Abstract
Fungi possess efficient mechanisms of pH and ion homeostasis, allowing them to grow over a wide range of environmental conditions. In this study, we addressed the role of the pH response transcription factor PacC in salt tolerance of the vascular wilt pathogen Fusarium oxysporum. Loss-of-function pacC+/− mutants showed increased sensitivity to Li+ and Na+ and accumulated higher levels of these cations than the wild type. In contrast, strains expressing a dominant activating pacCc allele were more salt tolerant and had lower intracellular Li+ and Na+ concentrations. Although the kinetics of Li+ influx were not altered by mutations in pacC, we found that Li+ efflux at an alkaline, but not at an acidic, ambient pH was significantly reduced in pacC+/− loss-of-function mutants. To explore the presence of a PacC-dependent efflux mechanism in F. oxysporum, we cloned ena1 encoding an orthologue of the yeast P-type Na+-ATPase ENA1. Northern analysis revealed that efficient transcriptional activation of ena1 in F. oxysporum required the presence of high Na+ concentrations and alkaline ambient pH and was dependent on PacC function. We propose a model in which PacC controls ion homeostasis in F. oxysporum at a high pH by activating expression of ena1 coordinately with a second Na+-responsive signaling pathway.
Fungi are a versatile class of organisms that have successfully occupied numerous ecological niches, including those of plant and animal pathogenesis. A striking property of fungi and a major determinant of their evolutionary success is their capacity to adapt to an extremely wide range of environmental conditions. This ability depends crucially on the presence of cellular sensory networks that monitor the environment and mediate changes in gene expression in response to shifts in the external conditions. We use the vascular wilt pathogen Fusarium oxysporum as a model to understand how environmental signals regulate gene expression in fungi and how these regulatory mechanisms determine fungal virulence.
A key factor in fungal growth and development is ambient pH. Fungi grow over a wide range of pH conditions and must thus be able to tailor gene expression to the particular pH of their growth environment. A conserved signaling cascade integrated by the products of the pal genes, whose terminal component is the zinc-finger transcription factor PacC/Rim101p, regulates gene expression in response to ambient pH (18). Upon shift to alkaline pH, an inactive PacC precursor is posttranscriptionally activated by proteolytic processing into a shorter functional form that activates genes preferentially expressed at alkaline pH and represses genes expressed under acidic growth conditions (18). The pacC orthologue of F. oxysporum was recently cloned, and the encoded protein was shown to regulate pH-dependent gene expression and to function as a negative regulator of virulence on plants (11). Thus, pacC+/− loss-of-function mutants of F. oxysporum mimic growth at acidic ambient pH and exhibit increased virulence, whereas pacCc strains expressing a dominant activating pacC allele mimic growth at alkaline pH and show significantly reduced virulence. At present, the downstream effector genes regulated by PacC in F. oxysporum remain largely unknown.
Yet another hallmark of fungal versatility is the capacity to grow over a wide range of salt concentrations. Generally, salt tolerance in living cells is conditioned by the capacity to maintain intracellular ion homeostasis. Fungi have developed extrusion systems to keep levels of intracellular sodium below concentrations toxic to the cell (9). In the best-studied system, that of Saccharomyces cerevisiae, the main Na+ efflux system is encoded by the ENA genes, a tandem array of four to five genes encoding nearly identical proteins. ENA1, the most important and the best-studied component of this system, is essential for ion homeostasis and salt tolerance in yeast (15, 23). The ENA1 protein works as a P-type Na+-ATPase but can also mediate Li+ or K+ efflux (8, 14, 15, 23). Expression of ENA1 in S. cerevisiae is tightly regulated by Na+ but also depends on alkaline ambient pH (for a review, see reference 20). Recent studies suggest that full expression of ENA1 at alkaline pH requires RIM101, the S. cerevisiae orthologue of PacC, providing further evidence for a functional link between pH signaling and ENA1 (17, 25).
In the present study we have addressed the role of pacC in salt tolerance of F. oxysporum. We provide evidence for the presence of a sodium efflux system based on an orthologue of the S. cerevisiae ENA1 gene. We further show that full transcriptional activation of the ena1 gene requires PacC and that both PacC and Ena1 play a fundamental role in the salt stress response of F. oxysporum.
