D, NTB-A (CD352) and 2B4 (CD244) [60] (Table 2). No specific ligands for activating KIRs on HIV-1-infected cells have been identified yet, but they are hypothesised to recognise HLA molecules presenting viral or host stress protein-derived peptides, with the interaction perhaps being dependent on a viral/host protein co-expressed on the cell surface (analogous to Ly49P-mediated recognition of murine cytomegalovirus-infected cells [61]). NKG2D interacts with unique-long 16 binding proteins (ULBPs)-1,-2 and -3, expression of which is up-regulated on infected cells by HIV-1 Vpr via a mechanism dependent on activation of the DNA damage/stress-sensing ataxia telangiectasia and rad-3-related (ATR) kinase [62, 63*]. Interaction of HIV-1 gp41 with a binding protein for the globular head domains of complement component C1q (gC1qR) on CD4+ T cells has also been shown to induce expression of a ligand for the activating NK receptor NKp44 [64], NK activation via which may contribute to immunopathogenic destruction of uninfected CD4+ T cells [65]. NK cells can also be targeted to combat HIV-1-infected cells via antibody-dependent cell-mediated cytotoxicity (ADCC), but although some of the first antibodies produced in AHI are thought to stimulate ADCC [66] this mechanism cannot operate prior to seroconversion.
Table 2.
Receptor-ligand interactions involved in innate NK cell recognition of HIV-1-infected cells and viral strategies for reducing NK triggering
| NK cell receptor | Activating or inhibitory? | (Putative) ligand on HIV-1- infected cells | How does HIV-1 modulate this interaction? |
|---|---|---|---|
| KIRs with short cytoplasmic tails e.g. KIR3DS1 | Activating | HLA molecules expressing peptides derived from viral or host stress proteins (perhaps together with a viral or host stress response protein on the cell membrane) | Expression of HLA-A and HLA-B alleles (which may be recognised by some activating KIRs) is down-modulated by Nef. Acquisition of viral mutations that confer escape from recognition by activating KIRs has not yet been documented but may occur? |
| NKG2D | Activating | ULBPs-1, 2 and 3 (up-regulated on HIV-1-infected cells by Vpr) | Other NKG2D ligands, e.g. MICA and MICB, are not up-regulated. |
| NTB-A (CD352) | Enhances activation | NTB-A (CD352) | Expression is down-modulated on HIV-1-infected cells by Vpu. |
| 2B4 (CD244) | Enhances activation | CD48 | Expression is down-modulated on HIV-1-infected cells. |
| NKp44 | Activating | Ligand induced on infected and uninfected CD4+ T cells following gp41 binding to gC1qR | Nef down-regulates expression of the gp41-induced NKp44L on infected cells. |
| KIRs with long cytoplasmic tails e.g. KIR3DL1 | Inhibitory | HLA molecules expressing peptides derived from normal host proteins | Although Nef down-modulates expression of HLA-A and HLA-B alleles to escape CD8+ T cell recognition, expression of HLA-C alleles is retained. Selection for mutations in HIV-1 that enhance recognition of peptide-MHC complexes by inhibitory KIRs has been documented, although whether this protects infected cells from NK activity is not clear. |
| NKG2A and NKG2C | Inhibitory Activating | HLA-E | HLA-E expression is not down-modulated by Nef. |
The importance of NK cells in HIV-1 infection is suggested by genetic associations between co-expression of KIR3DS1 or KIR3DL1 alleles encoding highly-expressed KIR3DL1 proteins and HLA-Bw480I, and slow HIV-1 disease progression [67, 68] (although similar associations are not observed in HIV-2 infection) [69]. KIR3DS1/HLA-Bw480I is associated with establishment of a low persisting viral load, indicating that it exerts protective effects during AHI [70]. KIRs are expressed by both NK cells and some CD8+ T cells, but the beneficial effects of the KIR/HLA compound genotypes likely involve effects on NK responses as KIR3DS1+ NK cells mediate highly potent control of HIV-1 replication in HLA-Bw480I+ target cells [59] and KIR3DL1+ NK cells from individuals with protective KIR/HLA compound genotypes exhibit enhanced functional potential in vitro [71*]. KIR3DS1 expression is also associated with enhanced NK cell functionality in AHI [72].
