Summary
Metastasis is the leading cause of cancer-associated death but has been difficult to study because it involves a series of rare, stochastic events. To capture these events, we developed a sensitive method to tag and track pancreatic epithelial cells in a mouse model of pancreatic cancer. Tagged cells invaded and entered the bloodstream unexpectedly early, before frank malignancy could be detected by rigorous histologic analysis; this behavior was widely associated with epithelial-tomesenchymal transition (EMT). Circulating pancreatic cells maintained a mesenchymal phenotype, exhibited stem cell properties, and seeded the liver. EMT and invasiveness were most abundant at inflammatory foci, and induction of pancreatitis increased the number of circulating pancreatic cells. Conversely, treatment with the immunosuppressive agent dexamethasone abolished dissemination. These results provide new insight into the earliest events of cellular invasion in situ and suggest that inflammation enhances cancer progression in part by facilitating EMT and entry into the circulation.
Introduction
Each step in the metastatic cascade is highly inefficient. Only a small fraction of cells from a primary tumor enter the circulation, and less than 0.01% of these develop into metastases (Gupta et al., 2005). It is thought that tumor cells pass through several stages during which they sequentially acquire the ability to invade through basement membrane(s), enter and exit the bloodstream, and survive and grow in distant organs. Because each of these events is rare, studies of the metastatic process have relied heavily upon cells that have been cultured and manipulated in vitro and re-introduced into recipient animals. As a result, there remains considerable uncertainty regarding the factors that influence each stage in vivo as well as the timing of dissemination itself.
Clinical observations, mainly in the field of breast cancer, have given rise to two major metastasis paradigms. The classical model treats metastasis as the final step in a progressive “Darwinian” sequence, in which tumors acquire mutations that promote invasive behavior and dissemination late in tumor evolution (Cairns, 1975). This model has several conceptual problems (Gupta et al., 2005; Klein, 2009) and fails to account for two clinical observations: the appearance of metastatic lesions years after resection of small tumors with no clinically evident metastases at diagnosis (Pantel et al., 2008) and metastases of unknown primary, which account for as many as 4–5% of all clinical metastases (Greco and Hainsworth, 2009). An alternative model has been proposed that envisions metastasis as an inherent feature of a tumor very early in its natural history (Hellman, 1994; Klein, 2009). Although direct evidence for this model is limited, recent studies of breast cancer are consistent with the notion that metastatic seeding may be mediated by cells that would not meet a standard definition of cancer (Husemann et al., 2008; Podsypanina et al., 2008). Furthermore, several small studies concluded that the presence of putative disseminated tumor cells in the bone marrow of patients with low grade mammary tumors or carcinoma in situ correlates with worse outcome (Ignatiadis et al., 2011; Sanger et al., 2011). The possibility that cellular dissemination leading to metastasis occurs prior to the formation of an identifiable primary tumor has significant clinical and biological implications.
One of the challenges in studying tumor cell dissemination has been the identification of markers that distinguish cancer cells from cells that normally reside in the bloodstream or at sites of seeding. During malignant progression, it has been proposed that carcinoma cells undergo an epithelial-to-mesenchymal transition (EMT), in which they lose epithelial characteristics and acquire invasive properties and stem cell-like features (Polyak and Weinberg, 2009). Although several studies support a physiologic role during tumor progression (Moody et al., 2005; Trimboli et al., 2008), most studies of EMT in the context of cancer biology have been conducted in vitro, and thus the relevance of EMT to carcinogenesis continues to be debated (Ledford, 2011). If EMT does play a crucial role in cancer cell spread in vivo, then detection methods that rely on cellular expression of epithelial markers alone are likely to provide an incomplete picture of metastasis.
To understand the early events that accompany invasive behavior, we developed a lineage labeling system to detect and isolate cells of pancreatic epithelial origin during stochastic tumor progression. This system allowed us to determine the kinetics of EMT and hematogenous dissemination during the natural evolution of pancreatic ductal adenocarcinoma (PDAC) in vivo and correlate cell phenotype with the acquisition of invasive and tumor-initiating properties.
