Abstract
Rationale
Electrophysiological studies have identified a scalp potential, the late positive potential (LPP), which is modulated by the emotional intensity of observed stimuli. Previous work has shown that the LPP reflects the modulation of activity in extrastriate visual cortical structures, but little is known about the source of that modulation.
Objectives
The present study investigated whether beta-adrenergic receptors are involved in the generation of the LPP.
Methods
We used a genetic individual differences approach (experiment 1) and a pharmacological manipulation (experiment 2) to test the hypothesis that the LPP is modulated by the activation of β-adrenergic receptors.
Results
In experiment 1, we found that LPP amplitude depends on allelic variation in the β1-receptor gene polymorphism. In experiment 2, we found that LPP amplitude was modulated by the β-blocker propranolol in a direction dependent on subjects' level of trait anxiety: In participants with lower trait anxiety, propranolol led to a (nonsignificant) decrease in the LPP modulation; in participants with higher trait anxiety, propranolol increased the emotion-related LPP modulation.
Conclusions
These results provide initial support for the hypothesis that the LPP reflects the downstream effects, in visual cortical areas, of β-receptor-mediated activation of the amygdala.
Keywords: ERP, Genetic, LPP, Neural basis, Norepinephrine
A key aspect of emotional stimuli is their emotional intensity or arousal; the degree to which the stimuli are pleasant or unpleasant. When participants view stimuli that vary in emotional arousal, a slow positive-going centroparietal event-related potential (ERP) component can be recorded at the scalp which begins roughly 400 ms after stimulus onset and continues until picture offset or even longer (Foti et al. 2009). The amplitude of this late positive potential (LPP) increases monotonically with participants' ratings of their subjective emotional arousal, regardless of whether the stimuli are experienced as pleasant or unpleasant (Cuthbert et al. 2000; Keil et al. 2002; Lang et al. 1997; Schupp et al. 2000). Even within the broad categories of “pleasant,” “neutral,” and “unpleasant” pictures, the LPP is larger for more arousing and motivationally salient pictures (Weinberg and Hajcak 2010). Because of its sensitivity to emotional arousal, the LPP is increasingly used as a tool for studying the role of emotion in social behavior (Hurtado et al. 2009; Ito et al. 1998) and for assessing emotional function in clinical populations (Foti et al. 2009; Leutgeb et al. 2009; Marissen et al. 2010).
Despite the increasing popularity of the LPP, little is known about its neural basis. There is a broad consensus that the LPP reflects the modulation of activity in occipital, inferotemporal, and parietal visual cortical structures (Keil et al. 2002; Sabatinelli et al. 2007). However, the source of this modulation is unclear. Although it remains to be substantiated, it has been suggested that the LPP modulation may index reentrant feedback from the amygdala to the visual cortex (Dolcos and Cabeza 2002; Sabatinelli et al. 2007). This hypothesis is consistent with the presence of direct anatomical projections from the amygdala to the ventral visual cortex (Amaral et al. 2003; Vuilleumier 2005). It is also consistent with the similar functional sensitivity of the LPP and amygdala to arousing stimuli; studies of amygdala activity in monkeys and humans suggest that, contrary to a widely held view, the amygdala is just as important for processing pleasant as it is for processing unpleasant stimuli (Murray 2007). Emotion regulation manipulations that increase or decrease the salience of aversive stimuli modulate both amygdala activity and LPP amplitude (Hajcak et al. 2010).
The enhanced amygdala response to arousing stimuli involves β-adrenergic receptors. For example, blockage with a β-adrenergic antagonist, such as propranolol, dampens the amygdala response to emotional stimuli (Hurlemann et al. 2010; Strange and Dolan 2004; Van Stegeren et al. 2005). β-adrenergic receptors are sensitive to norepinephrine (NE), a neuromodulator that is important in the regulation of emotional arousal (Aston-Jones et al. 1996). Most NE-containing cells in the human brain are located in a brainstem nucleus, the locus coeruleus (LC; Berridge and Waterhouse 2003). The LC has extensive connections with the amygdala, as well as other areas in the brain, and is the only source of NE to the hippocampus, amygdala, and neocortex. The LC shows a phasic response to both positive and negative salient stimuli, during which LC neurons rapidly increase their firing rates for a short period. As a result, the LC releases more NE in its projection areas, where β-adrenergic receptors are activated and temporarily modulate neural activity.
In experiment 1, we used a polymorphism of the β1-adrenergic receptor to test the hypothesis that activation of this receptor modulates the LPP. Three subtypes of β-adrenergic receptors have been identified. These subtypes are thought to mediate different functions although the specific functions subserved by each are not fully understood (Berridge and Waterhouse 2003). In the mammalian brain, most β-receptors are of the β1 subtype (Zill et al. 2003). Evidence from animal studies indicates that this is also the most abundant subtype in the amygdala (Tiong and Richardson 1990). The polymorphism we focused on is the β1-receptor polymorphism G1165C (Borjesson et al. 2000; Maqbool et al. 1999). The C-allele of this polymorphism is associated with an enhanced coupling to the stimulatory G protein and an increased response to the receptor agonists (Mason et al. 1999). We therefore expected C/C homozygotes to show enhanced LPP amplitudes compared to G/C heterozygotes and G/G homozygotes. This would provide initial evidence for the hypothesized β-adrenergic modulation of the LPP.
