Abstract
Objectives
To measure the incidence of disability in individuals aged 90 and older and examine factors that may increase risk of disability.
Design and Setting
The 90+ Study, a longitudinal study of aging initiated in January 2003 with follow-up through May 2009.
Participants
216 non-disabled, prospectively followed participants who were aged 90 or older at baseline.
Measurements
The incidence of disability, measured as needing help on one or more Activities of Daily Living, and calculated using person-years. Risk factors were examined using a Cox proportional hazards analysis.
Results
The overall incidence of disability was 16.4%per year (95% confidence interval, 13.3–20.0), and did not differ by gender. Disability incidence increased with age from 8.3%in the 90–94 age group to 25.7%in the 95 and older age group. Several factors were associated with increased risk of disability including history of congestive heart failure, history of depression poor self-rated quality of life and cognitive impairment.
Conclusion
Disability incidence is high and increases rapidly with age in the oldest-old, with rates essentially tripling between ages 90–94 and 95+. Some factors associated with increased risk of disability in younger elderly continue to be risk factors in the oldest-old. Because of the tremendous social and financial impact of disability and the rapid growth of the oldest-old, the development of strategies to delay disability in the elderly should be a priority for health care research.
Introduction
As life expectancy in the United States has risen, the number of elderly in the population has also grown. People aged 90 and older have become the fastest growing age group in the country and now comprise almost 2% of the United States population1. Disability is highly prevalent in the oldest-old2, reaching 97% in centenarians3. Although there is some evidence that the overall disability rate by age has declined in the United States4, there is evidence that this decline may have recently ended5. This cessation in decline seems to still be a matter in question6. Regardless, disability in the elderly has a major social and financial impact upon society7 and provides numerous challenges to the elderly, their families and their physicians.
Many researchers have examined factors that may be associated with the risk of disability in the elderly, but most have not included data on people aged 90 and older. These factors have ranged widely, including physical limitations8, level of physical activity9, depression10, cognitive status11, comorbidities12, falls13, self-rated health14 and social interaction15.
We estimated the incidence of disability in a sample of participants aged 90 and older, The 90+ Study. Additionally, in order to identify potentially modifiable risk factors for disability in the oldest-old, we examined a variety of factors and their effects on incident disability.
Subjects and Methods
Study Sample
In the early 1980s, a health survey was mailed to residents of Leisure World, a retirement community in southern California. The 13,978 residents who completed the survey became members of the Leisure World Cohort Study. Participants were mostly female, 99% Caucasian and well educated. These participants were followed longitudinally, and several follow-up surveys were completed. The 1150 participants who were alive and aged 90 or older on January 1, 2003 were invited to join The 90+ Study. As of December 31, 2007, 950 participants had enrolled.
Assessments
In order to be flexible with the wide range of health statuses among people aged 90 and older, participants of The 90+Study could participate at several different levels. Those who were able to underwent a biannual comprehensive in-person evaluation that included demographics, past medical history, family history, functional assessments, neurological examination, and a neuropsychological battery. However, those who were unable to be seen in person were still able to provide information via self-completed or informant-completed mailed questionnaires, which included demographics, past medical history, family history and functional assessments.
Determination of Disability
In order to determine disability status, participants in The 90+ Study were asked to identify an informant, a person who would best be able to provide researchers with information about their functional abilities via mail and telephone. While the participant was alive, a questionnaire was mailed annually to the informant asking about the participant’s functional abilities. In 57.8% of the cases, the informant was a son/daughter or son/daughter-in-law. Other informants included siblings, neighbors or paid caregivers (18.5%), spouses (10.2%) or other (13.5%). This mailed questionnaire included the Katz index of Activities of Daily Living (ADLs)16, which is one of the most widely used scales for measuring disability with well established reliability and validity17. Disability was defined as needing help from another person in order to perform one or more ADLs (feeding, dressing, bathing, toileting, transferring and walking indoors). Although this definition represents a somewhat later stage in the disablement process, by using the stricter definition of disability (needing help from another person), we were able to include more participants in the incidence analysis. Furthermore, our definition of disability did not include analysis of the Instrumental Activities of Daily Living (IADLs), and thus the participants classified as nondisabled may have had impairments in one or more IADLs.
Individuals that found one or more ADLs difficult to perform at baseline, but did not yet require help from another person, were included in the incidence analysis and were classified as having ADL difficulty at baseline. Written informed consent was obtained from all participants or their surrogates. The Institutional Review Board of the University of California, Irvine, approved all procedures.
Determination of Potential Risk Factors
A variety of factors that have been previously found to be related to disability in younger elderly were examined as potentially related to incident disability in this oldest-old cohort including medical histories, quality of life, level of exercise, and cognitive status. Medical history variables were obtained from either the participant or an informant at baseline and included history of dementia or memory loss, depression, stroke, transient ischemic attack (TIA), coronary artery disease, myocardial infarction, atrial fibrillation, heart valve disease, high cholesterol, hypertension, congestive heart failure (CHF), osteoarthritis, rheumatoid arthritis, diabetes, thyroid disease and any falls in the past year. Prevalent dementia at baseline for all participants in this cohort was established as previously described18.