Results
Enhanced detection of EMT using epithelial lineage tracing
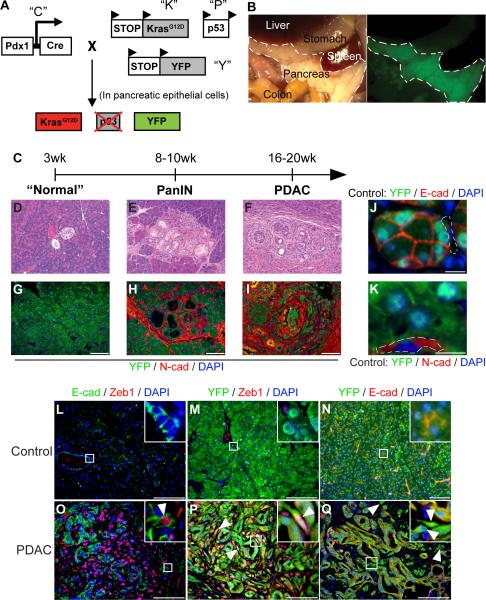
We used a Cre-lox based mouse model of PDAC to study the fate of pancreatic epithelial cells during various stages of tumor progression (Bardeesy et al., 2006). The model relies on the Pdx1-Cre transgenic strain (Gu et al., 2003) to generate pancreas-specific mutations in Kras and p53, genes that are mutated with high frequency in human pancreatic cancers (Hezel et al., 2006). In order to track pancreatic epithelial cells during tumor progression, we introduced a RosaYFP allele into the mutant background, resulting in highly specific and efficient (>95%) labeling (Fig. 1A–B). Animals containing all four alleles were referred to as PKCY mice. A second model, in which a single allele of p16Ink4a/Arf was deleted in place of p53 (IKCY; (Aguirre et al., 2003)), was also employed and yielded similar results (data not shown). The lineage-labeled mouse models displayed similar histology as non-labeled models, including the development of pancreatic intraepithelial neoplasias (PanINs), primary tumors, and metastases, with reproducible kinetics (Fig. 1C–I). Since the Pdx1 promoter is active only in endoderm-derived pancreatic cells (Gu et al., 2003), only the epithelium was tagged by this method. Importantly, mesenchymal cells were never labeled under control conditions in Pdx1-Cre; RosaYFP (CY) animals (Fig. 1J–K).
Figure 1. Lineage labeled mouse models of pancreatic cancer and detection of EMT.
(A) Schematic of the PKCY mouse model used in this study, which employs the KrasG12D (“K”), Pdx1-Cre (“C”), p53 (“P”) and RosaYFP (“Y”) alleles (see Experimental Procedures). Cre-mediated activation of Kras and deletion of one allele of the p53 tumor suppressor is accompanied by recombination of the YFP lineage label. (B) Brightfield and fluorescent images of midgut organs from a CY mouse showing robust and specific fluorescence of the pancreas (outlined); some labeling is also present in the duodenum. (C) Time course of malignant progression in PKCY mice. (D–F) Representative images of malignant progression. Prior to weaning, PKCY mice have histologically normal pancreata (D) but develop PanIN lesions (E) and eventually PDAC (F). (G–I) Images of pancreata from (D–F) stained with an antibody against YFP (green) and N-cadherin (N-cad, red); prior to weaning, scant N-cad staining is seen (G). (J–K) Fluorescent images of lineage-labeled cells derived from the pancreatic epithelium. In Control (CY; Pdx-Cre; YFP) pancreata, YFP+ cells express E-cadherin (E-cad; J) but not N-cad (K). Dotted lines indicate YFP− mesenchymal cells. (L–Q) Images of sections from control (CY; L–N) or PDAC (PKCY; O–Q) pancreata co-stained with E-cad and Zeb1 (L, O); YFP and Zeb1 (M, P); and YFP and E-cad (N, Q). Insets and arrowheads in O–Q show hi- magnification views of cells which co-express an epithelial and mesenchymal marker (O), epithelial-derived (YFP+) cells which have acquired expression of the mesenchymal marker Zeb1 (P) or epithelial-derived cells which have lost expression of the epithelial marker E-cad (Q). Scale bars denote 100μm in G–I and L–Q and 10μm in J–K. See also Figure S1.