MATERIALS AND METHODS
Fungal isolates and culture conditions.
F. oxysporum f.sp. lycopersici strain 4287 (race 2) was obtained from J. Tello, Universidad de Almería, Almería, Spain, and stored at −80°C with glycerol as a microconidial suspension (13). Construction of the pacC+/− loss-of-function mutant and the merodiploid strain carrying a dominant activating pacCc allele was described previously (11). For microconidia production, cultures were grown in potato dextrose broth (Difco, Detroit, Mich.) at 28°C as described previously (13).
For phenotypic analysis of colony growth, a 5-μl drop containing 2.5 × 105 freshly obtained microconidia was transferred on 1.5% (wt/vol) agar plates of synthetic medium (SM) (13) containing 1% (wt/vol) glucose, 0.1% NH4NO3, and different concentrations of NaCl or LiCl. Media were buffered with 100 mM Na2HPO4 at pH 4; 50 mM Na2HPO4, 50 mM NaH2PO4, and 50 mM NaCl at pH 6; and 100 mM NaH2PO4 and 100 mM NaCl at pH 8.
Determination of cation accumulation, influx, and efflux.
For determination of intracellular cation accumulation, microconidia were germinated in SM containing 1% (wt/vol) glucose and 0.1% NH4NO3, supplemented with 0.05, 0.1, or 0.15 mM LiCl or 0.5, 1, or 1.5 M NaCl. After 12 h, samples were filtered and processed for determination of intracellular ion content as previously reported (10, 21). Briefly, samples of cells were filtered, washed with 0.02 M MgCl2, and treated with 0.2 M HCl, and the cations were analyzed by atomic absorption spectrophotometry.
For determination of lithium and rubidium influx (rubidium was used as a transport analog of potassium), microconidia were germinated in SM containing 1% (wt/vol) glucose and 0.1% NH4NO3 for 12 h. At time zero, 0.1 M LiCl or RbCl was added to the growth medium, and samples were taken at regular time intervals, filtered immediately, and processed for determination of intracellular ion content (21).
For determination of the lithium efflux rate, microconidia germinated in SM were supplemented with 0.1 M LiCl (wild-type and pacC+/− strains) or 0.3 M LiCl (pacCc strain). After 12 h, microconidia were filtered, washed with sterile 0.02 M MgCl2, and resuspended in fresh SM buffered at pH 4.0 or 8.0 as described above. This medium was free of lithium and was supplemented with 0.05 M RbCl to trigger the efflux process. Samples were taken at regular time intervals, filtered, and processed for the determination of intracellular ion content (10, 21).
All experiments for determination of cation accumulation, influx, or efflux were performed at least three times with similar results (the maximum standard deviations were <10%).
Nucleic acid manipulations, cloning, and analysis of the ena1 gene.
For Northern analysis, microconidia were germinated for 12 h in SM without added Na+ and then transferred for different periods of time to SM buffered at the indicated pH values, with or without 0.5 M Na+. Total RNA was extracted from mycelium as reported elsewhere (12), and Northern analysis and probe labeling was performed as described previously (13) by using the nonisotopic digoxigenin labeling kit (Roche Diagnostic S.L, Barcelona, Spain). Southern and Northern analyses were carried out as described above.
Genomic DNA of F. oxysporum isolate 4287 was extracted as previously reported (19) and used for PCR amplification on a Perkin-Elmer GeneAmp System 2400 with the primers ena3 (5′-TGACAAGCGACGATCTTTCTTCCG-3′) and ena4 (5′-GGTGATGCCCTTGTGCTTGAAGAC-3′) derived from an F. oxysporum expressed sequence tag clone. The following PCR conditions were used: 35 cycles with denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. An initial denaturation step of 5 min at 94°C and a final elongation step at 72°C for 7 min were also performed. The amplified 250-bp DNA fragment was cloned into pGEM-T (Promega, Madison, Wis.), sequenced, and used to screen a lambda EMBL3 genomic library of F. oxysporum f.sp. lycopersici isolate 4287. Library screening, subcloning, and other routine procedures were performed as described in standard protocols (24). Sequencing of both DNA strands was performed at the Servicio Centralizado de Apoyo a la Investigación, University of Córdoba, by using the Dyedeoxy terminator cycle sequencing kit (PE Biosystems, Foster City, Calif.) on an ABI Prism 377 genetic analyzer apparatus (Applied Biosystems, Foster City, Calif.). DNA and protein sequence databases were searched by using the basic local alignment search tool (BLAST) algorithm (2) at the National Center for Biotechnology Information (Bethesda, Md.).