In experiment 2, we manipulated the activity of β-adrenergic receptors through the administration of the β-blocker propranolol and measured the effects on LPP amplitude. β-receptor blockage with propranolol modulates the amygdala response to emotional stimuli (Buffalari and Grace 2007; Strange and Dolan 2004; Van Stegeren et al. 2005). Accordingly, we predicted that β-receptor blockage would modulate the amplitude of the LPP evoked by emotional pictures. Because of the strong nonlinear relationship between baseline noradrenergic activity and phasic noradrenergic responses (Aston-Jones and Cohen 2005), the effects of noradrenergic drugs on behavioral (Coull 1994; Hartley et al. 1983; Luksys et al. 2009) and neural correlates of phasic noradrenergic responses (including, as we propose, the LPP) may critically depend on an individual's natural baseline level of noradrenergic activity. Therefore, we collected a measure of trait anxiety, which strongly correlates with baseline noradrenergic activity (Itoi and Sugimoto 2010; Ressler and Nemeroff 2000), and examined if the level of trait anxiety interacted with the effect of treatment.
Materials and methods
Participants
Participants in experiment 1 were 28 healthy students at Leiden University, aged 19–31 years, who were selected from a database with information on several polymorphisms related to (nor) adrenergic and dopaminergic signal transduction. They were paid 10 € for their participation. We selected 14 subjects who were homozygous for the C-allele (CC-genotype; seven men; M age = 23.1) and 14 subjects who were either homozygous for the G-allele (GG-genotype) or heterozygous (GC-genotype; seven men; M age = 23.3) for the G1165C polymorphism in the β1-adrenergic receptor. Data about other polymorphisms (DBH, ADRB1, ADRB2, NET, DRD1, DRD2, DRD4, DAT1, COMT, DARPP-32, GNAS, and BDNF) were not analyzed but is available upon request from the corresponding author. Informed consent was obtained from all participants before their inclusion in the study.
Participants in experiment 2 were 16 healthy young adults (eight women), aged 18–28 years, who took part in the experiment in return for 100 €. Only participants with a systolic blood pressure above 100 mmHg, a diastolic blood pressure above 60 mmHg, and a resting heart rate above 60 beats per minute were included in the study. All participants in experiment 2 underwent a medical screening, including a routine physical examination, and were considered to be in satisfactory health. The use of medication that could interfere with propranolol was stopped the day before testing. Participants received an oral dose of 80 mg propranolol or placebo in a randomized, double-blind, counterbalanced crossover design. Propranolol and placebo were administered to each participant on consecutive days (24 h in between administrations) and in both sessions EEG was recorded during the performance of a passive viewing task. The third and final session was 14 (±2) days after the first session and without any drug or placebo administration and participants performed a surprise recognition test (described in detail below). The data from one participant could not be collected because of severe side effects of propranolol (de Rover et al. 2010). The study was approved by the medical ethics committee of the Leiden University Medical Center and was conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants before their inclusion in the study.
Procedure
In experiment 2, each participant was tested at approximately the same time of the day. Drug or placebo was administered at 9:15–9:45 a.m. and the task started 90 min after administration (10:45–11:15 a.m.) because of the kinetics of propranolol's peak plasma concentration (1–2 h). Participants were instructed to abstain from caffeine, nicotine, alcohol, and other psycho-active substances from 15 h before the start of the first session until the end of the second session (the next day). After the medical screening, participants filled out the Liebowitz Social Anxiety Scale (LSAS; Liebowitz 1987)1 and then received a microcrystalline cellulose-filled capsule with either propranolol or placebo (t = 0). Propranolol has well-established antihypertensive properties; therefore, blood pressure and heart rate were monitored for participant safety. Measurements were taken at t = 0, t = 60, t = 90, t = 100, and t = 240 min. After completion of the tasks, the participants were debriefed and received the financial compensation.
Tasks
For experiment 1, we selected 120 pictures from the International Affective Picture System (IAPS; Lang et al. 2005): 40 highly arousing unpleasant pictures, 40 mildly arousing unpleasant pictures, and 40 neutral pictures. The normative valence ratings were: highly unpleasant 2.4, mildly unpleasant 3.1, and neutral 5.1. The normative arousal ratings were: highly unpleasant 6.2, mildly unpleasant 5.2, and neutral 2.8 (all ratings were based on a nine-point scale, Lang 1980). The stimuli were presented in a random order for 1,000 ms each. Each stimulus presentation was preceded by a fixation cross for 1,500 ms. Participants were instructed to view the pictures attentively.