Further evidence that NK cells exert pressure on HIV-1 replication in vivo is that HIV-1 has evolved multiple strategies for evading NK control (Table 2). Although surface expression of HLA-A/B molecules is reduced on HIV-1-infected cells, expression of HLA-C and HLA-E, which have a greater relative role in inhibiting NK activation, is retained [58]. Nef also down-regulates expression of the gp41-induced NKp44 ligand on HIV-1-infected cells [65]; likewise CD48 and NTB-A, ligands for 2B4 and NTB-A, are down-modulated [60], the latter by Vpu [73**]. Selection for mutations in HIV-1 that affect the interaction of peptide-HLA complexes with inhibitory KIRs has also been observed [74, 75], although as these mutations also reduce CD8+ T cell recognition it is unclear whether their selection is driven solely by T cells or also confers evasion by modulating NK activity.
HIV-1 may also induce abnormalities in innate functions that influence interactions between innate subsets and the induction of adaptive responses
pDCs, NK cells and cDCs mutually enhance oneanother’s activation via cytokine production and cell contact-dependent interactions. This is impaired during chronic HIV-1 infection [76, 77] but during AHI may conversely be enhanced [46, 50**], furthering immune activation and viral replication. NK cells also mediate DC editing, lysing immature cDCs in LNs to ensure that T cells interact with mature DCs capable of mediating effective priming. Again, this is disrupted during chronic HIV-1 infection, perhaps because NK functions are progressively impaired [78] and/or due to production of IL-10, which makes immature DCs resistant to NK lysis whilst increasing the sensitivity of mature DCs to NKG2D-dependent NK elimination [79*]. HIV-1-infected DCs are, however, rendered resistant to NK cell-mediated editing by a process that involves high-mobility group box 1 (HMGB1)-induced up-regulation of anti-apoptotic molecules [80*]. This prolongs HIV-1 persistence within DCs. cDCs with a partly-matured phenotype accumulate in LNs during AHI [81], although whether this is due to alterations in NK-mediated editing or effects of HIV-1 itself on DC maturation is unknown. HIV-1 infection of cDCs does not induce their maturation, and impairs their ability to prime CD4+ and CD8+ T cell responses in vitro [82]. Mechanisms involved may include interaction of virions with DC-SIGN, which triggers signalling that impairs subsequent DC maturation [11] and/or interaction of Env with other receptor(s) leading to activation of the mammalian target of rapamycin (mTOR) pathway and shut-down of autophagy, which down-regulates TLR responsiveness and impairs antigen presentation [83**]. HIV-1-exposed DCs also promote T cell exhaustion and stimulate induction of regulatory T (Treg) cell responses [84, 85*]. In addition, HIV-1 activates pDCs to induce Tregs via an indoleamine 2,3-dioxygenase (IDO)-dependent mechanism, and these Tregs can in turn inhibit the maturation of cDCs [86]. The extent to which these mechanisms impact on DC-T cell interactions in vivo remains unclear. IDO is up-regulated during acute SIV infection [42]; and IDO production is associated with a decline in TH17 cells and increase in Treg cell activity in chronic HIV-1 infection, supporting a role for this pathway in disease progression [87]. However HIV-1-specific CD8+ T cell responses with potent antiviral activity are elicited during AHI [3] and CD4+ T cell responses are transiently expanded [88], suggesting that at least a proportion of DCs retain functionality in vivo and/or that DC functions are not compromised rapidly enough to impair T cell priming. Vpu has also been shown to interfere with CD1d expression on HIV-1-infected DCs, which renders them poor stimulators of NKT cell activation in vitro [89*], although the in vivo importance of this is again unclear. Notably, cDCs express increased levels of B lymphocyte stimulator (BLyS) from AHI onwards, which, coupled with high expression of a proliferation-inducing ligand (APRIL), IL-6 and IL-10 may promote generalised B cell activation and dysfunction and contribute to the delay in neutralising antibody production in AHI [90**].
