Abstract
In view of the importance of Candida drug resistance protein (Cdr1p) in azole resistance, we have characterized it by overexpressing it as a green fluorescent protein (GFP)-tagged fusion protein (Cdr1p-GFP). The overexpressed Cdr1p-GFP in Saccharomyces cerevisiae is shown to be specifically labeled with the photoaffinity analogs iodoarylazidoprazosin (IAAP) and azidopine, which have been used to characterize the drug-binding sites on mammalian drug-transporting P-glycoproteins. While nystatin could compete for the binding of IAAP, miconazole specifically competed for azidopine binding, suggesting that IAAP and azidopine bind to separate sites on Cdr1p. Cdr1p was subjected to site-directed mutational analysis. Among many mutant variants of Cdr1p, the phenotypes of F774A and ΔF774 were particularly interesting. The analysis of GFP-tagged mutant variants of Cdr1p revealed that a conserved F774, in predicted transmembrane segment 6, when changed to alanine showed increased binding of both photoaffinity analogues, while its deletion (ΔF774), as revealed by confocal microscopic analyses, led to mislocalization of the protein. The mislocalized ΔF774 mutant Cdr1p could be rescued to the plasma membrane as a functional transporter by growth in the presence of a Cdr1p substrate, cycloheximide. Our data for the first time show that the drug substrate-binding sites of Cdr1p exhibit striking similarities with those of mammalian drug-transporting P-glycoproteins and despite differences in topological organization, the transmembrane segment 6 in Cdr1p is also a major contributor to drug substrate-binding site(s).
Candida albicans is an opportunistic diploid fungus that causes infections in immunocompromised and debilitated patients (34). Widespread and prolonged usage of azoles in recent years has led to the rapid development of the phenomenon of multidrug resistance (MDR), which poses a major hurdle in antifungal therapy. Various mechanisms which contribute towards the development of MDR have been implicated in Candida, and some of these include overexpression of or mutations in the target enzyme of azoles, lanosterol 14α-demethylase, and overexpression of drug efflux pumps (1, 33) belonging to the ATP-binding cassette (ABC) transporter channel superfamily (18) and to the major facilitator superfamily of transporters (MFS) (22, 35). Among ABC transporters, CDR1 has been shown to play a key role in azole resistance in C. albicans as deduced from its high level of expression found in several azole resistance clinical isolates recovered from patients receiving long-term antifungal therapy (41, 39). Additionally, high-level expression of CDR1 invariably contributes to an increased efflux of fluconazole, thus corroborating its direct involvement in drug efflux (24, 38). Cdr1p has not only acquired significant clinical importance but is considered an important player in any design of strategies to combat antifungal resistance.
The CDR1 gene encodes an integral plasma membrane (PM) protein of 1,501 amino acids, with a predicted molecular mass of 169.9 kDa. On the basis of its amino acid sequence, Cdr1p is predicted to consist of two homologous halves, each comprising one N-terminal hydrophilic domain followed by a C-terminal hydrophobic domain. The hydrophilic domain comprised a conserved ABC region, including the ATP-binding motifs known as the Walker A and Walker B motifs (48) and another highly conserved motif, ABC signature, preceding the Walker B motif (36). Cdr1p has a similar topology to its close homologues Pdr5p and Snq2p of Saccharomyces cerevisiae (36).
According to our current understanding, Cdr1p and Cdr2p drug extrusion proteins not only efflux azoles and its derivatives but also extrude a variety of structurally unrelated drugs. Overexpression of homologous ABC multidrug transporter proteins, human P-glycoprotein (P-gp) or the MDR-associated protein 1 (MRP1) is also responsible for the molecular basis of the MDR phenotype in tumor cells (3). The molecular mechanisms which govern Cdr1p functions are not well-known, and information is needed (i) to understand how the protein can bind a structurally diverse range of compounds, (ii) to define drug substrate binding, and (iii) to determine how ATP binding and hydrolysis are linked to drug transport.
In an effort to develop an understanding of the molecular details of drug binding and the importance of domains in Cdr1p, in this study we have overexpressed Cdr1p as a green fluorescent protein (GFP)-tagged fusion protein (Cdr1p-GFP) in a heterologous system and for the first time characterized it for drug and nucleotide binding. The GFP-tagged Cdr1p was functionally similar to its untagged version, as it imparted drug resistance to S. cerevisiae cells, showed ATPase activity, and effluxed Cdr1p substrates, such as rhodamine 6G. Photoaffinity P-gp substrate analogues were used to assess the drug substrate sites of Cdr1p. For this, we used iodoarylazidoprazosin (IAAP, a photoaffinity analogue of the P-gp substrate, prazosin) and azidopine (a dihydropyridine photoaffinity analogue), which are known to bind specifically to the human and murine drug transporting P-gps. Our study demonstrates that both IAAP and azidopine bind specifically to Cdr1p-GFP. Interestingly, IAAP binding was competed out by nystatin, while azidopine binding could only be competed out by miconazole, thus demonstrating the possibility of different drug-binding sites for the two analogues.
For functional analysis, mutations were introduced in predicted nucleotide-binding domain 1 (NBD1), transmembrane segment 6 (TMS6), NBD2, cytoplasmic loop 5 (CL5), and extracellular loop 6 (EL6). On the basis of phenotypic analysis, two mutants in TMS6 (F774A and ΔF774) were subjected to detailed analysis by overexpressing them as GFP-tagged proteins. Of note, the substitution of F774 with A in TMS6 resulted in a selective loss of functional activity of the mutated protein, while a deletion of the same phenylalanine (ΔF774) led to improper localization of the mutant protein. Our results further show that if mutant cells in which F774 had been deleted were grown in the presence of cycloheximide, it led to the appearance of a functional transporter at the cell surface. It appears that F774 in TMS6 is important for the localization of Cdr1p.
MATERIALS AND METHODS
Materials.
Anti-GFP monoclonal antibody was purchased from BD Biosciences Clontech, Palo Alto, Calif. DNA-modifying enzymes were purchased from Roche Molecular Biochemicals. Protease inhibitors, miconazole, nystatin, cycloheximide, anisomycin, rhodamine 6G, and other molecular grade chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.). The radiolabeled [125I]IAAP (2,200 Ci/mmol) was from Perkin-Elmer Life Sciences (Boston, Mass.). [α-32P]8-azido-ATP (15 to 20 Ci/mmol) was from Affinity Labeling Technologies, Inc. (Lexington, Ky.), and [3H]azidopine (60 Ci/mmol) was from Amersham Biosciences (Arlington Heights, Ill.). The [α-32P]8-azido-ATP showed no detectable contaminating [α-32P]8-azido-ADP using thin-layer chromatography with 0.8 M LiCl as the solvent. Fluconazole was kindly provided by Pfizer (Sandwich, Kent, United Kingdom) and Ranbaxy Laboratories, New Delhi, India.
Bacterial and yeast strains and growth media.
Plasmids were maintained in Escherichia coli XL-1 blue. E. coli was cultured in Luria-Bertani medium (Difco, BD Biosciences) to which ampicillin was added (100 μg/ml). The S. cerevisiae strains used were AD1234568 (provided by A Goffeau, Université Catholique de Louvain in Belgium), AD1-8u−, and AD1002 (provided by Richard D. Cannon, University of Otago, Dunedin, New Zealand). SS1 to SS18 were AD1234568 derivatives expressing mutant Cdr1ps (Table 1). PSCDR1-GFP, SS5G (F774A), and SS6G (ΔF774) were AD1-8u− derivatives expressing Cdr1p-GFP and its mutant proteins (mutant Cdr1p-GFPs) (Table 1). The yeast strains were cultured in yeast extract-peptone-dextrose (YEPD) broth (Bio 101, Vista, Calif.) or SD-URA medium (Bio 101). For agar plates, 2% (wt/vol) Bacto agar (Difco, BD Biosciences) was added to the medium.
TABLE 1.
Strains used in this study
| Strain | Genotype | Strain derivation | Reference |
|---|---|---|---|
| AD1234568 | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq2::hisG Δpdr5::hisG Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr15::hisG | Decottignies et al. (9) | |
| AD-CDR1 | AD1234568 transformant carrying cloned CDR1 with its native promoter in pYEURA3 (centromeric) vector | AD1234568 | This study |
| S6-20 | AD1234568 transformant carrying only the pYEURA3 (centromeric) vector | AD1234568 | This study |
| AD1-8u− | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq2::hisG Δpdr5::hisG Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG | Nakamura et al. (33) | |
| AD1002 | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq2::hisG Δpdr5::PDR5PROM-CDR1-CDRISTOP Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG | Nakamura et al. (33) | |
| PSCDR1-GFP | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq2::hisG Δpdr5::PDR5PROM-CDR1-GFP-GFPSTOP Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG | AD1-8u− | This study |
| SS1 to SS18 | AD1234568 transformant carrying cloned mutant CDR1 (SS1 to SS18 as described in the legend to Fig. 3b) with its native promoter in pYEURA3 (centromeric) vector | AD1234568 | This study |
| SS5G (F774A) | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq::hisG Δpdr5::PDR5PROM-CDR1(F774A)-GFP-GFPSTOP Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG | AD1-8u− | This study |
| SS6G (ΔF774) | MATapdr1-3 his1 ura3 Δyor1::hisG Δsnq::hisG Δpdr5::PDR5PROM-CDR1(ΔF774)-GFP-GFPSTOP Δpdr10::hisG Δpdr11::hisG Δycf1::hisG Δpdr3::hisG Δpdr15::hisG | AD1-8u− | This study |
Generation of polyclonal antibody to Cdr1p.
The peptide CQSNKISKKEKDDYVDY (amino acids 965 to 979, part of the putative NBD2), which represented the predicted most antigenic epitope of Cdr1p, was commercially synthesized and conjugated to keyhole limpet hemocyanin by Princeton Biomolecules. The antisera were raised in a New Zealand White rabbit after injecting 250 μg of conjugated peptide and giving a booster dose after 2 months. The antiserum was collected after 2 months (done by Covance Research Products, Inc.) and used at a dilution of 1:500 for Western blot analysis.
Molecular cloning.
A GFP tag was attached at the C-terminal end of CDR1. The GFP open reading frame (ORF) was amplified from plasmid pGFP31 (31) (a kind gift from Joachim Morschhauser, Zentrum für Infectionsforschung, Universität Würzburg, Würzburg, Germany) using the following primers. The forward primer was 5′-ACGCGTCGACATGAGTAAGGGAGAAGAA-3′ (the SalI site is shown in bold type), and the reverse primer was 5′-ACGCGTCGACGGACTAGTTTATTTGTATAGTTCATCCA-3′ (the SalI site is shown in bold type, and the SpeI site is shown in bold italic type). This GFP amplicon was digested with SalI restriction enzyme. The stop codon of CDR1 was replaced with a SalI site in p425-GPD-CDR1 (kind gift from Martine Raymond, Institut de Recherches Cliniques de Montréal, Montréal, Québec, Canada). It was then digested with SalI, and the digested GFP amplicon was ligated to the C-terminal end of CDR1. The resulting CDR1-GFP ORF was taken out of the vector p425-GPD-CDR1GFP by SpeI restriction enzyme and cloned in the vector pSK-PDR5 PPUS (33) (kind gift from R. D. Cannon) at the SpeI site resulting in plasmid pPSCDR1-GFP. After every cloning, the orientation was checked by restriction enzyme digestion and sequencing using the Big Dye Terminator cycle sequencing kit (ABI) and ABI 310 DNA sequencer.