In both sessions of experiment 2, participants were instructed to passively watch a series of 120 pictures. For the two sessions (sessions 1 and 2), two different but matched sets of 120 IAPS pictures were selected. In each set, 40 pictures depicted unpleasant events (e.g., mutilations, snakes), 40 pleasant events (e.g., sports, erotica), and 40 neutral events (e.g., houses, faces with a neutral expression). The normative valence ratings of the two sets of pictures were matched per picture type (average in sessions 1 and 2, respectively: unpleasant 2.4 and 2.5; neutral 5.1 and 4.9; pleasant 7.1 and 7.0; effect of session F 1, 39 = 0.6, p = 0.45; effect of picture type F 2, 78 = 1,070.7, p < 0.001; interaction between session and picture type F 2, 78 = 1.1, p = 0.35). Similarly, the normative arousal ratings of the two picture sets were matched (in sessions 1 and 2, respectively, unpleasant 6.2 and 6.2; neutral 2.8 and 2.9; pleasant 5.4 and 5.5; effect of session F 1, 39 = 0.4, p = 0.54; effect of picture type F 2, 78 = 632.2, p < 0.001; interaction between session and picture type: F 2, 78 = 0.2, p = 0.80). The 120 pictures were presented in random order for 1,000 ms each with a 1,500-ms blank screen in between.
Immediately after the passive viewing task, participants were shown 20 representative pictures from each type that they had seen in the preceding passive viewing task. Participants were instructed to rate the subjective arousal of each picture on a scale from 1 to 4 by pressing a corresponding button. These data could not be collected from two participants because of technical problems.
During the third session, 2 weeks after the first session, a surprise recognition test was administered. In this task, participants were shown 20 representative pictures of each picture type from session 1 and 20 from session 2 (not shown in the rating task) randomly intermixed with an equal number of new pictures. Participants were instructed to indicate by button press for each picture whether they had seen it before in one of the sessions (“old”), or whether they had not seen the picture in one of those sessions (“new”). Some studies have found that administration of 40 or 80 mg propranolol impairs encoding (and hence later recognition) of emotional stimuli (Cahill et al. 1994; Strange et al. 2003; van Stegeren et al. 2008); other studies have failed to replicate this effect (Strange and Dolan 2004; Tollenaar et al. 2009; Weymar et al. 2010). If we found a drug effect on recognition performance, this would provide evidence, in addition to peripheral drug effects on heart rate, that propranolol affected central nervous system activity.
EEG recording and analyses
We recorded EEG from 31 Ag/AgCl scalp electrodes (Fp1, AFz, Fz, F3, F7, FCz, FC3, FT7, Cz, C3, T7, CPz, CP3, TP7, Pz, P3, P7, POz, O1, O2, P8, P4, TP8, CP4, T8, C4, FT8, FC4, F8, F4, Fp2) and from the left and right mastoids. We measured the horizontal and vertical electrooculogram using bipolar recordings from electrodes placed approximately 1 cm lateral of the outer canthi of the two eyes and from electrodes placed approximately 1 cm above and below the participant's right eye.
The EEG signal was pre-amplified at the electrode to improve the signal-to-noise ratio and amplified with a gain of 16× by a BioSemi ActiveTwo system (BioSemi B.V., Amsterdam). The data were digitized at 24-bit resolution with a sampling rate of 512 Hz using a low-pass fifth-order sinc filter with a half-power cutoff of 102.4 Hz. Each active electrode was measured online with respect to a common mode sense active electrode producing a monopolar (non-differential) channel and was referenced offline to the average of the left and right mastoids. Ocular and eye blink artifacts were corrected using the method of Gratton and colleagues (Gratton et al. 1983). Epochs with other artifacts (spike artifacts [50 μV/2 ms] and slow drifts [200 μV/200 ms]) were also discarded. We extracted single-trial epochs for a period from 100 ms (experiment 1) or 200 ms (experiment 2) before until 1,000 ms after stimulus onset. Then, for each participant and stimulus type (experiment 1 highly unpleasant, mildly unpleasant, neutral; experiment 2 pleasant, unpleasant, neutral), we averaged the EEG epochs to create stimulus-locked ERPs. The average signal during the pre-stimulus baseline was subtracted from each ERP. LPP amplitude was defined as the average signal value in a time window from 400–1,000 ms after stimulus onset at the electrode, where the LPP modulation manifested its maximum amplitude, CPz in experiment 1 and Pz in experiment 2.
Results
Experiment 1: effect of beta receptor gene polymorphism on the LPP
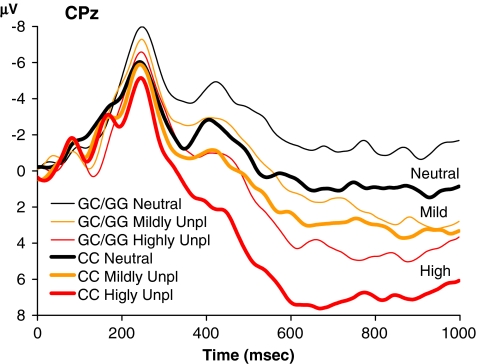
Figure 1 displays the grand average waveforms for each picture type for the β1-receptor genotype groups CC and GC/GG. As expected, we found a significant effect of picture type on LPP amplitude (F 2, 52 = 51.1, p < 0.001). Subsequent pairwise comparisons indicated that the order of the LPP amplitudes mirrored that of the arousal values: highly unpleasant pictures were associated with a larger LPP than mildly unpleasant pictures (F 1, 26 = 26.0, p < 0.001), and mildly unpleasant pictures were associated with a larger LPP than neutral pictures (F 1, 26 = 24.1, p < 0.001). Importantly, there was a significant difference in LPP amplitude between the two genotype groups (F 1, 26 = 5.2, p = 0.031). As predicted, C/C homozygotes showed enhanced LPP amplitudes compared to G/C heterozygotes and G/G homozygotes. We found no significant interaction of picture type and β1-receptor genotype (F 2, 52 = 1.2, p = 0.31), reflecting the finding that the effect of genotype was roughly similar for the three picture types.