Additional measures were obtained from the subset of participants who were seen in-person. Participants were asked how often they participated in vigorous exercise, dichotomized as < once per month or ≥ once per month. Self-rated quality of life (QOL) was determined by having participants complete the Quality of Life – Alzheimer’s Disease scale (QOL-AD)19 and scores less than the median (40 out of a total 52) were considered low self-rated QOL. Participants were asked how often they had social contact with friends and relatives either in-person or over the phone and were dichotomized as < weekly or ≥ weekly. Measures of cognitive status, including the Mini Mental StateExam20 (MMSE) and the California Verbal Learning Test II Short Form21 (CVLT) scores were also obtained from the subset of subjects seen in-person at baseline. The MMSE is a test of global cognition, whereas the CVLT is a test of verbal memory. AMMSE score of <23 and a CVLT long delay score < 4 were used to differentiate groups for analyses. These cut points were determined from previous studies in this cohort showing that a MMSE score of < 23 had high sensitivity and specificity for predicting dementia22, and from normative scores of the CVLT in this cohort23. If participants were impaired in any of the cognitive measures (history of dementia, prevalent dementia, low MMSE or low CVLT), they were included into an “any cognitive impairment” summary measure.
Statistical Analyses
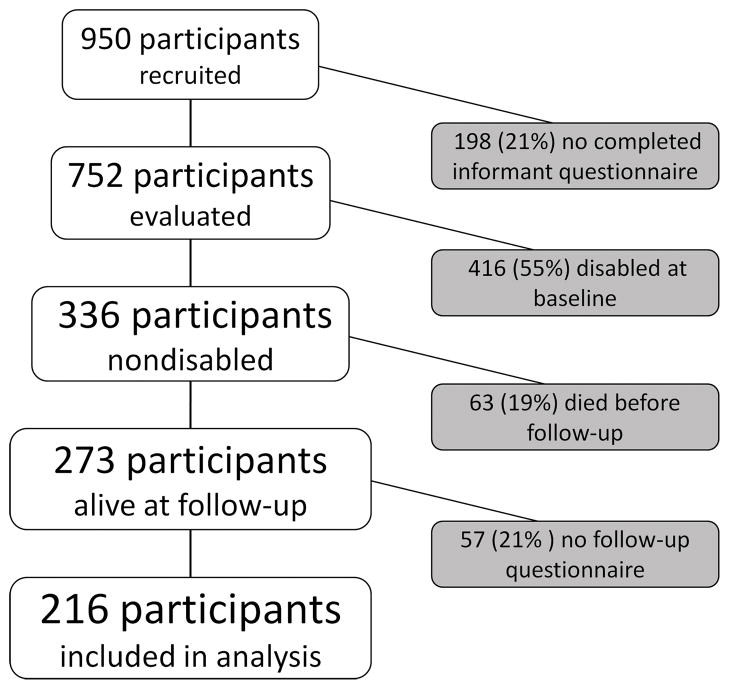
We restricted our analyses to the 216 participants who were not disabled at baseline, as ascertained by informant questionnaire, and had at least one additional follow-up informant questionnaire. Figure 1 shows a flow chart of participants included in the incidence estimates.
Figure 1.
Flow-chart of The 90+ Study participants included in the incident disability analysis. Participants were excluded if an informant questionnaire was not completed, if they were disabled at baseline, or if they had no follow-up.
Incidence rates were computed for strata of sex and 5-year age categories (90–94, 95+ years) using a person-years analysis. Participants were at risk and contributed person-years from the date of their baseline evaluation until the date of the follow-up evaluation when determined to have disabilityor the date of the last follow-up evaluation when determined to not have disability. A 95% confidence interval (CI) for the incidence rate was computed assuming a Poisson distribution for the number of incident cases in each age and gender strata. The relative risk (RR) of age was assessed by fitting a Poisson regression model. Risk factors for incident disability were examined using Cox regression models, with age as the time scale. A P value of less than 0.05 was considered statistically significant. All analyses were done using SAS 9.2, SPSS Statistics 17.0, and STATA 7.0 for Windows.
Results
Table 1 shows the baseline characteristics of the participants. Participants were highly educated, 40.7% having at least a college degree. Although the age range was from 90–101, 76.4% were younger than 95 at baseline. Almost half of all participants already had difficulty with 1 or more ADLs at baseline. Participants who developed disability were significantly older, less likely to live at home alone, and much more likely to have ADL difficulty at baseline.
Table 1.