RESULTS
PacC controls salt tolerance and intacellular cation levels.
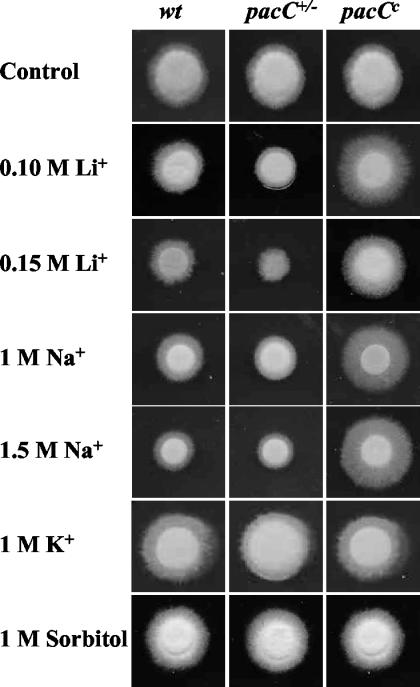
To test the hypothesis that pacC may be involved in the salt stress response of F. oxysporum, 2 × 105 freshly obtained microconidia of the wild-type strain, a pacC+/− loss-of-function or a pacCc dominant activating mutant (11) were inoculated on plates containing SM (pH 6.0) supplemented with different concentrations of salts or sorbitol. The results depicted in Fig. 1 show that the pacC+/− loss-of-function mutant displayed a clear Li+ sensitivity phenotype and a somewhat more subtle Na+ sensitivity phenotype, whereas the dominant activating pacCc allele conferred increased tolerance to these toxic cations. Importantly, pacC mutations did not affect growth at high concentrations of the nontoxic cation K+ or sorbitol, suggesting that PacC is specifically involved in the regulation of salt stress but not osmotic stress. Reintroduction of a functional pacC copy into the pacC+/− mutant restored the wild-type growth phenotype, suggesting that the increased Li+ and Na+ sensitivity of the mutant was caused solely by the absence of a functional pacC allele (data not shown).
FIG. 1.
F. oxysporum pacC mutants are affected in salt tolerance. (A) Wild-type strain 4287, loss-of-function mutant pacC+/−, and dominant activating mutant pacCc were grown for 3 days on plates with SM buffered at pH 6.0 and supplemented with the indicated compounds.
To determine whether the differences in sensitivity to Na+ or Li+ observed in the three strains were due to differential accumulation of these cations, we measured the intracellular levels of Li+ and Na+ in the different strains grown in liquid medium (pH 6.0) containing increasing levels of LiCl or NaCl. The results (Fig. 2) confirmed that increased sensitivity to Li+ and Na+ of the pacC+/− mutant was related to higher internal levels of the two cations. Moreover, the increased tolerance observed in the pacCc dominant activating mutant correlated with a lower intracellular accumulation of Li+ and Na+. Importantly, the three strains contained similar amounts of internal K+, ruling out the possibility that higher sensitivity or tolerance to Li+ and Na+ was caused by differences in the inhibition of K+ uptake by the toxic cations (Fig. 2C).
FIG. 2.
Mutations in pacC affect ion homeostasis. Intracellular accumulation of cations was determined in germlings of the F. oxysporum wild-type strain (□), loss-of-function mutant pacC+/− (▧), or dominant activating mutant pacCc (▪) grown for 12 h in the presence of the indicated concentrations of Li+ (A and C) or Na+ (B).
pacC is required for efficient cation efflux at alkaline ambient pH.