Roles of innate responses during the later stages of AHI as viremia is contained
Systemic innate responses are maximally activated during the viral expansion phase: as viremia decreases and stabilises later in AHI plasma cytokine/chemokine levels decline [43], circulating DC numbers begin to increase [50*] and peripheral blood NK frequencies normalise [56] (Figure 2). However although not as highly-stimulated as during the acute viral burst, innate responses remain in a heightened state of activation.
Adaptive responses play a dominant role in containment of viremia during the later stages of AHI, but innate responses likely also contribute to control of virus replication. The major common genetic determinants of the persisting viral load established in early HIV-1 infection map to the major histocompatibility complex [91], polymorphisms in which can influence innate as well as adaptive responses. For example the beneficial effects of the KIR3DS1/HLA-Bw480I compound genotype likely reflect an important effector role for KIR3DS1+ NK cells in control of HIV-1 replication [59, 67, 70]; and the detrimental effects of HLA-B35-Px alleles may be due to their ability to bind with high affinity to immunoglobulin-like transcript 4 (ILT4), an inhibitory receptor on DCs, and promote DC dysfunction [92]. Importantly, maintenance of innate activation after control of viremia in AHI also has detrimental consequences, promoting viral replication and enhancing CD4+ T cell loss.
Ongoing stimulation of pDC production of IFN-1 and other cytokines may be particularly harmful, as IFN-1 promote the activation of innate and adaptive cells, have direct pro-apoptotic effects and also up-regulate TNF-related apoptosis-inducing ligand (TRAIL) on CD4+ T cells and pDCs, indirectly furthering CD4+ cell apoptosis [93, 94]. pDCs from women produce more IFN-1 following TLR7 ligation than pDCs from men, and women undergo faster HIV-1 disease progression than men with similar viral loads, supporting a detrimental role for pDC activation and IFN-1 production in chronic infection [95]. Furthermore, although a strong IFN-1 response is activated during the acute phase of both pathogenic (e.g. HIV-1 infection of humans and SIV infection of rhesus macaques) and non-pathogenic (e.g. SIVsmm infection of sooty mangabeys and SIVagm infection of African green monkeys) immunodeficiency virus infections, IFN-1 production is down-regulated following acute infection in the non-pathogenic infections, whilst high levels of ISG expression are sustained into chronic infection in the pathogenic infections [51, 96–99]. Likewise in HIV-2 infection, which is typically associated with slower CD4 decline and disease progression than HIV-1 infection, ISG expression levels during chronic infection are lower than those in HIV-1 infection [100]. The mechanisms responsible for the differential regulation of immune activation in pathogenic and non-pathogenic primate immunodeficiency virus infections are unclear, but are of priority to understand.
Conclusion
Interactions between HIV-1 and the innate immune system determine critical events at all stages of AHI. Modulation of innate responses thus represents a promising approach for combating HIV-1 infection, although the complex combination of protective/pathogenic effects mediated by innate responses during AHI suggests that intervention strategies will need to act selectively. Definition of the ISGs that mediate the in vivo antiviral activity of IFN-1 could enable their activity to be invoked to enhance HIV-1 control. Likewise identification of receptor-ligand interactions involved in NK recognition of HIV-1-infected cells could enable the development of strategies for enhancing NK-mediated containment of viral replication. Conversely dissection of the pathways via which HIV-1 stimulates pDC-mediated production of high levels of inflammatory mediators and understanding of mechanisms by which innate responses are down-regulated following virus containment in non-pathogenic primate immunodeficiency virus infections may enable development of prophylactic or therapeutic strategies to combat HIV-1 infection via down-modulation of immunopathogenic innate activation. Finally, the impact of HIV-1-induced abnormalities in innate functions on the induction/maintenance of adaptive responses in acute/early infection is poorly understood, but counteraction of early innate dysfunction could also provide a means of enhancing HIV-1 control by adaptive responses.