Site-specific mutagenesis and development of transformants.
Site-directed mutagenesis was performed by using the QuikChange mutagenesis system from Stratagene (La Jolla, Calif.). The mutations were introduced into plasmid pS12-35 and pPSCDR1-GFP according to the manufacturer's instructions, and the desired nucleotide sequence alterations were confirmed by DNA sequencing of the ORF. The mutated plasmid pS12-35 or the mutated pPSCDR1-GFP after linearizing with XbaI was used to transform AD1234568 or AD1-8u− cells, respectively, for uracil prototrophy by the lithium acetate transformation protocol (2).
Genomic DNA extraction and Southern analysis of CDR1 gene in S. cerevisiae.
Plasmid or genomic DNA was isolated from S. cerevisiae cells as described previously (19, 47). Genomic DNA was digested with restriction endonuclease (EcoRV, BamHI, and PstI; Roche Biochemicals). Plasmid DNA (5 μg) or digested genomic DNA (10 μg) was separated on a 1% agarose gel and transferred to a Hybond+ nylon membrane (Amersham). Membranes were hybridized with [α-32P]dATP-labeled CDR1-specific probe (ORF nucleotides 1 to 280) under high-stringency conditions (40).
Immunodetection of Cdr1p in PM.
Crude membranes (CM) were prepared from S. cerevisiae cells grown in YEPD to late exponential phase. The cells were broken with glass beads by vortexing the cells four times for 30 s each time, followed by a 30-s interval on ice. The homogenization medium contained 50 mM Tris (pH 7.5), 2.5 mM EDTA, and the protease inhibitor cocktail (1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, pepstatin A, and aprotinin). The CM were recovered by centrifuging at 1, 000 × g to remove unbroken cells and pelleting the CM by ultracentrifugation at 100,000 × g for 1 h. The CM were resuspended in resuspension buffer (10 mM Tris [pH 7.5], 0.5 mM EDTA, 10% glycerol). The CM were applied to a discontinuous gradient made of an equal volume of 53.5% (wt/vol) sucrose and 43.5% (wt/vol) sucrose. Following centrifugation for 5 h at 100,000 × g in a Beckman SW 28 rotor, the purified PMs were recovered at the interface of the 43.5 and 53.5% sucrose layers by the method of Monk et al. (30). The protein samples (20 μg) were separated on sodium dodecyl sulfate (SDS)-8% polyacrylamide gels and either stained with colloidal Coomassie blue G250 or electroblotted (40 V, 1 h, 4°C) onto nitrocellulose membranes (Invitrogen Life Technologies). The membranes were incubated with anti-Cdr1p polyclonal antibody (diluted 1:500), anti-Pma1p polyclonal antibody (diluted 1:10,000), or anti-GFP monoclonal antibody (diluted 1:1,000). Immunoreactivity of Cdr1p and Pma1p antibodies was detected with horseradish peroxidase (HRP)-labeled goat anti-rabbit antibody, which was diluted 1:20,000 in 20% fat-free milk. Immunoreactivity of GFP antibody was detected with an anti-mouse antibody, which was diluted 1:10,000 in 5% fat-free milk. Proteins on immunoblots were visualized using the enhanced chemiluminescence assay system (ECL kit; Amersham Biosciences).
Drug susceptibility of S. cerevisiae.
The susceptibilities of S. cerevisiae cells to different drugs were determined by microtiter and spot assays as described earlier (32).
ATPase assay.
Cdr1p-associated ATPase activity of the purified PM was measured as an oligomycin-sensitive release of inorganic phosphate. Membrane suspension (10 μg of PM protein as determined by amido black B protein estimation) (46) was incubated at 30°C in 0.1 ml of a medium containing 59 mM Tris (pH 7.5) and 7 mM MgCl2 (ATPase assay buffer) and 20 μM oligomycin where indicated. To eliminate possible contributions from nonspecific phosphatases and vacuolar and mitochondrial ATPases, 0.2 mM ammonium molybdate, 50 mM KNO3, and 10 mM NaN3, respectively, were included in the reaction mixture. The reaction was started by the addition of 5 mM ATP and was stopped by the addition of 0.1 ml of 5% SDS solution. The amount of inorganic phosphate released was immediately determined as described previously (42).
Rhodamine 6G efflux.
The efflux of rhodamine 6G was determined essentially by a previously described protocol (29). Approximately 107 cells from a culture grown overnight were inoculated in 250 ml of YEPD and grown for 5 h at 30°C. The cells were pelleted and washed three times with phosphate-buffered saline (PBS) without glucose. The cells were subsequently resuspended as a 2% cell suspension in PBS to which rhodamine 6G was added to a final concentration of 10 μM and incubated for 2 h at 30°C. The cells were then washed and resuspended in PBS with 2% glucose. An aliquot of 1 ml was taken after 45 min and centrifuged at 9,000 × g for 2 min. The absorbance of the supernatant was measured at 527 nm.
Confocal microscopy.
The cells were grown in SD-URA medium to late log phase. The drugs were added 4 h after the inoculation as indicated. The cells were washed and resuspended in an appropriate volume of 50 mM HEPES (pH 7.0). The cells were placed on the glass slides, and a drop of antifade reagent (Fluoroguard high-performance antifade reagent; Bio-Rad, Hercules, Calif.) was added to prevent photobleaching. The cells were directly viewed with a Bio-Rad confocal microscope (MRC 1024) with a 100× oil immersion objective.
Flow cytometry of the cells.
Flow cytometric analysis of the Cdr1p-GFP-carrying S. cerevisiae cells was performed with a FACSort flow cytometer (Becton-Dickinson Immunocytometry Systems, San Jose, Calif.). Cells were grown to mid-log phase, and 106 cells were harvested and washed with 50 mM HEPES (pH 7.0). Cells were resuspended in 500 μl of 50 mM HEPES (pH 7.0). Ten thousand cells were analyzed in acquisition. Analysis was performed with CellQuest software (Becton-Dickinson Immunocytometry Systems). The mean fluorescence intensity was calculated using the histogram stat program.
Photoaffinity labeling with IAAP.
The PM proteins (15 μg) were incubated with the indicated competing drug for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). The samples were brought to room temperature, and 3 to 6 nM [125I]IAAP (2,200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp assembly (PGC Scientifics, Gaithersburg, Md.) fitted with two black light (self-filtering) UV-A long-wavelength F15T8BLB tubes (365-nm wavelength) for 10 min at room temperature (21 to 23°C). Following SDS-polyacrylamide gel electrophoresis (SDS-PAGE) on an 8% Tris-glycine gel at constant voltage, gels were dried and exposed to Bio-Max MR film (Eastman Kodak, Rochester, N.Y.) at −80°C for 12 to 24 h. The radioactivity incorporated into the Cdr1p band was quantified using the STORM 860 PhosphorImager system (Molecular Dynamics, Sunnyvale, Calif.) and the software ImageQuaNT as described previously (44).
Photoaffinity labeling with [3H]azidopine.
The PM proteins (30 μg) were incubated with the indicated competing drug for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). The samples were brought to room temperature and treated with 0.5 μM [3H]azidopine (60 Ci/mmol) for 5 min and then photo-cross-linked at 365 nm at room temperature (21 to 23°C) for 10 min, and 5× SDS sample buffer was added. Following electrophoresis, the gel was incubated with Fluro-Hance (Research Products Inc.) for 30 to 45 min and dried under vacuum, and the dried gel was exposed to an X-ray film for 3 to 6 days at −80°C. The radioactivity incorporated into the Cdr1p band was quantified as described above.
Binding of [α-32P]8-azido-ATP.
The PM protein (15 μg) was incubated in the ATPase assay buffer containing 10 μM [α-32P]8-azido-ATP (10 μCi/nmol) in the dark at 4°C for 5 min in the presence or absence of 10 mM ATP. The samples were then illuminated with a UV lamp assembly (365-nm wavelength) for 10 min on ice (4°C) as described previously (45). Following SDS-PAGE on an 8% Tris-glycine gel at constant voltage, the gels were dried and exposed to Bio-Max MR film at −80°C for 12 to 24 h. The radioactivity incorporated into the Cdr1p band was quantified as described above.
RESULTS
The Candida drug resistance protein (Cdr1p), a member of the ABC superfamily, causes MDR by an active efflux mechanism, which keeps the intracellular level of antimycotic compounds, such as azoles, below a cell-killing threshold. Similar to other well-characterized multidrug ABC transporters, Cdr1p (∼170 kDa) comprises two NBDs and 12 TMSs. NBDs are the hub of ATP hydrolysis activity and are considered critical for ABC protein-mediated drug efflux. In an attempt to functionally characterize the Cdr1p in terms of drug and nucleotide binding and the importance of domains in drug interactions, in this study we have overexpressed Cdr1p as a GFP-tagged protein. The functional characterization of wild-type Cdr1p-GFP and of the mutant variants generated by site-directed mutagenesis are the subject of the following experiments.
Overexpressed Cdr1p-GFP is fully functional.
In order to confirm the PM localization of Cdr1p and for biochemical characterization, we used an expression system developed by Nakamura et al. (33), which was a generous gift from R. D. Cannon, where Cdr1p was stably overexpressed from a genomic PDR5 locus in an S. cerevisiae mutant, AD1-8u−. The AD1-8u− mutant was derived from a pdr1-3 mutant strain with a gain-of-function mutation in the transcription factor Pdr1p, resulting in a constitutive hyperinduction of the PDR5 promoter. The high-level expression of Cdr1p was obtained by integration of the CDR1 ORF to the PDR5 promoter in AD1-8u−, and the resulting strain was designated AD1002 (33). We tagged the GFP gene at the C-terminal end of CDR1, which was overexpressed as a fusion protein in the PSCDR1-GFP strain (Fig. 1a). A single-copy integration of GFP-tagged CDR1 was ensured by Southern hybridization of restricted genomic DNA with a CDR1-specific probe as described earlier (33) (data not shown).