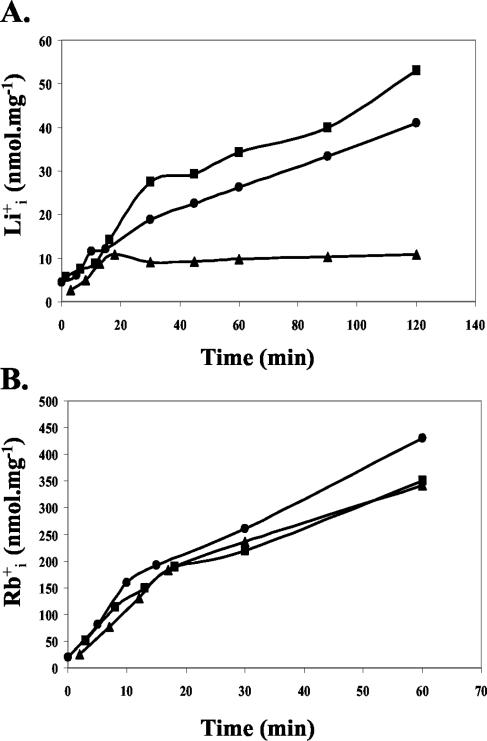
Based on these initial results, we decided to explore the effect of PacC on cation fluxes. To determine Li+ influx in the wild-type strain and the pacC mutants, mycelia were transferred to 0.1 M LiCl, and the time course of Li+ uptake was monitored for several hours. During the first minutes, the three strains accumulated Li+ at a very similar rate, suggesting that the kinetics of Li+ influx was not altered in the pacC mutants (Fig. 3A). After a few minutes, the internal level of Li+ in the salt-tolerant pacCc strain stabilized at ca. 10 nmol mg−1 and did not increase further, whereas levels continued rising in the wild-type strain and reached even higher levels in the salt-sensitive pacC+/− mutant. Because it is generally assumed that most of the Na+ and Li+ influx in fungi under standard laboratory conditions takes place through the K+ influx system (22), we studied the time course of influx of the K+ transport analog Rb+. We found that even after 1 h in the presence of 0.1 M RbCl, the kinetics of uptake and the internal levels of Rb+ were highly similar in all of the strains (Fig. 3B). Taken together, these results support the view that mutations in pacC do not affect alkali cation influx, thus indicating that efflux of these toxic cations may be affected in the pacC mutants.
FIG. 3.
Mutations in pacC do not affect kinetics of cation import. Kinetics of cation import was determined in germlings of the F. oxysporum wild-type strain (•), loss-of-function mutant pacC+/− strain (▪), or dominant activating mutant pacCc strain (▴). Samples were taken at the indicated times after the addition of 0.1 M LiCl (A) or 0.1 M RbCl (B).
In fungi, two Na+ and Li+ efflux systems have been described, a sodium/proton antiporter working at acidic pH and a P-type sodium ATPase functioning at a higher pH (20). To test whether one or both of these efflux systems was affected in the pacC mutants, microconidia of the three strains were germinated for 12 h in liquid medium containing 0.1 M LiCl (wild type and the pacC+/− strain) or 0.3 M LiCl (pacCc strain), transferred to lithium-free medium buffered either at pH 4.0 or 8.0, and the time course of Li+ efflux was determined. The results shown in Fig. 4 clearly indicate that, although at pH 4.0 the three strains extruded lithium with the same efficiency (Fig. 4A), at pH 8.0 the pacC+/− loss-of-function mutant displayed a strongly reduced Li+ efflux compared to the wild type and the pacCc strain (Fig. 4B). These results are in agreement with the increased Li+ accumulation and sensitivity of the pacC+/− loss-of-function mutant. Although we did not detect differences between the wild type and the pacCc strain in terms of kinetics of Li+ efflux, the fact that absolute concentrations of internal Li+ in the pacCc strain were significantly lower than those of the wild type (see Fig. 2A) suggests that Li+ efflux was more efficient in the pacCc strain. In summary, our results indicate that PacC controls salt tolerance in F. oxysporum by regulating an efflux system functional at an alkaline pH but not at an acidic pH. In support of this view, differences in salt tolerance between the wild type and the pacC mutants were almost negligible when strains were grown on plates at pH 4.0 (results not shown).