Fig. 1.
Grand average ERP waveforms in experiment 1 for β1-receptor polymorphism groups CC and GC/GG in the neutral and threatening conditions
Experiment 2: effect of propranolol on the LPP
Cardiovascular measurements
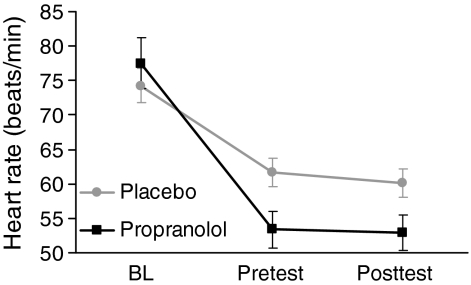
Heart rate was used as a marker to check for successful β-receptor blockade by propranolol (Fig. 2). Heart rate was registered at baseline, 90 min later, just before the start of the passive viewing task (pretest), and immediately after the end of the passive viewing task (posttest). During the experiment, there was a significant decrease in heart rate (F 2, 24 = 63.2, p < 0.001), which was significantly larger for propranolol than for placebo treatment (interaction time × treatment F 2, 24 = 26.8, p < 0.001). Subsequent pairwise comparisons showed that baseline heart rate did not differ between propranolol and placebo treatment (t 14 = 1.4, p = 0.18), whereas heart rate was significantly lower in the propranolol condition than the placebo condition at pretest (t 14 = 3.3, p = 0.006) and at posttest measurements (t 14 = 2.8, p = 0.015).
Fig. 2.
Cardiovascular measurements in experiment 2. BL baseline, Pretest 90 min after baseline right before the start of the passive viewing task, Posttest right after the end of the passive viewing task. Error bars indicate standard errors of the means
Behavior
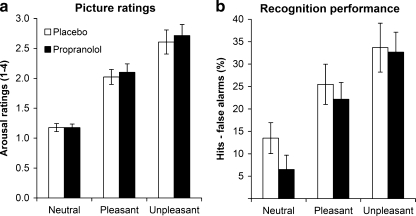
Consistent with previous studies (Van Stegeren et al. 2005; Weymar et al. 2010; see also Cahill et al. 1995), the mean rating of the pictures was similar after propranolol and placebo treatment (Fig. 3a; F 1, 12 = 1.0, p = 0.33). The effect of picture type on rating was significant (F 2, 24 = 58.4, p < 0.001). In line with the normative arousal values, subsequent pairwise comparisons indicated that unpleasant pictures were rated as more arousing than pleasant pictures (F 1, 12 = 17.8, p = 0.001) and that pleasant pictures were rated as more arousing than neutral pictures (F 1, 12 = 78.9, p < 0.001). There was no significant interaction between treatment and picture type (F 2, 28 = 0.5, p = 0.62).
Fig. 3.
Behavioral results in experiment 2. a Mean subjective arousal ratings for neutral, pleasant, and unpleasant pictures in the placebo and propranolol condition. b Average recognition performance (hits [% correct old pictures]–false alarms [% incorrect new pictures]) for neutral, pleasant, and unpleasant pictures in the placebo and propranolol condition. Error bars indicate standard errors of the means
Recognition performance did not differ between placebo and propranolol treatment (Fig. 3b; F 1, 14 = 1.9, p = 0.19). The effect of picture type on recognition performance was significant (F 2, 28 = 13.8, p < 0.001). Subsequent pairwise comparisons indicated that recognition performance mirrored the pattern of arousal ratings: participants recognized more unpleasant pictures than pleasant pictures (F 1, 14 = 5.4, p = 0.036) and more pleasant pictures than neutral pictures (F 1, 14 = 10.0, p = 0.007). There was no significant interaction between treatment and picture type (F 2, 28 = 0.9, p = 0.42).