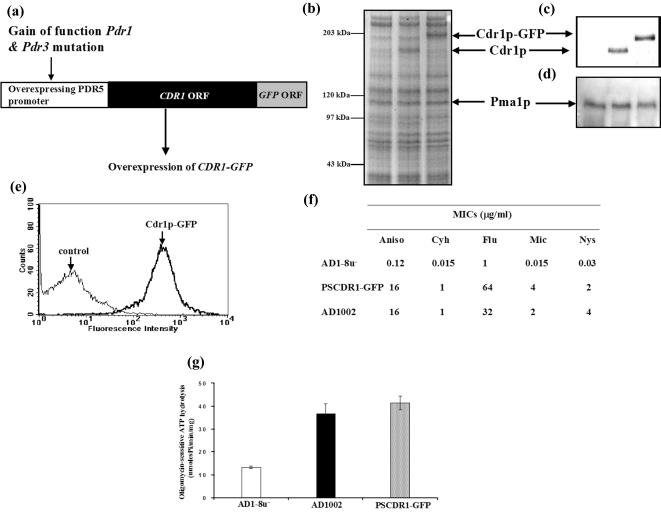
FIG. 1.
Comparison of Cdr1p and Cdr1p-GFP localization and functions. (a) Cartoon showing the overexpression cassette of Cdr1p-GFP. A gain-of-function mutation in the transcription factor genes PDR1 and PDR3 resulted in a constitutive hyperinduction of the PDR5 promoter. CDR1 with a GFP tag at the C-terminal end was integrated under the control of this promoter, which resulted in the overexpression of Cdr1p-GFP. (b and c) Expression of Cdr1p and Cdr1p-GFP in S. cerevisiae AD1-8u− cells. The PM proteins (20 μg) from AD1-8u−, AD1002 (wild-type Cdr1p), and PSCDR1-GFP (Cdr1p-GFP) cells were separated on an SDS-8% polyacrylamide gel (b), stained with colloidal Coomassie blue G-250 (c), electroblotted onto a nitrocellulose membrane and incubated with rabbit polyclonal anti-Cdr1p antibody (diluted 1:500). Proteins were detected with HRP-labeled anti-rabbit immunoglobulin using an ECL kit. (d) Expression of Pma1p. The PM proteins (20 μg) from AD1-8u−, AD1002 (wild-type Cdr1p), and PSCDR1-GFP (Cdr1p-GFP) cells were separated on an SDS-8% polyacrylamide gel and probed with rabbit polyclonal anti-Pma1p antibody (diluted 1:10,000) to detect Pma1p expression as described above. The position of the corresponding 100-kDa band of Pma1p on the gel is marked by an arrow. (e) Flow cytometry of cells expressing Cdr1p-GFP. AD1-8u− (control) and PSCDR1-GFP (Cdr1p-GFP) cells were grown to mid-log phase and used for FACS analysis as detailed in Materials and Methods. Analysis was performed with CellQuest software as described previously (44). (f) Drug resistance profiles of AD1-8u−, PSCDR1-GFP, and AD1002 cells. The MIC80s (in micrograms per milliliter) of the indicated drugs, anisomycin (Aniso), cycloheximide (Cyh), fluconazole (Flu), miconazole (Mic), and nystatin (Nys), for AD1-8u−, PSCDR1-GFP (Cdr1p-GFP), and AD1002 (wild-type Cdr1p) cells were determined as described in Materials and Methods. The results are typical of one determination, which was confirmed by three independent experiments. (g) Oligomycin-sensitive Cdr1p ATPase activity in purified PMs from AD1-8u−, PSCDR1-GFP (Cdr1p-GFP), and AD1002 (wild-type Cdr1p) cells. ATPase assay was performed by using PM proteins (100 μg/ml) of AD1-8u−, AD1002, and PSCDR1-GFP cells at 30°C for 10 min as described in Materials and Methods. The oligomycin-sensitive activity was determined as the difference in the ATPase activity in the presence or absence of 20 μM oligomycin. The values are given as the means ± standard deviations (error bars) for three independent experiments.
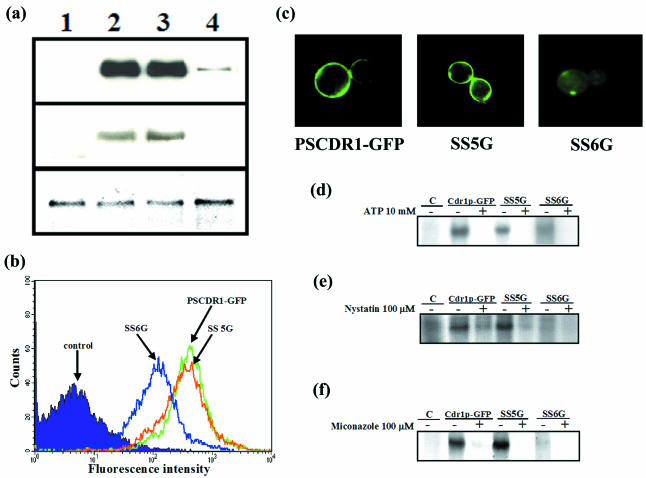
The presence of larger amounts of Cdr1p and Cdr1p-GFP in the purified PM fractions of AD1002 and PSCDR1-GFP cells could be detected on an SDS-polyacrylamide gel when stained by Coomassie brilliant blue G250 dye (Fig. 1b), which was further confirmed by Western blot analysis of the PM proteins using Cdr1p polyclonal antibody. Figure 1c confirms that the Cdr1p and Cdr1p-GFP were expressed in PM fractions to the same levels. There was an expected ∼30-kDa difference in the observed molecular mass of Cdr1p-GFP compared to that of Cdr1p because of the GFP tag attached to the C-terminal end of Cdr1p (Fig. 1c). The Pma1 antibodies were used as a PM marker to check the purity of the purified membranes (Fig. 1d). The intrinsic fluorescence of GFP imparted to the fusion protein also enabled us to detect the wild-type and mutant Cdr1p proteins in live cells. Cdr1p-GFP expression at the cell periphery also confirmed that the GFP tagging of Cdr1p did not interfere with its localization (data not shown). The expression of Cdr1p-GFP was further confirmed by fluorescence-activated cell sorting (FACS) analysis in which Cdr1p-GFP variant cells showed enhanced fluorescence intensity (Fig. 1e).
We ensured that GFP tagging and overexpression did not impair functional activity by analyzing drug susceptibilities of cells harboring CDR1 or the GFP-tagged variant. It was found that the cells expressing Cdr1p or Cdr1p-GFP had similar levels of resistance to the drugs tested (Fig. 1f). The purified PM from PSCDR1-GFP cells containing Cdr1p-GFP also showed oligomycin-sensitive ATPase activity which was almost threefold higher (and comparable to the activity of overexpressed Cdr1p) than the activity of the PM protein isolated from the AD1-8u− control cells (Fig. 1g). Taken together, it was thus established that overexpressing Cdr1p and its GFP-tagged variant is properly localized and functional. In the following experiments, these proteins were further examined for drug- and nucleotide-binding properties.
Photoaffinity analogues of ATP and drug substrates bind to Cdr1p.
Cdr1p utilizes ATP as an energy source by catalyzing its hydrolysis and coupling the energy released to the efflux of the drugs. Therefore, the binding of ATP to this protein can also be checked as a means of determining the functionality of this protein. The photoaffinity analogue, [α-32P]8-azido-ATP, has been used routinely for studying the interactions of nucleotide with the human P-gp, MRP1, and other ABC transporter proteins (4, 12, 45). The binding of [α-32P]8-azido-ATP to Cdr1p was examined to see the interactions of ATP with the protein as detailed in Materials and Methods. Figure 2a shows that the incubation of purified PM proteins (15 μg) from AD1-8u−, AD1002, and PSCDR1-GFP cells with 10 μM [α-32P]8-azido-ATP (10 μCi/nmol) followed by UV irradiation led to covalent incorporation of this analogue (Fig. 2a, lane 3). The labeling was competed out by 1,000-fold excess ATP (10 mM), confirming that the observed binding was specific (Fig. 2a, lane 4). The binding of [α-32P]8-azido-ATP to the Cdr1p-GFP variant protein was similar to that of native Cdr1p, which was also competed out by excess ATP (Fig. 2a, lanes 5 and 6). The control PM fraction from AD1-8u− cells did not show any band at the corresponding position (Fig. 2a, lanes 1 and 2). The specificity of 8-azido-ATP-, IAAP-, and azidopine-labeled bands (described below) corresponding to Cdr1p and Cdr1p-GFP in Fig. 2 was ensured by Western blot analysis. The PM fraction labeled with either photoreactive analogue and/or substrate was run on a gel, which was then transferred to a nylon membrane. The blot was probed with Cdr1p antibodies, and the same blot was used to expose X-ray film. The film was overlaid with the blot to confirm that the bands corresponded to Cdr1p and Cdr1p-GFP (data not shown). The binding of 8-azido-ATP analogue confirmed that Cdr1p or its variant Cdr1p-GFP overexpressed in this heterologous system is functionally active in terms of its ability to bind ATP.
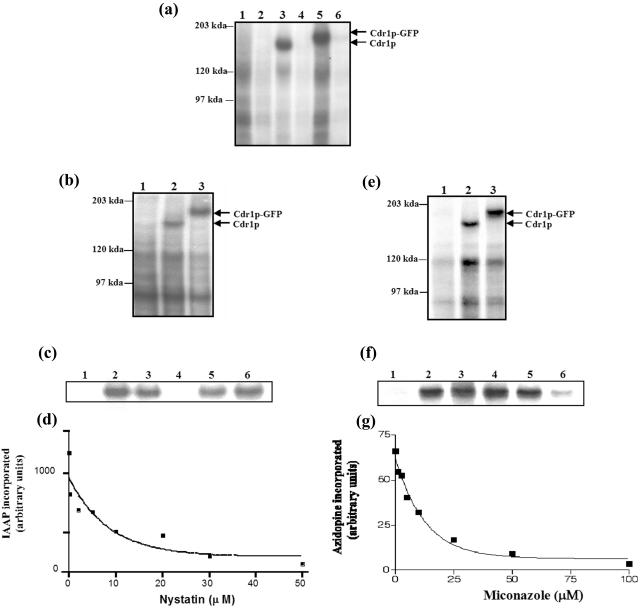
FIG. 2.