FIG. 4.
Mutations in pacC affect cation efflux at alkaline but not at acidic ambient pH. Kinetics of cation export was determined in germlings of the F. oxysporum wild-type strain (•), loss-of-function mutant pacC+/− strain (▪), or dominant activating mutant pacCc strain (▴). Microconidia were germinated for 12 h in SM containing 0.1 M Li+ (wild type and pacC+/− mutant) or 0.3 M Li+ (pacCc mutant), washed, and transferred to Li+-free medium buffered at pH 4.0 (A) or 8.0 (B), and samples were taken at the indicated times.
PacC controls transcriptional activation of ena1 encoding a P-type Na+-ATPase of F. oxysporum.
The results shown in Fig. 4 suggest that, like S. cerevisiae, F. oxysporum has at least two different Na+ (Li+) efflux systems: one functioning at pH 4.0 that is still present in the pacC+/− loss-of-function mutant and the other one working at pH 8.0 that is not present in the mutant. Because the main alkaline Na+ and Li+ efflux system in yeast and filamentous fungi is based on P-type Na+-ATPases encoded by the ENA genes (1, 3, 6, 7, 15), we decided to clone the ENA1 orthologue in F. oxysporum. PCR amplification with degenerate primers derived from highly conserved regions of the Neurospora crassa ena1 gene produced a fragment of the expected size (250 bp) that was cloned and sequenced. After we confirmed its homology with N. crassa ena1, we used the fragment to probe a λEMBL3 genomic library of F. oxysporum isolate 4287. Sequencing of a hybridizing genomic clones revealed the presence of an open reading frame of 3,261 nucleotides encoding a predicted protein of 1,087 amino acids. Sequence alignment of F. oxysporum ena1 with fungal ena1 genes in the databases suggested the presence of three introns 215, 51, and 49 nucleotides in length, respectively. The 5′-flanking sequence of the ena1 gene contains four copies of the PacC consensus binding sequence 5′-GCCARG-3′ (26) at positions −549, −474, −382, and −321. The sequence data have been submitted to the DDBJ/EMBL/GenBank databases under accession number AY345588. The deduced F. oxysporum Ena1 protein has 70 and 46% amino acid identity with the Ena1 proteins of N. crassa and S. cerevisiae, respectively, and contains nine putative transmembrane regions that are highly conserved between fungal P-type Na+-ATPases (Fig. 5). Southern analysis of genomic DNA digested with different restriction enzymes suggested that ena1 is present as a single copy in the F. oxysporum genome. Moreover, a BLAST search of the F. graminearum complete genome sequence with ena1 only produced one significant match (results not shown).
FIG. 5.
Amino acid sequence alignment of the predicted F. oxysporum ena1 gene product with fungal P-type Na+-ATPases. Deduced Ena1 proteins from F. oxysporum (EMBL accession no. AY345588), N. crassa (AJ243520), and S. cerevisiae (U24069) are shown. Identical amino acids are highlighted on a shaded background. Dashes indicate gaps in the alignments. Nine predicted transmembrane domains are indicated by solid bars.
To study the mechanisms controlling expression of ena1 in F. oxysporum, Northern hybridization analysis was performed with total RNA from mycelia of the wild-type strain transferred for 2 h to SM buffered at different pH values and containing either 0.5 M Na+ or no added Na+. No ena1 transcript was detected in mycelia grown in the absence of Na+ (Fig. 6A). In contrast, ena1 expression was strongly induced in the presence of Na+ at pH 8.0 but not at pH 4.0. Thus, a combination of Na+ and alkaline pH is required to trigger the expression of ena1 gene.
FIG. 6.
