Abstract
BACKGROUND
Musculoskeletal symptoms are common adverse effects of statins, yet little is known about the prevalence of musculoskeletal pain and statin use in the general population.
METHODS
We conducted a cross sectional study of the National Health and Nutrition Examination Survey (NHANES) 1999–2004. We estimated the prevalence of self-reported musculoskeletal pain according to statin use and calculated prevalence ratio estimates of musculoskeletal pain obtained from adjusted multiple logistic regression modeling.
RESULTS
Among 5,170 participants without arthritis, the unadjusted prevalence of musculoskeletal pain was significantly higher for statin users reporting pain in any region (23% among statin users, 95%CI: 19–27% compared to 18% among those not using statins, 95%CI: 17–20%; p=0.02) and in the lower extremities (12% among statin users, 95%CI: 8–16% compared to 8% among those not using statins, 95%CI: 7–9%; p=0.02). Conversely, among 3,058 participants with arthritis, statin use was not associated with higher musculoskeletal pain in any region. After controlling for confounders, among those without arthritis, statin use was associated with a significantly higher prevalence of musculoskeletal pain in any region, the lower back, and the lower extremities (adjusted prevalence ratios: 1.33 [1.06, 1.67]; 1.47 [1.02, 2.13]; 1.59 [1.12, 2.22], respectively). Among participants with arthritis, no association was observed between musculoskeletal pain and statin use on adjusted analyses.
CONCLUSIONS
In this population-based study, statin use was associated with a higher prevalence of musculoskeletal pain, particularly in the lower extremities, among individuals without arthritis. Evidence that statin use was associated with musculoskeletal pain among those with arthritis was lacking.
Keywords: Statins, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Musculoskeletal Diseases, Arthritis
Introduction
Statins, or 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, reduce LDL cholesterol, cardiovascular morbidity and mortality, and all cause mortality.1–3 They are one of the most widely prescribed medications in the U.S. and globally4; in 2003–2004, it was estimated that 24 million U.S. individuals received a prescription for statin medication.5 Although statins are usually well tolerated, musculoskeletal side effects (e.g., muscle aches, pain, weakness, cramps and/or creatinine kinase (CK) elevations) are the most common adverse effect associated with statin use.6 Because myalgia in the absence of significant CK elevation was not commonly evaluated in randomized controlled trials of statins,7 systematically collected data on muscle symptoms among statin users remains sparse.
We previously showed an association between statin use and musculoskeletal pain among individuals without arthritis enrolled in a population based study, the National Health and Nutrition Examination Survey (NHANES) 1999–2002.8 However, the relatively small sample size limited our ability to determine if statins added to the burden of those with arthritis. The aims of the current study were to better assess the relation between statin use and musculoskeletal pain in the general population, including those with and without arthritis, and to determine if arthritis affects the relationship, using 6 years of NHANES data.
Methods
Study population
We included 8,228 adults aged 40 years and older who participated in the NHANES 1999–2004. The NHANES is a cross-sectional, continuous survey administered by the National Center for Health Statistics (NCHS).9 The survey uses a complex, multistage, probability sampling design to select participants representative of the civilian, non-institutionalized U.S. population with oversampling of certain population subgroups to increase the reliability and precision of estimates for these groups. Public use data files are released in two-year increments. Consent was obtained from participants by NCHS after approval by the NHANES Institutional Review Board/ NCHS Research Ethics Review Board.10 Our analyses were approved for exemption by our institutional review board based on 45 CFR 46.101(b) (4) because of de-identified data.
Assessment of Musculoskeletal Pain
Participants were asked, "During the past month, have you had a problem with pain that lasted more than 24 hours?” Those who answered “yes” were asked to specify the location of their pain. We assessed reporting of pain at 4 different musculoskeletal regions including neck or upper back, upper extremities (shoulder, arm, wrist or hand), lower back, and lower extremities (buttock, leg, or foot). Any musculoskeletal pain was defined as pain in any one of these regions.