Key points.
Interactions between HIV-1 and the innate immune system are pivotal determinants of critical events at all stages of AHI.
During the eclipse phase of AHI HIV-1 subverts dendritic cell functions to overcome the problem of limited CD4+ target cell availability at mucosal infection sites, and employs multiple strategies to evade local control by type 1 interferons.
As systemic virus dissemination occurs HIV-1 stimulates widespread activation of innate responses to fuel its replication, and to minimise the protective effects of innate responses it resists control by natural killer cells and may impair innate regulation of adaptive responses.
In non-pathogenic primate immunodeficiency virus infections innate responses are down-regulated after the acute phase of infection, but they remain chronically activated after virus containment in acute HIV-1 infection, which is thought to drive HIV-1 pathogenesis.
Increased understanding of mechanisms involved in innate control of HIV-1 and pathways regulating innate activation during HIV-1 infection could facilitate development of novel approaches to combating this infection.
Acknowledgments
The author is supported by funding from the National Institutes of Health (Center for HIV/AIDS Vaccine Immunology (CHAVI) # U19 AI067854-06), and is a Jenner Institute Investigator.
The author’s work is supported by funding from the National Institutes of Health, National Institute of Allergy and Infectious Disease, Division of AIDS, Center for HIV/AIDS Vaccine Immunology (CHAVI) (# U19 AI067854-06) and by the Grand Challenges in Global Health Program of the Bill and Melinda Gates Foundation (# 37874). The author is a Jenner Institute Investigator.
Footnotes
The author has no conflicts of interest.
References
Papers of particular interest, published within the annual period of review (2010–2011) have been highlighted as:
*of special interest
**of outstanding interest
- 1.Turnbull EL, Wong M, Wang S, et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol. 2009;182:7131–45. doi: 10.4049/jimmunol.0803658. [DOI] [PubMed] [Google Scholar]
- 2.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–63. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–72. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HY, Giorgi EE, Keele BF, et al. Modeling sequence evolution in acute HIV-1 infection. J Theor Biol. 2009;261:341–60. doi: 10.1016/j.jtbi.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. An excellent review of events in the earliest stages of HIV-1 infection. [DOI] [PubMed] [Google Scholar]
- 6*.Liu J, Keele BF, Li H, et al. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–12. doi: 10.1128/JVI.01155-10. This study and reference [7] show that mucosal exposure to low doses of SIV results in establishment of infection by a limited number of founder viruses and is associated with a longer eclipse phase before systemic virus dissemination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7*.Stone M, Keele BF, Ma ZM, et al. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–95. doi: 10.1128/JVI.00481-10. See annotation to reference [6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 11.Hodges A, Sharrocks K, Edelmann M, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007;8:569–77. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 12.Gringhuis SI, den Dunnen J, Litjens M, et al. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–8. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 13*.Gringhuis SI, van der Vlist M, van den Berg LM, et al. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–26. doi: 10.1038/ni.1858. This study describes how HIV-1 subverts signalling through two pattern-recognition receptors to enable productive infection of cDCs. [DOI] [PubMed] [Google Scholar]
- 14.Lambert AA, Gilbert C, Richard M, et al. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112:1299–307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arrighi JF, Pion M, Garcia E, et al. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J Exp Med. 2004;200:1279–88. doi: 10.1084/jem.20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez N, Bermejo M, Calonge E, et al. SDF-1/CXCL12 production by mature dendritic cells inhibits the propagation of X4-tropic HIV-1 isolates at the dendritic cell-T-cell infectious synapse. J Virol. 2010;84:4341–51. doi: 10.1128/JVI.02449-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Schoggins JW, Wilson SJ, Panis M, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011 doi: 10.1038/nature09907. In this paper a high-throughput screening approach is used to identify ISGs that mediate antiviral activity against HIV-1 and several other viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Kirchhoff F. Immune evasion and counteraction of restriction factors by HIV-1 and other primate lentiviruses. Cell Host Microbe. 2010;8:55–67. doi: 10.1016/j.chom.2010.06.004. An insightful review of primate lentivirus restriction factors and viral strategies for counteracting their activity. [DOI] [PubMed] [Google Scholar]
