SUMMARY
Hepatocellular carcinoma (HCC) is sexually dimorphic in both rodents and humans, with significantly higher incidence in males, an effect that is dependent on sex hormones. The molecular mechanisms by which estrogens prevent and androgens promote liver cancer remain unclear. Here, we discover that sexually-dimorphic HCC is completely reversed in Foxa1- and Foxa2-deficient mice after diethylnitrosamine-induced hepatocarcinogenesis. Co-regulation of target genes by Foxa1/a2 and either the estrogen receptor (ERα) or the androgen receptor (AR) was increased during hepatocarcinogenesis in normal female or male mice, respectively, but was lost in Foxa1/2-deficient mice. Thus, both estrogen-dependent resistance to and androgen-mediated facilitation of HCC depend on Foxa1/2. Strikingly, single nucleotide polymorphisms at FOXA2 binding sites reduce binding of both FOXA2 and ERα to their targets in human liver, and correlate with HCC development in women. Thus, Foxa factors and their targets are central for the sexual dimorphism of HCC.
INTRODUCTION
Sexual dimorphism is the biological inequality between females and males, favoring females in a variety of conditions including resistance to nutrient deprivation, prevention of premature aging, and resistance to diseases such as vascular and heart disease, brain disorders, and hepatocellular carcinoma (HCC) (Genazzani et al., 2007; Kalra et al., 2008; Stice et al., 2009). Sex hormones, i.e. estrogens in females and androgens in males, are the drivers of sexual dimorphism.
HCC is the fifth most common cancer and ranks third in annual mortality worldwide (Parkin et al., 2005). Women show significantly lower incidence of HCC than men (Parkin et al., 2005). Female rodents are also resistant to HCC compared to males during chemically-induced carcinogenesis (Kalra et al., 2008). Male mice treated with estrogen develop fewer liver tumors than control males, and ovariectomized females develop more liver tumors than normal females during chemically-induced carcinogenesis (Naugler et al., 2007; Shimizu et al., 1998; Tsutsui et al., 1992; Yamamoto et al., 1991). In addition, female mice deficient for the estrogen receptor alpha (ERα) lose their resistance to HCC (Naugler et al., 2007), while reduced incidence of HCC was observed in male mice lacking the androgen receptor (AR) (Ma et al., 2008; Wu et al., 2010). These data demonstrate that both protective effects of estrogens and deleterious effects of androgens contribute to the sexual dimorphism in HCC incidence. However, the molecular mechanisms of how this is achieved remain to be determined.
A recent report suggested that estrogens prevent HCC through inhibition of IL-6 expression in Kupffer cells, the resident macrophages in the liver, and that this in turn affects hepatocyte proliferation (Naugler et al., 2007). However, follow-up studies employing IL-6 antagonists or estrogen and its analogs to prevent HCC indicate that other mechanisms must also be involved in estrogen-mediated protection from liver cancer (Di Maio et al., 2006; Kalra et al., 2008; Lawrence et al., 2007). Comparatively little is known about the mechanism by which androgen signaling promotes HCC in males, although a recent study suggests that androgens enhance DNA damage and oxidative stress during hepatocarcinogenesis (Ma et al., 2008).
The vertebrate forkhead box A (Foxa) gene family of transcription factors consists of three members, Foxa1, Foxa2 and Foxa3, which are encoded by individual genes in mammals (Kaestner, 2010). Previous gene ablation studies of Foxa factors in mice have shown that Foxa1 and Foxa2 redundantly regulate liver development and metabolism, whereas the role of Foxa3 in the liver is limited (Bochkis et al., 2008; Friedman and Kaestner, 2006; Kaestner, 2005; Kaestner et al., 1998; Kaestner et al., 1999; Lee et al., 2005; Li et al., 2009; Shen et al., 2001; Sund et al., 2000). Though no liver forms in mice when both Foxa1 and Foxa2 are ablated in foregut endoderm following gastrulation (Lee et al., 2005), ablation of both Foxa1 and Foxa2 after liver specification does not affect hepatocyte development or proliferation (Li et al., 2009).
Genome-wide location analyses have revealed that FOXA1 and ERα or AR frequently bind to adjacent cis-regulatory elements in their target genes in human breast or prostate cancer cell lines, respectively, and that the recruitment of ERα or AR to their targets depends on FOXA1 (Carroll et al., 2005; Gao et al., 2003; Lupien et al., 2008; Yu et al., 2005). Hence, FOXA1 plays an essential role in estrogen and androgen signaling in breast and prostate epithelia.
We hypothesized that the effects of estrogens and androgens on HCC development are dependent upon Foxa factors. Using liver-specific gene ablation, we demonstrate that sexually-dimorphic HCC is completely dependent on Foxa1/2, and establish, using genome-wide analyses, how the winged helix transcription factors modulate the responses of the sex hormones in hepatic carcinogenesis.
RESULTS
Foxa1/2 Protect Female Mice from HCC and Promote HCC in Male Mice
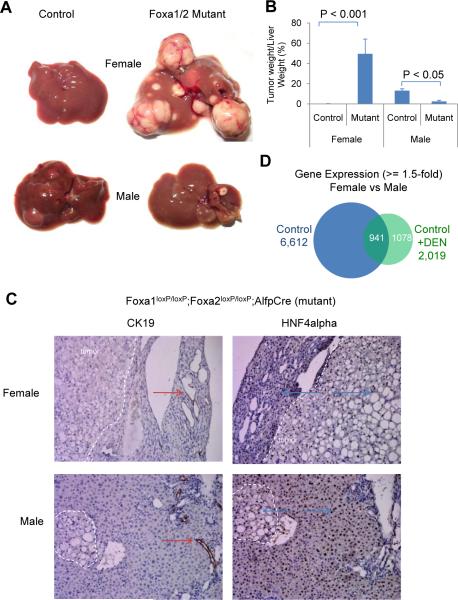
To investigate the role of Foxa factors in hepatocarcinogenesis, we induced liver tumors in control and liver-specific Foxa1/2-deficient mice (mutant) of both genders. We employed the established N,N-diethylnitrosamine (DEN) followed by TCPOBOP two-step strategy to induce rapid HCC formation in mice. Consistent with previous reports (Kalra et al., 2008), male control mice developed severe HCC following carcinogen treatment, whereas female control mice were resistant to hepatocarcinogenesis, as evidenced by both direct visualization and NMR quantification of tumor nodules (Figure 1A and 1B). Strikingly, multiple and large tumors were found in female Foxa1/2-deficient livers after carcinogen treatment, while tumor growth in male mutants was reduced when compared to controls (Figure 1A and 1B). Thus, sexual dimorphic HCC is completely dependent on the Foxa factors in mice. Immunostaining for Cytokeratin 19 (CK19) and hepatic nuclear factor 4 alpha (HNF4alpha), markers of cholangiocytes and hepatocytes, respectively, confirmed that the tumors in Foxa1/2-deficient livers were HCC and not cholangiocarcinomas (Figure 1C), consistent with the genotoxic action of DEN on hepatocytes. In the two-step strategy for hepatocarcinogenesis, phenobarbital-like inducers are commonly used for `boosting' tumor growth following tumor initiation by DEN (Oliver and Roberts, 2002; Qatanani and Moore, 2005). These phenobarbital-like inducers are CAR (constitutive androstane receptor, or Nr1i3) and PXR (pregnane X receptor, or Nr1i2) agonists. We had included the phenobarbital-like inducer TCPOBOP in our protocol, leading to the question of whether Foxa1/2-dependent differential tumor growth was related to CAR/PXR signaling. However, neither CAR nor PXR expression levels were correlated with tumor load in our mice; in fact we saw the lowest levels of CAR/PXR in female mutant mice treated with carcinogen, which had the highest tumor load (Figure S1A).
