Abstract
Background
The treatment of endometrial cancer in young women who desire future fertility poses several challenges. Oral progestin and progestin-releasing intrauterine devices (IUDs) have been shown to result in regression of endometrial hyperplasia and grade 1 endometrioid endometrial carcinoma. However, limited data are available on the use of these methods in women with grade 2 disease.
Case
An 18 year-old nulliparous woman was diagnosed with a grade 2 endometrial adenocarcinoma. She desired future fertility and therefore underwent placement of a levonorgestrel-releasing IUD. The patient subsequently underwent endometrial sampling every 3 months, and remained disease-free 13 months after initial IUD placement.
Conclusion
A progestin-releasing IUD may be a valid treatment option for grade 2 endometrial cancer in young individuals who desire to retain fertility.
Introduction
Endometrial cancer in women under 40 years of age is relatively uncommon, accounting for only 2 to 14% of reported cases [1-3]. The treatment of endometrial cancer in these young women is challenging, as many have not yet completed childbearing and desire to preserve fertility.
As an alternative to the standard surgical treatment with hysterectomy, oral progestins have been proposed as a treatment option for women with stage I, grade 1 endometrial cancer desiring to retain fertility [3-5]. These patients require close observation to ensure that lesions respond and normalize with progestin therapy. Typical surveillance includes endometrial sampling every three months, although no clear guidelines exist. Once childbearing is completed, it is recommended that these women proceed with definitive treatment with hysterectomy.
Recent studies have proposed using progestin-releasing intrauterine devices (IUDs) as an alternative to oral systemic progestin for the treatment of patients with complex atypical hyperplasia as well as early stage, grade 1 endometrial cancer [6-8]. A recent meta-analysis by Gallos et al. [8] reported that these devices may be superior to oral progestin, with higher rates of regression noted in patients with complex and atypical endometrial hyperplasia. One such device is the Mirena® (levonorgestrel) IUD which releases levonorgestrel at a rate of 20 micrograms daily. This device is Food and Drug Administration (FDA) approved for up to 5 years of usage after which time the hormone release rate decreases by approximately 50%. It is hypothesized that these devices may be safer and better tolerated when compared to oral agents. The devices provide local progestin therapy to the endometrium, and spare the patient most of the systemic effects encountered with oral progestins including weight gain and increased risk of venous thromboembolic events. However, some patients experience abnormal vaginal bleeding, amennorhea and nausea associated with the IUD. Because of the steady hormone dosing and ability for long-term use, these devices are thought to improve patient compliance and may provide a safe and effective way of managing grade 1 endometrial cancer among women desiring to preserve fertility. However, limited data are available on the use of these methods in women with grade 2 disease.
Case
An 18 year-old nulliparous woman was noted to have a polyp protruding through her cervical os on routine gynecologic examination. A polypectomy was performed which revealed endometrioid adenocarcinoma International Federation of Gynecology and Obstetrics (FIGO) grade 2. The patient’s medical history was significant for polycystic ovarian syndrome (PCOS) and type II diabetes mellitus, both diagnosed during late adolescence. Her body mass index (BMI) at diagnosis was 47.7 kg/m2. Her family history was significant for a maternal first cousin diagnosed with uterine cancer at age 22, but she had no family history of colon or ovarian cancer.
The patient underwent endometrial biopsy and endocervical curettage, both showing endometrioid adenocarcinoma, FIGO grade 2, in a background of complex endometrial hyperplasia with atypia (Figure 1). Magnetic resonance imaging (MRI) of the pelvis showed a polypoid mass in the lower uterine segment. There was no evidence of myometrial invasion. In addition, a nonspecific left external iliac lymph node measuring 2.1 × 0.9 cm was noted. Computed tomography (CT) guided biopsy of this lymph node was negative for malignancy. On pathology review, it was unclear if the tumor was endocervical or endometrial in origin. The patient therefore underwent a cold knife conization (CKC) of the cervix, endocervical curettage (ECC), as well as hysteroscopy with dilation and curettage (D&C). Pathology from the conization was benign. Endocervical and endometrial curettings showed persistent endometrial endometrioid adenocarcinoma, FIGO grade 2. The patient desired future fertility and conservative management of her cancer, and therefore underwent levonorgestrel-releasing IUD placement.
Figure 1.