Characteristics of Participants in The 90+ Study
| Disability status at follow-up* | |||
|---|---|---|---|
| Characteristic | All Participants (n=216) | Not Disabled (n=120) | Disabled (n=96) |
| No. (%) | No. (%) | ||
| Sex | |||
| Men | 56 (25.9) | 36 (30) | 20 (20.8) |
| Women | 160 (74.1) | 84 (70) | 76 (79.2) |
| χ2(1) = 2.3; P = 0.16 | |||
| Age category at baseline, y | |||
| 90–94 | 165 (76.4) | 101 (84.2) | 64 (66.7) |
| 95+ | 51 (23.6) | 19 (15.8) | 32 (33.3) |
| χ2(1) = 9.1; P = 0.004 | |||
| Education | |||
| < High School | 17 (7.9) | 8 (6.7) | 9 (9.4) |
| High School to Some College | 111 (51.4) | 61 (50.8) | 50 (52.1) |
| College Degree or More | 88 (40.7) | 51 (42.5) | 37 (38.5) |
| χ2(2) = 0.7; P = 0.70 | |||
| Living situation at baseline** | |||
| Living alone | 123 (56.9) | 78 (66.7) | 45 (53.6) |
| In household with spouse, relatives or caregiver | 54 (25) | 32 (27.4) | 22 (26.2) |
| Group quarters | 23 (10.6) | 7 (6.0) | 16 (19.0) |
| Nursing home | 1 (0.5) | 0 (0) | 1 (1.2) |
| χ2(3) = 18.9; P = 0.008 | |||
| ADL Difficulty at Baseline | |||
| Any ADL | 104 (48.1) | 41 (34.2) | 63 (65.6) |
| χ2(1) = 21.1; P < 0.001 | |||
| Feeding | 29 (13.4) | 7(5.8) | 22 (22.9) |
| Bathing | 35 (16.2) | 9 (7.5) | 26 (27.1) |
| Dressing | 39 (18.1) | 9 (7.5) | 30 (31.3) |
| Transferring | 58 (26.9) | 18 (15.0) | 40 (41.7) |
| Toileting | 28 (13.0) | 8 (8.7) | 20 (20.8) |
| Walking | 71 (32.9) | 28 (23.3) | 43 (44.8) |
| Mean (SD) | Mean (SD) | ||
| Age at baseline, y | 93.7 (2.4) | 93.2 (1.9) | 94.2 (2.8) |
| t(161.5) = 2.9; P = 0.004 | |||
| Follow-up, y | 2.7 (1.3) | 2.8 (1.4) | 2.6 (1.3) |
| t(214) = 1.5; P = 0.13 | |||
| MMSE Score† | 25.6 (3.7) | 26.7 (2.5) | 24.4 (4.5) |
| t(104.5) = 3.9; P < 0.001 | |||
| CVLT Long Delay Score‡ | 4.7 (2.8) | 5.3(2.6) | 3.9 (2.9) |
| t(115) = 2.7; P = 0.009 | |||
P values are from χ2 tests for categorical variables and from t-tests for continuous variables
Living situation information was unknown for 15 participants
MMSE was completed by 151 participants;
CVLT was completed by 117 participants
ADL=Activities of Daily Living, SD=Standard Deviation, MMSE=Mini Mental State Exam, CVLT=California Verbal Learning Test-Short Form
Of the 216 participants, 96 cases of incident disability were identified, resulting in an incidence rate of 16.4% per year (Table 2). Incidence rates increased across age categories (RR=3.07, 95%CI=1.96–4.82), rising from 8.3% per year for ages 90–94 to 25.7% in the 95 and older age group. There was no significant interaction between age and gender in the prediction of incident disability.
Table 2.
Age-and Gender-Specific Disability Incidence Rates in The 90+ Study
| Gender | Age | Number of New Cases | Person Years | Incidence per 100 person-years (95% CI) |
|---|---|---|---|---|
| Both | 90–94 | 26 | 312.13 | 8.3 (5.4–12.2) |
| 95+ | 70 | 272.17 | 25.7 (20.1–32.5) | |
| All ages | 96 | 584.30 | 16.4 (13.3–20.0) | |
| Men | 90–94 | 7 | 84.44 | 8.3 (3.4–17.2) |
| 95+ | 13 | 65.66 | 19.8 (10.5–33.7) | |
| All ages | 20 | 150.11 | 13.3 (8.1–20.6) | |
| Women | 90–94 | 19 | 227.69 | 8.3 (5.0–13.0) |
| 95+ | 57 | 206.51 | 27.6 (20.9–35.7) | |
| All ages | 76 | 434.20 | 17.5 (13.8–21.9) |
Each predictor variable was examined independently, using a Cox regression model (Table 3). The strongest risk factors for incident disability were history of CHF (HR=2.68, 95%CI=1.41–5.10), baseline difficulty with any ADL (HR=2.56, 95%CI=1.67–3.94) and a history of depression (HR=2.42, 95%CI=1.37–4.29). Having a low self-rated quality of life (HR=2.24, 95% CI=1.25–4.03) or rare contact with family and friends (HR=2.23, 95%CI=1.10–4.53) also predicted incident disability. Baseline cognition was strongly related to disability as a MMSE score below 23 (HR=2.09, 95%CI=1.17–3.73), a history of dementia or memory loss (HR=2.05, 95%CI=1.11–3.78), and a CVLT long delay score below four (HR=1.86, 95%CI=1.07–3.26) were all associated with increased disability incidence. Aside from history of depression and congestive heart failure, no other medical history variables were associated with incident disability. Level of vigorous exercise was also not related to disability. In order to ensure that the risk factors were not themselves the result of ADL difficulty as baseline, we examined a second regression model that adjusted for number of ADLs found difficult at baseline. The effects of all significant predictors of incident disability remained significant and were similar in magnitude after this adjustment, with the exception of history of dementia or memory loss at baseline (HR=1.40, 95%CI=0.72–2.70).
Table 3.