Ascertainment of sociodemographics, health habits and health conditions
Participants reported age, sex, race/ethnicity, smoking status, physical activity, and health status. Smoking status was defined as past, current, or never. Physical activity was based on activity during the last 30 days and was defined as vigorous if participants reported any “vigorous activity for a least 10 minutes that caused heavy sweating or large increases in breathing or heart rate,” moderate if participants reported “moderate activities for at least 10 minutes that cause only light sweating or a slight to moderate increase in breathing or heart rate,” or sedentary if neither vigorous nor moderate activity was reported. Self-rated health status was defined as excellent/very good, good, and fair/poor.
Prevalence of arthritis and cancer (excluding cervical cancer and non-melanoma skin cancer) was based on self-report of a doctor or other health-professional's diagnosis, i.e., “Has a doctor or other health professional ever told you that you had [condition]?” Coronary heart disease was defined by a self-report of a doctor or health care provider's diagnosis of coronary heart disease, angina, or a previous heart attack. Prevalence of diabetes was determined by self-report of a doctor or health care provider's diagnosis, a randomly measured glucose level of greater than 200 mg/dl, or use of an anti-diabetic medication. Serum total cholesterol and systolic blood pressure were measured. Body mass index (BMI) was calculated using measured height and weight. Ankle brachial index (ABI) was obtained by comparing the means of two blood pressure measurements in the right arm with that of each ankle. We used the lower of the two ABI values and defined peripheral artery disease as an ABI ≤ 0.97.
Medication use
To determine prescription medication use, survey participants were asked if they had taken a medication in the past month for which they needed a prescription. Participants who answered “yes” to this question were asked to show their medication containers during the in-home interview so the complete name of the product could be obtained. No container was available for approximately 15% of the medications, in which case a verbal recollection was reported to the interviewer.
Statistical Methods
All analyses were weighted to represent the U.S. population, to account for oversampling in specific demographic subgroups, and to account for nonresponses. We assessed participants’ characteristics, first according to arthritis status, and then by statin use. To compare the groups, t-tests were calculated for continuous measurements, and chi-square tests for categorical variables. We calculated the unadjusted prevalence of musculoskeletal pain in different body regions among those without and with arthritis.
To assess our primary outcomes, we obtained adjusted prevalence ratio estimates of musculoskeletal pain directly from the logistic regression modeling using the SUDAAN PREDMARG statement. This calculates the model-adjusted prevalence ratio based on estimates of the average marginal prediction for each level of a categorical variable or continuous variable, with confidence intervals obtained using the Taylor linearization method.11 Adjusted prevalence ratios and 95% confidence intervals are presented according to body regions. All analyses were adjusted for the following list of potential confounders that were specified a priori: age, sex, race/ethnicity, smoking status, health status, health status, coronary heart disease, ABI (≤.90, 91–.99, 1.00–1.29, ≥1.29, and missing), systolic blood pressure, diabetes, total cholesterol (segregated into quintiles), BMI, and cancer. To examine nonlinear effects of continuous variables, we included quadratic terms for age, BMI, and systolic blood pressure. Other factors were examined but not included in our final models because they were not associated with musculoskeletal pain and did not appear to confound the relation of statin use with musculoskeletal pain, including education, physical activity, and alcohol intake.
We evaluated whether the association between statin use and musculoskeletal pain was different among those with and without arthritis by including an interaction term between presence of arthritis and statin use in the final logistic regression model, and used a likelihood ratio test to examine its statistical significance. After confirming a significant interaction, we calculated the weighted mean of the marginal predicted probability for the overall effect of statin use in the entire population by fitting a model with the main effects of statin and arthritis and their interaction term. We used the SUDAAN statement PRED_EFF and the linear contrast for statin to obtain the weighted average statin effect and to account for the proportion of those with and without arthritis in the population. Due to the significant interaction we also present stratified analyses by arthritis status. We calculated the number needed to harm (NNH) as the reciprocal of the absolute difference in prevalence risk between statin use and no statin use for the primary outcome of musculoskeletal pain.
As a post hoc analysis, we evaluated whether use of prescription medications that might affect pain (non-steroidal anti-inflammatory drugs, opioids, corticosteroids or muscle relaxants), altered the results. To assess trends of statin use over the study period we included the data release year as a covariate in the model.
Statistical analyses were performed using SAS (version: 9.2; SAS Institute, Cary, NC) and SUDAAN (version 10.0.1; Research Triangle Institute, Research Triangle Park, NC).