Figure 1. Foxa1 and Foxa2 Protect Female Mice from HCC and Promote HCC in Male Mice.
(A) Livers from Foxa1loxP/loxP;Foxa2loxP/loxP (control) and Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre (mutant) mice 18 weeks after exposure to hepatic carcinogens.
(B) The percentage of tumor weight to liver weight assayed by NMR. 5–6 mice were analyzed in each group.
(C) Immunostaining of cytokeratin 19 (CK19) and hepatic nuclear factor 4 alpha (HNF4alpha) in livers from Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre mice. Tumors are delimited by dotted white lines. Arrows indicate positive staining for CK19 (red) and HNF4alpha (blue).
(D) Gender-differential gene expression in control mice with or without carcinogen (DEN) administration. Purified hepatic RNA from four groups of mice (female and male, exposed to DEN or not) were processed for expression profiling. Over 6,000 genes are expressed in gender-specific fashion in control mice, but only ~ 2,000 in mice exposed to carcinogen.
See also Figure S1.
Enhanced Co-Regulation of Target Genes by Foxa1/2 and ERα in the Female Liver during Hepatocarcinogenesis
To investigate the mechanisms of relative HCC resistance in female mice, we characterized gender-specific gene expression in mice with or without carcinogen treatment using microarray analysis. 6,612 genes exhibited gender-specific differential expression in control mice without carcinogen treatment (Figure 1D). Interestingly, only 2,019 genes were differentially expressed between female and male control mice after carcinogen treatment, among which 1,078 genes were expressed in a gender-specific fashion only after DEN treatment (Figure 1D). This latter group is comprised of the gender-specific and carcinogen-responsive genes, which may play a role in the resistance to carcinogenesis in female mice and/or the promotion of carcinogenesis in male mice.
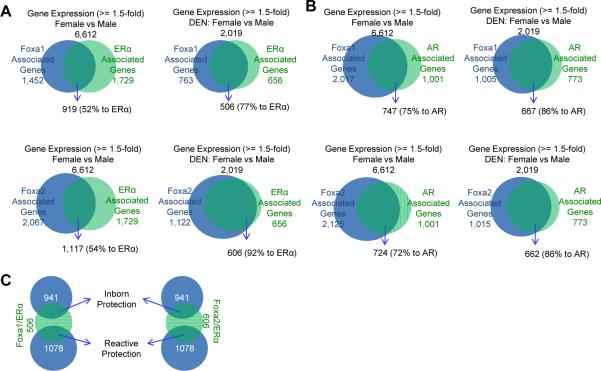
To identify Foxa and ERα target genes in the liver, we determined genome-wide binding sites of Foxa1, Foxa2, and ERα in control female livers without carcinogen treatment by ChIP-Seq. We also analyzed target genes associated with these binding sites (Table S1). By intersecting these target genes with the 6,612 genes showing gender-specific expression identified above (Figure 1D), we found 52% and 54% (919 and 1,117 genes) of ERα-associated genes associated with Foxa1 and Foxa2 binding sites, respectively (Figure 2A), suggesting co-regulation of Foxa1/2 and ERα of the majority of genes modulating gender dimorphism in females.
Figure 2. Enhanced Co-Regulation of Foxa and ERα/AR during Hepatocarcinogenesis.
Livers were collected from Foxa1loxP/loxP;Foxa2loxP/loxP (control) and Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre (mutant) mice with/without carcinogen exposure for 18 weeks and processed for ChIP-Seq analysis.
(A, B) Genome-wide co-regulation by Foxa1/2 and ERα (A) or AR (B) in control liver with or without carcinogen treatment. Genes associated with Foxa1/2, ERα, or AR were identified from ChIP-Seq analyses.
(C) Foxa/ERα dual-target genes potentially involved in resistance to hepatocellular carcinoma (HCC) in the liver of female mice before and after carcinogen administration, defined as inborn and reactive protection, respectively.
See also Figure S2, and Table S1 and S2.
Next, we asked whether regulation of gender-dimorphic gene expression was also dependent on the Foxa factors and ERα in livers of female mice exposed to carcinogen. As shown above, over 2,000 genes are expressed in a gender-specific fashion in the liver of mice exposed to DEN (Figure 1D). When we analyzed transcription factor binding in livers of female mice exposed to carcinogen (Table S1), we found co-occupancy of the Foxa factors with ERα greatly increased compared to the situation in livers of untreated females, with 77% and 92% of the ERα-bound genes also being targets of Foxa1 and Foxa2, respectively (Figure 2A), indicating enhanced co-regulation of Foxa1/2 and ERα in females during carcinogenesis. This finding suggests that the protection of females from hepatocarcinogenesis might result from co-regulation of protective target genes by both Foxa1/2 and ERα. Further analysis revealed that the Foxa/ERα dual target genes were almost equally distributed between those that were expressed in gender-specific fashion before and after carcinogen-treatment, which we define as `inborn protection' and `reactive protection', respectively (Figure 2C).
Resistance to HCC in Females by Estrogen Signaling Depends on Foxa1/2
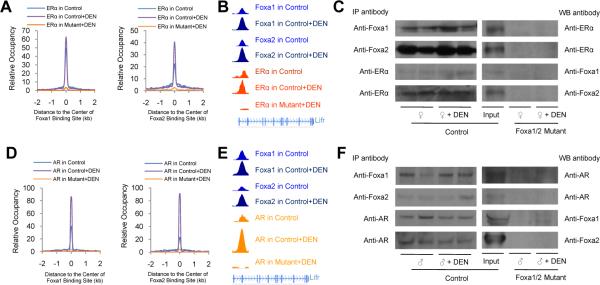
Next, we analyzed genome-wide co-localization of Foxa1/2 and ERα binding sites by calculating the distribution of ERα binding sites relative to the center of the Foxa1 or Foxa2 binding sites. Foxa1/2 and ERα binding sites were close to each other at the regulatory regions of dual target genes in control females (Figure 3A and 3B), suggesting co-regulation and/or co-operative binding Foxa1/2 and ERα. Remarkably, Foxa1/2 and ERα co-occupancy dramatically increased following carcinogen treatment in female livers, paralleling the increased number of binding sites of Foxa1, Foxa2, and ERα (Table S1, and Figure 3A and 3B), further supporting the notion that enhanced co-regulation of Foxa1/2 and ERα in response to carcinogen exposure plays a critical role in protecting female mice from HCC. More importantly, when we analyzed binding sites of ERα genome-wide in Foxa1/a2 mutant livers, we found ERα binding dramatically reduced (Table S1, and Figure 3A and 3B), providing genetic evidence that the recruitment of ERα to its binding sites depends to a very large extent on Foxa1/2.