[α-32P]8-azido-ATP, [125I]IAAP, and [3H]azidopine bind specifically to Cdr1p and Cdr1p-GFP. (a) Binding of [α-32P]8-azido-ATP to Cdr1p and Cdr1p-GFP. The PM proteins (300 μg/ml) were photoaffinity labeled with 10 μM [α-32P]8-azido-ATP (10 μCi/nmol) under subdued light as described in Materials and Methods. Ice-cold ATP (10 mM) was added to displace excess bound [α-32P]8-azido-ATP where indicated. Lane 1, AD1-8u− cells; lane 2, AD1-8u− cells plus 10 mM ATP; lane 3, AD1002 cells; lane 4, AD1002 cells plus 10 mM ATP; lane 5, PSCDR1-GFP cells; lane 6, PSCDR1-GFP cells plus 10 mM ATP. (b) Binding of [125I]IAAP to Cdr1p and Cdr1p-GFP. The PM proteins (300 μg/ml) from AD1-8u− (lane 1), AD1002 (lane 2), and PSCDR1-GFP (lane 3) cells were incubated with 3 to 6 nM [125I]IAAP (2200 Ci/mmol) for 5 min under subdued light and processed as described in Materials and Methods. (c) Effects of Cdr1p substrates on the binding of IAAP to Cdr1p-GFP. The PM proteins (15 μg/50 μl) from AD1-8u− (control) (lane 1) or PSCDR1-GFP (lanes 2 to 6) cells were incubated with 100 μM concentrations of the following drug substrates for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). Lane 2, no drug; lane 3, cycloheximide; lane 4, nystatin; lane 5, anisomycin; lane 6, miconazole. The samples were brought to room temperature, and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp (365-nm wavelength) for 10 min and processed as described in Materials and Methods. (d) Concentration-dependent inhibition of IAAP binding to Cdr1p-GFP by nystatin. The PM proteins (15 μg/50 μl) of PSCDR1-GFP cells were incubated with increasing concentrations (0.25 to 50 μM) of nystatin for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). The samples were brought to room temperature, and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were processed and the radioactivity incorporated into the Cdr1p band was quantified as described in Materials and Methods. The data were fitted using the software GRAPHPAD PRISM 2.0 for the PowerPC Macintosh and are representative of three independent experiments. (e) Photoaffinity labeling of Cdr1p and Cdr1p-GFP with [3H]azidopine. The PM proteins (30 μg) from AD1-8u− (lane 1), AD1002 (lane 2), and PSCDR1-GFP (lane 3) cells were incubated with 0.5 μM [3H]azidopine (60 Ci/mmol) for 5 min under subdued light. The samples were processed and analyzed as described in Materials and Methods. (f) Effects of Cdr1p substrates on the binding of [3H]azidopine to Cdr1p-GFP. The PM proteins (30 μg) of AD1-8u− (control) (lane 1) or PSCDR1-GFP (lanes 2 to 6) cells were incubated with 100 μM concentrations of the following drug substrates for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). Lane 2, no drug; lane 3,cycloheximide; lane 4, nystatin; lane 5, anisomycin; lane 6, miconazole. The samples were brought to room temperature, and 0.5 μM [3H]azidopine was added and incubated for an additional 5 min, and the samples were processed as described in Materials and Methods. (g) Concentration-dependent inhibition of [3H]azidopine binding to Cdr1p-GFP by miconazole. The PM proteins (30 μg) from PSCDR1-GFP cells were incubated with increasing concentrations (0.25 to 100 μM) of miconazole for 10 min at 37°C. The samples were brought to room temperature, and 0.5 μM [3H]azidopine was added and incubated for an additional 5 min. The samples were processed and quantitated as described in Materials and Methods and the legend to Fig. 2d.
In order to monitor drug-binding sites on human P-gp, radiolabeled photoactive analogs of prazosin and dihydropyridine have been used successfully (16, 6). Such studies have revealed two major areas, one on each homologous half of the protein, as primary sites of drug interaction (6). In this study, we used IAAP for analyzing interactions with Cdr1p. To analyze interactions with Cdr1p, 15 μg of purified PM proteins from AD1-8u−, AD1002, and PSCDR1-GFP cells was incubated with 3 to 6 nM [125I]IAAP at room temperature for 5 min followed by UV cross-linking as detailed in Materials and Methods. Figure 2b (lane 2) shows that while IAAP specifically labeled Cdr1p, there was no binding of IAAP to the PM fraction prepared from control AD1-8u− cells (Fig. 2b, lane 1). The binding of IAAP of Cdr1p-GFP variant PM was similar to that of native Cdr1p (Fig. 2b, lane 3). In order to test whether the IAAP binding was specific, we performed binding with Cdr1p-GFP in the presence of molar excess cycloheximide, nystatin, anisomycin, and miconazole. Interestingly, IAAP was competed out by nystatin (100 μM), a known substrate of Cdr1p (Fig. 2c, lane 4), while the binding remained unaffected by the presence of the other drugs (Fig. 2c, lanes 3, 5, and 6).
Azidopine, a dihydropyridine photoaffinity analog, has been shown to bind specifically to mammalian drug-transporting P-gps (37, 20, 26, 27) and is a valuable tool used in determining the substrate-binding sites on these multidrug transporters. We explored this possibility by examining the ability of azidopine to bind to Cdr1p. The PM proteins (30 μg) from AD1-8u−, AD1002, and PSCDR1-GFP cells were incubated with 0.5 μM [3H]azidopine for 5 min at room temperature and then photo-cross-linked. The incorporation of radioactivity in protein bands corresponding to Cdr1p and Cdr1p-GFP showed similar intensities, implying that [3H]azidopine could bind to both proteins (Fig. 2e). The specificity of binding was assessed by the addition of a 100 μM concentration of cycloheximide, nystatin, anisomycin, or miconazole. Interestingly, [3H]azidopine binding to Cdr1p-GFP was not inhibited by nystatin, as was the case with IAAP binding, but was instead found to be competed out by miconazole (Fig. 2f). Of note, the competition by both the substrates was concentration dependent, which showed saturation and thus was specific. We used substrate concentrations for routine experiments, which showed maximum inhibition or competition (Fig. 2d and g).
Construction and expression of mutant variants of Cdr1p.
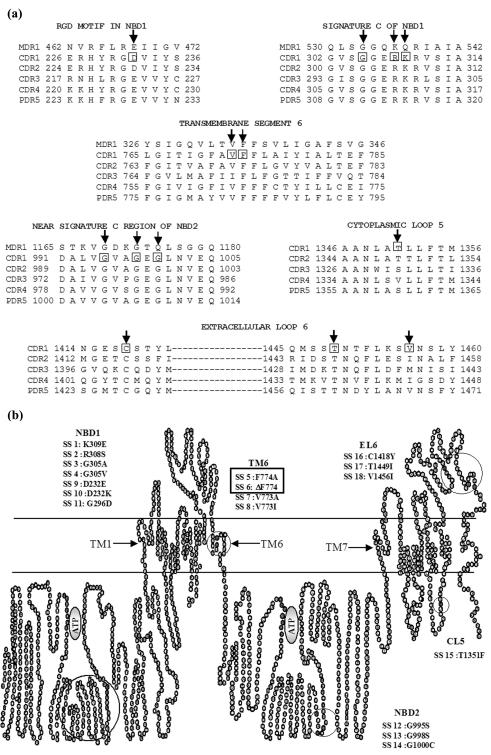
A multiple-sequence alignment of human P-gp, the ABC drug transporter of S. cerevisiae (Pdr5p), and C. albicans Cdr1p, Cdr2p, Cdr3p, and Cdr4p highlights the conservation of certain amino acid residues within TMSs and NBDs which may have a role in the functioning of these transporters (Fig. 3a). Considering the functional importance of amino acid residues determined for other ABC drug transporters, particularly of human P-gp and Pdr5p of S. cerevisiae, in this study we selected the equivalent of the most homologous or conserved residues of Cdr1p for point mutations (Fig. 3b) as indicated. This was achieved either by replacing a conserved residue by another amino acid, such as in SS1, SS2, SS3, SS4, SS5, SS7, SS8, SS9, SS10, SS11, SS12, SS13, SS14, SS15, SS16, SS17, or SS18, or by deleting the residue altogether, as in SS6. In total, 18 mutations (SS1 to SS18) were introduced in the different predicted domains spanning the entire length of Cdr1 and are highlighted in Fig. 3b.
FIG. 3.
Alignment of protein sequences of various domains and the positions of mutations in a predicted two-dimensional topology model of Cdr1p. (a) The alignment of the highly conserved regions NBDs, TMS6, CL5, and EL6 of human Mdr1, Cdr1, Cdr2, Cdr3, Cdr4, and Pdr5 proteins. The amino acids are numbered according to their positions in the proteins. The positions of mutated residues in Cdr1p with respect to other proteins are marked with arrows, and the residues mutated in Cdr1p are boxed. Since there is no homology in the protein sequences of Mdr1 and Cdr1 to Cdr4 proteins in CL5 and EL6, the Mdr1 protein sequence has not been included in these alignments. (b) Locations of the mutations introduced into CDR1. The amino acid substitutions introduced by site-directed mutagenesis, the designation, and the location of mutation are indicated in the predicted topology model in Cdr1p. The mutants, which were tagged with GFP at the C-terminal end and overexpressed in S. cerevisiae, are boxed.
Initially, all the mutations were introduced into the CDR1 gene driven by its native promoter cloned in nonintegrative pYEURA3 plasmid (pS12-35) (36). The hypersensitive AD1234568 cells were then transformed with the mutated plasmids, and the positive clones were selected by their ability to grow on SD-URA plates. The positive transformants were further confirmed by Southern hybridization (data not shown). At least two positive clones of every mutant were selected for initial screening to rule out clonal variations.
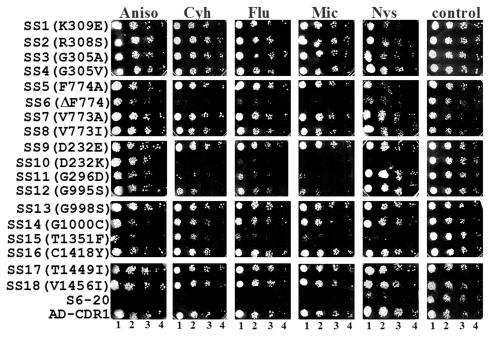
Drug resistance profiles of mutant Cdr1ps.
Confirmed positive mutants designated SS1 to SS18 were screened for their sensitivity to drugs by determining the lowest drug concentration that gave >80% inhibition (MIC80) (Table 2) and also by spot test (Fig. 4). The mutants were classified into three groups according to their sensitivity to the drugs tested. Substitutions such as D232K (SS10) and T1351F (SS15) and deletion of phenylalanine, ΔF774 (SS6), resulted in a general drug-sensitive phenotype towards all the drugs tested, while strains with mutations F774A (SS5), G296D (SS11), G995S (SS12), G1000C (SS14), C1418Y (SS16), T1449I (SS17), and V1456I (SS18) were selectively sensitive to one or more drugs compared to the wild type. Mutants, such as K309E (SS1), R308S (SS2), G305A (SS3), G305V (SS4), V773A (SS7), V773I (SS8), D232E (SS9), and G998S (SS13), retained the hyperresistant phenotype comparable to wild-type Cdr1p-expressing cells (AD-CDR1).
TABLE 2.