Initially, we looked for cancer cells that co-expressed an epithelial marker and a mesenchymal marker, a standard approach used to detect cells at an “intermediate stage” of EMT. In the course of these studies, we used several mesenchymal markers (Supp. Fig. 1) but focused our analysis on Zeb1 and Fsp1 as these markers serve as independent predictors of mortality in patients with pancreatic cancer (Brabletz et al., 2011; Wang et al., 2007). Using this method, we detected tumor cells in tumor-bearing mice (“PDAC mice”) that co-expressed either Zeb1 or Fsp1 and the epithelial marker E-cadherin (E-cad; Fig. 1O), indicating that such “bi-phenotypic” cells exist, albeit at a low frequency (<10%).
We then used the YFP lineage label to identify PDAC cells that had completed an EMT. Since labeling was limited to cells of epithelial origin, we defined EMT as having occurred if a cell co-expressed YFP and either Zeb1 (Fig. 1P) or Fsp-1 (Supp Fig. 1D) and/or lacked E-cad (Fig. 1Q) expression. Using this approach, we observed that 42% of the lineage labeled YFP+ cells in PKCY tumors had undergone EMT (Fig. 1P); higher rates of EMT (68% of all YFP+ cells) were found in the IKCY model (data not shown). EMT was not detected in lineage-labeled CY control mice by either method (Figs 1L–N). Thus, genetic lineage marking is a sensitive tool for distinguishing cells of epithelial and mesenchymal origin and for the detection of EMT.
EMT in premalignant lesions
EMT has been proposed to be a prerequisite for invasion and dissemination of carcinoma cells (Hanahan and Weinberg, 2011). To determine when EMT first occurs during PanIN-to-carcinoma progression, we analyzed 8–10 wk-old PKCY mice. At these time points, only precancerous PanIN lesions were present and there was no histological evidence of PDAC based on extensive H&E analysis (n=18); these animals were referred to as “PanIN mice” solely to reflect the histological state of the pancreas at these time points.
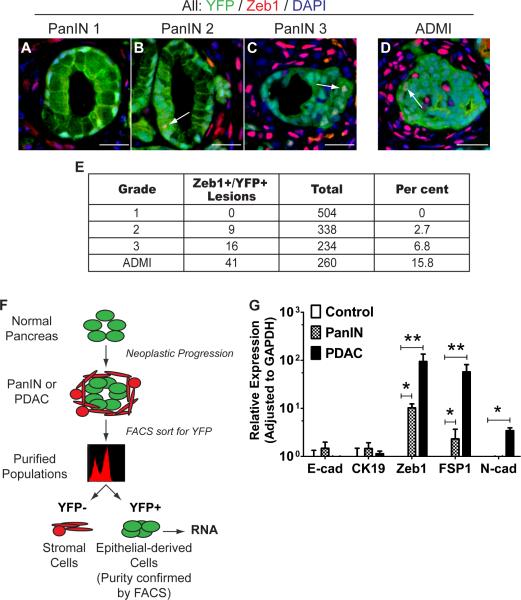
EMT was identified in premalignant lesions from both models (Fig. 2B–C, Supp Fig 1BC, data not shown). 2.7% and 6.8% of all PanIN 2 and 3 lesions, respectively, contained at least one YFP+Zeb1+ cell, while EMT was never observed in PanIN 1 lesions (Fig. 2A, E). Similar results were noted with other mesenchymal markers, including Fsp1, Slug, Snail1, and Sip1 (Supp. Fig. 1). EMT was also prevalent in areas of acinar-to-ductal metaplasia (ADM), particularly in lesions surrounded by abundant inflammatory cells (Fig. 2D, Supp. Fig. 2A). We refer to these areas as ADMIs (acinar-to-ductal metaplasia with inflammation) and determined that 15.8% of ADMIs had evidence of EMT in 8–10 wk old PKCY PanIN mice (Fig. 2E).