Figure 3. Both Estrogen Signaling Preventing HCC and Androgen Signaling Promoting HCC Depend on Foxa1/2.
Livers were collected from Foxa1loxP/loxP;Foxa2loxP/loxP (control) and Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre (mutant) mice with/without carcinogen (DEN) treatment for 18 weeks and processed for ChIP-Seq analysis.
(A, D) Genome-wide distribution of ERα and AR binding sites relative to the center of Foxa1 or Foxa2 binding sites, respectively.
(B, E) Examples of Foxa1/2 and ERα or AR co-occupancy at the intronic region (~ 10 kb from the transcription start site) of the leukemia inhibitory factor receptor (Lifr) gene.
(C, F) The interactions between ERα/AR and Foxa1/2 were analyzed by immunoprecipitation (IP) followed by western blotting (WB), which were abolished in mutant liver. Liver homogenates were made with liver pieces lacking visible tumor nodules.
See also Figure S3.
FOXA1 has been found to directly interact with ERα in human breast cancer cell lines (Carroll et al., 2005; Gao et al., 2003). To investigate the mechanism by which Foxa1/2 and ERα modulated tumor progression in the liver, we employed immunoprecipitation with anti-Foxa1, anti-Foxa2, or anti-ERα antibodies followed by Western blot analysis. We found that both Foxa1 and Foxa2 are complexed with ERα in the female mouse liver with or without carcinogen administration (Figure 3C), further supporting the notion of co-regulation of target genes by Foxa1/2 and ERα. As expected, the interaction between Foxa1/2 and ERα was abolished in Foxa1/2 mutant livers, confirming the specificity of the immunoprecipitation assay (Figure 3C).
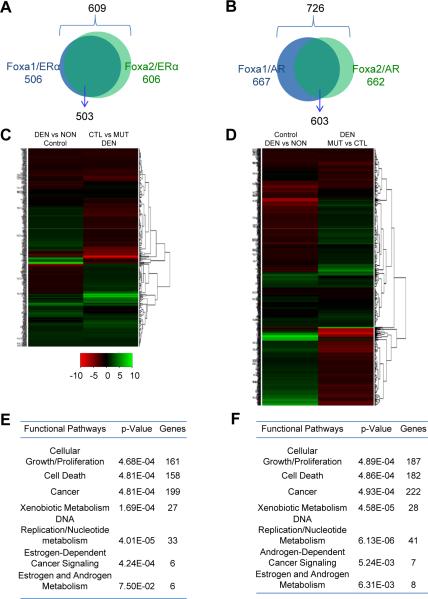
Genomic distribution analysis showed that Foxa1, Foxa2, and ERα binding sites were widespread in the genome, with preference at enhancer regions (intergenic and intron regions) (Table S1 and S2). In addition, the common targets of Foxa1/ERα were largely overlapping with those of Foxa2/ERα in the female liver (Figure 4A). In total, we found 609 Foxa/ERα common targets in genes for which gene expression changes in response to carcinogen exposure (Figure 4A).
Figure 4. Comprehensive Protection from Hepatocarcinogenesis by Foxa1/2 and ERα.
Livers were collected from Foxa1loxP/loxP;Foxa2loxP/loxP (control) and Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre (mutant) mice with/without carcinogen (DEN) treatment for 18 weeks.
(A, B) Foxa/ERα (A) or Foxa/AR (B) dual targets potentially responsive to liver cancer resistance or promotion in female or male mice, respectively.
(C, D) The change of gene expression of Foxa/ERα (C) or Foxa/AR (D) dual targets in female or male control mice was mostly reversed in mutant mice during hepatocarcinogenesis, respectively. DEN, mice with carcinogen; NON, mice without carcinogen. MUT, mutant mice; CTL, control mice.
(E, F) Pathway analysis of all Foxa/ERα (E) or Foxa/AR (F) dual targets during hepatocarcinogenesis.
See also Figure S4 and S7.
Next, we analyzed the gene expression profile of these 609 Foxa/ERα dual target genes in female control and Foxa1/a2 mutant liver following exposure to carcinogen. Strikingly, we found that the change in gene expression for these Foxa/ERα dual targets following DEN treatment was largely (for ~77% of the genes in question) reversed between control and mutant female mice (Figure 4B). Thus, deficiency of Foxa1/2 caused differential expression of Foxa/ERα dual targets, indicating that the co-regulation of Foxa/ERα dual target genes depends on Foxa1/2.
To investigate the mechanism that caused enhanced co-regulation of Foxa and ERα, we analyzed the target occupancy of each factor (Foxa1, Foxa2, or ERα) before and after carcinogen treatment. We observed enhanced occupancy of Foxa1, Foxa2, and ERα in livers of female mice exposed to carcinogen (Figure S2A). To confirm this observation, we performed sequential ChIP-qPCR assays with anti-Foxa2 and anti-ERα antibodies for 20 Foxa2/ERα targets identified by ChIP-Seq analysis. 16 targets were confirmed for both Foxa2 and ERα with at least 2-fold enrichment in liver of female control mice not treated with carcinogen (Figure S3A). As expected, none of these genes were ERα-bound in male mice, although 13 of them were bound by Foxa2 (Figure S3C). All 20 targets showed significant binding for both Foxa2 and ERα in livers of female mice after carcinogen administration (Figure S3A). Linear regression analysis showed that carcinogen exposure significantly enhanced binding of Foxa2 and ERα to these common targets (Figure S3B). Interestingly, enhanced Foxa2 binding at these loci was also observed in male mice after carcinogen administration, although these sites lacked ERα binding as expected (Figure S3C). Strikingly, we found that ERα binding to all these targets was eliminated in Foxa1/2-deficient livers, regardless of exposure to carcinogen (Figure S3A), further demonstrating that ERα binding at these dual targets is completely dependent on the presence of Foxa factors. In conclusion, these findings suggest that Foxa1/2 and ERα cooperate to regulate gene expression in the female liver, and that Foxa1/2 is essential for estrogen signaling in the protection of female mice from chemically-induced hepatocarcinogenesis.