Risk Factors for Disability
| Risk Factors at Baseline | Total N in analysis | N with risk factor | Model 1 HR†(95% CI) | Χ2 (P)§ | Model 2 HR‡(95% CI) | Χ2 (P) § |
|---|---|---|---|---|---|---|
| Previous Functional Loss | ||||||
| Any difficulty with ADLs | 216 | 104 | 2.56 (1.67–3.94)* | 18.41(0.001) | NA | NA |
| Demographic | ||||||
| Being female | 216 | 160 | 1.31 (0.80–2.16) | 1.12(0.29) | 1.11 (0.67–1.85) | 0.17 (0.68) |
| Cognitive | ||||||
| MMSE Score <23 | 151 | 21 | 2.09 (1.17–3.73)* | 6.26 (0.01) | 2.22 (1.24–3.99)* | 7.13 (0.008) |
| History of Dementia or Memory Loss | 176 | 20 | 2.05 (1.11–3.78) | 5.26 (0.02) | 1.40 (0.72–2.70) | 0.99 (0.32) |
| Prevalent Dementia | 216 | 24 | 1.69 (0.98–2.91) | 3.52 (0.06) | 1.44 (0.83–2.50) | 1.70 (0.19) |
| CVLT Long Delay Score <4 | 117 | 42 | 1.86 (1.07–3.26) | 4.83 (0.03) | 2.16 (1.21–3.84)* | 6.80 (0.009) |
| Any cognitive impairment | 216 | 96 | 1.80(1.20–2.71)* | 8.02(0.005) | 1.69(1.12–2.55)* | 6.32(0.01) |
| Behavioral | ||||||
| Novigorous exercise | 177 | 72 | 1.31 (0.82–2.09) | 1.28 (.026) | 1.29 (0.81–2.07) | 1.14 (0.29) |
| Rare contact with family or friends (< weekly) | 176 | 21 | 2.23 (1.10–4.53) | 4.92 (0.03) | 2.28 (1.11–4.68) | 5.03 (0.03) |
| Low self-rated quality of life | 111 | 51 | 2.24 (1.25–4.03)* | 7.30 (0.007) | 1.97 (1.07–3.63) | 4.70 (0.03) |
| Medical History | ||||||
| More than 1 Co-morbidity | 215 | 133 | 1.02 (0.66–1.55) | 0.005 (0.95) | 0.95 (0.62–1.46) | 0.05 (0.82) |
| History of | ||||||
| Depression | 207 | 22 | 2.43 (1.37–4.30)* | 9.30 (0.002) | 1.99 (1.12–3.53) | 5.48 (0.02) |
| Strokes | 209 | 12 | 1.58 (0.76–3.31) | 1.49 (0.22) | 1.88 (0.90–3.92) | 2.81 (0.09) |
| TIAs | 199 | 29 | 1.34 (0.75–2.38) | 0.99 (0.32) | 0.99 (0.54–1.81) | 0.0007 (0.98) |
| Any falls in the past year | 216 | 78 | 1.34 (0.88–2.02) | 1.88 (0.17) | 1.12 (0.74–1.72) | 0.29 (0.59) |
| Coronary Artery Disease | 201 | 20 | 1.35 (0.70–2.63) | 0.80 (0.37) | 1.29 (0.66–2.51) | 0.57 (0.45) |
| Myocardial Infarction | 208 | 21 | 0.83 (0.38–1.80) | 0.21 (0.64) | 0.78 (0.36–1.70) | 0.39 (0.53) |
| Atrial Fibrillation | 204 | 49 | 0.75 (0.44–1.28) | 1.14 (0.29) | 0.76 (0.45–1.31) | 0.96 (0.33) |
| Heart Valve Disease | 206 | 13 | 1.19 (0.51–2.75) | 0.16 (0.69) | 0.98 (0.42–2.28) | 0.002 (0.96) |
| High Cholesterol | 196 | 55 | 1.22 (0.75–2.00) | 0.64 (0.42) | 1.43 (0.86–2.37) | 1.94 (0.16) |
| Hypertension | 209 | 116 | 1.12 (0.73–1.70) | 0.26 (0.61) | 1.06 (0.69–1.62) | 0.07 (0.79) |
| Congestive Heart Failure | 207 | 16 | 2.68 (1.41–5.10)* | 8.97 (0.003) | 1.99 (1.03–3.85) | 4.23 (0.04) |
| Osteoarthritis | 199 | 74 | 1.09 (0.70–1.69) | 0.14 (0.71) | 1.00 (0.65–1.56) | 0.0003 (0.99) |
| Rheumatoid Arthritis | 202 | 27 | 0.64 (0.33–1.24) | 1.79 (0.18) | 0.76 (0.39–1.49) | 0.65 (0.42) |
| Diabetes | 206 | 10 | 1.71 (0.62–4.74) | 1.06 (0.30) | 1.74 (0.62–4.84) | 1.11 (0.29) |
| Thyroid Disease | 202 | 50 | 1.03 (0.64–1.66) | 0.02 (0.88) | 1.07 (0.66–1.71) | 0.07 (0.80) |
P ≤ 0.01
Adjusted for age
Adjusted for age and difficulty with ADLs at baseline
ADL=Activities of Daily Living, MMSE=Mini Metal Status Exam, CVLT= California Verbal Learning Test –Short Form, TIA=transient ischemic attack, HR=Hazard Ratio
All tests had 1 degree of freedom.
In order to determine if disability in this cohort was a dynamic process in which recovery was possible, we examined how many participants transitioned from nondisabled to disabled and back to nondisabled on a subsequent follow-up visit. Of the 96 participants who became disabled during follow-up, only seven (7.3%) were considered nondisabled on a subsequent visit. Because so few participants recovered, disability among those aged 90 and older may be a less dynamic process involving injury and recovery, but instead represent a more chronic permanent decline in function.