Figure 2. EMT precedes tumor formation.
(A–D) In pancreata taken from 8–10 wk-old PKCY mice, EMT is observed in regions of acinarto-ductal metaplasia with inflammation (ADMI; D), PanIN 2 (B), PanIN 3 (C), but not in PanIN 1 lesions (A). Arrows show individual YFP+ cells (green) that also express Zeb1 (red). (E) Quantification of observations from A–D, showing the percentage of each type of lesion having at least one cell that has undergone EMT; numbers reflect at least 10 medium-powered fields from each of five PanIN mice. (F) Strategy for isolating YFP+ epithelially-derived cells from the pancreas; the purity of the YFP+ population was confirmed by a repeat FACS analysis. (G) Transcriptional analysis of sorted YFP+ pancreas cells from lineage-labeled CY control (n=4), PanIN (n=6), and PDAC (n=5) pancreata. Bar graph data are presented as mean ± SD in this and subsequent figures. *, p<0.01; **, p<0.001 by two-tailed Student's t-test in this and subsequent figures, unless otherwise noted. Scale bars, 20μm. See also Figure S2.
We confirmed that epithelium-derived pancreatic cells activated a mesenchymal program at the transcriptional level by sorting YFP+ cells and performing qPCR (Fig. 2F). Transcripts for Zeb1, Fsp-1 and N-cadherin were found in YFP+ cells from tumor-bearing PKCY animals and PanIN animals but not in YFP+ cells from CY control mice (Fig. 2G; p<0.01). These data indicate that EMT occurs in PanIN lesions and ADMIs prior to tumor formation.
Pancreatic epithelial cells spread before tumor formation
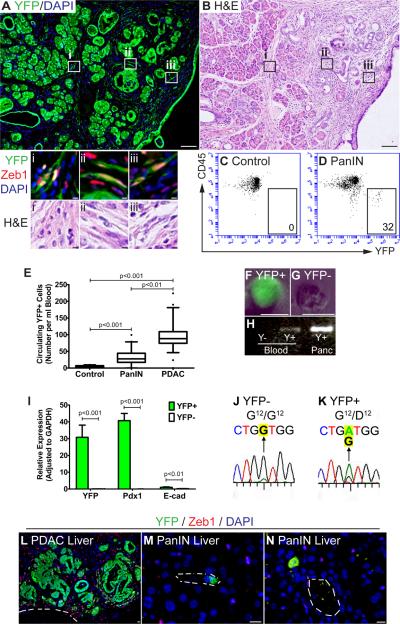
Cells that have undergone EMT acquire an invasive phenotype in vitro (Polyak and Weinberg, 2009). Thus, we hypothesized that cells which undergo EMT in PanIN mice might also have invasive properties. Consistent with this notion, we identified individual YFP+ cells that had traversed the basement membrane and dissociated from any discernible pancreatic epithelial structure (a process we refer to as “delamination”) in mice bearing PanIN 2 and PanIN 3 lesions (Fig. 3A, Supp. Fig. 2A). Most of these cells expressed Zeb1 (Fig. 3Ai–iii) and had acquired a fibroblast-like morphology, making them indistinguishable from surrounding stromal cells by conventional histology (Fig. 3A–B; Supp. Fig. 2); a fraction of the delaminated YFP+ cells also expressed Fsp1 (Supp. Fig. 2A, insets). To rule out the possibility that the Pdx1 promoter might be ectopically activated in mesenchymal cells during premalignant progression, we performed lineage tracing in Mist1CreERT2 mice whose acinar cells had been labeled by tamoxifen pulse. In the setting of experimental pancreatitis (as described in greater detail below), Mist1CreERT2; KrasG12D; RosaYFP pancreata contained fibroblast-like YFP+ cells that lacked E-cad expression (Supp. Fig. 2B). Because Mist1CreERT2 mediates labeling solely at the time of tamoxifen administration (Habbe et al., 2008), this experiment demonstrates unambiguously that the labeled mesenchymal cells were derived from pancreatic acinar cells in vivo.