- 19.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–30. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 20*.Fitzpatrick K, Skasko M, Deerinck TJ, et al. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6:e1000701. doi: 10.1371/journal.ppat.1000701. This paper and references [21] and [22] describe mechanisms by which tetherin exerts antiviral activity against HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Perez-Caballero D, Zang T, Ebrahimi A, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. See annotation to reference [20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Zhang J, Liang C. BST-2 diminishes HIV-1 infectivity. J Virol. 2010;84:12336–43. doi: 10.1128/JVI.01228-10. See annotation to reference [20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Dube M, Roy BB, Guiot-Guillain P, et al. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 2010;6:e1000856. doi: 10.1371/journal.ppat.1000856. The mechanisms by which Vpu antagonises tetherin activity are described in this study and references [24] and [25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Hauser H, Lopez LA, Yang SJ, et al. HIV-1 Vpu and HIV-2 Env counteract BST-2/tetherin by sequestration in a perinuclear compartment. Retrovirology. 2010;7:51. doi: 10.1186/1742-4690-7-51. See annotation to reference [23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25*.Iwabu Y, Fujita H, Kinomoto M, et al. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J Biol Chem. 2009;284:35060–72. doi: 10.1074/jbc.M109.058305. See annotation to reference [23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Casartelli N, Sourisseau M, Feldmann J, et al. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. This study and references [27] and [28] address how levels of tetherin expression on infected cells and target cells affect cell-to-cell transmission of HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Kuhl BD, Sloan RD, Donahue DA, et al. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology. 2010;7:115. doi: 10.1186/1742-4690-7-115. See annotation to reference [26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–99. doi: 10.1128/JVI.01447-10. See annotation to reference [26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Lu J, Pan Q, Rong L, et al. The IFITM proteins inhibit HIV-1 infection. J Virol. 2011;85:2126–37. doi: 10.1128/JVI.01531-10. This paper and references [30] and [31] describe ISGs that exert antiviral activity against HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Pincetic A, Kuang Z, Seo EJ, Leis J. The interferon-induced gene ISG15 blocks retrovirus release from cells late in the budding process. J Virol. 2010;84:4725–36. doi: 10.1128/JVI.02478-09. See annotation to reference [29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Singh R, Gaiha G, Werner L, et al. Association of TRIM22 with the type 1 interferon response and viral control during primary HIV-1 infection. J Virol. 2011;85:208–16. doi: 10.1128/JVI.01810-10. See annotation to reference [29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol. 2010;84:9254–66. doi: 10.1128/JVI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheney KM, McKnight A. Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication. PLoS One. 2010;5:e13521. doi: 10.1371/journal.pone.0013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Manel N, Hogstad B, Wang Y, et al. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–7. doi: 10.1038/nature09337. HIV-1 does not undergo productive replication within cDCs very efficiently, but this study shows that under conditions where replication can occur DCs are activated to produce IFN-1 via a novel mechanism involving interaction of viral capsids with cyclophilin A followed by IRF3 activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui Y, Zhu Q, Gagnon S, et al. Innate and adaptive immune correlates of vaccine and adjuvant-induced control of mucosal transmission of SIV in macaques. Proc Natl Acad Sci U S A. 2010;107:9843–8. doi: 10.1073/pnas.0911932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Bergmeier LA, Stebbings R, et al. Mucosal immunization in macaques upregulates the innate APOBEC 3G anti-viral factor in CD4(+) memory T cells. Vaccine. 2009;27:870–81. doi: 10.1016/j.vaccine.2008.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Solis M, Nakhaei P, Jalalirad M, et al. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. J Virol. 2011;85:1224–36. doi: 10.1128/JVI.01635-10. This paper and reference [38] describe mechanisms that prevent recognition of HIV-1 RNA and DNA by cytoplasmic nucleic acid sensors, which enable HIV-1 to avoid triggering IFN-1 production in the cells it infects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Yan N, Regalado-Magdos AD, Stiggelbout B, et al. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–13. doi: 10.1038/ni.1941. See annotation to reference [37] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doehle BP, Hladik F, McNevin JP, et al. Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J Virol. 2009;83:10395–405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Harman AN, Lai J, Turville S, et al. HIV infection of dendritic cells subverts the interferon induction pathway via IRF1 and inhibits type 1 interferon production. Blood. 2011 doi: 10.1182/blood-2010-07-297721. HIV-1 does not stimulate IFN-1 production when it replicates in cDCs, but this manuscript reports that IRF1 expression is up-regulated, which induces a subset of ISGs and simultaneously enhances HIV-1 replication. It is also shown that Vpr blocks IRF3 activation in HIV-1-infected DCs without inducing its degradation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abel K, Rocke DM, Chohan B, et al. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–72. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malleret B, Maneglier B, Karlsson I, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 43.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–33. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Lepelley A, Louis S, Sourisseau M, et al. Innate Sensing of HIV-Infected Cells. PLoS Pathog. 2011;7:e1001284. doi: 10.1371/journal.ppat.1001284. This paper describes two mechanisms by which pDCs can be triggered to produce IFN-1 following contact with HIV-1-infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.O’Brien M, Manches O, Sabado RL, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–101. doi: 10.1172/JCI44960. This study addresses how HIV-1 stimulation of pDCs is able to elicit persistent IFN-1 production, and shows that this is due to accumulation of virions in early endosomes, which drives a partly-matured, persistently IFN-1-secreting phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Kramer HB, Lavender KJ, Qin L, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010;6:e1000893. doi: 10.1371/journal.ppat.1000893. In this study a proteomics-based approach was used to analyse unique plasma sample timecourses spanning the eclipse and viral expansion phases of AHI, and the earliest systemic perturbation in analyte levels was shown to be an increase in plasma acute-phase proteins with antiviral activity that occurs prior to detection of viremia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sander LE, Sackett SD, Dierssen U, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Sabado RL, O’Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–52. doi: 10.1182/blood-2010-03-273763. This study describes frequency, phenotype and functions of the pDCs and cDCs in the blood during acute and early HIV-1 infection, and makes the important observation that DCs from AHI subjects exhibit hyper-responsiveness to stimulation with TLR ligands, which may participate in driving immunopathological immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris LD, Tabb B, Sodora DL, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. 2010;84:7886–91. doi: 10.1128/JVI.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39:235–42. doi: 10.1111/j.1600-0684.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehmann C, Lafferty M, Garzino-Demo A, et al. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS One. 2010;5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dillon SM, Friedlander LJ, Rogers LM, et al. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Wijewardana V, Soloff AC, Liu X, et al. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6:e1001235. doi: 10.1371/journal.ppat.1001235. This paper reports that during acute SIV infection activated cDCs are recruited from blood to lymph nodes, where they undergo apoptosis; and shows that the reduction in blood cDC numbers during early infection is predictive of the subsequent rate of disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–9. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 57.Alter G, Rihn S, Walter K, et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J Virol. 2009;83:6798–805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–94. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 59.