Event-related potentials
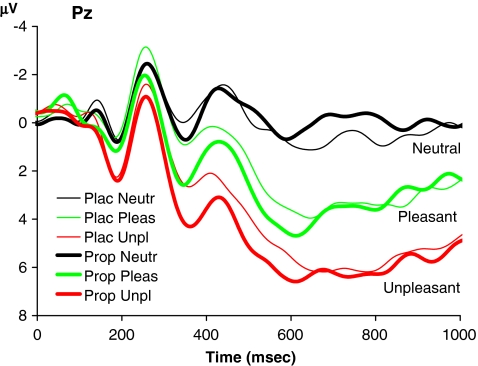
Both pleasant and unpleasant pictures elicited a large LPP compared to neutral pictures (Fig. 4). This modulation started around 400 ms and lasted until at least 1,000 ms after picture onset. Inspection of the scalp topography maps showed that the modulation was largest at Pz in both the propranolol and placebo condition. There was a significant effect of picture type on mean LPP amplitude in this interval (F 2, 28 = 61.3, p < 0.001). Subsequent pairwise comparisons indicated that the order of the LPP amplitudes mirrored that of the normative and rated arousal values and performance on the recognition memory test: unpleasant pictures were associated with a larger LPP than pleasant pictures (F 1, 14 = 39.0, p < 0.001), and pleasant pictures were associated with a larger LPP than neutral pictures (F 1, 14 = 39.7, p < 0.001). The LPP amplitude was not affected by treatment (F 1, 14 = 0.1, p = 0.75) and there was no interaction between picture type and treatment (F 2, 28 = 1.0, p = 0.38).
Fig. 4.
Grand average ERP waveforms in experiment 2 associated with neutral, pleasant, and unpleasant pictures in the placebo and propranolol condition
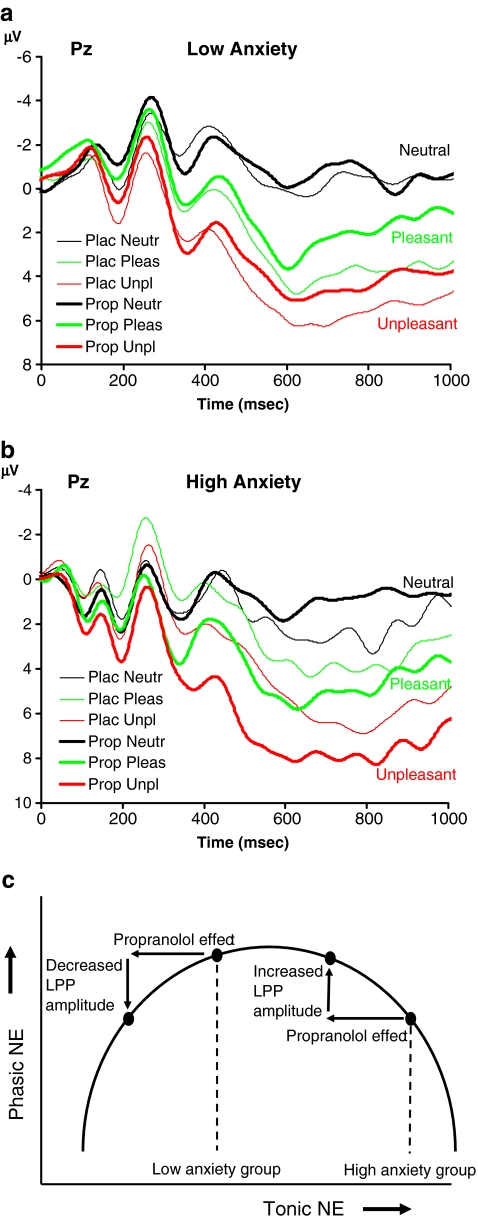
To examine if the main effect of treatment on LPP amplitude was obscured by a crossover interaction with trait anxiety levels, we repeated the analysis while including LSAS score (high or low) as an additional between-subject variable. LSAS data from one participant were unavailable. The remaining 14 participants were classified in a high-anxiety group (N = 7, six women; LSAS score M = 22.9, SD = 9.6) and low-anxiety group (N = 7, one woman; LSAS score M = 9.9, SD = 2.7) based on a median split analysis. Anxiety level did not show a reliable main effect or two-way interactions with treatment and picture type. Importantly, however, the three variables showed a significant three-way interaction (F 2, 24 = 5.6, p = 0.01). We investigated this interaction further using separate ANOVAs for the two anxiety groups. For the low-anxiety participants, there was no reliable main effect of treatment (F 1, 6 = 1.2, p = 0.31) and no interaction between treatment and picture type (F 2, 12 = 1.8, p = 0.21). For low-anxious participants (Fig. 5a), propranolol treatment led to numerically smaller LPPs to pleasant (placebo 3.2 μV, propranolol 1.7 μV) and unpleasant stimuli (5.1 vs. 3.9 μV), but did not essentially change the LPP to neutral stimuli (−0.6 vs. −0.8 μV).
Fig. 5.