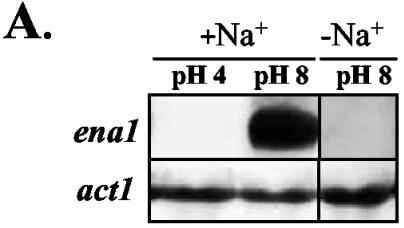
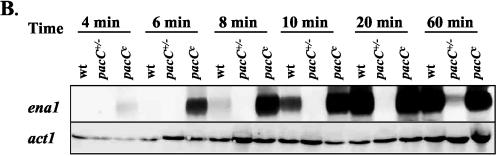
Activation of ena1 transcription requires high salt concentrations, alkaline ambient pH, and PacC. Transcript levels of ena1 in the F. oxysporum wild-type strain, pacC+/− mutant, and pacCc mutant were analyzed by northern hybridization analysis. (A) Microconidia of the wild-type strain were germinated for 12 h in SM without added Na+ and then transferred for 2 h to SM buffered at the indicated pH values, with or without 0.5 M Na+. Total RNA was extracted, fractionated on an agarose gel, blotted onto a nylon membrane and hybridized with the ena1 probe. As a loading control, a probe corresponding to the F. oxysporum actin gene (act1) was hybridized to the same RNA samples. (B) Microconidia of the wild-type strain, the pacC+/− mutant and the pacCc mutant were germinated as in panel A and transferred to SM buffered at pH 8.0 and containing 0.5 M Na+. Total RNA was extracted from samples obtained at the indicated time points, fractionated, blotted, and hybridized with the ena1 or act1 probes.
We next studied the kinetics of ena1 activation in the wild type and the pacC mutants upon transfer of mycelia to SM buffered at pH 8.0 containing 0.5 M Na+. In the wild-type strain, the ena1 transcript was detectable after 10 min and increased further until 20 min after transfer (Fig. 6B). In the pacCc− mutant, ena1 transcript levels increased much earlier and reached higher levels than in the wild-type strain. In both strains, transcript levels remained high until 60 min after transfer and declined slightly after 90 min (data not shown). In contrast, in the pacC+/− mutant ena1 expression was induced much later, only 60 to 90 min after the transfer, and remained at much lower levels. These results suggest that PacC positively controls transcriptional activation of ena1 in response to Na+ at alkaline pH.
DISCUSSION
The transcription factor PacC regulates expression of alkaline- and acid-expressed genes in F. oxysporum (11). We show here that this factor plays an essential role in salt tolerance of F. oxysporum because strains that lack PacC are highly sensitive to Na+ and Li+. Our data strongly suggest that salt sensitivity in these mutants is caused by a defect in the Na+ and Li+ efflux process. Conversely, dominant activating pacCc mutants show increased salt tolerance, correlating with rapid and increased expression of a sodium efflux system in this strain. In a previous study, we proposed the existence of an Na+-(Li+)-ATPase in F. oxysporum as the main system involved in the efflux of these cations (10). We identify here ena1, an F. oxysporum orthologue of the ENA1 genes from yeast and N. crassa (3, 6, 7, 15, 23). We show that expression of ena1 requires a combination of high Na+ concentrations and alkaline pH. Although the presence of Na+ alone is sufficient to induce expression of P-type Na+-ATPases in certain cases (1), our results are in agreement with most previous studies demonstrating that full expression of ENA genes requires a combination of Na+ and high pH (1, 3).