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–36. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward J, Bonaparte M, Sacks J, et al. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–14. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kielczewska A, Pyzik M, Sun T, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–23. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Richard J, Sindhu S, Pham TN, et al. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell-mediated killing. Blood. 2010;115:1354–63. doi: 10.1182/blood-2009-08-237370. This study and reference [63] show that the HIV-1 Vpr protein activates a stress response in infected cells that leads to up-regulation of expression of ligands for the activating NK cell receptor NKG2D and promotes NK lysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63*.Ward J, Davis Z, DeHart J, et al. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog. 2009;5:e1000613. doi: 10.1371/journal.ppat.1000613. See annotation to reference [62] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fausther-Bovendo H, Vieillard V, Sagan S, et al. HIV gp41 engages gC1qR on CD4+ T cells to induce the expression of an NK ligand through the PIP3/H2O2 pathway. PLoS Pathog. 2010;6:e1000975. doi: 10.1371/journal.ppat.1000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fausther-Bovendo H, Sol-Foulon N, Candotti D, et al. HIV escape from natural killer cytotoxicity: Nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23:1077–87. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 66.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol. 2001;75:6953–61. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 68.Martin MP, Qi Y, Gao X, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–40. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yindom LM, Leligdowicz A, Martin MP, et al. Influence of HLA class I and HLA-KIR compound genotypes on HIV-2 infection and markers of disease progression in a Manjako community in West Africa. J Virol. 2010;84:8202–8208. doi: 10.1128/JVI.00116-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi Y, Martin MP, Gao X, et al. KIR/HLA pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathog. 2006;2:e79. doi: 10.1371/journal.ppat.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71*.Boulet S, Song R, Kamya P, et al. HIV protective KIR3DL1 and HLA-B genotypes influence NK cell function following stimulation with HLA-devoid cells. J Immunol. 2010;184:2057–64. doi: 10.4049/jimmunol.0902621. This manuscript reports that KIR3DL1+ NK cells from subjects with KIR3DL1/HLA compound genotypes associated with good prognosis in HIV-1 infection exhibit enhanced functional potential in vitro. [DOI] [PubMed] [Google Scholar]
- 72.Long BR, Ndhlovu LC, Oksenberg JR, et al. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J Virol. 2008;82:4785–92. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73**.Shah AH, Sowrirajan B, Davis ZB, et al. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8:397–409. doi: 10.1016/j.chom.2010.10.008. This study describes a novel mechanism by which Vpu increases the resistance of HIV-1-infected cells to NK lysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brackenridge S, Evans EJ, Toebes M, et al. An early HIV mutation within a HLA-B*57-restricted T cell epitope abrogates binding to the killer inhibitory receptor 3DL1 (KIR3DL1) J Virol. 2011 doi: 10.1128/JVI.00238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thananchai H, Makadzange T, Maenaka K, et al. Reciprocal recognition of an HLA-Cw4-restricted HIV-1 gp120 epitope by CD8+ T cells and NK cells. AIDS. 2009;23:189–93. doi: 10.1097/QAD.0b013e32831fb55a. [DOI] [PubMed] [Google Scholar]
- 76.Conry SJ, Milkovich KA, Yonkers NL, et al. Impaired plasmacytoid dendritic cell (PDC)-NK cell activity in viremic human immunodeficiency virus infection attributable to impairments in both PDC and NK cell function. J Virol. 2009;83:11175–87. doi: 10.1128/JVI.00753-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reitano KN, Kottilil S, Gille CM, et al. Defective plasmacytoid dendritic cell-NK cell cross-talk in HIV infection. AIDS Res Hum Retroviruses. 2009;25:1029–37. doi: 10.1089/aid.2008.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mavilio D, Lombardo G, Kinter A, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J Exp Med. 2006;203:2339–50. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79*.Alter G, Kavanagh D, Rihn S, et al. IL-10 induces aberrant deletion of dendritic cells by natural killer cells in the context of HIV infection. J Clin Invest. 