Results
Among 8,228 NHANES participants aged 40 years and over (representing 113 million US individuals), including those with and without arthritis, 1,306 participants (representing 17 million US individuals) reported using a statin in the past 30 days. Statin use increased significantly over time, with prevalence of use in the last 30 days estimated to be 13%, 15%, and 18% for study periods 1999–2000, 2001–2002, and 2003–2004, respectively (p for trend <0.001).
In Table 1 we summarize characteristics of participants according to arthritis status and statin use. Among those with or without arthritis, individuals who used statins were older, more likely to be former smokers and reported poorer self-rated health, compared to non-users. Among those without arthritis, individuals who used statins were more likely to be male, while among participants with arthritis, those who used statins were more likely to be female.
Table 1.
Characteristics of the Study Participants, NHANES 1999–2004
| Without Arthritis, n=5170 | With Arthritis, n=3058 | |||||
|---|---|---|---|---|---|---|
| No Statin Use n=4,499 |
Statin Use n=671 |
p-value | No Statin Use n=2,423 |
Statin Use n=635 |
p-value | |
| Age, mean (years) | 52.9 | 61.1 | <0.001 | 60.6 | 65.2 | <0.001 |
| Female, % | 50.1 | 41.4 | 0.002 | 63.1 | 53.8 | 0.007 |
| Race/Ethnicity, % | <0.001 | <0.001 | ||||
| Non-Hispanic White | 75.0 | 82.9 | 79.6 | 86.0 | ||
| Non-Hispanic Black | 9.9 | 6.2 | 9.8 | 5.7 | ||
| Mexican American | 5.7 | 2.7 | 3.3 | 2.1 | ||
| Other | 9.4 | 8.1 | 7.3 | 6.2 | ||
| Education, % | 0.24 | 0.052 | ||||
| <High school diploma | 17.9 | 14.9 | 26.9 | 31.0 | ||
| High school diploma | 25.1 | 27.6 | 26.3 | 30.1 | ||
| >High school diploma | 57.0 | 57.4 | 46.8 | 38.9 | ||
| Physical activity status, % | 0.136 | 0.091 | ||||
| Sedentary | 36.8 | 35.3 | 47.2 | 44.6 | ||
| Moderate | 32.1 | 37.1 | 31.4 | 38.3 | ||
| Vigorous | 31.1 | 27.6 | 21.4 | 21.4 | ||
| Smoking status, % | <0.001 | 0.002 | ||||
| Never | 49.0 | 44.3 | 45.0 | 38.0 | ||
| Former | 29.1 | 41.3 | 34.4 | 45.5 | ||
| Current | 21.9 | 14.4 | 20.7 | 16.6 | ||
| Alcohol (drinks/day), % | 0.004 | 0.001 | ||||
| <1 | 59.9 | 67.8 | 68.3 | 79.8 | ||
| 1–2 | 34.9 | 30.1 | 27.7 | 17.8 | ||
| >2 | 5.2 | 2.1 | 4.0 | 2.5 | ||
| Self-reported health status, % | <0.001 | 0.004 | ||||