Drug susceptibility of S. cerevisiae cells expressing the wild type and mutant variant of Cdr1p
| Strain | Amino acid change | MICa (μg/ml)
|
||||
|---|---|---|---|---|---|---|
| Aniso | Cyh | Flu | Mic | Nys | ||
| SS1 | K309E | 8 | 0.15 | 16 | 2 | 0.5 |
| SS2 | R308S | 16 | 0.075 | 16 | 2 | 0.5 |
| SS3 | G305A | 16 | 0.15 | 32 | 4 | 0.25 |
| SS4 | G305V | 8 | 0.15 | 32 | 4 | 0.5 |
| SS5 | F774A | 16 | 0.037 | 8 | 4 | 0.12 |
| SS6 | ΔF774 | 4 | 0.018 | 4 | 0.5 | 0.03 |
| SS7 | V773A | 16 | 0.15 | 32 | 8 | 1 |
| SS8 | V773I | 16 | 0.15 | 32 | 16 | 1 |
| SS9 | D232E | 16 | 0.15 | 64 | 16 | 1 |
| SS10 | D232K | 2 | 0.018 | 4 | 1 | 0.03 |
| SS11 | G296D | 8 | 0.037 | 8 | 0.5 | 0.5 |
| SS12 | G995S | 8 | 0.037 | 8 | 8 | 0.5 |
| SS13 | G998S | 32 | 0.075 | 64 | 32 | 1 |
| SS14 | G1000C | 16 | 0.075 | 128 | 0.5 | 1 |
| SS15 | T1351F | 0.5 | 0.008 | 8 | 0.12 | 0.03 |
| SS16 | C1418Y | 32 | 0.15 | 64 | 1 | 1 |
| SS17 | T1449I | 8 | 0.15 | 64 | 1 | 0.5 |
| SS18 | V1456I | 16 | 0.075 | 64 | 1 | 0.5 |
| S6-20 | 1 | 0.004 | 2 | 0.25 | 0.015 | |
| AD-CDR1 | 32 | 0.3 | 64 | 8 | 1 | |
MICs were determined by a microdilution method as described in Materials and Methods. Aniso, anisomycin; Cyh, cycloheximide; Flu, fluconazole; Mic, miconazole; Nys, nystatin. The MIC test end point was defined as the MIC80.
FIG. 4.
Drug resistance profiles of wild-type and mutant CDR1 strains determined by the spot and MIC assays. S6-20 (control) or AD-CDR1 (expressing wild-type Cdr1p) cells or the Cdr1p mutants (SS1 to SS18) created by site-directed mutagenesis were grown overnight on SD-URA plates at 30°C. The cells were then resuspended in PBS to an A600 of 0.1. Five microliters of fivefold serial dilutions, namely, 1:5 (lanes 1), 1:25 (lanes 2), 1:125 (lanes 3), and 1:625 (lanes 4) dilutions of each strain were spotted onto SD-URA plates in the absence (control) or presence of the following drugs: anisomycin (Aniso) (1 μg/ml), cycloheximide (Cyh) (20 ng/ml), fluconazole (Flu) (2 μg/ml), miconazole (Mic) (250 ng/ml), and nystatin (Nys) (500 ng/ml). Growth differences were recorded after incubation of the plates for 48 h at 30°C. Growth was not affected by the presence of the solvents used for the drugs (data not shown).
In order to ascertain whether the introduced mutation in Cdr1p and the observed altered drug susceptibility were not due to poor expression or localization of the protein, we analyzed its expression in PM proteins by immunoblotting. It was observed that all the mutants except ΔF774 (SS6) (discussed below) expressed the mutant variants of Cdr1ps to the same level (data not shown).
As shown in Fig. 4 and Table 2, the mutant variant SS5 (F774A) was sensitive to nystatin, cycloheximide, and fluconazole but showed marginal or no alterations in drug susceptibilities towards other drugs tested (Table 2). However, the Cdr1p-harboring ΔF774 variant in SS6 was found to be sensitive to all the drugs tested.
Site-directed mutagenesis in the overexpressed Cdr1p.
In order to understand the molecular bases of the observed phenotypes of the two variant mutant Cdr1p-expressing cells (SS5 [F774A] and SS6 [ΔF774]), they were analyzed further in greater detail. To do this, the same mutations were introduced in the Cdr1p-GFP-overexpressing plasmids (pPSCDR1-GFP). The mutated plasmids were integrated in S. cerevisiae (AD1-8u−) as described above. Southern hybridization was done using a CDR1-specific probe to ensure that the gene was inserted as a single copy at the genomic PDR5 locus (data not shown). The new overexpressing strains harboring mutant Cdr1p-GFP were designated SS5G (F774A) and SS6G (ΔF774).
Drug susceptibility of SS5G (F774A) and SS6G (ΔF774).
The cells overexpressing GFP-tagged Cdr1p variants were checked for their sensitivity to drugs. As shown in Table 3, the SS6G (ΔF774) strain was significantly sensitive to all drugs tested, while the SS5G (F774A) strain showed significant sensitivity to only nystatin. It should be pointed out that the difference in the fold sensitivity or resistance depicted in Tables 2 and 3 cannot be compared due to different expression strategies employed in these experiments (Table 2 depicts data of a low-copy-number plasmid expression system, while Table 3 shows MICs of an integrative overexpression system).
TABLE 3.
Drug susceptibility of S. cerevisiae cells overexpressing the wild type and mutant variant of Cdr1p
| Strain | MICa (μg/ml)
|
||||
|---|---|---|---|---|---|
| Aniso | Cyh | Flu | Mic | Nys | |
| AD1-8u− | 0.12 | 0.015 | 1 | 0.015 | 0.03 |
| PSCDR1-GFP | 16 | 1 | 64 | 4 | 2 |
| SS5G (F774A) | 8 | 0.5 | 16 | 1 | 0.25 |
| SS6G (ΔF774) | 0.12 | 0.015 | 2 | 0.015 | 0.03 |
MICs were determined by a microdilution method as described in Materials and Methods. Aniso, anisomycin; Cyh, cycloheximide; Flu, fluconazole; Mic, miconazole; Nys, nystatin. The MIC test end point was defined as the MIC80.
ATPase activity of mutant Cdr1p-GFPs.
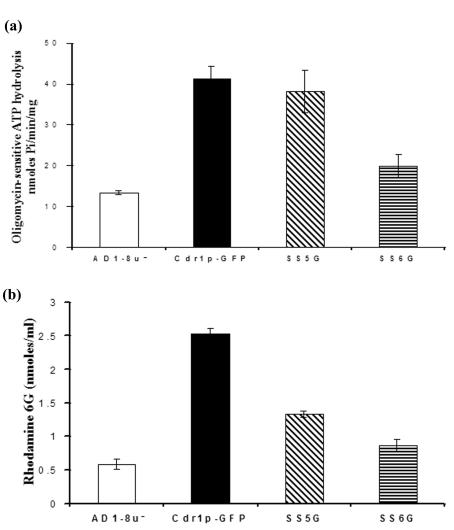
The purified PM proteins from SS5G (F774A) and SS6G (ΔF774) cells were analyzed for their oligomycin-sensitive ATPase activity. Of note, the oligomycin-sensitive ATPase activity was higher in all the PM fractions from cells expressing either native or mutant Cdr1p-GFP than in control AD1-8u− cells (Fig. 5a). However, the oligomycin-sensitive ATPase activity of SS5G (F774A) was comparable to that of native Cdr1p-GFP, while the specific activity of SS6G (ΔF774) was ∼50% lower (Fig. 5a). However, considering the fact that the expression of the mutant Cdr1p-GFP in SS6G (ΔF774) was ∼5% of the wild-type Cdr1p-GFP (Fig. 6a), the specific activity of mutant protein could actually be higher. It is important to mention that we were unable to detect drug-stimulated ATPase activity, although that would have been a correct measure of the ATPase activity contributed by the active protein. In fact, we and others have so far been unable to demonstrate drug-stimulated ATPase activity of any of the yeast ABC drug transporters (10, 33).
FIG. 5.
Functional characterization of the Cdr1p-GFP and mutant Cdr1p-GFPs. (a) Oligomycin-sensitive ATPase activity in the PM proteins of cells expressing wild-type and mutant Cdr1p-GFPs. The ATPase activity of the wild-type and mutant SS5G (F774A) and SS6G (ΔF774) Cdr1p-GFPs was determined in the PM fraction as described in the legend to Fig. 1g. The results are the means ± standard deviations (error bars) for three independent experiments. (b) Rhodamine 6G efflux from the wild-type, SS5G (F774A), and SS6G (ΔF774) cells. Rhodamine 6G efflux from AD1-8u−, wild-type, SS5G (F774A), and SS6G (ΔF774) cells was determined as described in Materials and Methods. The values are the means ± standard deviations (error bars) for four independent experiments.
FIG. 6.
Expression of mutant Cdr1p-GFPs and ATP- and substrate analog-binding characteristics, localizations, and FACS analysis of mutant Cdr1p-GFPs. (a) Expression of Cdr1p-GFP and mutant Cdr1p-GFPs in S. cerevisiae. The PM proteins (20 μg) from AD1-8u− (lane 1), PSCDR1-GFP (lane 2), SS5G (F774A) (lane 3), and SS6G (ΔF774) (lane 4) cells were separated on an SDS-8% polyacrylamide gel, electroblotted onto a nitrocellulose membrane, and incubated with mouse monoclonal anti-GFP antibody (diluted 1:1,000) (upper panel), rabbit polyclonal anti-Cdr1p antibody (diluted 1:500) (middle panel), and rabbit polyclonal anti-Pma1p antibody (diluted 1:10,000) (lower panel). Proteins were immunodetected as described in Materials and Methods. (b) Flow cytometry of S. cerevisiae SS5G (F774A) and SS6G (ΔF774) cells. The flow cytometry of the S. cerevisiae cells was performed as described in the legend to Fig. 1e. The histogram derived from the CellQuest program depicts fluorescence intensities for AD1-8u− (control) (purple), SS5G (F774A) (orange), SS6G (ΔF774) (blue), and PSCDR1-GFP (green) cells. (c) Confocal pictures of S. cerevisiae cells expressing GFP-tagged wild-type and mutant Cdr1ps SS5G (F774A) and SS6G (ΔF774). Cells were grown in SD-URA medium to late log phase. The cells were washed and resuspended in an appropriate volume of 50 mM HEPES (pH 7.0). The cells were directly viewed on a glass slide with a drop of antifade reagent to prevent photobleaching, with a 100× oil immersion objective on a Bio-Rad confocal microscope (MRC 1024). (d) [α-32P]8-azido-ATP labeling of AD1-8u− (control), wild-type, SS5G (F774A), and SS6G (ΔF774) mutant Cdr1p-GFPs. The PM proteins (15 μg/50 μl) were photoaffinity labeled with 10 μM [α-32P]8-azido-ATP (10 μCi/nmol) in the absence (−) or presence (+) of 10 mM ATP as described in Materials and Methods. (e) [125I]IAAP labeling of AD 1-8u− (control [C]), wild-type (Cdr1p-GFP), and mutant Cdr1p-GFP from SS5G (F774A) and SS6G (ΔF774). The PM proteins (15 μg) were labeled with 3 to 6 nM [125I]IAAP (2,200 Ci/mmol) and competed as described in Materials and Methods. A 100 μM concentration of nystatin as indicated (+) was added to compete IAAP binding. (f) [3H]azidopine labeling of AD1-8u− (control [C]) and wild-type (Cdr1p-GFP) cells and mutant Cdr1p-GFPs from strains SS5G (F774A) and SS6G (ΔF774). The PM proteins (30 μg) were labeled with [3H]azidopine as described in Materials and Methods in the presence (+) or absence (−) of 100 μM miconazole as indicated.