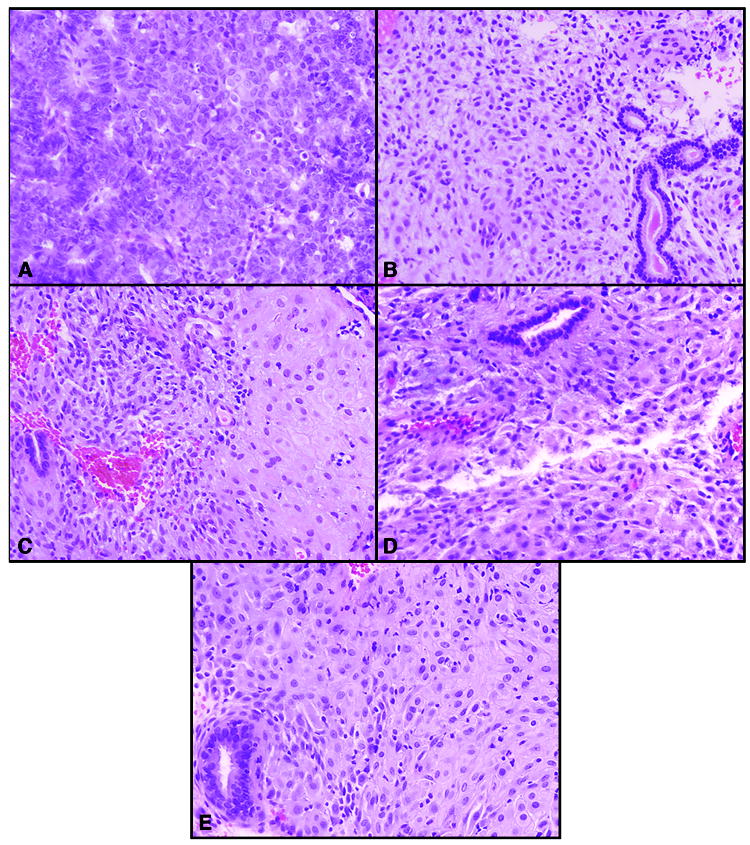
Endometrial biopsies obtained before treatment (A) and 3 (B), 6 (C), 11 (D), and 13 (E) months post-treatment. All photomicrographs are hematoxylin and eosin (H&E), 20X magnification. The initial biopsy (A) demonstrated an endometrial endometrioid adenocarcinoma, FIGO grade 2. Subsequent endometrial biopsies (B-E) obtained after treatment yielded benign endometrium with stromal cell pseudo-decidualization and inactive endometrial glands, microscopic changes that are characteristically associated with exposure to progestins.
Endometrial sampling three months following IUD placement showed complete regression of the hyperplasia and carcinoma. The patient has subsequently undergone endometrial sampling every three months, alternating between office biopsy with IUD in place and hysteroscopy with D&C and IUD replacement. All specimens have been negative to date. Two follow-up MRI scans have been performed showing resolution of the enlarged pelvic lymph node and no other evidence of intrauterine or metastatic disease. The patient remained without evidence of disease 13 months after initial IUD placement.
Given the patient’s young age at diagnosis, she was referred for genetic counseling. Immunohistochemical studies and microsatellite instability testing was performed on the patient’s endometrial tumor to test for Lynch syndrome/Hereditary Non-Polyposis Colorectal Cancer (HNPCC). Immunohistochemical analysis demonstrated intact expression of the MLH1, MSH2, MSH6, and PMS2 proteins. Microsatellite instability analysis showed no allelic shift in the seven tested microsatellite markers, confirming a microsatellite stable tumor and no evidence of Lynch syndrome/HNPCC. The patient subsequently underwent a clinical evaluation for Cowden’s syndrome, which was also negative.
Comment
The use of oral progestins for the conservative management of early stage, grade 1 endometrial cancer in women desiring to maintain fertility is well established [4, 5, 9]. Chiva et al. [5] performed a review of the literature reporting on 133 women with endometrial cancer treated with hormonal therapy. They noted a complete response in 76% of patients. Of those, 66% had a lasting complete response and 34% relapsed. Most patients responded within 12 weeks of initiating treatment. Among the 133 patients reviewed, 53 (39.9%) went on to have successful pregnancies. In this review and others, there are limited data regarding the use of oral progestin therapy in patients with grade 2 endometrial cancer.
Recent studies have also reported on the use of progestin-releasing IUDs in the management of early grade endometrial cancer [6, 7]. Montz et al. [6] examined response rates in 12 women with stage IA, FIGO grade 1 endometrial cancer treated with a progesterone-releasing IUD (Progestasert®). In this study, no residual carcinoma was identified in 6 of 12 women at 3 months, 7 of 11 women at 6 months, 7 of 9 women at 9 months, and 6 of 8 women at 12 months after IUD placement (some patients were lost to follow-up or chose to proceed to hysterectomy during the study). Further recurrence of endometrial cancer was not found in any of the 6 women who had complete regression and continued treatment with progesterone-releasing IUD for as long as 36 months.