Figure 3. Hematogenous spread and liver seeding precede tumor formation.
(A and B) Images showing individual YFP+ cells (green) intermingled with stromal cells prior to tumor formation in a 10 wk-old PKCY PanIN mouse (A). Delaminated YFP+ cells have a spindle-shaped morphology and express Zeb1 (boxes i–iii); they are indistinguishable from surrounding Zeb1+YFP− stromal cells by H&E staining of an adjacent section (B). (C and D) FACS analysis of blood samples from age-matched CY Control (C) and PKCY PanIN mice (D). YFP fluorescence and a stain for the leukocyte marker CD45 are depicted on the X- and Y-axes of the FACS plot. YFP+CD45− cells were seen in the blood of PanIN (D) and PDAC (not shown) animals (boxed area indicates representative gating and absolute number of YFP+ cells). (E) Quantification of circulating YFP+ pancreatic cells (CPCs). Mean CPC numbers (per ml blood) were 3.65±3.76 (CY control, n=13), 32.8±26.2 (PanIN, n=17), and 97.3±48.9 (PDAC, n=18) (p<0.001). (F–G) Phase-fluorescent images showing epifluorescence of a sorted YFP+ cell. (H) Genomic PCR showing the presence of the recombined YFP allele in YFP+ cells but not YFP− cells. Pancreatic DNA containing the recombined allele was included as a positive control. (I) Expression of transcripts encoding YFP, Pdx1, and E-cad, comparing sorted YFP+ and YFP− cells and measured by qPCR (±SD). (J–K) Sanger sequencing after PCR amplification of cDNA showing that YFP+ CPCs express a mutant Kras allele which harbors an altered codon 12 (G→A, highlighted). (L–N) CPCs from 8–10 wk-old PKCY animals seed the liver. (L) Micrometastasis in a liver from a tumor-bearing mouse (“PDAC Liver”). (M and N) Individual CPCs seed the liver at the PanIN stage (“PanIN Liver”); vascular lumens are outlined. Scale bar, 40μm for A–B; 5μm for L–N. See also Figure S3.
To extend these studies, we performed immunostaining for the Pdx1 transcription factor. Pdx1 is normally expressed at high levels during pancreatic development and in adult β–cells, and it is commonly “re-activated” in human PanIN lesions and in PDAC (Park et al., 2011). Pdx1 was widely expressed in PanIN lesions and in a subset of YFP+ cells that had delaminated in PanIN mice (Supp. Fig. 3A). Consistent with these data, sections of human pancreas that contained PanIN lesions (but no tumor) exhibited scattered Pdx1+ cells that were separated from any defined epithelial structure (Supp. Fig. 3B–D). Thus, human pancreatic cells may delaminate from PanIN lesions as they do in the mouse model.
Since lineage tracing demonstrated that pancreatic cells can cross the basement membrane before invasive behavior is detectable by standard histology, we asked whether these cells could also enter the bloodstream prior to tumor formation. In tumor-bearing PDAC mice, YFP+ circulating pancreatic cells (CPCs) were readily detected in the blood by flow cytometry (Fig. 3E). Surprisingly, CPCs were also abundant in the bloodstream of 8–10 wk-old PKCY PanIN mice (Fig. 3C–G). Sorted YFP+ cells contained the recombined YFP allele (Fig. 3H), expressed transcripts for YFP, Pdx1, and E-cad (Fig. 3I) and carried the Gly→Asp mutation at codon 12 of the Kras cDNA (Fig. 3J–K). Thus, cells derived from the pancreatic epithelium are present in the circulation of mice with no evidence of carcinoma.