| Very good/excellent | 56.6 | 47.0 | 35.8 | 26.5 | ||
| Good | 29.7 | 31.7 | 31.9 | 34.4 | ||
| Fair/poor | 13.8 | 21.3 | 32.3 | 39.1 | ||
| Co-morbidities, % | ||||||
| Coronary heart disease | 4.1 | 25.7 | <0.001 | 10.8 | 35.2 | <0.001 |
| Diabetes | 6.6 | 21.0 | <0.001 | 12.5 | 29.3 | <0.001 |
| Cancer | 5.9 | 10.0 | 0.002 | 11.6 | 14.2 | 0.227 |
| Peripheral artery disease | 7.8 | 14.7 | <0.001 | 11.6 | 18.3 | <0.001 |
| Body mass index, mean (kg/m2) | 28 | 28.6 | 0.016 | 29.3 | 30.3 | 0.002 |
| Systolic blood pressure, mean (mm Hg) | 126.5 | 129.5 | 0.002 | 131.6 | 134.1 | 0.083 |
| Total Cholesterol, mean (mg/dL) | 213.6 | 193.1 | <0.001 | 214.7 | 196.7 | <0.001 |
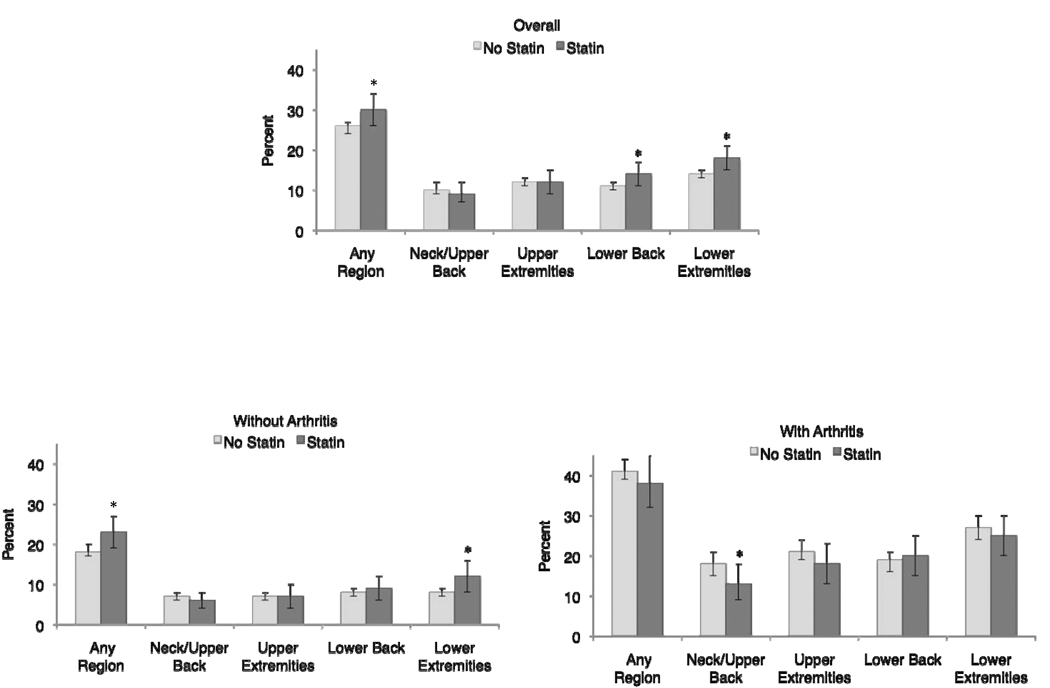
Figure 1 shows the estimated prevalence of musculoskeletal pain in different body regions during the last 30 days among adults 40 years and older, in the overall sample and in those with and without arthritis, according to statin use. In the overall sample, the unadjusted prevalence of musculoskeletal pain was significantly higher among statin users reporting pain in any region (30% among statin users, 95%CI: 26–34%, compared to 26% among those not using statins, 95%CI: 24–27%; p=0.007), in the lower extremities (18% among statin users, 95%CI: 15–21%, compared to 14% among those not using statins, 95%CI: 13–15%; p=0.008), and in the lower back (14% among statin users, 95%CI: 11–17%, compared to 11% among those not using statins, 95%CI: 10–12%; p=0.02).
Figure 1.
Unadjusted weighted prevalence estimates (percent) and 95% confidence intervals of any musculoskeletal pain and musculoskeletal pain at 4 different anatomical regions according to statin use among all participants (p=0.007, p=0.36, p=0.82, p=0.02, p=0.008, respectively), those without arthritis (p=0.02, p=0.40, p=0.88, p=0.31, p=0.02, respectively), and those with arthritis (p=0.32, p=0.04, p=0.23, p=0.69, p=0.45, respectively).
Among those without arthritis, the unadjusted prevalence of musculoskeletal pain was significantly higher for statin users reporting pain in any region (23% among statin users, 95%CI: 19–27% compared to 18% among those not using statins, 95%CI: 17–20%; p=0.02) and musculoskeletal pain in the lower extremities (12% among statin users, 95%CI: 8–16% compared to 8% among those not using statins, 95%CI: 7–9%; p=0.02).