Mutant Cdr1p-GFPs elicit reduced efflux of rhodamine 6G.
We checked the rhodamine 6G efflux and found that both the mutants overexpressing GFP-tagged Cdr1p variants exhibited reduced efflux compared to cells expressing native protein. (Fig. 5b).
Expression of mutant Cdr1p-GFPs.
In order to ensure that the observed changes in drug susceptibilities and efflux activities of SS5G (F774A) and SS6G (ΔF774) were due to alterations in protein functions rather than due to altered expression levels, the steady-state levels of Cdr1p-GFP variants were compared to those of the native protein by Western blot analysis. The Western blot of these two overexpressing mutant Cdr1p-GFP PM proteins with anti-Cdr1p polyclonal antibody showed that SS5G (F774A) expressed mutant Cdr1p-GFPs to the same level as that of wild type (Fig. 6a, middle panel). Hence, the observed functional defects observed in SS5G (F774A) expressing a mutant variant of Cdr1p were not due to an effect on the level of the synthesis or trafficking of these proteins. The PM fractions were probed for PM-ATPase with its antibodies on a Western blot, which showed equal amounts of protein in all the PM fractions (Fig. 6a, lower panel). As revealed by immunoblotting with a monoclonal antibody against GFP, the mutant Cdr1p-GFP of SS6G (ΔF774) was poorly expressed at the cell surface (Fig. 6a, upper panel, lane 4). Of note, this faint band of mutant Cdr1p-GFP from SS6G was picked up consistently when we used monoclonal antibody against GFP. The FACS analysis of the SS6G (ΔF774) cells also showed poor cellular expression of mutant Cdr1p-GFP (Fig. 6b). Confocal microscopy confirmed that there was considerably reduced cell surface expression of mutant Cdr1p-GFP in SS6G (ΔF774) cells (Fig. 6c). Thus, ΔF774 Cdr1p-GFP mutant variant protein was consistently detected at a reduced steady-state level on the cell surface relative to native Cdr1p-GFP, suggesting that this mutation might harbor a defect which affects its surface localization (discussed below).
Characterization of ATP- and substrate-binding sites of mutant Cdr1p-GFP variants.
In order to explore whether the observed differences in drug susceptibilities accompanied by increased drug sensitivity and efflux activities were not due to any aberration in nucleotide or drug binding, these mutants were analyzed for their ATP- and drug-binding abilities by using [α-32P]8-azido-ATP, [125I]IAAP, and [3H]azidopine.
[α-32P]8-azido-ATP binding.
The radiolabeled analogue of ATP, [α-32P]8-azido-ATP, was used to assess its binding to mutant Cdr1p-GFPs using the purified PM fractions from SS5G (F774A) and SS6G (ΔF774) cells. It is clear from Fig. 6d that there was no significant difference in the ATP binding to the PM fraction of wild type and SS5G (F774A) cells. There was very little binding of [α-32P]8-azido-ATP to the PM of SS6G (ΔF774) cells, which correlated well with its poor expression in these cells. The binding of [α-32P]8-azido-ATP was, however, specific in all the mutants carrying Cdr1p variants, as was evident from its competition by ATP (10 mM) (Fig. 6d).
[125I]IAAP and [3H]azidopine binding to mutant Cdr1p-GFPs.
It was found that although mutant Cdr1p-GFP of SS5G (F774A) could bind [125I]IAAP, the variant showed 2.3-fold-greater binding than the native Cdr1p-GFP (Fig. 6e). [125I]IAAP binding was considerably lower in SS6G (ΔF774) cells. Further, as in the case of native Cdr1p, [125I]IAAP binding was also competed out by nystatin (100 μM) for the mutants.
The drug-binding domains of mutant Cdr1ps were further probed by examining the binding of [3H]azidopine. It was observed that although [3H]azidopine binds to mutant Cdr1p-GFP from SS5G (F774A), [3H]azidopine binding to mutant Cdr1p-GFP from SS5G (F774A) was 1.8-fold higher than that for wild-type Cdr1p-GFP (Fig. 6f). On the other hand, the mutant Cdr1p-GFP from SS6G (ΔF774) showed a very faint specific band, which corresponded to very little protein at the cell surface of this mutant protein. Irrespective of the extent of [3H]azidopine binding among mutant variants of Cdr1p-GFP, it could be competed out by miconazole (100 μM).
The deletion of F774 leads to poor cell surface localization of the mutant Cdr1p-GFP in SS6G.
The confocal images showed that while the expression of Cdr1p-GFP in the wild-type cells and of mutant Cdr1p-GFP in SS5G (F774A) cells at the cell surface (Fig. 6c) was comparable, it was minimal in SS6G (ΔF774) cells. Although some of the fluorescent protein was visible in the cytoplasm of SS6G (ΔF774) cells, very small patches of fluorescence were seen on the cell surface, which showed that probably the mutant Cdr1p-GFP in SS6G (ΔF774) cells was not properly localized on the cell surface. These results were corroborated by Western blot analysis where PM of SS6G (ΔF774) cells also showed significantly reduced expression of mutant Cdr1p-GFP (Fig. 6a). FACS analysis of the live cells showed fluorescence in SS5G (F774A) cells that was comparable to that of the wild-type PSCDR1-GFP cells. SS6G (ΔF774) cells, however, showed lower fluorescence intensity, but it was still higher than that of the control cells (AD1-8u−) (Fig. 6b). The mean fluorescence intensities (in arbitrary units) for wild-type PSCDR1-GFP, SS5G (F774A), SS6G (ΔF774), and AD1-8u− cells were 500, 482, 160, and 11, respectively. Taken together, these results suggest that there was only marginal expression of mutant Cdr1p-GFP in the PM of SS6G (ΔF774) cells, which was probably sufficient to show some functional activity (Fig. 5a and b and Fig. 6); the functional activity was lower than that of the wild type but not sufficient to confer drug resistance (Fig. 4 and Table 2).
Mutant ΔF774 Cdr1p-GFP can be brought to the cell surface by growing cells in the presence of the Cdr1p substrate.
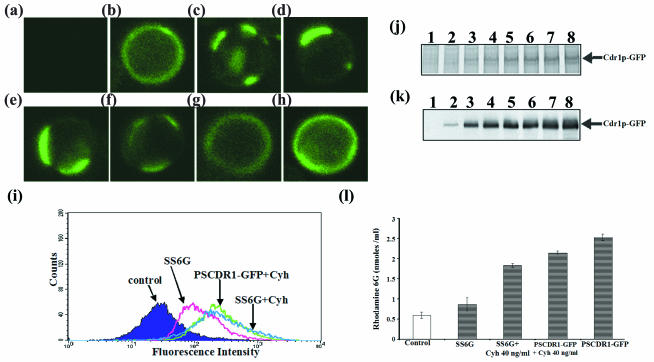
In the following experiment, we explored various possibilities related to poor surface expression of the SS6G (ΔF774) protein variant. The poor surface expression of the SS6G (ΔF774) protein could be due either to poor localization, reduced expression of protein, or rapid degradation of protein. We checked whether the localization of mutant Cdr1p-GFP in SS6G (ΔF774) cells could be improved. To do this, we added different drugs, which are substrates of Cdr1p, such as cycloheximide, miconazole, anisomycin, and nystatin (at concentrations lower than their respective MICs), just after the lag phase of the SS6G (ΔF774) cells (4 to 5 h). The cells were then grown for 12 more hours. Thereafter, cells were checked by confocal microscopy for the localization of the protein. It was observed that the mutant Cdr1p-GFP protein showed improved surface localization with increasing concentrations of cycloheximide. The surface expression of mutant Cdr1p-GFP was almost identical to that of native Cdr1p-GFP at 40 ng of cycloheximide per ml (Fig. 7a to h). FACS analysis also confirmed that the surface expression of SS6G (ΔF774)-expressing Cdr1p-GFP mutant grown in the presence of 40 ng of cycloheximide per ml was similar to that of the wild type (Fig. 7i). Interestingly, PM protein isolated from the SS6G (ΔF774) cells, which were grown in increasing concentrations of cycloheximide and subjected to SDS-PAGE and Western blot analysis (probed with monoclonal anti-GFP antibody), also showed a concentration-dependent increase in the amount of Cdr1p-GFP in the PM fraction, which peaked at 40 and 50 ng/ml (Fig. 7j and k, lanes 6 and 7). Under these conditions, SS6G (ΔF774) cells expressing mutant variant distinctly showed improvement in surface localization, while the presence of drug had no effect in cells expressing native Cdr1p-GFP (Fig. 7b, h, and i). The observed rescuing effect was not specific to cycloheximide alone. Other substrates of Cdr1p, such as anisomycin and nystatin, could also rescue the ΔF774 protein to the PM (data not shown).
FIG. 7.
Properties of SS6G (ΔF774) mutant Cdr1p-GFP cells. (a to h) Confocal pictures of AD1-8u− cells and cells expressing SS6G (ΔF774) mutant Cdr1p-GFP grown in the presence of increasing concentrations of cycloheximide. (a) AD1-8u− cells; (b) PSCDR1-GFP cells expressing wild-type Cdr1p-GFP grown with 50 ng of cycloheximide per ml; (c to h) SS6G (ΔF774) cells expressing mutant Cdr1p-GFP grown without cycloheximide (c) or in the presence of the following concentrations of cycloheximide: 10 ng/ml (d), 20 ng/ml (e), 30 ng/ml (f), 40 ng/ml (g), or 50 ng/ml (h). (i) Flow cytometry of SS6G (ΔF774) cells grown in the presence of cycloheximide. The control (AD1-8u−), SS6G (ΔF774), and PSCDR1-GFP cells grown in the presence or absence of cycloheximide (Cyh) (40 ng/ml) as indicated were analyzed by flow cytometry as described in Materials and Methods. (j and k) Expression of mutant Cdr1p-GFP from SS6G (ΔF774) cells grown in the presence of cycloheximide. The PM proteins from AD1-8u− (lane 1), SS6G (ΔF774) (lane 2), and SS6G (ΔF774) cells grown in the presence of increasing concentrations of cycloheximide (lanes 3 to 7) and PSCDR1-GFP cells grown in the presence of cycloheximide (50 ng/ml) (lane 8) were separated on an SDS-8% polyacrylamide gel and stained with colloidal Coomassie blue G250 (j) or electroblotted onto a nitrocellulose membrane (k) and incubated with mouse anti-GFP monoclonal antibody (diluted 1:1,000). The SS6G (ΔF774) cells were grown in the presence of the following increasing concentrations of cycloheximide: 10 ng/ml (lane 3), 20 ng/ml (lane 4), 30 ng/ml (lane 5), 40 ng/ml (lane 6), and 50 ng/ml (lane 7). Proteins were detected with HRP-labeled anti-mouse immunoglobulin using an ECL kit. (k) Rhodamine 6G efflux from SS6G (ΔF774) cells grown in the presence of cycloheximide. The rhodamine 6G efflux by AD1-8u−, SS6G (ΔF774), and PSCDR1-GFP (with or without cycloheximide) cells was determined as described in Materials and Methods. The results are the means ± standard deviations (error bars) for three independent experiments.