These data raised the possibility that CPCs from PanIN mice might seed distant organs. To assess this possibility, we first examined PDAC mice as a positive control. Bright-field stereomicroscopy permitted detection of liver and lung metastases in 8/20 animals; use of the YFP lineage label enhanced detection, revealing micro-metastases in 16/20 PDAC mice (Fig. 3L). Next, we analyzed 8–10 wk-old PKCY PanIN mice. Although no animals had macro- or micro-metastases, liver seeding by YFP+ cells was detected in 4/11 PanIN mice (Fig. 3M–N); most were single cells located near blood vessels and expressed neither Zeb1 nor E-cad (Fig. 3M–N). By contrast, 0/10 livers from lineage-labeled control CY mice harbored YFP+ cells when examined by the same technique.
Characterization of CPCs
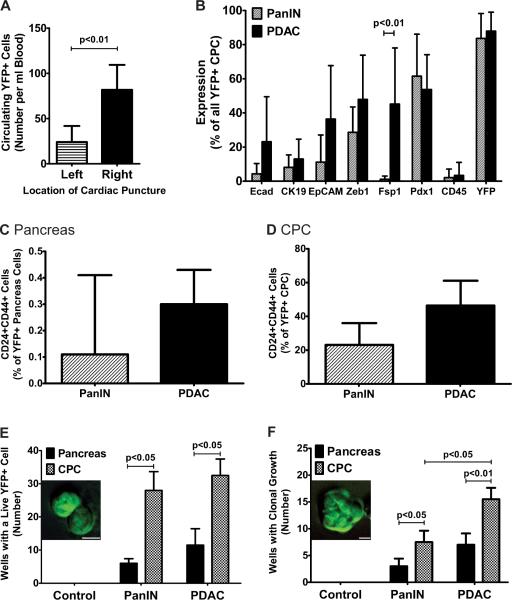
The number of YFP+ CPCs from PDAC mice depended on the location of blood collection, with a roughly three-fold increase in abundance in the right side of the heart compared to the left side (Fig. 4A). Only 2/9 PDAC mice had evidence of lung metastases, suggesting that the vast majority of CPCs do not survive passage through the pulmonary circulation. To determine whether cells in the circulation exhibited an epithelial, mesenchymal, or mixed phenotype, we stained CPCs with a variety of markers in a flow cytometric assay. The epithelial markers E-cad, CK19, and EpCAM were detected in fewer than 20% of PanIN CPCs and fewer than 40% of PDAC CPCs (Fig. 4B). With the exception of Fsp1 – which was detected in only 1.1% of all PanIN CPCs compared to 45.2% in PDAC CPCs (p<0.01) – there were no statistically significant differences in cell surface phenotype between CPCs from the two groups (Fig. 4B). Co-immunofluorescence for Zeb1 and EpCAM revealed that approximately 40% of PDAC CPCs were Zeb1+, 27% were EpCAM+, and 18% were double positive (Supp. Fig. 4), suggesting that most CPCs do not exhibit a “mixed” epithelial-mesenchymal phenotype. These data indicate that CPCs from PDAC and PanIN animals are phenotypically similar and that a large fraction maintains a mesenchymal phenotype in the circulation.
Figure 4. CPC characterization.
(A) Quantification of CPCs after sampling from the left atrium or ventricle (Left) or right atrium or ventricle (Right) of the same animal (n=3). (B) Quantification of FACS staining for epithelial and mesenchymal markers in CPCs obtained from PanIN or PDAC mice (n=6–8 for each data point). (C and D) Quantification of YFP+ cells from the pancreas (C) and circulation (D) in PKCY PanIN and PDAC mice that stained positive for the putative pancreatic cancer stem cell markers CD24 and CD44. (E and F) Quantification of survival (E) or clonal growth (F) of YFP+ cells obtained from lineage-labeled Control (CY), PanIN and PDAC mice in ultra-low attachment wells. Bar graphs show the number of wells (out of 96 wells seeded with a single cell) exhibiting any live YFP+ cells (E, inset) or evidence of clonal growth (F, inset) after 7d. p-values for paired 2-tailed Student's t-tests are shown. Scale bars, 10μm. See also Figure S4.