Comprehensive Protection from Hepatocarcinogenesis by Foxa1/2 and ERα
Detailed analysis of the Foxa/ERα dual targets suggested that these genes modulate multiple pathways in resistance to HCC, as indicated by their gene annotations (Figure 4C). One of these targets is the oncogene Myc. We found multiple Foxa2 and ERα binding sites in Myc promoter and enhancer regions, and confirmed Foxa2 and ERα co-occupancy at these sites by sequential ChIP (Figure S4A). In addition, we found that Myc expression was significantly higher in female mutant livers compared to controls after carcinogen treatment (Figure S4B). When we suppressed Myc expression in isolated hepatocytes from DEN-treated Foxa1/2 mutant mice using siRNA, hepatocyte proliferation was significantly reduced as shown by Ki67 expression (Figure S4C). These findings implicate Myc as an important target for repression by Foxa2/ERα in the livers of female mice. However, as mentioned above, multiple genes shown to be involved in cell cycle control and carcinogenesis are Foxa/ERα dual targets, and multiple co-binding sites of Foxa1/2 and ERα were also observed at regulatory regions of the `HCC-resistant genes' as defined above. These genes are involved in pathways related to cancer resistance, including xenobiotic metabolism and detoxification, DNA biosynthesis and replication, and cell cycling and proliferation (Figure 4C and Figure S6). Thus, Foxa1/2 and ERα co-regulate multiple pathways of hepatocarcinogenesis.
A prior study had proposed IL-6 as an essential mediator of gender-specific hepatocarcinogenesis (Naugler et al., 2007). We had found previously that gene ablation of Foxa1/2 after liver specification led to mildly elevated IL-6 expression and bile duct hyperplasia in the adult mouse liver (Li et al., 2009). Based on this model, we expected that Foxa1/2-deficient livers would develop more tumors than controls in both genders, because serum IL-6 levels were elevated similarly in male and female Foxa1/2 mutant mice (Figure S5). However, this was not the case, as female Foxa1/2 mutant mice developed dramatically more and larger tumors than male mutants (Figure 1A). Furthermore, while we observed higher serum IL-6 levels in male mice compared to female control mice after DEN administration, IL-6 levels in DEN-treated mutant mice did not correlate with carcinogenesis in males and females (Figure 1A and Figure S5). In addition, the tumor load did not correlate with the levels of IL-6 among all four groups (Figure 1A and Figure S5). Therefore, we conclude that IL-6 is not a major factor regulating sexual dimorphism of HCC.
Androgen Signaling Promoting HCC in Males Depends on Foxa1/2
Remarkably, Foxa1/2 deficiency significantly reduced tumor load in male mice after DEN administration (Figure 1A and 1B). To investigate the molecular basis of this phenomenon, we analyzed genome-wide binding sites and the corresponding genes for Foxa1, Foxa2, and AR in control male livers without carcinogen treatment by ChIP-Seq (Table S1). Genomic distribution analysis showed that Foxa1, Foxa2, and AR binding sites were widespread in the genome, with preference at enhancer regions (intergenic and intronic regions) (Table S1). The numbers of Foxa1, Foxa2, and AR binding sites were greatly increased in livers of male mice exposed to carcinogen as compared to controls (Table S1, Figure S2B). Next, we intersected Foxa1/2 and AR targets with the 6,612 genes showing gender-specific expression in control mice without carcinogen treatment (see Figure 1D). Among the intersect group, 75% or 72% (747 or 727 genes) of AR-associated genes were also bound be Foxa1 or Foxa2, respectively (Figure 2B), indicating potential co-regulation of Foxa1/2 and AR in controlling gender dimorphic gene expression in the male liver. We also analyzed genome-wide binding sites of Foxa1, Foxa2, and AR in livers of male mice exposed to DEN by ChIP-Seq (Table S1). Among the genes (2,019) differentially expressed in the livers of male and female mice following carcinogen treatment, Foxa1/2 and AR target co-occupancy was even higher than among the control group, with 86% and 86% of the AR-bound genes also being targets of Foxa1 and Foxa2, respectively (Figure 2B).
Next, we analyzed genome-wide co-localization of Foxa1/2 and AR binding sites from ChIP-Seq by calculating the distribution of AR binding sites relative to the center of the Foxa1 or Foxa2 binding sites. Foxa1/2 and AR binding sites were very close to each other at the regulatory regions of their common target genes (Figure 3D and 3E). Remarkably, Foxa1/2 and AR co-occupancy dramatically increased following carcinogen treatment in male livers (Figure 3D and 3E), further supporting the notion that enhanced co-regulation of Foxa1/2 and AR plays a critical role in promoting HCC in male mice. Strikingly, when we analyzed target binding by AR in Foxa1/2 mutant livers, we found AR binding to the common Foxa/AR targets almost completely abolished (Table S1, and Figure 2D and 2E), demonstrating that the recruitment of AR to its binding sites depends on Foxa1/2.
Both FOXA1 and FOXA2 have been found to directly interact with the AR in human prostate cancer cells (Gao et al., 2003; Yu et al., 2005). By immunoprecipitation assays, we found that both Foxa1 and Foxa2 were bound to AR in the male mouse liver, regardless of exposure to DEN (Figure 3F). As expected, the interaction between Foxa1/2 and AR was completely abolished in Foxa1/a2 mutant livers, confirming the specificity of our immunoprecipitation assay (Figure 3F).
In addition, the common Foxa1/AR targets were largely overlapping with those of Foxa2/AR in the male liver and were mostly found at putative enhancer regions (Figure 4D and Table S2). Just as was the case for the Foxa/ERa common targets, we found that the difference in gene expression of Foxa/AR dual targets between DEN-treated and -untreated control mice was reversed for most (~80%) genes in the livers of male Foxa1/a2 mutant male mice (Figure 4E). Functional annotation of Foxa/AR dual target genes revealed that Foxa1/2 and AR promote tumor growth through multiple pathways, including DNA replication, cell cycling, and cell growth and proliferation (Figure 4F). In conclusion, these findings demonstrate that Foxa1/2 and AR cooperate to regulate gene expression in the male liver, and that Foxa1/2 is essential for androgen signaling in promoting hepatocarcinogenesis in male mice.
Good and Bad Estrogen
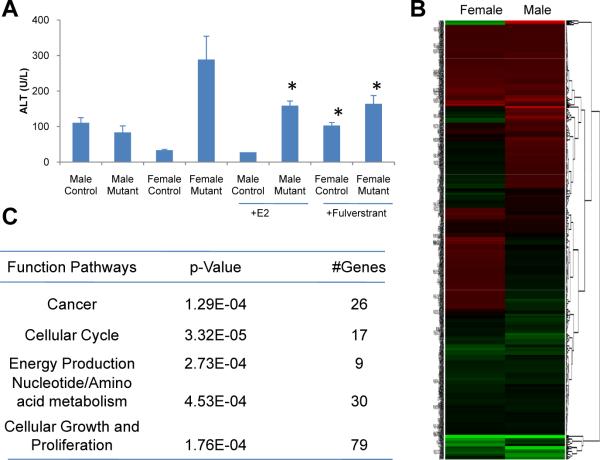
Hepatocyte injury is considered the first step in hepatocarcinogenesis induced by DEN. Administration of estrogen to male mice treated with DEN prevents liver injury and carcinogenesis (Kalra et al., 2008), which we confirmed in our own study (Figure 5A). In addition, administration of the ERα-specific antagonist, fulverstrant (also called Faslodex), to female control mice led to loss of protection from DEN-mediated liver injury, confirming the importance of estrogen signaling in preventing HCC (Figure 5A). However, we found that estrogen enhanced liver injury in DEN-treated Foxa1/2-deficient male mice (Figure 5A).