Compared to those without arthritis, those with arthritis showed nearly twofold the prevalence of reported musculoskeletal pain in all regions on weighted, unadjusted analyses. However, statin use was not associated with higher musculoskeletal pain in any body region among those with arthritis, instead statin use was significantly associated with lower musculoskeletal pain in the neck/upper back region (13% among statin users, 95%CI: 9–18% compared to 18% among those not using statins, 95%CI: 15–21%; p=0.04).
In multivariable adjusted analyses, statin use was only associated with musculoskeletal pain among subjects without arthritis and the likelihood ratio test for the interaction between statin use and arthritis was highly statistically significant (p=0.0001). Because of this interaction, we estimated the average effect of statin on musculoskeletal pain in the overall population, taking into account the interaction and the proportion of those with arthritis in the population. In this analysis, the estimated adjusted marginal prevalence ratio was 1.15, with 95% CI 1.01–1.30, and NNH=25. We then stratified multivariable analyses by arthritis status. Table 2 provides multivariable-adjusted prevalence ratios and 95% confidence intervals for musculoskeletal pain according to arthritis status. After adjustment, statin use among those without arthritis remained significantly associated with higher prevalence of musculoskeletal pain, particularly in the lower extremities and lower back, with a NNH equal to 17 for musculoskeletal pain in any region.
Table 2.
Adjusted* Prevalence Ratios and 95% Confidence Intervals of the Effect of Statin Use on Musculoskeletal Pain, by Arthritis Status
| Region | Without Arthritis | With Arthritis |
|---|---|---|
| Any Region | 1.33 (1.06, 1.67) | 0.96 (0.81, 1.15) |
| Neck/Upper Back | 0.88 (0.53, 1.45) | 0.81 (0.61, 1.08) |
| Upper Extremities | 0.82 (0.49, 1.35) | 0.84 (0.62, 1.15) |
| Lower Back | 1.47 (1.02, 2.13) | 1.05 (0.81, 1.37) |
| Lower Extremities | 1.59 (1.12, 2.22) | 0.96 (0.76, 1.22) |
Adjusted for age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, others), smoking status (never, former, current), self reported health (excellent/very good, good, poor/fair), coronary heart disease, diabetes, cancer, systolic blood pressure (SBP and SBP2), body mass index (BMI and BMI2), total cholesterol (quintiles), and ankle brachial index (≤0.90, 0.91–0.98, 0.99–1.08, 1.09–1.29, ≥1.30, and missing).
Among those with arthritis, no association was observed between musculoskeletal pain and statin use. Additional adjustment for prescription pain medications did not materially alter the results in models among those with or without arthritis.
Discussion
In this cross-sectional study of 8,228 individuals representative of the general U.S. population, we showed that use of statins is associated with musculoskeletal pain among individuals without arthritis, but that statin use does not add to the pain burden of individuals with arthritis. Among those without arthritis, statin use is significantly associated with a 33% higher prevalence of reporting musculoskeletal pain in any region after adjusting for multiple confounders (NNH=17), with regions most commonly associated being the lower extremities and lower back. These findings are consistent with our prior study performed among those without arthritis,8 which showed higher prevalence of musculoskeletal pain in these same regions, and support previous studies in clinical settings showing pain associated with statin use to be more common in the lower than upper extremities.12–14These same regions have been noted to be affected in some other drug induced myopathies,15, 16 and could be due to these regions containing large muscles of the body, which carry greater weight, compared to upper body muscles.
Our study findings are in contrast with data from randomized clinical trials of statin therapy, which showed no significant association of statins with myalgia.17 This may be attributed to the differing demographics of participants in randomized clinical trials compared to patients using statins in the general population. While several studies have evaluated the association of myopathy and statin use,7, 17–19 myalgia, or more specifically muscle symptoms without significant elevations in CK, were rarely investigated in clinical trials.7
Among those with arthritis, our data did not show higher prevalence of musculoskeletal pain among statin users. Although we could not determine causal factors for our finding of effect modification of musculoskeletal pain and statin use by arthritis status, our finding may represent differences in clinical practices and/or pathophyisiologic processes related to arthritis and statin use. Arthritis commonly typifies a more prominent pain, and thus could mask statin-associated pain, hence mitigating the association between statin-related pain and arthritis. Furthermore, individuals with substantial musculoskeletal symptoms, and doctors who care for such patients, may refrain from starting a statin or prematurely stop statins due to fear of worsening musculoskeletal symptoms, and could represent a bias unique to this group that would appear as if those with arthritis not using statins had worse pain than those using statins. Lastly, it is possible that some statin users who develop musculoskeletal symptoms of the lower extremities or lower back as a side effect of their statin may have been incorrectly diagnosed with arthritis. However, this type of misclassification bias would increase the positive association between statin use and musculoskeletal pain in this subgroup.