A subsequent prospective observational study by Minig and colleagues [7] utilized a levonorgestrel-releasing IUD for one year concurrently with a 6 month course of a GnRh analogue in 20 patients with atypical endometrial hyperplasia (AEH) and 14 patients with early stage, grade 1 endometrial cancer. They noted a 95% complete response rate in patients with AEH and a 57% complete response rate with grade 1 endometrial cancer. Progression of disease was noted in 5% of the AEH group and 28% of the grade 1 endometrial cancer group. Twenty percent of the AEH group and 14% of the grade 1 endometrial cancer group had disease recurrence, with an average relapse time of 36 months.
The patient in the current case was diagnosed with endometrial cancer at a strikingly young age. She therefore underwent evaluation and testing for hereditary cancer syndromes, including Lynch syndrome/HNPCC and Cowden syndrome with negative results. Lynch syndrome/HNPCC is an autosomal dominant hereditary cancer syndrome with increased risk of colon, endometrial, and ovarian cancer. This syndrome is usually diagnosed through the occurrence of one of the above cancers between the ages of 40-56 years old. Women diagnosed with Lynch syndrome have a 40-60% risk of developing endometrial cancer during their lifetime. Cowden syndrome is a rare autosomal dominant genetic disorder resulting in intestinal hamartomas, mucocutaneous lesions and an increased risk of endometrial, breast, and thyroid cancer. Women diagnosed with Cowden syndrome have a 6% lifetime risk of developing endometrial cancer, typically between the ages of 38-59 [10, 11].
Endometrial cancer in young women may also be attributed to other underlying conditions such as PCOS or other hyper-estrogenic states that result in unopposed endometrial stimulation [1-3]. This unopposed endometrial stimulation may lead to hyperplasia and ultimately result in cancer. Duska et al. [1] reported on women under 40 years of age with endometrial cancer. They noted that 44% of patients were obese and 60% were nulliparous. Similarly, a study by Gallup and colleagues [2] found that 44% of young women diagnosed with endometrial cancer were obese, 44% were nulliparous, and 31% had PCOS. The current case reports on a nulliparous adolescent with morbid obesity, PCOS, and diabetes, yet no known predisposing hereditary cancer syndrome. It is hypothesized that her endometrial cancer resulted from unopposed estrogen stimulation of the endometrium secondary to her morbid obesity and PCOS. However, it remains unclear why she developed endometrial cancer at such a strikingly young age.
Conservative management of early stage, low-grade endometrial cancer with hormonal therapy has allowed many women to complete childbearing prior to proceeding to definitive treatment with hysterectomy [5, 9]. This method is not without risks, namely the risks of disease progression and lymph node metastasis. Accordingly, patients must be carefully counseled regarding the dangers inherent in delaying hysterectomy. Furthermore, patients require close monitoring following placement of a progestin-releasing IUD. Although the current patient has had a complete response to this therapy, she is being monitored with endometrial sampling every three months, alternating between office endometrial biopsy and hysteroscopy with D&C.
Despite these risks, insertion of a progestin-releasing IUD may provide a viable management option for young women desiring to maintain fertility. Given the rising obesity in the United States and worldwide, there is a clear need to identify successful conservative treatments in this population. Further study is needed to confirm if a progestin-releasing IUD represents a viable alternative to young women with early stage endometrial cancer.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83:388–93. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 2.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–20. [PubMed] [Google Scholar]
- 3.Ota T, Yoshida M, Kimura M, Kinoshita K. Clinicopathologic study of uterine endometrial carcinoma in young women aged 40 years and younger. Int J Gynecol Cancer. 2005;15:657–62. doi: 10.1111/j.1525-1438.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- 4.Jadoul P, Donnez J. Conservative treatment may be beneficial for young women with atypical endometrial hyperplasia or endometrial adenocarcinoma. Fertil Steril. 2003;80:1315–24. doi: 10.1016/s0015-0282(03)01183-x. [DOI] [PubMed] [Google Scholar]
- 5.Chiva L, Lapuente F, Gonzalez-Cortijo L, Carballo N, Garcia JF, Rojo A, Gonzalez-Martin A. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008;111:S101–4. doi: 10.1016/j.ygyno.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 6.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–7. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 7.Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924–7. doi: 10.1016/j.ygyno.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2003;547:e1–10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–8. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Starink TM, van der Veen JP, Arwert F, de Waal LP, de Lange GG, Gille JJ, Eriksson AW. The Cowden syndrome: a clinical and genetic study in 21 patients. Clin Genet. 1986;29:222–33. doi: 10.1111/j.1399-0004.1986.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 11.Schmeler KM, Daniels MS, Brandt AC, Lu KH. Endometrial cancer in an adolescent: a possible manifestation of Cowden syndrome. Obstet Gynecol. 2009;114:477–9. doi: 10.1097/AOG.0b013e31819dade8. [DOI] [PubMed] [Google Scholar]