Figure 5. Good and Bad Estrogen.
(A) Liver injury as indicated by alanine aminotransferase (ALT) plasma levels in mice following DEN administration. Male mice were treated with 100 mg/kg estrogen (E2) and female mice were treated with 50 mg/kg fulverstrant, a specific inhibitor of ERα. *, p < 0.05 from comparison between female mutants with and without fulverstrant, between female controls with and without fulverstrant, and between male mutants with and without E2.
(B) ERα-unique target genes show opposite effects on female and male mutant mice after carcinogen treatment.
(C) Functional pathway analysis of ERα-unique targets during hepatocarcinogenesis.
See also Figure S5.
The data on tumor load in Foxa1/2-mutant mice present a new puzzle (Figure 1A,B). If estrogen had only hepatoprotective effects, one would expect female Foxa1/2-mutant mice to develop exactly the same tumor load as male controls after exposure to DEN. However, as shown above, the tumor load in female mutants far exceeded that of male mice (Figure 1A,B). This suggests that in the absence of Foxa1/2, estrogens exert an additional tumor-promoting effect on hepatocytes. Estrogen itself has been found to act as a carcinogen due to the genotoxicity of its metabolites, such as 16 alpha-hydroxy estrone and 2- and 4-hydroxycatechol estrogens (Yager and Liehr, 1996). When we blocked estrogen signaling with fulverstrant, DEN-induced liver injury was significantly attenuated in female Foxa1/2 mutant mice (Figure 5A). However, this tumor-promoting effect of estrogen was only observed when Foxa1/2 was absent, indicating that the protective effect of estrogens is normally dominant over its tumor-promoting action. Estrogens have been employed clinically to treat many cancers with mixed results: in some cases, estrogens were found to prevent cancer progression; whereas in other cases, estrogens were found to promote tumor growth (Di Maio et al., 2006; Kalra et al., 2008; Lawrence et al., 2007). Our findings regarding the role of Foxa1/2 in estrogen carcinogenesis provide a possible explanation for these findings, in that estrogens prevent cancer progression when Foxa1/2 are present, but promote tumor growth in the absence of the Foxa factors.
In addition to the Foxa/ERα dual targets discussed above, there are also many genes solely bound by ERα in the female liver (Table S1). These are candidate genes to explain the cancer-promoting effects of estrogens in female mice lacking Foxa1/a2. Interestingly, most of these ERα-unique target genes showed opposite changes between female and male mutant mice after carcinogen treatment (Figure 5B). Pathway analysis revealed that most of them are involved in cell cycle, cancer development, and nucleotide/amino acid metabolism (Figure 5C). Thus, estrogen and its metabolites enhance hepatocarcinogenesis through both their genotoxicity and estrogen signaling when Foxa1 and Foxa2 are absent.
Foxa1/2-guided Genomic Landscapes for Sex Hormone Gene Regulation
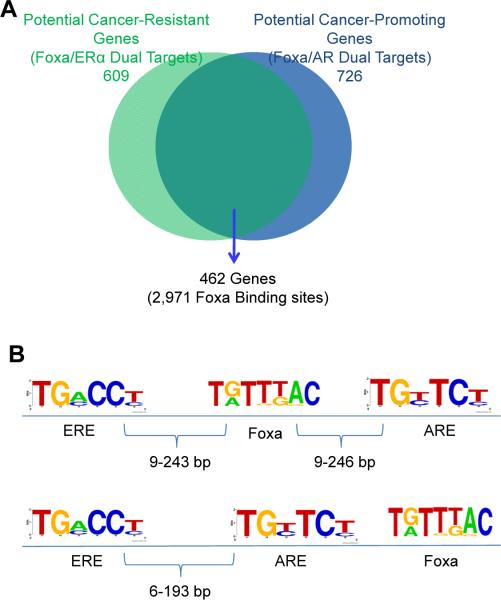
As shown above, we discovered gender-specific recruitment of ERα and AR to Foxa1/a2 binding sites in female and male liver, respectively. In addition, we did not observe significant differences of either Foxa1 or Foxa2 binding between males and females, or of their core consensus sequences (Figure S2C and S2D). Next, we compared the genomic locations of the 609 Foxa/ERα dual target genes that are likely mediators of carcinogenesis resistance in females with the 726 Foxa/AR dual target genes that are likely promoting carcinogenesis in male mice. Strikingly, we found that the vast majority of the targets (76% of Foxa/ERα and 64% of Foxa/AR) were overlapping between females and males (Figure 6A). For example, leukemia inhibitory factor receptor (Lifr) was bound by Foxa/ERα or Foxa/AR at the same location in female or male liver, respectively (Figure 3B and 3E). De novo motif analysis showed the presence of a strong consensus Foxa motif at these sites, as expected (Figure 6B). Next, we searched for estrogen response elements (ERE) and androgen response elements (ARE) in proximity (± 250 bp) to these Foxa binding sites. We found that the distribution of ERE/ARE pairs was random at these Foxa binding sites, with half of the ERE/ARE pairs clustered on either side of the Foxa core element, and the other half separated by the Foxa motif (Figure 6B). These data suggest that adjacent localization of Foxa binding sites with both steroid hormone receptor binding elements (ERE and ARE) enables gender-specific regulation of the same set of genes by each hormone in the liver.
Figure 6. Foxa-guided Genomic Landscape for Sex Hormone Gene Regulation.
(A) Genes regulating both cancer resistance in females and cancer promotion in males that are targeted by Foxa and ERα, Foxa and AR, or both.
(B) Genomic distribution of estrogen response elements (ERE) and androgen response elements (ARE) near Foxa binding sites. Genomic regions near Foxa binding elements (± 250 bp) were used to search ERE and ARE. Two groups of ERE-ARE pairs near Foxa2 binding elements at each locus were found: half were separated by Foxa motifs (upper panel); the other half were paired on either side of Foxa binding sites (bottom panel).