Study limitations
Portions of our analyses rely on self-reported data including, most importantly, self-reported doctor or other health care provider’s diagnosis of arthritis. In clinical practice a presumptive diagnosis of arthritis, generally osteoarthritis, may be given to a patient who presents with joint pain.20, 21 Often little is pursued to confirm the diagnosis, which could lead to misclassification.20, 21 A previous validation study assessing “self-reported doctor's diagnosis of arthritis” against a standardized clinical history and physical examination showed that a self-reported doctor's diagnosis had 80% specificity and 53–70% sensitivity of identifying clinically significant arthritis,22 indicating greater reliability in the classification of having no arthritis than with the classification of having arthritis.
Other than region of pain, we lacked details on the characteristics of musculoskeletal pain. Obtaining information about severity, quality, and interference with activities due to musculoskeletal symptoms as well as site of arthritis would be important to include in future studies to better define and understand musculoskeletal pain associated with statin use.
In this study, those with arthritis used more prescription pain medications than those without arthritis, however, adjusting our models for use of these medications did not change the results of our findings. We had limited ability to fully adjust for over-the-counter analgesic use, as questions asked about these medications did not describe reasons for use. Therefore, we could not differentiate between aspirin use for analgesia versus cardiovascular disease prevention, nor acetaminophen or ibuprofen use for musculoskeletal pain versus use in a combination cold/flu treatment.
Our study is further limited by its cross sectional design and therefore cannot address causality nor account for those who may have previously started and stopped a statin due to musculoskeletal pain. The sample is representative of the U.S. population with the majority of participants self reporting non-Hispanic-white race/ethnicity. However, being of Asian ancestry was not collected. Such information would be of interest, as studies have shown this group may be at higher risk for statin musculoskeletal side effects compared to other groups.23, 24 Lastly, despite attempts to adjust for several important confounders, our results in this observational study could be limited by residual and unmeasurable confounding
Conclusions
In summary, in this population based cross sectional study, we found that among those without arthritis, statins are associated with a higher prevalence of musculoskeletal pain, particularly in the lower extremities. These data did not demonstrate an association of a higher prevalence of musculoskeletal pain among those with arthritis.
Acknowledgments
Funding: This study was supported by Grant Number K23AR055664 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors of this study had no conflicts of interest as pertaining to this study. No pharmaceutical industry funds were received for the preparation of this manuscript or any related research.
Authorship: All the authors verify having access to the data and have directly participated in the planning, execution, or analysis of this study.
Contributor Information
Catherine Buettner, Instructor of Medicine, Harvard Medical School, Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215, Phone: 617-735-2461, Phone: 617-735-2833, cbuettne@bidmc.harvard.edu.
Matthew J. Rippberger, Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215.
Julie K. Smith, Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215.
Suzanne G. Leveille, Professor, College of Nursing and Health Sciences, University of Massachusetts Boston, 100 Morrissey Blvd, Boston, MA 02125-3393, Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215.
Roger B. Davis, Associate Professor, Department of Biostatistics, Harvard School of Public Health, Associate Professor of Medicine, Harvard Medical School, Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215.
Murray A. Mittleman, Associate Professor, Harvard School of Public Health, Associate Professor of Medicine, Harvard Medical School, Director, Cardiovascular Epidemiology Research Unit, Beth Israel Deaconess Medical Center, 375 Longwood Avenue, Boston, MA 02215.