Discussion
In this study, we found very high levels of incident disability in the oldest-old, with incidence rates essentially tripling with 5 years of age. Our incidence estimates for 90–94 year old participants are slightly higher than previous studies that examined somewhat younger cohorts24, 25, suggesting that our findings are consistent with the literature. Incidence rates of disability in these younger cohorts (aged 80+) roughly double after 5 years of age. Because our incidence rates tripled after 5 years of age, there might be an acceleration of disability incidence after age 90. We have previously shown that the prevalence of disability also increases with age in this cohort3. Therefore, both the prevalence and incidence of disability increase with age past age 90. However, it is important to note that the incidence rate of the 95+ group was 3 times higher than the 90–94 group, whereas the prevalence of the 95+ group was only 65% higher than the 90–94 group3. These findings suggest a decreased duration of disability with increased age, likely due to the increases in mortality.
This study found no difference in the incidence of disability between men and women. Previous studies have described conflicting results; some found no gender differences in disability incidence26, whereas others have noted that women have a greater incidence than men27. In this cohort, women had a greater prevalence of disability than men3 without increased incidence of disability. An explanation may be that men are more likely to recover from disability than women are; however, our findings suggest that recovery from disability in this age group is uncommon. It is more likely that women are remaining disabled longer than men and thus disabled women have decreased mortality compared to disabled men, which has been described before in disabled elderly stroke patients28.
Cognitive impairment was one of the largest predictors of incident disability. The summary measure had an odds ratio of similar magnitude to each individual cognitive measure, despite identifying many more individuals than any one measure alone. This suggests that the method of determining cognitive impairment may not be critical. Specifically, having a history of dementia or memory loss or having a low MMSE score at baseline more than doubled the risk of disability. These findings confirm the link between cognitive and physical health in the elderly29, 30. There are several potential mechanisms for this relationship. Cognitive impairment may lead to disability through the direct inability to perform ADLs due to cognition, for example, being unable to dress because one has forgotten how zippers and buttons operate. Whereas this is possible, other ADLs, such as transferring, rely heavily on physical rather than cognitive ability. Another explanation is that cognitive impairment may lead to disability through the loss of physical, not cognitive, health. Those with cognitive impairment are less likely to maintain physical health31, which in turn may lead to disability. Therefore, cognitive impairment may be the cause of the physical decline.
Another possibility is that the participants with cognitive impairment may already have difficulty performing ADLs, but not enough to require help. Thus, in this case, our cognitive impairment measure may simply be identifying participants with prior ADL difficulty, which is itself a risk factor for incident disability. When adjusted for ADL difficulty at baseline, history of dementia or memory loss no longer significantly predicted disability. This measure may have simply represented those participants who already had some ADL difficulty at baseline. However, after adjustment for ADL difficulty, low MMSE or CVLT scores still predicted disability, suggesting that direct measures of global cognition and memory predict disability incidence even after adjusting for baseline functional loss.
Our findings suggest that cognitive impairment is associated with a doubling of the risk of disability past age 90, whereas studies in younger cohorts have found smaller magnitude effects32. Therefore, our findings agree with others that have found that cognitive impairment may become a more important predictor of disability with increasing age29. Overall, it appears that cognitive impairment likely predicts incident disability and any preventive measures taken to reduce dementia in the oldest-old may have the added benefit of reducing disability levels also.
Several medical history variables were associated with incident disability, including history of CHF. This confirms previous findings that CHF sufferers have an increased likelihood of subsequent disability and poorly perceived health33. Additionally, cross-sectional studies have shown people with CHF have a lower overall quality of life34. With the increased aging of the population and increased survival after myocardial infarctions, the prevalence and incidence of CHF is rising in the United States35. It will be important to prepare for a potential increase in disability as a result. Reduction of risk factors for CHF, which is already a prioroity of modem health care, could possibly have the added benefit of reducing incident disability in the oldest-old as well. A history of depression was also associated with an increased risk of disability. Studies measuring depressive symptoms directly have found increases in risk of disability as well11, 36. Depression, whether current or by history, presents an important risk for incident disability in the elderly, and thus a potential therapeutic target. Because of the likelihood that baseline ADL difficulty could cause depression, we adjusted for the number of ADLs found to be difficult, and found that history of depression was still associated with disability. However, it is still unknown if reducing depression in the elderly will be helpful in reducing disability incidence. Studies examining antidepressant medications and incident disability will be helpful in understanding this relationship.
Of all the other medical history variables examined, most were not associated with increased disability, including history of stroke, TIA and myocardial infarction. Finding that cognitive impairment, but not co-morbidity is associated with disability is common30, 37, but may simply be the result of the way in which the co-morbidity is measured. Because these events are typically acute, they likely cause disability immediately, and a history of such an event may not predict disability in a person who is nondisabled at baseline. Therefore, new occurrences (i.e. after baseline) of these events may be more associated with incident disability.