Mutant Cdr1p-GFP from SS6G (ΔF774) cells grown in the presence of cycloheximide is functional.
In order to test whether improved localization led to the resumption of transport functions, the efflux of rhodamine 6G was measured in cells grown in the presence of cycloheximide. The control (AD1-8u−), PSCDR1-GFP, and SS6G (ΔF774) cells were grown in the presence or absence of cycloheximide (40 ng/ml) for 12 to 14 h, and rhodamine 6G efflux was studied as described in Materials and Methods. Figure 7l shows that the SS6G (ΔF774) cells grown in the presence of cycloheximide showed considerable increase in rhodamine 6G efflux over the same cells grown in the absence of cycloheximide. The level of efflux was comparable to that of native PSCDR1-GFP cells grown at the same concentration of cycloheximide. There was some reduction in the efflux of rhodamine 6G by PSCDR1-GFP cells grown in the presence of cycloheximide. The decrease in efflux by the cells expressing native Cdr1p could be due to the fact that cycloheximide, which is also a substrate for the Cdr1p transporter, probably competes with rhodamine 6G.
DISCUSSION
Cdr1p was first cloned and identified as a protein capable of effluxing a variety of unrelated cytotoxic drugs and subsequently demonstrated to be a transporter involved in azole resistance of C. albicans (24, 36). Further studies from our laboratory as well as from other research groups established that the efflux of drugs by Cdr1p represents one of the major determinants of azole resistance in clinical isolates of C. albicans (24, 38). Functional analysis of Cdr1p further revealed that in addition to drug extrusion activity, the protein is also capable of effluxing human steroid hormones and can translocate membrane phospholipids between the two monolayers of PM of C. albicans cells (13, 24). The promiscuity of Cdr1p towards substrates and its ability to mediate several functions suggest that the protein might contain mutually exclusive substrate-binding sites that allow the efflux of a variety of unrelated compounds with striking physical and chemical diversity. The molecular basis for the broad range of this transport capacity is largely unknown for Cdr1p as well as for other ABC drug transporters of yeast. Delineating the architecture of the drug-binding sites of Cdr1p for the substrates will be invaluable not only to understand how drugs interact with this protein but also to design more useful and specific inhibitors of Cdr1p. In this study, we have attempted to examine the relationship between structure and function for Cdr1p.
Cdr1p is fully active as a GFP-tagged protein.
For detailed functional analysis, Cdr1p tagged with GFP was overexpressed from a genomic PDR5 locus in a S. cerevisiae AD1-8u− mutant derived from a pdr1-3 mutant strain with a gain-of-function mutation in the gene encoding transcription factor PDR1, resulting in a constitutive hyperinduction of the PDR5 promoter (33). Thus, Cdr1p-GFP expression driven by hyperinduced PDR5 promoter provided a sufficient level of expression, confirmed by confocal microscopy, flow cytometry analysis, and immunoblotting, for functional analysis. That GFP-tagged Cdr1p was functional like native Cdr1p was established by assaying the MICs of various drugs for S. cerevisiae host cells. The fluorescence imparted by GFP-tagged Cdr1p visualized with a confocal microscope confirmed its proper localization to PM.
Cdr1p harbors multiple drug-binding sites.
Cdr1p, Pdr5p, human P-gp, and some of the MRPs function as drug extrusion pumps. Cdr1p differs structurally from the human full-length ABC transporters, since it possesses inverted domain organization [(NBD-TMD)2] (36) compared to human P-gp [(TMD-NBD)2] (15). However, for the human ABCG2 (the mitoxantrone resistance-associated protein MXR, the breast cancer resistance protein BCRP, or the placenta ABC protein ABCP), the domain organization of the half transporter is similar to that of Cdr1p (25), and the functional unit of this transporter is a dimer [(NBD-TMD)2]. Notwithstanding these domain-based differences and low level of sequence identity outside conserved stretches, the binding characteristics of Cdr1p have many commonalties with those of human P-gp. The binding of IAAP as well as azidopine to Cdr1p is one such case of similarity. Both analogues bind to human and murine drug-transporting P-gps and Cdr1p (this study), where it has been used to map drug-binding sites (17). The fact that IAAP binding to Cdr1p is competed out by nystatin, while miconazole did not have any effect, and the fact that azidopine binding is competed out only by miconazole (Fig. 2c and f) suggest that IAAP and azidopine share two different binding sites in Cdr1p. We had earlier observed that the deletion of a 79-amino-acid stretch from the C-terminal end, which encompasses the TMS12 of this transporter, leads to selective impairment of drug resistance (23). The expression of ΔCdr1p led to decreased resistance to nystatin, while resistance to miconazole was retained. Recently, by using a variety of novel substrates of Pdr5p, Golin et al. (14) have reported that this ABC drug transporter from S. cerevisiae has at least three drug-binding sites and suggested that some substrates might even interact at more than one site. Taken together, our results suggest that Cdr1p harbors different drug-binding sites, which recognize structurally dissimilar drugs. Of note, selective sensitivity to nystatin and miconazole revealed upon mutational analysis further reaffirmed that Cdr1p has multiple drug-binding sites. While variant F774A retained the resistance to miconazole compared to native Cdr1p, its sensitivity towards nystatin was increased considerably (Fig. 4 and Table 2). This duality of Cdr1p towards these two drugs can be explained if one considers different binding sites. Interestingly, a similar conclusion can be drawn from IAAP and azidopine binding. While the former gets competed out by nystatin, the latter is affected by miconazole. Taken together, this would suggest that nystatin and IAAP share common binding site(s), while azidopine and miconazole bind to another site. The concept of multiple drug-binding sites is further illustrated by the fact that the binding of IAAP and azidopine are not competed out by drugs, such as cycloheximide, anisomycin, and fluconazole, which may bind to an as yet unidentified site(s).
Notwithstanding topological differences, the photoaffinity analogues (IAAP and azidopine) could specifically label Cdr1p as well as human P-gp. The extent of binding of these photoaffinity analogues to Cdr1p revealed even more similarity between the two ABC drug transporters. For example, both IAAP and azidopine showed enhanced binding to Cdr1p-GFP variant of SS5G (F774A). Similar observations were reported by Chen et al. (7), who observed that the deletion of F335 led to enhanced IAAP binding to P-gp. In another study, Loo and Clarke (26) observed that the replacement of F335 by A of human P-gp also led to enhanced binding of azidopine. Taken together, these results suggest that F335 in TMS6 of mammalian P-gp and its equivalent F774 in TMS6 of Cdr1p behave similarly and play an important role in determining substrate specificity.
ΔF774 in SS6G leads to poor localization of mutated Cdr1p.
In order to analyze some of the interesting mutant variants of Cdr1p, F774A and ΔF774 mutations were introduced into the Cdr1p-GFP expression system driven by hyperinduced PDR5 promoter (discussed above) for detailed analysis. It was observed that in the SS6G (ΔF774) strain, in which F774 of the predicted TMS6 was deleted, showed very low expression of mutant Cdr1p-GFP in the PM fraction when analyzed by Western blot analysis. While mutant Cdr1p-GFP in SS6G (ΔF774) cells was poorly expressed in PM fractions isolated from these cells, Western blotting did not show any steady-state expression defect in SS5G (F774A) membranes. Confocal microscopy and FACS analysis also confirmed poor expression of SS6G (ΔF774) mutant protein.
Cell surface expression of mutant Cdr1p (ΔF774) protein could be rescued by growth in the presence of cycloheximide.
In the case of the SS6 mutant, one cannot exclude the possibility of poor protein localization in the PM due to either low protein levels or its degradation. However, since the protein can be rescued to the cell surface by growing the mutant cells in the presence of substrates, such as cycloheximide (at a concentration that does not affect growth and protein synthesis), it is probable that the mutant protein is not properly localized. Of note, the mutant protein of ΔF774 which could be directed to the PM in the presence of cycloheximide was not only found to be at the cell surface but also became functionally active, as determined by its ability to efflux rhodamine 6G. These results suggest that the deletion mutation ΔF774 in TMS6 inhibits the transporter from folding into an active conformation, but the protein can be made to fold into an active conformation if synthesized in the presence of cycloheximide.
Earlier misprocessing of ABC transporters has also been observed. For example, the deletion of phenylalanine 508 in NBD1 of the cystic fibrosis transport receptor (CFTR) causes its improper localization on the cell surface (8). Deletion of an equivalent phenylalanine residue in NBD1 of Yor1p of S. cerevisiae also led to a variant protein, which was retained in the endoplasmic reticulum (21). Interestingly, a number of low-molecular-weight molecules have been shown to rescue the processing defect of mutant CFTR protein (5). In addition, even lower temperatures or the presence of glycerol, trimethylamine N-oxide, or deuterated water has been shown to rescue the ΔF508 CFTR mutant misprocessing phenotype (11, 43). Similarly, the TMS6 mutant of human P-gp expressing misfolded protein was rescued when grown in the presence of substrates, such as cyclosporine A, capsaicin, vinblastine, or verapamil (28). In such rescues, an early interaction between the drug substrate and TMD is observed; this stabilizes the folding intermediates in a native conformation, thus escaping cell quality control mechanism. The exact mechanism by which cycloheximide rescues the misprocessed ΔF774 mutant variant of Cdr1p is not clear at present. However, given the clinical importance of this protein, such residues, which affect the localization, could be exploited in rationally designing antifungal agents.
Taken together, in this study, we have characterized Cdr1p by overexpressing it as a GFP-tagged protein in S. cerevisiae. We show that the photoaffinity analogs IAAP and azidopine specifically bind to Cdr1p. The analysis of GFP-tagged mutant variants of Cdr1p revealed that the deletion of a conserved F774 in predicted TM6 led to poor surface localization of the protein. The mislocalized ΔF774 mutant Cdr1p could be rescued to the PM as a functional transporter by growth in the presence of a Cdr1p substrate, cycloheximide. In addition, our study demonstrates that there is a conserved functional homology between Cdr1p and human P-gp. For example, similar to Pgp, TMS6 in Cdr1p also appears to be a major contributor to drug substrate-binding sites. Considering the clinical importance of MDR in cancer patients and fungal infections, structure-and-function study of Cdr1p should provide additional information about the mechanism of action of these yeast and mammalian drug transporters.
Acknowledgments
We thank R. D. Cannon, A. Goffeau, and M. Raymond for the plasmid and strain gifts. We are grateful to R. Serrano for the kind gift of PM-ATPase antibodies. We thank Pfizer Inc., Kent, United Kingdom, and Ranbaxy Laboratories Limited, New Delhi, India, for providing fluconazole. We sincerely thank Zuben E. Sauna for helping with photoaffinity labeling experiments.