A Role of FOXA1/2 in Human HCC
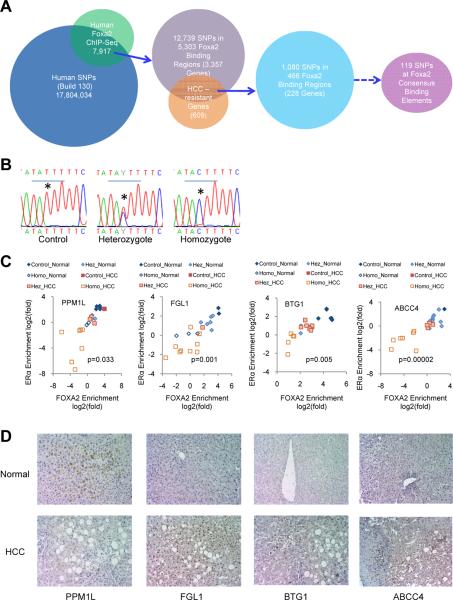
Mutations in gene coding regions are the predominating cause of cancer. Where analyzed to date, hepatic FOXA1/2 levels did not differ significantly in HCC patients compared to controls (Chen et al., 2002; Lee et al., 2004; Lee et al., 2006), in concordance with our results regarding Foxa1 and Foxa2 expression in mice treated with DEN (Figure S1C) and previous reports in mice during hepatocarcinogenesis (Kalra et al., 2008). However, our findings suggest the possibility that mutations in cis-regulatory elements binding FOXA1 and/or FOXA2 could predispose humans to HCC. To investigate this possibility, we analyzed genome-wide FOXA2 binding in healthy human livers by ChIP-Seq and found 7,917 FOXA2 binding sites. Next, we retrieved single nucleotide polymorphisms (SNP) from the human GWAS database (NCBI, build 130). By intersecting these two datasets, we found 12,739 SNPs, associated with 3,357 genes, located within 5,303 FOXA2 binding regions (Figure 7A). We intersected this human gene list with the 609 dual Foxa/ERα targets (potential liver cancer-resistance genes) identified above in the mouse liver (Figure 4A). We obtained 228 potential `HCC-resistance genes' associated with 466 FOXA2 binding sites that contained 1,080 SNPs in the human orthologous loci (Figure 7A). Using the MATCH program to search the binding elements of transcription factors based on the positional weight matrices from the Transfac database (Kel et al., 2003), we further identified FOXA2 consensus binding elements in those 466 FOXA2 ChIP-Seq regions and found that 119 of them contained SNPs within the FOXA2 core binding element (TRTTT, R=G or A) (Figure 7A).
Figure 7. The Relationship Between Hepatocellular Carcinoma (HCC) and Single Nucleotide Polymorphisms (SNPs) at FOXA2 Binding Sites in Women.
(A) Computational procedure for identifying SNPs at FOXA2 binding sites. Human SNPs were downloaded from the NCBI GWAS database (Build 130). FOXA2 binding sites in normal human livers were identified by FOXA2 ChIP-Seq. Potential HCC-resistance genes were identified from our mouse models described above (Figure 1D).
(B) Examples of sequencing results for SNPs at FOXA2 binding sites associated with BTG1. *, the nucleotide with SNP from T to C.
(C) Synergic reductions of FOXA2 and ERα binding at FOXA2 binding sites containing SNPs are associated with increased incidence of HCC. FOXA2 binding regions with potential SNPs were sequenced in 11 healthy (normal) and 11 HCC livers from women. ChIP assays with anti-FOXA2 or anti-ERα antibodies revealed that mutations at core FOXA2 binding sites were associated with impaired binding of both FOXA2 and ERα, shown as ChIP enrichment in livers from women. The occupancy of both FOXA2 and ERα (ChIP enrichment) in all samples was highly correlated with the number of mutation in both alleles. Hez, heterozygous SNP; Homo, homozygous SNPs. These mutations were found at FOXA2 binding sites associated with four genes PPM1L (protein phosphatase, Mg2+/Mn2+ dependent, 1L), FGL1 (fibrinogen-like 1), BTG1 (B-cell translocation gene 1), and ABCC4 (ATP-binding cassette, sub-family C (CFTR/MRP), member 4). The co-occupancy of FOXA2 and ERα is significantly reduced in HCC livers compared to normal controls. P values are from the comparison of co-occupancy of FOXA2 and ERα between 11 normal and 11 HCC livers by t-test.
(D) Immunohistochemical detection of PPM1L, FGL1, BTG1 and ABCC4 in the livers of normal and HCC women. Magnification × 200.
See also Figure S6.
In order to investigate the impact of FOXA2 SNPs on estrogen signaling, we determined FOXA2 and ERα binding at regions of ± 200 bp surrounding these FOXA2 core binding elements by ChIP-qPCR in samples from four healthy and four HCC livers from women. Strikingly, among the 113 FOXA2 binding sites tested, 18 sites showed significant reduction in binding to both FOXA2 and ERα in some or all HCC livers compared to controls (Figure S6). To identify SNPs at these FOXA2 binding sites, we sequenced these 18 loci in the HCC samples and found multiple point mutations, deletions, and insertions that were either heterozygous or homozygous (Figures S6 and Figure 7B). In most cases, SNPs at FOXA2 binding sites were associated with impaired FOXA2 and ERα binding (Figures S6). In particular, the BTG1 (B-cell translocation gene 1), FGL1 (fibrinogen-like 1), and ABCC4 (ATP-binding cassette, sub-family C (CFTR/MRP), member 4) loci had mutations at one or two alleles causing loss of binding of both FOXA2 and ERα in all four HCC patients, while three out of four PPM1L (protein phosphatase, Mg2+/Mn2+ dependent, 1L) loci contained mutations at one or both alleles (Figure S6B) and all loci with mutations also showed impaired binding of both FOXA2 and ERα (Figure S6B). BTG1 is an anti-proliferative factor (Rouault et al., 1992), FGL1 is an acute phase reactant for inflammatory response (Liu and Ukomadu, 2008), ABCC4 mediates the membrane transport of various molecules in multi-drug resistance (Russel et al., 2008; Vlaming et al., 2006), and PPM1L is a tumor suppressor (Thean et al., 2010). To confirm this observation, we analyzed additional seven normal and seven HCC livers from women. When analyzing all 22 samples, we found that both FOXA2 and ERα binding were highly correlated with the sequence of the FOXA2 binding elements (Figure S6C), and that FOXA2/ERα dual occupancy was significantly different between normal and HCC livers (Figure 7C). Impaired binding of both FOXA2 and ERα occurred predominantly in female HCC livers (Figure 7C), which we postulated would lead to altered gene expression of BTG1, FGL1, ABCC4 and PPM1L, which in turn might contribute to the development of HCC. Indeed, when we performed immunostaining of these four proteins for liver sections from female HCC patients and normal controls, we found that PPM1L expression was reduced and FGL1, BTG1 and ABCC4 expression was increased in HCC livers compared to normal controls (Figure 7D). These data suggest that impaired regulation by FOXA2 and ERα contributed to altered expression of these dual target genes in HCC and supports the notion that SNPs at FOXA2 binding sites could contribute to HCC risk in women.