Exercise has previously been associated with decreased disability incidence in the younger elderly38. Although exercise was not significantly associated with disability in our study, in frequent exercise was in the direction of being associated with increased disability incidence. There are several possible reasons why this study was unable to replicate previous findings. The definition of exercise may differ between the oldest-old and younger old ages, such that what oldest-old subjects consider vigorous exercise may not be of sufficient intensity or duration to modify their risk of becoming disabled. Similarly, our measure of exercise may not be sensitive enough to distinguish the subtle differences in physical activity that may modify disability risk in the oldest-old. Conversely, the protective effects of exercise may simply be exclusive to younger ages, and by age 90, exercise of any intensity may only have minimal benefits in preventing disability. These distinctions will be increasingly important as many exercise-based interventions are currently being examined for the prevention of disability in the elderly.
The concept of QOL has previously been suggested to be highly related with disability in the elderly39. In our study, a low QOL was predictive of incident disability, even after adjusting for baseline ADL difficulty. Individuals with a low QOL may feel less motivated to maintain their health, which could lead to disability. Thus, in the oldest-old, self-reports of QOL may be an effective way for clinicians to evaluate the risk of disability in the future. Furthermore, it may be possible to delay disability onset by modifying QOL and thus further research is warranted.
Participants in this study who had less than weekly contact with family or friends were more than twice as likely to become disabled. Social contact has previously been associated with decreased levels of depression, increased QOL, and decreased disability40. Infrequent social contact could be acting as a proxy for depression or low QOL, however, our data suggest that infrequent social contact predicts disability incidence independent of these other measures. Additional analyses were performed to assess the relationship between social contact and incident dementia after adjusting for history of depression and low QOL, and rare social contact remained a significant predictor of incident disability (HR=3.65, 95%CI=1.50–8.91). The biological mechanisms explaining this effect are still largely unknown and further research is warranted.
Several limitations to this study require discussion. First, although The 90+ Study is one of the largest studies dedicated to the oldest-old, the population is predominantly white and highly educated. Therefore, this population may have limited generalizability to other populations of people aged 90 and older. However, despite the modest representation of minority subjects in this cohort, these numbers reflect the composition of the oldest-old in Orange County, California41 and the US as a whole. A second limitation is that disability status was established using data from informants, not from the participants themselves. Informant-based measures of disability tend to overestimate disability, especially in the elderly42. However, the study used an informant-based questionnaire in order to be able to assess functional status in participants who may to be unable to fill out a questionnaire themselves due to sensory or cognitive impairments. Third, because 120 participants (36% of those non-disabled at baseline) had no follow-up information, we were unable to determine their disability incidence. About half of these participants died within one year of their baseline and about half did not have follow-up information for another reason, such as a reliable informant could no longer be reached. For those subjects that died before a follow-up, it is possible that they became disabled before death. Nevertheless, because those without follow-up could differ from those included in the analysis, the resulting sample may be biased. We compared those who had follow-up information to those who did not, and found that the two groups did not differ in gender, level of education or baseline scores on the MMSE or CVLT. However, participants without follow-up were older, more likely to live in an institution and more likely to have ADL difficulty at baseline. Additionally, those who died after at least one follow-up visit (and were non-disabled at their last follow-up) were assumed nondisabled until death. Therefore, in this way we may have underestimated disability as some subjects may have become disabled between our last follow-up and death. Because of these differences, it is possible that this study has underestimated the incidence of disability in those aged 90 and older, and the real incidence may in fact be even higher than the values presented. Fourth, when examining the risk factors for disability, several of the predictors (i.e. MMSE, CVLT) were only available on the subset of participants who were examined in-person. Although this could be a potential source of selection bias, there were no differences between participants with or without a MMSE in age, education or baseline ADL difficulty. Finally, the analysis of potential risk factors made use of multiple comparisons, which greatly increases the potential for type 1 errors. Although we adjusted our alpha value to 0.01, the chance for a type 1 error is still elevated. However, all of the risk factors for disability were selected from factors previously reported as associated with disability and therefore represent hypothesis driven analyses.
This investigation details the alarming increase in disability with age in the oldest-old. The rapid growth of this age group and their high rates of disability will have a tremendous financial impact throughout the United States as people living with disability have much higher health care expenditures than those who are not disabled43. A recent study found that health care costs of people with difficulty in 5–6 ADLs are almost 30 times greater than those with no ADL difficulty. Moreover, subjects with cognitive impairment as well as disability have health care costs over 50 times greater44. Medicare costs for people aged 85 and older already exceed $80 billion45, and are increasing with the rapid expansion of the elderly in the population. The current study has identified several risk factors associated with increased disability incidence in the oldest-old, several of which may be modifiable. More research is needed to see if modifying these risk factors would result in a reduction in disability incidence. Even a modest reduction in incidence of disability through risk factor modification could result in significant savings in healthcare spending.
In addition to the tremendous financial toll that disability takes on the elderly and their families, there is an equally devastating social cost of disability. Although not as easily measured, the loss of functional ability leads to a variety of negative outcomes for all those affected. Whether it is the physician who is unable to provide proper treatment because of a patient’s inability to reach the clinic, or the caregiver who suffers from anxiety due to the stress of a caring for a disabled parent, disability is a basic social issue with wide reaching effects. One of the goals of this study was to quantify the extremely high levels of incident disability seen in the oldest-old, as well as aid in the formation of potential interventions to prevent this disability onset. Educating physicians, patients, families and caregivers in the lifestyle factors associated with disability onset (social isolation, depression, low QOL), it gives them the tools to both identify risk factors and potentially reduce the risk of disability.