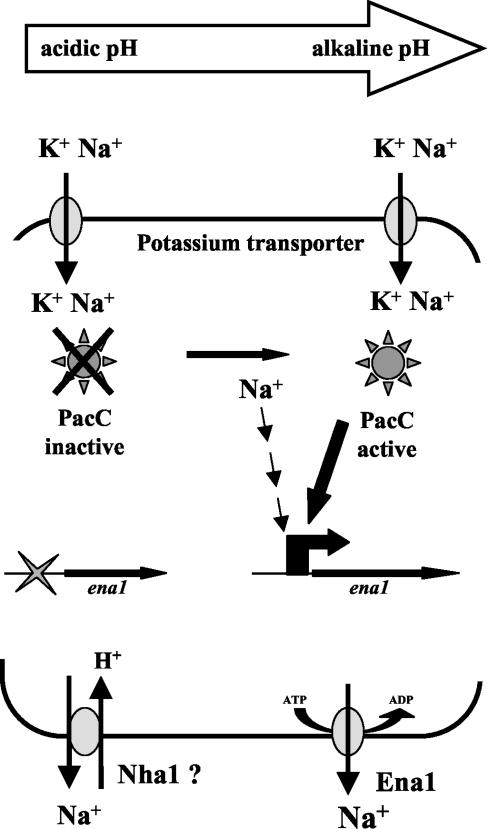
The changes in salt tolerance and intracellular Na+ and Li+ levels observed in the pacC mutants suggested a functional link between ambient pH, PacC, and ena1 expression in F. oxysporum. Our data support a positive role of PacC in the regulation of ena1, since pacC+/− loss-of-function mutants show strongly delayed and reduced expression of ena1, whereas pacCc strains expressing a dominant activating pacC allele induce ena1 expression more rapidly and to higher levels than did the wild type. Our results are similar to those reported in S. cerevisiae, where the pacC orthologue RIM101 was shown to control the expression of ENA1 (17, 25). Further supporting this view, we found that the 5′-flanking sequence of the ena1 gene of F. oxysporum contains four copies of the PacC consensus binding sequence 5′-GCCARG-3′. In Fig. 7 we present a model summarizing the regulation of ena1 expression by PacC in F. oxysporum. According to this model, PacC activates ena1 expression at alkaline ambient pH coordinately with a second factor that responds to high Na+ levels. Conversely, at an acidic ambient pH the Na+ efflux process would be mediated by a Na+/H+ antiporter acting independently of PacC. In S. cerevisiae, a Na+/H+ antiporter system encoded by the NHA1 gene mediates Na+ tolerance at acidic pH values (4, 5, 16). We have detected the existence in F. oxysporum of an orthologue of NHA, a plasma membrane Na+/H+ antiporter from fungi and plants (Z. Caracuel et al., unpublished data).
FIG. 7.
Model for the role of PacC in the control of ion homeostasis in F. oxysporum. High external concentrations of Na+ result in an increased influx of the cation, elevating intracellular Na+ levels beyond a threshold. This triggers transcriptional activation of the ena1 gene encoding a P-type Na+-ATPase via an unknown signaling mechanism. In addition, expression of ena1 also requires binding of the activated form of PacC transcription factor to its cognate binding sites in the promoter. Proteolytic activation of PacC occurs predominantly at alkaline ambient pH; therefore, both high Na+ concentrations and high pH are required for transcriptional activation of ena1. Conversely, at acidic pH an alternative, PacC-independent Na+ efflux system is active, possibly based on a Na+/H+ antiporter orthologous to Nha1 of S. cerevisiae. Besides activation of ena1, PacC may have additional regulatory effects on ion homeostasis.
The results of the present study suggest a major role for PacC in salt tolerance of F. oxysporum. Although most of the changes in Na+ sensitivity and accumulation observed in the pacC mutants could be explained by differences in ena1 expression with the consequent effects on cation efflux, such a function for ena1 in F. oxysporum remains to be demonstrated. Moreover, a role of additional regulatory mechanisms controlled by PacC cannot be ruled out. Thus, the fact that even after prolonged times of exposure, absolute concentrations of internal Li+ in the pacC+/− and pacCc mutants stabilized at significantly higher and lower levels than in the wild-type strain, respectively, suggests that mutations in pacC may not only affect cation efflux but also intracellular cation sensing and homeostasis, although the underlying mechanisms remain to be elucidated.
Acknowledgments
We gratefully acknowledge Eduardo Espeso, CIB, CSIC, Madrid, Spain, for helpful suggestions and discussions.
This research was supported by grants BIO2001-2601 to M.I.G.R. and BMC2002-04011-C05 to J.R. from the Spanish Ministerio de Ciencia y Tecnología. Z. C. has a Ph.D. fellowship from Ministerio de Ciencia y Tecnología. A.D.P. is the recipient of a Ramón y Cajal grant from Ministerio de Ciencia y Tecnología.