CPCs exhibit features of cancer stem cells
Cancer stem cells are functionally defined as cells that have enhanced tumor initiating capacity upon transplantation into a permissive host. In human pancreatic tumors, this activity may be contained within a CD24+CD44+ population of cells, among others (Hermann et al., 2007; Jimeno et al., 2009; Li et al., 2007). Since EMT in primary cells has been associated with the acquisition of stem cell-like characteristics (Mani et al., 2008), we hypothesized that CPCs might also exhibit features of cancer stem cells. We compared the relative abundance of CD24+CD44+ cells in pancreata and CPCs from PanIN and PDAC mice. By FACS analysis, 0.11±0.32% and 0.30±0.13% of sorted YFP+ cells from PanIN and PDAC pancreata, respectively, expressed both CD24 and CD44 (Fig. 4C). By contrast, 23.1±12.9% and 46.4±14.7% of sorted YFP+ CPCs from PanIN and PDAC samples were found to be CD24+CD44+, representing a greater than 100-fold enrichment when compared to the source pancreas (Fig. 4D).
We next assessed the survival and self-renewal properties of CPCs directly by employing an in vitro pancreatosphere assay, in which single YFP+ cells were cultured in attachment-free conditions (Rovira et al., 2010). In both PanIN and PDAC mice, YFP+ CPCs had significantly greater rates of clonal survival and growth compared to YFP+ pancreas cells from the same animal (Fig. 4E–F; p<0.05). Taken together, these data suggest that during tumor evolution in vivo, bloodstream entry is associated with enrichment of cells that have phenotypic and functional features associated with pancreatic cancer stem cells.
Cells that have undergone EMT have tumor initiating properties
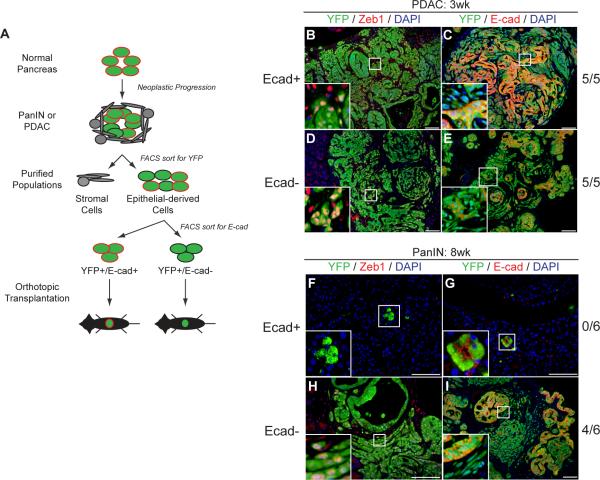
Although previous work has suggested a link between EMT and increased tumor aggressiveness, most studies have relied on in vitro manipulation of cancer cell lines to induce EMT (Weinberg, 2008). Such treatments could have a direct effect on cell behavior apart from their EMT-inducing activity, and thus a direct demonstration of the role of EMT in tumor progression is lacking. We used our lineage labeling system to isolate cells that had either lost or retained E-cadherin expression to determine whether an EMT in vivo is associated with tumor-initiating capacity (Fig. 5A).
Figure 5. Epithelial and mesenchymal states are plastic.
(A) Schematic of orthotopic transplantation experiments. (B–E) Fluorescent images taken 3 wks following transplantation of YFP+ cells from PDAC mice into NOD/SCID hosts. Tumors form in all mice regardless of E-cad status (n=5 for each condition). YFP+E-cad+ and YFP+E-cad− cells are present in both conditions (C and E), as are YFP+Zeb1+ and YFP+Zeb1− cells (B and D). (F–I) Fluorescent images taken 8 wks following transplantation of YFP+ cells from PanIN mice into NOD/SCID hosts. After transplantation of YFP+E-cad+ cells, no tumors are found (n=6); the few transplanted YFP+ cells that remain are Zeb1− and E-cad+ (F, G). Transplantation of YFP+E-cad− cells results in tumor formation (H, I). Tumors contain both E-cad+ and E-cad− cells (I) as well as Zeb1+ and Zeb1− cells (H), providing direct evidence for MET.