Grand average ERP waveforms in experiment 2 associated with neutral, pleasant, and unpleasant pictures in the placebo and propranolol condition for a low-anxiety participants and b high-anxiety participants. c Schematic illustration of a potential mechanism underlying the observed interaction between treatment and trait anxiety
In contrast, for the high-anxiety participants, there was no main effect of treatment (F 1, 6 = 3.4, p = 0.11), but there was a significant interaction between treatment and picture type (F 2, 12 = 4.3, p = 0.040). In the high-anxiety group (Fig. 5b), propranolol treatment led to numerically larger LPPs to pleasant (placebo 3.1 μV, propranolol 4.3 μV) and unpleasant stimuli (5.2 vs. 7.2 μV) and smaller LPPs to neutral stimuli (1.9 vs. 0.8 μV). Follow-up two-sided t tests in the high-anxiety group showed a significant treatment effect on the LPP amplitude associated with unpleasant pictures (t 6 = 2.7, p = 0.038) but not pleasant (p = 0.14) and neutral pictures (p = 0.21).2
Discussion
The LPP holds great promise as a noninvasive neural measure of emotional arousal in healthy and clinical populations and offers a temporal resolution that cannot be achieved with functional magnetic resonance imaging. However, the neural basis of the LPP is still poorly understood. Here, we tested the hypothesis that the LPP reflects the indirect effects, in the visual cortex, of β-receptor activation in the amygdala. The prediction derived from that hypothesis is that LPP amplitude should be dependent on changes in β-receptor activation. The results presented here provide tentative support for our hypothesis. In experiment 1, we found that LPP amplitude depended on individual differences in a β1-receptor gene polymorphism (G1165C), thus suggesting that the generation of the LPP is mediated, at least in part, by the activation of β1-receptors. In experiment 2, we found that LPP amplitude was modulated by the nonselective beta-blocker propranolol in a direction dependent on individual differences in trait anxiety, a marker of baseline noradrenergic activity. These results leave open the possibility that β2-receptors are also involved in the generation of the LPP. More research, including in animal models, is needed to determine the exact pathways and mechanisms by which β-receptor activation modulates the LPP at the scalp.
In experiment 1, we found that C/C homozygotes, with increased sensitivity of the β1-receptor to NE, showed enhanced LPP amplitudes compared to G/C heterozygotes and G/G homozygotes. This effect was similar for the three picture types; it was not specific to the emotionally arousing pictures. It is important to note that the LPP elicited by neutral pictures is more than merely a “baseline” against which the LPP elicited by emotional pictures can be compared; several studies have found meaningful variation in the amplitude of the LPP elicited by neutral pictures. For example, more arousing neutral pictures elicit a larger LPP (Weinberg and Hajcak 2010) and interfere more with ongoing behavior (Weinberg and Hajcak 2011) than less-arousing neutral pictures. This suggests that the LPP is a continuous measure of arousal and may explain why increased sensitivity of the β1-receptor to NE is associated with a greater neural response to all stimuli. Future studies might further examine whether individuals with the C/C genotype show increased behavioral interference from visual distractors independent of picture type.
Previous fMRI studies have found that 40 (Strange and Dolan 2004) and 80 mg propranolol (Van Stegeren et al. 2005) reliably reduced amygdala activation to emotional stimuli. In experiment 2, we investigated the effect of 80 mg propranolol on the LPP, which according to our hypothesis reflects the modulatory control of the amygdala over the visual cortex. Propranolol treatment did not result in marked changes in arousal ratings and recognition of the pictures, but did slow heart rate significantly. The timing of this heart rate effect indicated that propranolol was effective while participants were watching the IAPS pictures. In line with our hypothesis, propranolol modulated LPP amplitude, but the modulation went in opposite directions, depending on the participants' trait anxiety—a correlate of baseline noradrenergic activity. In participants with lower trait anxiety, propranolol resulted in the predicted (but nonsignificant) decrease in LPP amplitudes associated with emotional stimuli. In participants with higher trait anxiety, propranolol significantly enhanced the emotion-related LPP modulation.
A possible explanation for this interaction between propranolol and trait anxiety is that propranolol decreases tonic LC activity through actions at β2-receptors in the LC (Ampatzis and Dermon 2010; Berridge and Waterhouse 2003). Depending on a subject's baseline level of tonic LC activity (which correlates with trait anxiety), this shifts the subject to a higher or lower point on the inverted U curve describing the strength of phasic LC responses (which according to our proposal underlie LPP generation) as a function of tonic LC activity. Subjects with a higher trait anxiety (right from the center) shift to larger LC responses, whereas subjects with a lower trait anxiety (center) shift to smaller LC responses (Fig. 5c). Similar quantitative interactions have been reported in the dopamine literature: the effect of dopaminergic drugs on performance in various types of tasks goes in opposite directions, depending on trait impulsivity (which correlates with dopamine receptor binding; Cools et al. 2007) and baseline levels of dopamine synthesis (Cools et al. 2009).
While we were collecting the data of experiment 2, Weymar and colleagues (2010) published results that seem in line with our findings in experiment 2. They used a parallel-group design to study the effects of propranolol vs. placebo on ERP correlates of memory for neutral and emotional IAPS pictures. Although the study focused on the memory retrieval phase, the authors also reported ERP waveforms for the encoding phase, which indicated no effect of propranolol on the LPP. It is possible that the participants in that study also included a subgroup of participants with lower trait anxiety in which propranolol decreased the LPP and a subgroup of participants with higher trait anxiety in which propranolol increased the LPP. To our knowledge, the authors did not collect anxiety measures.3 Our results suggest that future studies should more explicitly take into account genotype, personality, and other individual differences factors that may mediate the effects of propranolol.