2010;120:1905–13. doi: 10.1172/JCI40913. This paper describes a mechanism via which persistent up-regulation of IL-10 production during HIV-1 infection may skew NK editing of DCs and potentially impact on the induction/maintenance of HIV-1-specific T cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80*.Melki MT, Saidi H, Dufour A, et al. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1. PLoS Pathog. 2010;6:e1000862. doi: 10.1371/journal.ppat.1000862. This study shows that anti-apoptotic molecules are up-regulated in HIV-1-infected DCs via a HMGB1-dependent mechanism, rendering these cells resistant to TRAIL-mediated destruction by NK cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lore K, Sonnerborg A, Brostrom C, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–92. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 82.Granelli-Piperno A, Golebiowska A, Trumpfheller C, et al. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004;101:7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83**.Blanchet FP, Moris A, Nikolic DS, et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32:654–69. doi: 10.1016/j.immuni.2010.04.011. This study describes a novel mechanism by which HIV-1 may impair the functions of cDCs during infection: via down-regulation of autophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Che KF, Sabado RL, Shankar EM, et al. HIV-1 impairs in vitro priming of naive T cells and gives rise to contact-dependent suppressor T cells. Eur J Immunol. 2010;40:2248–58. doi: 10.1002/eji.201040377. This paper and reference [84] show that exposure of cDCs to HIV-1 in vitro impairs T cell priming and promotes Treg cell induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Shankar EM, Che KF, Messmer D, et al. Expression of a broad array of negative costimulatory molecules and Blimp-1 in T cells following priming by HIV-1 pulsed dendritic cells. Mol Med. 2011;17:229–240. doi: 10.2119/molmed.2010.00175. See annotation to reference [83] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–9. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2, -dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oxenius A, Fidler S, Brady M, et al. Variable fate of virus-specific CD4(+) T cells during primary HIV-1 infection. Eur J Immunol. 2001;31:3782–8. doi: 10.1002/1521-4141(200112)31:12<3782::aid-immu3782>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 89*.Moll M, Andersson SK, Smed-Sorensen A, Sandberg JK. Inhibition of lipid antigen presentation in dendritic cells by HIV-1 Vpu interference with CD1d recycling from endosomal compartments. Blood. 2010;116:1876–84. doi: 10.1182/blood-2009-09-243667. This paper describes a mechanism via which interaction between HIV-1-infected cDCs and CD1d-restricted NKT cells can be subverted by HIV-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90**.Fontaine J, Chagnon-Choquet J, Valcke HS, et al. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood. 2011;117:145–55. doi: 10.1182/blood-2010-08-301887. This paper reports that expression of BLyS is up-regulated on cDCs during AHI and remains elevated throughout infection in viremic subjects, which is associated with polyclonal B cell activation and hyperglobulinemia. [DOI] [PubMed] [Google Scholar]
- 91.Fellay J, Shianna KV, Ge D, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–7. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J, Goedert JJ, Sundberg EJ, et al. HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J Exp Med. 2009;206:2959–66. doi: 10.1084/jem.20091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herbeuval JP, Hardy AW, Boasso A, et al. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2005;102:13974–9. doi: 10.1073/pnas.0505251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stary G, Klein I, Kohlhofer S, et al. Plasmacytoid dendritic cells express TRAIL and induce CD4+ T-cell apoptosis in HIV-1 viremic patients. Blood. 2009;114:3854–63. doi: 10.1182/blood-2009-04-217927. [DOI] [PubMed] [Google Scholar]
- 95.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bosinger SE, Li Q, Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–72. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–55. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lederer S, Favre D, Walters KA, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Q, Smith AJ, Schacker TW, et al. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol. 2009;183:1975–82. doi: 10.4049/jimmunol.0803222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cavaleiro R, Baptista AP, Soares RS, et al. Major depletion of plasmacytoid dendritic cells in HIV-2 infection, an attenuated form of HIV disease. PLoS Pathog. 2009;5:e1000667. doi: 10.1371/journal.ppat.1000667. [DOI] [PMC free article] [PubMed] [Google Scholar]