DISCUSSION
Hepatocellular carcinoma is an often lethal malignancy with limited treatment options. Here, we have uncovered a central role for the winged helix transcription factors Foxa1 and Foxa2 in controlling estrogen and androgen signaling through recruitment of ERα and AR to their relevant targets in the liver, thereby explaining the sexual dimorphism of liver cancer in mammals. Not only are the Foxa factors required for the sex hormone receptors to bind to many of their targets, importantly, tumor growth is also strongly dependent on Foxa1/2. Thus, tumor load is dramatically increased in the livers of female Foxa1/2 mutant mice, and decreased in the livers of male Foxa1/2 mutants exposed to hepatocarcinogens. Our current view of how the Foxa1/2 factors control multiple aspects of hepatocarcinogenesis is summarized in the schema provided in Supplementary Figure S7.
The Foxa factors play a dominant role in determining the gender specificity of HCC development. Interestingly, without carcinogen treatment, Foxa1/2 mutant mice maintain much of the sexual dimorphic gene expression profile that is present in control livers (data not shown). This suggests that the loss of gender specificity in Foxa1/2-deficient mice occurs with the onset of carcinogen exposure. Given the fact that multiple physiological and pathophysiological processes are sexually dimorphic in the organs expressing Foxa proteins, we speculate that other stresses, like dietary deprivation and aging, might also invoke gender-specific responses from Foxa factors, which we aim to address in the future.
A previous study had attributed the disparity in liver cancer between the sexes to differences in IL-6 production by Kupffer cells in response to chemical carcinogens (Naugler et al., 2007). In fact, DEN-induced tumor incidence was reduced in both male and female IL-6 null mice. However, while we confirmed higher IL-6 plasma levels in wild type male mice treated with DEN compared to female controls, IL-6 levels did not correlate with tumor load in Foxa1/2-deficient mice (Figure 1A and Figure S5). In addition, because Foxa1/2 were not ablated in Kupffer cells but in hepatocytes in our model, the effect of the sex hormones on tumor susceptibility shown here is exclusively due to direct action of the Foxa factors on the parenchymal cells in the liver.
Estrogen promotes and estrogen antagonists (such as tamoxifen) prevent the growth of breast cancer cells, at least in estrogen receptor-positive tumors (Hollingsworth et al., 1998; Zumoff, 1998). Androgen promotes and androgen deprivation prevents the growth of prostate cancer cells (Paulson, 1984; Smolev et al., 1977). As discussed above, in both cancers, binding of the sex hormone receptor occurs near FOXA1 binding sites. However, in liver cancer, a different scenario has been recognized for years, in that estrogen prevents cancer development, opposite to the situation in the mammary gland. As we have shown here, both the effects of androgens and estrogens are Foxa1/2-dependent in the liver. Thus, a puzzle emerges: how can it be that estrogen signaling, dependent on Foxa factors in both the mammary gland and the liver, is tumor-promoting in the former, and tumor-preventing in the latter? This paradox suggests the existence of tissue-specific targets or tissue-specific co-regulation of the Foxa/ERα axis in the two tissues, an issue that will be of great interest for future investigation.
Thus far, no mutations in the FOXA1 or FOXA2 gene have been linked to human HCC. This might not be too surprising given the redundant function of the two proteins, which suggests that mutations in one or the other might not be sufficient to affect tumor initiation or progression. However, strikingly, we found multiple examples of single nucleotide polymorphisms in FOXA binding sites that affect FOXA and ERα occupancy, which were significantly more frequent in HCC samples than in normal livers from women. Not only does this finding provide supporting evidence that the co-regulation of target genes by FOXA and ERα extends from mice to humans, but it also suggests that SNPs in FOXA binding sites could contribute to the risk of hepatocarcinogenesis in women. Future large-scale studies investigating HCC risk in women with respect to these SNPs appear warranted.
We previously found that glucocorticoid receptor (GR)-mediated transcriptional regulation also depends on Foxa1/2 (Li et al., 2009; Zhang et al., 2005). Full activation of certain genes in the liver in response to a prolonged fast, and engagement of their cis-regulatory elements by GR requires Foxa2 (Zhang et al., 2005). Likewise, repression of the IL-6 promoter by GR is dependent on the Foxa factors in hepatocytes (Li et al., 2009). Why are Foxa factors required for steroid hormone signaling? Our current study suggests that Foxa factors serve as a scaffold for steroid hormone receptors to regulate gene transcription in the liver on a genome-wide scale, and extends to at least three nuclear hormone receptors. Our finding that two sets of sex hormone cis-regulatory elements, AREs and EREs, are found close to the same Foxa binding site indicates that Foxa-dependent estrogen and androgen signaling regulates the same set of genes in the liver (Figure S7). More importantly, our studies showed that ERα and AR had opposite effects on this gene set between females and males (Figure 4 and Figure S7), which provides a molecular explanation for previous findings that estrogen and androgen were able to reverse HCC incidence in the opposite genders (Naugler et al., 2007; Shimizu et al., 1998; Tsutsui et al., 1992; Yamamoto et al., 1991). In conclusion, we have identified a set of regulatory units in which juxtaposition of Foxa binding sites next to both EREs and AREs allows for the mediation of the gender-specific effects of the sex hormones in the liver.
EXPERIMENTAL PROCEDURES
The derivation of Foxa1loxP/loxP;Foxa2loxP/loxP (control) and Foxa1loxP/loxP;Foxa2loxP/loxP;AlfpCre (mutant) mice has been reported previously (Gao et al., 2008; Li et al., 2009; Sund et al., 2000). A two-step strategy was employed for hepatocarcinogenesis for 5–6 mice in each group. 32 microarray analyses were performed in livers from control and mutant mice of both genders with or without carcinogen treatment (four mice in each group for eight groups) for gene expression profiling. 17 ChIP-Seq experiments were performed and analyzed, for Foxa1, Foxa2, ERα, AR in control and Foxa1/a2 mutant livers of both genders with or without carcinogen treatment, and FOXA2 in normal human liver. Detailed experimental procedures are provided in supplemental information.
Supplementary Material
HIGHLIGHTS
Estrogen/androgen signaling prevents/promotes liver cancer in females/males, respectively.
ERα-dependent prevention and AR-mediated promotion of liver cancer depend on Foxa1/2.
Foxa1/2 and ERα/AR co-regulate multiple pathways of hepatocellular carcinogenesis.
Human SNPs at FOXA2 binding sites correlate with HCC in women.
ACKNOWLEDGEMENTS
We thank Drs. Jeff Albrecht, Linda Greenbaum, and Snorri Thorgeirsson for valuable comments on the manuscript. We gratefully acknowledge Alan Fox, Olga Smirnova, Karrie Brondell, Amber Riblett, James LaRossa and Reina Aoki for their excellent technical support. We also thank Dr. Erik Knudsen for sharing his hepatocarcinogenesis protocol. This study was supported by the NIDDK (P01-DK049210 to KHK). Z. Li was supported by NSREC and JDRF postdoctoral fellowship awards. We thank the University of Pennsylvania Diabetes and Endocrinology Center (DERC) for the use of the Functional Genomics Core (P30-DK19525).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental Information includes seven supplemental figures, two supplemental tables and extended experimental procedures.