Not only β-receptors but also α2-receptors play a role in the increased amygdala activation and enhanced memory associated with emotional stimuli (de Quervain et al. 2007; Rasch et al. 2009). These receptors, and the polymorphisms that influence them, may also be important in the generation of the LPP. Furthermore, there may be other neuromodulatory influences on the LPP. For example, it is possible that the LPP is modulated by the activity of the serotonergic system, which is known to play an important role in regulating emotion (Cools et al. 2008). One study has examined the effects on the LPP of polymorphisms of the serotonin transporter and tryptophan hydroxylase-2 genes, two key regulators of serotonergic activity (Herrmann et al. 2007). Although both polymorphisms affected the EPN, they were not significantly related to the (early portion of the) LPP (315–745 ms). Further research is needed to examine if the LPP is related to other polymorphisms associated with serotonergic function. Furthermore, the LPP may be modulated by the activity of the dopaminergic system, which is also known to be involved in the modulation of affect (Ashby et al. 1999). Franken and colleagues investigated the effects of dopaminergic drugs on the LPP and found a nonspecific effect of the D2 agonist bromocriptine on LPP amplitude (Franken et al. 2008), an effect similar to that of the beta1-receptor polymorphism in our experiment 1. This may reflect negative feedback interactions between the dopaminergic midbrain and the locus coeruleus (Beckstead et al. 1979). Franken and colleagues found no effect of the D2 antagonist haloperidol.
Finally, it is worth noting a study that examined the effects of the selective noradrenergic reuptake inhibitor reboxetine (4 mg) and the selective serotonergic reuptake inhibitor citalopram (20 mg) on the LPP to neutral and emotional faces (Kerestes et al. 2009). This study might have provided valuable information about neuromodulatory influences on the LPP. Unfortunately, the study found no effect of facial expression on the LPP, and therefore also no drug effects. The absence of an emotion-related LPP modulation was probably due to the use of happy and sad faces, which in previous work have also failed to modulate the LPP (in contrast to threatening faces; e.g., Foti et al. 2010).
In sum, previous work has shown that the LPP reflects the modulation of activity in extrastriate visual cortical structures (Keil et al. 2002; Sabatinelli et al. 2007). However, not much is known about the source of that modulation. Our results support the hypothesis that one source of the modulation is the LC-mediated activation of β-receptors in the amygdala.
Acknowledgments
This work was supported by the Netherlands Organization for Scientific Research and a Marie Curie European Reintegration Grant within the 7th European Community Framework Programme. We thank Zoe Samara and Andriani Kyriklaki for their assistance in the data collection.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
The Liebowitz Social Anxiety Scale is a trait measure designed to measure social anxiety. This measure was originally included in our study for purposes unrelated to the present report. Importantly, LSAS scores correlate highly with scores on the Hamilton Anxiety Scale, a more general measure of trait anxiety (r = 0.48 Heimberg et al. 1999; r = 0.55 Kummer et al. 2008).
Besides the LPP, we also examined the early posterior negativity (EPN), defined as the average signal value across electrodes O1 and O2 in a window from 180 to 250 ms after the stimulus. However, in experiments 1 and 2, there was no significant effect of genotype or treatment on EPN amplitude and no significant interaction between picture type and genotype/treatment.
An interesting aspect of the Weymar et al. (2010) study was that only male participants were included. It is well established that men generally report less fear and anxiety than women (McLean and Anderson 2009), a finding replicated in the LSAS scores of our participants (men 10.3 ± 1.2; women 22.4 ± 3.8; t 12 = 3.0, p = 0.01). This suggests that the participants in the study of Weymar et al. were probably more similar to our low-anxiety group.
References
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/S0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Ampatzis K, Dermon CR. Regional distribution and cellular localization of beta2-adrenoceptors in the adult zebrafish brain (Danio rerio) J Comp Neurol. 2010;518:1418–1441. doi: 10.1002/cne.22278. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529–550. doi: 10.1037/0033-295X.106.3.529. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/S0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJH. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Borjesson M, Magnusson Y, Hjalmarson A, Andersson B. A novel polymorphism in the gene coding for the beta(1)-adrenergic receptor associated with survival in patients with heart failure. Eur Heart J. 2000;21:1853–1858. doi: 10.1053/euhj.1999.1994. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. β-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. The amygdala and emotional memory. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Roberts AC, Robbins TW. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci. 2008;12:31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT (1994) Monoamergic modulation of human attentional and executive function. Dissertation, University of Cambridge