Acknowledgments
This manuscript was funded by a grant from the National Institutes of Health (R01AG21055) and the Al and Trish Nichols Chair in Clinical Neuroscience. We thank all participants and their relatives, testers and examiners of The 90+ Study.
Funding Source: NIA grant R01AG21055and the Al and Trish Nichols Chair in Clinical Neuroscience.
Footnotes
No disclosures to report.
This research was previously presented at the 2010 American Academy of Neurology meeting in Toronto, OT, Canada, April 12-16, 2010.
References
- 1.US Census Bureau. Annual Estimates of Population by Sex and Five-Year Age Groups for the United States: April 1, 2000 to July 1, 2007. 2008. [Accessed May 5, 2009];2008 May 1; http://www.census.gov/popest/mational/asrh/NC-EST2007-sa.html.
- 2.Waidmann TA, Liu K. Disability trends among elderly persons and implications for the future. J Gerontol B Psychol Sci Soc Sci. 2000 Sep;55(5):S298–307. doi: 10.1093/geronb/55.5.s298. [DOI] [PubMed] [Google Scholar]
- 3.Berlau DJ, Corrada MM, Kawas C. The prevalence of disability in the oldest-old is high and continues to increase with age: findings from The 90+ Study. Int J Geriatr Psychiatry. 2009 Nov;24(11):1217–1225. doi: 10.1002/gps.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman VA, Crimmins E, Schoeni RF, et al. Resolving inconsistencies in trends in old-age disability: report from a technical working group. Demography. 2004 Aug;41(3):417–441. doi: 10.1353/dem.2004.0022. [DOI] [PubMed] [Google Scholar]
- 5.Fuller-Thomson E, Yu B, Nuru-Jeter A, Guralnik JM, Minkler M. Basic ADL disability and functional limitation rates among older Americans from 2000–2005: the end of the decline? J Gerontol A Biol Sci Med Sci. 2009 Dec;64(12):1333–1336. doi: 10.1093/gerona/glp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin LG, Schoeni RF, Andreski PM. Challenges in estimating trends in late-life disability from the American Community Survey. J Gerontol A Biol Sci Med Sci. May;65(5):517–518. doi: 10.1093/gerona/glp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997 Jan;45(1):92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balzi D, Lauretani F, Barchielli A, et al. Risk factors for disability in older persons over 3-year follow-up. Age Ageing. 2010 Jan;39(1):92–98. doi: 10.1093/ageing/afp209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kivela SL, Pahkala K. Depressive disorder as a predictor of physical disability in old age. J Am Geriatr Soc. 2001 Mar;49(3):290–296. doi: 10.1046/j.1532-5415.2001.4930290.x. [DOI] [PubMed] [Google Scholar]
- 11.Mehta KM, Yaffe K, Covinsky KE. Cognitive impairment, depressive symptoms, and functional decline in older people. J Am Geriatr Soc. 2002 Jun;50(6):1045–1050. doi: 10.1046/j.1532-5415.2002.50259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Elzen WP, Willems JM, Westendorp RG, de Craen AJ, Assendelft WJ, Gussekloo J. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus Study. Cmaj. 2009 Aug 4;181(3–4):151–157. doi: 10.1503/cmaj.090040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA. 1995 May 3;273(17):1348–1353. [PubMed] [Google Scholar]
- 14.Idler EL, Kasl SV. Self-ratings of health: do they also predict change in functional ability? J Gerontol B Psychol Sci Soc Sci. 1995 Nov;50(6):S344–353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- 15.Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. Am J Epidemiol. 1995 Jan 1;141(1):41–49. doi: 10.1093/oxfordjournals.aje.a117344. [DOI] [PubMed] [Google Scholar]
- 16.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963 Sep;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 17.Sheikh K, Smith DS, Meade TW, Goldenberg E, Brennan PJ, Kinsella G. Repeatability and validity of a modified activities of daily living (ADL) index in studies of chronic disability. Int Rehabil Med. 1979;1(2):51–58. doi: 10.3109/03790797909164024. [DOI] [PubMed] [Google Scholar]
- 18.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from the 90+ study. Neurology. 2008 Jul 29;71(5):337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 19.Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002 May–Jun;64(3):510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Adult version (CVLT-II-A): Manual. 2. San Antonio, TX: 2000. [Google Scholar]
- 22.Kahle-Wrobleski K, Corrada MM, Li B, Kawas CH. Sensitivity and specificity of the mini-mental state examination for identifying dementia in the oldest-old: the 90+ study. J Am Geriatr Soc. 2007 Feb;55(2):284–289. doi: 10.1111/j.1532-5415.2007.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittle C, Corrada MM, Dick M, et al. Neuropsychological data in nondemented oldest old: the 90+ Study. J Clin Exp Neuropsychol. 2007 Apr;29(3):290–299. doi: 10.1080/13803390600678038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braungart Fauth E, Zarit SH, Malmberg B, Johansson B. Physical, cognitive, and psychosocial variables from the Disablement Process Model predict patterns of independence and the transition into disability for the oldest-old. Gerontologist. 2007 Oct;47(5):613–624. doi: 10.1093/geront/47.5.613. [DOI] [PubMed] [Google Scholar]