REFERENCES
- 1.Almagro, A., C. Prista, B. Benito, M. C. Loureiro-Dias, and J. Ramos. 2001. Cloning and expression of two genes coding for sodium pumps in the salt-tolerant yeast Debaryomyces hansenii. J. Bacteriol. 183:3251-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, C. W. Myers, and D. L. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bañuelos, M. A., and A. Rodríguez-Navarro. 1998. P-type ATPases mediate sodium and potassium effluxes in Schwanniomyces occidentalis. J. Biol. Chem. 273:1640-1646. [DOI] [PubMed] [Google Scholar]
- 4.Bañuelos, M. A., M. C. Ruiz, A. Jiménez, J. L. Souciet, S. Potier, and J. Ramos. 2002. Role of the Nha1 antiporter in regulating K+ influx in Saccharomyces cerevisiae. Yeast 19:9-15. [DOI] [PubMed] [Google Scholar]
- 5.Bañuelos, M. A., H. Sychrov , C. Bleykasten-Grosshans, J. L. Souciet, and S. Potier. 1998. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology 144:2749-2758. [DOI] [PubMed] [Google Scholar]
- 6.Benito, B., B. Garciadebl s, and A. Rodríguez-Navarro. 2000. Molecular cloning of the calcium and sodium ATPases in Neurospora crassa. Mol. Microbiol. 35:1079-1088. [DOI] [PubMed] [Google Scholar]
- 7.Benito, B., B. Garciadeblas, and A. Rodriguez-Navarro. 2002. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 148:933-941. [DOI] [PubMed] [Google Scholar]
- 8.Benito, B., F. J. Quintero, A. and Rodríguez-Navarro. 1997. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: conditions for phosphorylation from ATP and Pi. Biochim. Biophys. Acta 1328:214-225. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg, A., and L. Adler. 1993. Tolerance of fungi to NaCl, p. 209-232. In D. H. Jenings (ed.), Stress tolerance of fungi. Marcel Dekker, Inc., New York, N.Y.
- 10.Cabello-Hurtado, F., G. Blasco, and J. Ramos. 2000. Potassium and sodium fluxes in the phytopathogenic fungus Fusarium oxysporum var. lini. Curr. Microbiol. 41:363-367. [DOI] [PubMed] [Google Scholar]
- 11.Caracuel, Z., M. I. G. Roncero, E. A. Espeso, C. Gonz lez-Verdejo, F. I. García-Maceira, and A. Di Pietro. 2003. The pH signalling transcription factor PacC controls virulence in the plant pathogen Fusarium oxysporum. Mol. Microbiol. 48:765-779. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156. [DOI] [PubMed] [Google Scholar]
- 13.Di Pietro, A., and M. I. G. Roncero. 1998. Cloning, expression, and role in pathogenicity of pg1 encoding the major extracellular endopolygalacturonase of the vascular wilt pathogen Fusarium oxysporum. Mol. Plant Microbe Interact. 11:91-98. [DOI] [PubMed] [Google Scholar]
- 14.Garciadeblas, B., F. Rubio, F. J. Quintero, M. A. Bañuelos, R. Haro, and A. Rodríguez-Navarro. 1993. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol. Gen. Genet. 236:363-368. [DOI] [PubMed] [Google Scholar]
- 15.Haro, R., B. Garciadeblas, and A. Rodríguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 16.Kinclová, O., J. Ramos, S. Potier, and H. Sychrov . 2001. Functional study of the Saccharomyces cerevisiae Nha1p C terminus. Mol. Microbiol. 40:656-668. [DOI] [PubMed] [Google Scholar]
- 17.Lamb, TM., W. Xu, A. Diamond, and AP. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 18.Peñalva, M. A., and H. N. Arst, Jr. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66:426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raeder, U., and P. Broda. 1985. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1:17-20. [Google Scholar]
- 20.Ramos, J. 1999. Contrasting salt tolerance mechanisms in Saccharomyces cerevisiae and Debaryomyces hansenii, p. 377-390. In S. G. Pandalai (ed.), Recent research developments in microbiology, vol. 3. Research Signpost, Trivandrum, India.
- 21.Ramos, J., and A. Rodríguez-Navarro. 1985. Rubidium transport in Neurospora crassa. Biochim. Biophys. Acta 815:97-101. [Google Scholar]
- 22.Rodríguez-Navarro, A. 2000. Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469:1-30. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. A. LeVitre, L. S. Davidow, J. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of Ca2+ ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J., and D. W. Russell. 2001. Molecular cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Ariño. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 26.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Peñalva, and H. N. Arst, Jr. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]