References
- 1.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. Journal of the American College of Cardiology. 2006;48(3):438. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 2.Cheung BMY, Lauder IJ, Lau CP, Kumana CR. Meta analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. British journal of clinical pharmacology. 2004;57(5):640–651. doi: 10.1111/j.1365-2125.2003.02060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonarow GC, Watson KE. Effective strategies for long-term statin use. The American journal of cardiology. 2003;92(1):27–34. doi: 10.1016/s0002-9149(03)00506-x. [DOI] [PubMed] [Google Scholar]
- 4.Maggon K. Best-selling human medicines 2002–2004. Drug Discovery Today. 2005;10(11):739–742. doi: 10.1016/S1359-6446(05)03468-9. [DOI] [PubMed] [Google Scholar]
- 5.Mann D, Reynolds K, Smith D, Muntner P. Trends in Statin Use and Low-Density Lipoprotein Cholesterol Levels Among US Adults: Impact of the 2001 National Cholesterol Education Program Guidelines (September) The Annals of pharmacotherapy. 2008 doi: 10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 6.McKenney JM, Davidson MH, Jacobson TA, Guyton JR. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006 Apr 17;97(8A):89C–94C. doi: 10.1016/j.amjcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003 Apr 2;289(13):1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 8.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. Journal of general internal medicine. 2008;23(8):1182–1186. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health and Nutrition Examination Survey. [Accessed February 2, 2011];Centers for Disease Control and Prevention. http://www.cdc.gov/nchs/nhanes/about_nhanes.htm, accessed February 2, 2011. [ http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 10.National Health and Nutrition Examination Survey, NCHS Research Ethics Review Board (ERB) [accessed February 2, 2011]; Approval, http://www.cdc.gov/nchs/nhanes/irba98.htm.
- 11.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. American journal of epidemiology. 2010;171:618–623. doi: 10.1093/aje/kwp440. [DOI] [PubMed] [Google Scholar]
- 12.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients the PRIMO study. Cardiovascular Drugs and Therapy. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 13.Franc S, Dejager S, Bruckert E, Chauvenet M, Giral P, Turpin G. A comprehensive description of muscle symptoms associated with lipid-lowering drugs. Cardiovasc Drugs Ther. 2003 Sep–Nov;17(5–6):459–465. doi: 10.1023/b:card.0000015861.26111.ab. [DOI] [PubMed] [Google Scholar]
- 14.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005 Dec 12–26;165(22):2671–2676. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 15.Frieze DA. Musculoskeletal pain associated with corticosteroid therapy in cancer. Curr Pain Headache Rep. Aug;14(4):256–260. doi: 10.1007/s11916-010-0120-z. [DOI] [PubMed] [Google Scholar]
- 16.Kuncl RW, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. N Engl J Med. 1987 Jun 18;316(25):1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 17.Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006 Dec 19;114(25):2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 18.Silva M, Matthews ML, Jarvis C, et al. Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy. Clinical therapeutics. 2007;29(2):253–260. doi: 10.1016/j.clinthera.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clinical therapeutics. 2006;28(1):26–35. doi: 10.1016/j.clinthera.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Ladouceur M, Rahme E, Pineau CA, Joseph L. Robustness of prevalence estimates derived from misclassified data from administrative databases. Biometrics. 2007;63(1):272–279. doi: 10.1111/j.1541-0420.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 21.Losina E, Barrett J, Baron JA, Katz JN. Accuracy of Medicare claims data for rheumatologic diagnoses in total hip replacement recipients. Journal of clinical epidemiology. 2003;56(6):515–519. doi: 10.1016/s0895-4356(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 22.Sacks JJ, Harrold LR, Helmick CG, Gurwitz JH, Emani S, Yood RA. Validation of a surveillance case definition for arthritis. J Rheumatol. 2005 Feb;32(2):340–347. [PubMed] [Google Scholar]
- 23.FDA Drug Safety Communication. Ongoing safety review of high-dose Zocor (simvastatin) and increased risk of muscle injury, Postmarket Drug Safety Information for Patients and Providers. [accessed February 2, 2011]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm204882.htm.
- 24.FDA Public Health Advisory for Crestor (rosuvastatin) Postmarket Drug Safety Information for Patients and Providers. [accessed February 2, 2011]; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051756.htm.