The work presented in this paper has been supported in part by grants to one of us (R.P.) from the Department of Biotechnology of the Indian government (BT/PR1110/MED/09/186/98), the Volkswagen Foundation in Germany (1/76 798), and the European Commission (QLK-CT-2001-02377). S.S., P.S., and S.J. acknowledge the Council of Scientific and Industrial Research of India for fellowship awards in the form of senior research fellowships. Part of this work was done by S.S. while working as a visiting predoctoral fellow at the NCI, NIH.
REFERENCES
- 1.Albertson, G. D., M. Niimi, R. D. Cannon, and H. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altherr, M. R., L. A. Quinn, C. I. Kado, and R. L. Rodroguez. 1983. Transformation and storage of competent yeast cells. Genet. Eng. Eukaryot. 1983:33-36.
- 3.Ambudkar, S. V., S. Dey, C. A. Hrycyna, M. Ramachandran, I. Pastan, and M. M. Gottesman. 1999. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 39:361-398. [DOI] [PubMed] [Google Scholar]
- 4.Booth, C. L., L. Pulaski, M. M. Gottesman, and I. Pastan. 2000. Analysis of the properties of the N-terminal nucleotide-binding domain of human P-glycoprotein. Biochemistry 39:5518-5526. [DOI] [PubMed] [Google Scholar]
- 5.Brown, C. R., and W. J. Welch. 1996. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones 1:109-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruggemann, E. P., U. A. Germann, M. M. Gottesman, and I. Pastan. 1989. Two different regions of phosphoglycoprotein are photoaffinity-labeled by azidopine. J. Biol. Chem. 264:15483-15488. [PubMed] [Google Scholar]
- 7.Chen, G., G. E. Duran, K. A. Steger, N. J. Lacayo, J. P. Jaffrezou, C. Dumontet, and B. I. Sikic. 1997. Multidrug-resistant human sarcoma cells with a mutant P-glycoprotein, altered phenotype, and resistance to cyclosporins. J. Biol. Chem. 272:5974-5982. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, S. H., R. J. Gregory, J. Marshall, S. Paul, D. W. Souza, G. A. White, C. R. O'Riordan, and A. E. Smith. 1990. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827-834. [DOI] [PubMed] [Google Scholar]
- 9.Decottignies, A., A. M. Grant, J. W. Nichols, H. De Wet, D. B. McIntosh, and A. Goffeau. 1998. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 273:12612-12622. [DOI] [PubMed] [Google Scholar]
- 10.Decottignies, A., M. Kolaczkowski, E. Balzi, and A. Goffeau. 1994. Solubilisation and characterisation of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast. J. Biol. Chem. 269:12797-12803. [PubMed] [Google Scholar]
- 11.Denning, G. M., M. P. Anderson, J. F. Amara, J. Marshall, A. E. Smith, and M. J. Welsh. 1992. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 358:761-764. [DOI] [PubMed] [Google Scholar]
- 12.Dey, S., M. Ramachandran, I. Pastan, M. M. Gottesman, and S. V. Ambudkar. 1998. Photoaffinity labeling of human P-glycoprotein: effect of modulator interaction and ATP hydrolysis on substrate binding. Methods Enzymol. 292:318-328. [DOI] [PubMed] [Google Scholar]
- 13.Dogra, S., S. Krishnamurthy, V. Gupta, B. L. Dixit, C. M. Gupta, D. Sanglard, and R. Prasad. 1999. Asymmetric distribution of phosphatidylethanolamine in C. albicans: possible mediation by CDR1, a multidrug transporter belonging to ATP binding cassette (ABC) superfamily. Yeast 15:111-121. [DOI] [PubMed] [Google Scholar]
- 14.Golin, J., S. V. Ambudkar, M. M. Gottesman, A. Habib, J. Sczepanski, W. Ziccardi, and L. May. 2003. Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J Biol. Chem. 278:5963-5969. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman, M. M., C. A. Hrycyna, P. V. Schoenlein, U. A. Germann, and I. Pastan. 1995. Genetic analysis of the multidrug transporter. Annu. Rev. Genet. 29:607-649. [DOI] [PubMed] [Google Scholar]
- 16.Greenberger, L. M. 1993. Major photoaffinity drug labeling sites for iodoaryl azidoprasin in P-glycoprotein are within, or immediately C-terminal to transmembrane domains 6 and 12. J. Biol. Chem. 268:11417-11425. [PubMed] [Google Scholar]
- 17.Greenberger, L. M., C. J. Lisanti, J. T. Silva, and S. B. Horwitz. 1991. Domain mapping of the photoaffinity drug-binding sites in P-glycoprotein encoded by mouse mdr1b. J. Biol. Chem. 266:20744-20751. [PubMed] [Google Scholar]
- 18.Higgins, C. F. 1993. The ABC transporter channel superfamily—an overview. Semin. Cell Biol. 4:1-5. [Google Scholar]
- 19.Hoffman, C. S., and F. Winston. 1987. A ten minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 20.Kajiji, S., F. Talbot, K. Grizzuti, V. Van Dyke-Phillips, M. Agresti, A. R. Safa, and P. Gros. 1993. Functional analysis of P-glycoprotein mutants identifies predicted transmembrane domain 11 as a putative drug binding site. Biochemistry 32:4185-4194. [DOI] [PubMed] [Google Scholar]
- 21.Katzmann, D. J., E. A. Epping, and W. S. Moye-Rowley. 1999. Mutational disruption of plasma membrane trafficking of Saccharomyces cerevisiae Yor1p, a homologue of mammalian multidrug resistance protein. Mol. Cell. Biol. 19:2998-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keppler, D., Y. Cui, J. Konig, I. Leier, and A. Nies. 1999. Export pumps for anionic conjugates encoded by MRP genes. Adv. Enzyme Regul. 39:237-246. [DOI] [PubMed] [Google Scholar]
- 23.Krishnamurthy, S., U. Chatterjee, V. Gupta, R. Prasad, P. Das, P. Snehlata, S. E. Hasnain, and R. Prasad. 1998. Deletion of transmembrane domain 12 of CDR1, a multidrug transporter from Candida albicans, leads to altered drug specificity: expression of a yeast multidrug transporter in baculovirus expression system. Yeast 14:535-550. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamurthy, S., V. Gupta, P. Snehlata, and R. Prasad. 1998. Characterisation of human steroid hormone transport mediated by Cdr1p, multidrug transporter of Candida albicans, belonging to the ATP binding cassette superfamily. FEMS Microbiol. Lett. 158:69-74. [DOI] [PubMed] [Google Scholar]
- 25.Litman, T., T. E. Druley, W. D. Stein, and S. E. Bates. 2001. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol. Life Sci. 58:931-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo, T. W., and D. M. Clarke. 1993. Functional consequences of phenylalanine mutations in the predicted transmembrane domain of P-glycoprotein. J. Biol. Chem. 268:19965-19972. [PubMed] [Google Scholar]
- 27.Loo, T. W., and D. M. Clarke. 1994. Mutations to amino acids located in predicted transmembrane segment 6 (TM6) modulate the activity and substrate specificity of human P-glycoprotein. Biochemistry 33:14049-14057. [DOI] [PubMed] [Google Scholar]
- 28.Loo, T. W., and D. M. Clarke. 1997. Correction of defective protein kinesis of human P-glycoprotein mutants by substrates and modulators. J. Biol. Chem. 272:709-712. [DOI] [PubMed] [Google Scholar]
- 29.Maesaki, S., P. Marichal, H. V. Bossche, D. Sanglard, and S. Kohno. 1999. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 44:27-31. [DOI] [PubMed] [Google Scholar]
- 30.Monk, B. C., M. B. Kurtz, J. A. Marrinan, and D. S. Perlin. 1991. Cloning and characterization of the plasma membrane H+-ATPase from Candida albicans. J. Bacteriol. 173:6826-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morschhauser, J., S. Michel, and J. Hacker. 1998. Expression of a chromosomally integrated, single-copy GFP gene in Candida albicans, and its use as a reporter of gene regulation. Mol. Gen. Genet. 257:420. [DOI] [PubMed] [Google Scholar]
- 32.Mukhopadhyay, K., A. Kohli, and R. Prasad. 2002. Drug susceptibilities of yeast cells are affected by membrane lipid composition. Antimicrob. Agents Chemother. 46:3695-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura, K., M. Niimi, K. Niimi, A. R. Holmes, J. E. Yates, A. Decottignies, B. C. Monk, A. Goffeau, and R. D. Cannon. 2002. Functional expression of Candida albicans drug efflux pump Cdr1p in a Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 45:3366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odds, F. C. 1988. Candida and candidosis: a review and bibliography. Ballière Tindall, London, United Kingdom.
- 35.Pao, S. S., I. T. Paulsen, and M. H. Saier, Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prasad, R., P. D. Worgifosse, A. Goffeau, and E. Balzi. 1995. Molecular cloning and characterisation of a novel gene of C. albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr. Genet. 27:320-329. [DOI] [PubMed] [Google Scholar]
- 37.Safa, A. R. 1988. Photoaffinity labelling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc. Natl. Acad. Sci. USA 85:7187-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanglard, D., F. Ischer, D. Calabrese, P. A. Majcherczyk, and J. Bille. 1999. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 43:2753-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of Candida albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1997. Cloning of Candida albicans genes conferring resistance to azole antifungal agents: characterisation of CDR2, a new multidrug ABC transporter gene. Microbiology 143:405-416. [DOI] [PubMed] [Google Scholar]
- 41.Sanglard, D., K. Kuchler, F. Ischer, J.-L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkadi, B., E. M. Price, R. C. Boucher, U. A. Germann, and G. A. Scarborough. 1992. Expression of the human multidrug resistant cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 267:4854-4858. [PubMed] [Google Scholar]
- 43.Sato, S., C. L. Ward, M. E. Krouse, J. J. Wine, and R. R. Kopito. 1996. Glycerol reverses the misfolding phenotype of the most common cystic fibrosis mutation. J. Biol. Chem. 271:635-638. [DOI] [PubMed] [Google Scholar]
- 44.Sauna, Z. E., and S. V. Ambudkar. 2000. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc. Natl. Acad. Sci. USA 97:2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauna, Z. E., and S. V. Ambudkar. 2001. Characterization of the catalytic cycle of ATP hydrolysis by human P-glycoprotein. J. Biol. Chem. 276:11653-11661. [DOI] [PubMed] [Google Scholar]
- 46.Schaffner, W., and C. Weissmann. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502-514. [DOI] [PubMed] [Google Scholar]
- 47.Scherer, S., and D. A. Stevens. 1987. Application of DNA typing methods to epidemiology and taxonomy of Candida albicans. J. Clin. Microbiol. 25:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker, J. E., M. Sarsate, M. Runswick, and N. J. Gay. 1982. Distantly related sequences in the a- and b-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]