REFERENCES
- Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14:828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio M, De Maio E, Morabito A, D'Aniello R, De Feo G, Gallo C, Perrone F. Hormonal treatment of human hepatocellular carcinoma. Ann N Y Acad Sci. 2006;1089:252–261. doi: 10.1196/annals.1386.007. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, Jin R, Gupta A, Rennie PS, Matusik RJ. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17:1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Pluchino N, Luisi S, Luisi M. Estrogen, cognition and female ageing. Hum Reprod Update. 2007;13:175–187. doi: 10.1093/humupd/dml042. [DOI] [PubMed] [Google Scholar]
- Gevry N, Hardy S, Jacques PE, Laflamme L, Svotelis A, Robert F, Gaudreau L. Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev. 2009;23:1522–1533. doi: 10.1101/gad.1787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth AB, Lerner MR, Lightfoot SA, Wilkerson KB, Hanas JS, McCay PB, Brackett DJ. Prevention of DMBA-induced rat mammary carcinomas comparing leuprolide, oophorectomy, and tamoxifen. Breast Cancer Res Treat. 1998;47:63–70. doi: 10.1023/a:1005872132373. [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The making of the liver: developmental competence in foregut endoderm and induction of the hepatogenic program. Cell Cycle. 2005;4:1146–1148. doi: 10.4161/cc.4.9.2033. [DOI] [PubMed] [Google Scholar]
- Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev. 2010;20:527–532. doi: 10.1016/j.gde.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Hiemisch H, Schutz G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol. 1998;18:4245–4251. doi: 10.1128/mcb.18.7.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G. Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev. 1999;13:495–504. doi: 10.1101/gad.13.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol. 2008;14:5945–5961. doi: 10.3748/wjg.14.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kel AE, Gossling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, Wingender E. MATCH: A tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res. 2003;31:3576–3579. doi: 10.1093/nar/gkg585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T, Hageman T, Balkwill F. Cancer. Sex, cytokines, and cancer. Science. 2007;317:51–52. doi: 10.1126/science.1146052. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- Li Z, White P, Tuteja G, Rubins N, Sackett S, Kaestner KH. Foxa1 and Foxa2 regulate bile duct development in mice. J Clin Invest. 2009;119:1537–1545. doi: 10.1172/JCI38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ukomadu C. Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem Biophys Res Commun. 2008;365:729–734. doi: 10.1016/j.bbrc.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, Jou YS, Chen CW, Yeh S, Chang C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–955. 955, e941–945. doi: 10.1053/j.gastro.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Oliver JD, Roberts RA. Receptor-mediated hepatocarcinogenesis: role of hepatocyte proliferation and apoptosis. Pharmacol Toxicol. 2002;91:1–7. doi: 10.1034/j.1600-0773.2002.910101.x. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Paulson DF. Carcinoma of the prostate: the therapeutic dilemma. Annu Rev Med. 1984;35:341–372. doi: 10.1146/annurev.me.35.020184.002013. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD. CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab. 2005;6:329–339. doi: 10.2174/1389200054633899. [DOI] [PubMed] [Google Scholar]
- Rouault JP, Rimokh R, Tessa C, Paranhos G, Ffrench M, Duret L, Garoccio M, Germain D, Samarut J, Magaud JP. BTG1, a member of a new family of antiproliferative genes. EMBO J. 1992;11:1663–1670. doi: 10.1002/j.1460-2075.1992.tb05213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Shen W, Scearce LM, Brestelli JE, Sund NJ, Kaestner KH. Foxa3 (hepatocyte nuclear factor 3gamma) is required for the regulation of hepatic GLUT2 expression and the maintenance of glucose homeostasis during a prolonged fast. J Biol Chem. 2001;276:42812–42817. doi: 10.1074/jbc.M106344200. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Yasuda M, Mizobuchi Y, Ma YR, Liu F, Shiba M, Horie T, Ito S. Suppressive effect of oestradiol on chemical hepatocarcinogenesis in rats. Gut. 1998;42:112–119. doi: 10.1136/gut.42.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolev JK, Coffey DS, Scott WW. Experimental models for the study of prostatic adenocarcinoma. J Urol. 1977;118:216–220. doi: 10.1016/s0022-5347(17)57949-5. [DOI] [PubMed] [Google Scholar]
- Stice JP, Lee JS, Pechenino AS, Knowlton AA. Estrogen, aging and the cardiovascular system. Future Cardiol. 2009;5:93–103. doi: 10.2217/14796678.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund NJ, Ang SL, Sackett SD, Shen W, Daigle N, Magnuson MA, Kaestner KH. Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol Cell Biol. 2000;20:5175–5183. doi: 10.1128/mcb.20.14.5175-5183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thean LF, Loi C, Ho KS, Koh PK, Eu KW, Cheah PY. Genome-wide scan identifies a copy number variable region at 3q26 that regulates PPM1L in APC mutation-negative familial colorectal cancer patients. Genes Chromosomes Cancer. 2010;49:99–106. doi: 10.1002/gcc.20724. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Yamamoto R, Iishi H, Tatsuta M, Tsuji M, Terada N. Promoting effect of ovariectomy on hepatocellular tumorigenesis induced in mice by 3'-methyl-4-dimethylaminoazobenzene. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62:371–375. doi: 10.1007/BF02899706. [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Mohrmann K, Wagenaar E, de Waart DR, Elferink RP, Lagas JS, van Tellingen O, Vainchtein LD, Rosing H, Beijnen JH, et al. Carcinogen and anticancer drug transport by Mrp2 in vivo: studies using Mrp2 (Abcc2) knockout mice. J Pharmacol Exp Ther. 2006;318:319–327. doi: 10.1124/jpet.106.101774. [DOI] [PubMed] [Google Scholar]
- Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, Hung YC, Yeh S, Chang C. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Iishi H, Tatsuta M, Tsuji M, Terada N. Roles of ovaries and testes in hepatocellular tumorigenesis induced in mice by 3'-methyl-4-dimethylaminoazobenzene. Int J Cancer. 1991;49:83–88. doi: 10.1002/ijc.2910490116. [DOI] [PubMed] [Google Scholar]
- Yu X, Gupta A, Wang Y, Suzuki K, Mirosevich J, Orgebin-Crist MC, Matusik RJ. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Ann N Y Acad Sci. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- Zaret K. Developmental competence of the gut endoderm: genetic potentiation by GATA and HNF3/fork head proteins. Dev Biol. 1999;209:1–10. doi: 10.1006/dbio.1999.9228. [DOI] [PubMed] [Google Scholar]
- Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2005;2:141–148. doi: 10.1016/j.cmet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Zumoff B. Does postmenopausal estrogen administration increase the risk of breast cancer? Contributions of animal, biochemical, and clinical investigative studies to a resolution of the controversy. Proc Soc Exp Biol Med. 1998;217:30–37. doi: 10.3181/00379727-217-44202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.