- 25.Covinsky KE, Hilton J, Lindquist K, Dudley RA. Development and validation of an index to predict activity of daily living dependence in community-dwelling elders. Med Care. 2006 Feb;44(2):149–157. doi: 10.1097/01.mlr.0000196955.99704.64. [DOI] [PubMed] [Google Scholar]
- 26.Strawbridge WJ, Kaplan GA, Camacho T, Cohen RD. The dynamics of disability and functional change in an elderly cohort: results from the Alameda County Study. J Am Geriatr Soc. 1992 Aug;40(8):799–806. doi: 10.1111/j.1532-5415.1992.tb01852.x. [DOI] [PubMed] [Google Scholar]
- 27.Hardy SE, Allore HG, Guo Z, Gill TM. Explaining the effect of gender on functional transitions in older persons. Gerontology. 2008;54(2):79–86. doi: 10.1159/000115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh MS, Yu KH, Roh JK, Lee BC. Gender differences in the mortality and outcome of stroke patients in Korea. Cerebrovasc Dis. 2009;28(5):427–434. doi: 10.1159/000235986. [DOI] [PubMed] [Google Scholar]
- 29.Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive impairment as a strong predictor of incident disability in specific ADL-IADL tasks among community-dwelling elders: the Azuchi Study. Gerontologist. 2005 Apr;45(2):222–230. doi: 10.1093/geront/45.2.222. [DOI] [PubMed] [Google Scholar]
- 30.Scanlan JM, Binkin N, Michieletto F, Lessig M, Zuhr E, Borson S. Cognitive impairment, chronic disease burden, and functional disability: a population study of older Italians. Am J Geriatr Psychiatry. 2007 Aug;15(8):716–724. doi: 10.1097/JGP.0b013e3180487cd7. [DOI] [PubMed] [Google Scholar]
- 31.Verdery RB. Clinical evaluation of failure to thrive in older people. Clin Geriatr Med. 1997 Nov;13(4):769–778. [PubMed] [Google Scholar]
- 32.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007 Oct;62(10):1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J Am Geriatr Soc. 1997 Mar;45(3):276–280. doi: 10.1111/j.1532-5415.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 34.Hobbs FD, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life: a cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Eur Heart J. 2002 Dec;23(23):1867–1876. doi: 10.1053/euhj.2002.3255. [DOI] [PubMed] [Google Scholar]
- 35.Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997 Aug;45(8):968–974. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 36.Dalle Carbonare L, Maggi S, Noale M, et al. Physical disability and depressive symptomatology in an elderly population: a complex relationship. The Italian Longitudinal Study on Aging (ILSA) Am J Geriatr Psychiatry. 2009 Feb;17(2):144–154. doi: 10.1097/jgp.0b013e31818af817. [DOI] [PubMed] [Google Scholar]
- 37.Orsitto G, Cascavilla L, Franceschi M, et al. Influence of cognitive impairment and comorbidity on disability in hospitalized elderly patients. J Nutr Health Aging. 2005;9(3):194–198. [PubMed] [Google Scholar]
- 38.Cress ME, Buchner DM, Prohaska T, et al. Best practices for physical activity programs and behavior counseling in older adult populations. J Aging Phys Act. 2005 Jan;13(1):61–74. doi: 10.1123/japa.13.1.61. [DOI] [PubMed] [Google Scholar]
- 39.Maddux RE, Delrahim KK, Rapaport MH. Quality of life in geriatric patients with mood and anxiety disorders. CNS Spectr. 2003 Dec;8(12 Suppl 3):35–47. doi: 10.1017/s1092852900008269. [DOI] [PubMed] [Google Scholar]
- 40.Golden J, Conroy RM, Lawlor BA. Social support network structure in older people: underlying dimensions and association with psychological and physical health. Psychol Health Med. 2009 May;14(3):280–290. doi: 10.1080/13548500902730135. [DOI] [PubMed] [Google Scholar]
- 41.US Census Bureau. Population Estimates of Orange County, California by Age and Race from the Census 2000 Summary File 1 (SF 1) 100-Percent Data 2000. [Accessed April 7, 2010]; http://factfinder.census.gov/servlet/MetadataBrowserServlet?type=dataset&id=DEC_2000_SF1_U.
- 42.Todorov A, Kirchner C. Bias in proxies’ reports of disability: data from the National Health Interview Survey on disability. Am J Public Health. 2000 Aug;90(8):1248–1253. doi: 10.2105/ajph.90.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitra S, Findley PA, Sambamoorthi U. Health Care Expenditures of Living With a Disability: Total Expenditures, Out-of-Pocket Expenses, and Burden, 1996 to 2004. Arch Phys Med Rehabil. 2009 Sep;90(9):1532–1540. doi: 10.1016/j.apmr.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Taylor DH, Jr, Schenkman M, Zhou J, Sloan FA. The relative effect of Alzheimer’s disease and related dementias, disability, and comorbidities on cost of care for elderly persons. J Gerontol B Psychol Sci Soc Sci. 2001 Sep;56(5):S285–293. doi: 10.1093/geronb/56.5.s285. [DOI] [PubMed] [Google Scholar]
- 45.Manton KG, Lamb VL, Gu X. Medicare cost effects of recent U.S. disability trends in the elderly: future implications. J Aging Health. 2007 Jun;19(3):359–381. doi: 10.1177/0898264307300186. [DOI] [PubMed] [Google Scholar]