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- de Rover M, Van Noorden MS, Nieuwenhuis S, Van der Wee NJ. Cardiorespiratory arrest in a healthy volunteer after a single oral dose of 80 mg of the beta-blocker propranolol. Neurobiol Learn Mem. 2010;94:576–577. doi: 10.1016/j.nlm.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: encoding pleasant, unpleasant, and neutral pictures. Cogn Affect Behav Neurosci. 2002;2:252–263. doi: 10.3758/CABN.2.3.252. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Nijs I, Pepplinkhuizen L. Effects of dopaminergic modulation on electrophysiological brain response to affective stimuli. Psychopharmacology. 2008;195:537–546. doi: 10.1007/s00213-007-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol. 2010;35:129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hartley LR, Ungapen S, Davie I, Spencer DJ. The effect of beta adrenergic blocking drugs on speakers’ performance and memory. Br J Psychiatry. 1983;142:512–517. doi: 10.1192/bjp.142.5.512. [DOI] [PubMed] [Google Scholar]
- Heimberg RG, Horner KJ, Juster HR, Safren SA, Brown EJ, Schneier FR, Liebowitz MR. Psychometric properties of the Liebowitz Social Anxiety Scale. Psychol Med. 1999;29:199–212. doi: 10.1017/S0033291798007879. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Huter T, Müller F, Mühlberger A, Pauli P, Reif A, Renner T, Canli T, Fallgatter AJ, Lesch K-P. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cereb Cortex. 2007;17:1160–1163. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Schnell K, Musshoff F, Keysers C, Maier W, Kendrick KM, Onur OA. Human amygdala reactivity is diminished by the β-noradrenergic antagonist propranolol. Psychol Med. 2010;40:1839–1848. doi: 10.1017/S0033291709992376. [DOI] [PubMed] [Google Scholar]
- Hurtado E, Haye A, González R, Manes F, Ibáñez A. Contextual blending of ingroup/outgroup face stimuli and word valence: LPP modulation and convergence of measure. BMC Neurosci. 2009;10:69. doi: 10.1186/1471-2202-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998;75:887–900. doi: 10.1037/0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1111/1469-8986.3950641. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Labuschagne I, Croft RJ, O’Neill BV, Bhagwagar Z, Phan KL, Nathan PJ. Evidence for modulation of facial emotional processing bias during emotional expression decoding by serotonergic and noradrenergic antidepressants: an event-related potential (ERP) study. Psychopharmacol. 2009;202:621–634. doi: 10.1007/s00213-008-1340-3. [DOI] [PubMed] [Google Scholar]
- Kummer A, Cardoso F, Teixeira AL. Frequency of social phobia and psychometric properties of the Liebowitz social anxiety scale in Parkinson’s disease. Mov Disord. 2008;23:1739–1743. doi: 10.1002/mds.22221. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in mental health care delivery systems. Norwood: Ablex; 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: affect, activation, and action. Mahwah: Erlbaum; 1997. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-6. Gainesville: University of Florida; 2005. [Google Scholar]
- Leutgeb V, Schäfer A, Schienle A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biol Psychol. 2009;82:293–300. doi: 10.1016/j.biopsycho.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- Luksys G, Gerstner W, Sandi C. Stress, genotype and norepinephrine in the prediction of mouse behavior using reinforcement learning. Nat Neurosci. 2009;12:1180–1186. doi: 10.1038/nn.2374. [DOI] [PubMed] [Google Scholar]
- Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of the β1-adrenoceptor: identification and rapid screening assay. Lancet. 1999;353:897. doi: 10.1016/S0140-6736(99)00549-8. [DOI] [PubMed] [Google Scholar]
- Marissen MA, Meuleman L, Franken IH. Altered emotional processing in borderline personality disorder: an electrophysiological study. Psychiatry Res. 2010;181:226–232. doi: 10.1016/j.pscychresns.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-rotein coupling domain of the human beta1-adrenergic receptor. J Biol Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev. 2009;29:496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DJ. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci U S A. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: correlation of functional MRI and event-related potentials. Cereb Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. doi: 10.1111/1469-8986.3720257. [DOI] [PubMed] [Google Scholar]
- Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci U S A. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proc Natl Acad Sci U S A. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong AH, Richardson JS. The characterization of beta adrenoceptor subtypes in the rat amygdala and hippocampus. Int J Neurosci. 1990;54:231–244. doi: 10.3109/00207459008986639. [DOI] [PubMed] [Google Scholar]
- Tollenaar MS, Elzinga BM, Spinhoven P, Everaerd W. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiol Learn Mem. 2009;91:23–31. doi: 10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Van Stegeren AH, Goekoop R, Everaerd W, Scheltens P, Barkhof F, Kuijer JPA, Rombouts SARB. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. NeuroImage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Everaerd W, Rombouts SA. Interaction of endogenous cortisol and noradrenaline in the human amygdala. Prog Brain Res. 2008;167:263–268. doi: 10.1016/S0079-6123(07)67020-4. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9:585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G (2011) The late positive potential predicts subsequent interference with target processing. J Cogn Neurosci. doi:10.1162/jocn.2011.21630 [DOI] [PubMed]
- Weymar M, Löw A, Modess C, Engel G, Grundling M, Petersmann A, Siegmund W, Hamm AO. Propranolol selectively blocks the enhanced parietal old/new effect during long-term recollection of unpleasant pictures: a high density ERP study. NeuroImage. 2010;49:2800–2806. doi: 10.1016/j.neuroimage.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Zill P, Baghai TC, Engel R, Zwanzger P, Schüle C, Minov C, Behrens S, Bottlender R, Jäger M, Rupprecht R, Moller HJ, Ackenheil M, Bondy B. Beta-1-adrenergic receptor gene in major depression: influence on antidepressant treatment response. Am J Med Genet B Neuropsychiatr Genet. 2003;120:85–89. doi: 10.1002/ajmg.b.20017. [DOI] [PubMed] [Google Scholar]