First, we transplanted 100,000 YFP+E-cad+ or YFP+E-cad− pancreatic cells from PDAC mice into the pancreata of NOD/SCID animals (n=5 for each group). After three weeks, all transplants gave rise to large tumors with local invasion and distant metastasis regardless of E-cad status at the time of transplantation; tumors were histologically similar and YFP+ cells co-expressing either Zeb1 or E-cad were found at comparable proportions in both groups (Fig. 5B–E). This result suggests that tumor-derived E-cad+ and E-cad− cells can each form tumors and that there is significant plasticity between epithelial and mesenchymal states.
By contrast, we observed a dramatic effect of E-cad status on tumor formation when cells from PanIN mice were transplanted: 4/6 animals transplanted with 100,000 YFP+E-cad− cells formed tumors after two months while 0/6 animals transplanted with 100,000 YFP+E-cad+ cells formed tumors over the same time period (Fig. 5F–I). Tumors derived from E-cad− PanIN cells were heterogeneous with respect to E-cad and Zeb1 expression (Fig. 5H–I). By comparison, E-cad+ transplanted animals had few detectable YFP+ cells at the two-month time point, and almost all of the YFP+ cells detected were Zeb1− (Fig. 5F). Mice transplanted with E-cad+ PanIN cells eventually developed tumors (with a mean latency of four months after transplantation) that were indistinguishable from the PDAC-derived tumors. Thus, functional analysis of tumor-initiating capacity revealed that E-cad− cells that have undergone EMT have a marked advantage at the PanIN stage.
Inflammation promotes EMT, invasiveness, and dissemination
The emergence of PanIN lesions is associated with the appearance of an inflammatory stroma characterized by activated fibroblasts and myeloid-derived cells (Aguirre et al., 2003; Clark et al., 2007). Inflammation is commonly correlated with tumor initiation and progression (Coussens and Werb, 2002; Grivennikov et al., 2010) and accelerates pancreatic carcinogenesis in adult Kras mutant mice (Guerra et al., 2007; Guerra et al., 2011). Our observation that ADMIs have a high prevalence of EMT (Fig. 3) led us to hypothesize that inflammation contributes to EMT and dissemination at the PanIN stage.
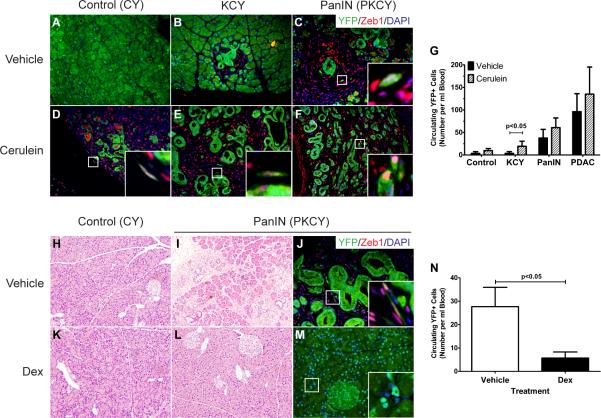
To address this possibility, we induced acute pancreatitis with cerulein, a cholecystokinin analog that induces acinar cell death (Siveke et al., 2008). Initially, KCY mice were analyzed to determine if expression of oncogenic Kras alone facilitated EMT and dissemination in response to acute pancreatitis. At two months of age, KCY mice had few PanIN lesions or inflammation at baseline; maximum PanIN grade found at this stage was PanIN 1 (Fig. 6B). As expected, cerulein treatment resulted in the formation of ADMIs and accelerated PanIN formation (compare Fig. 6B and E; Supp. Fig. 5A–D). Surprisingly, cerulein treatment also resulted in a marked elevation in circulating cells, such that CPCs in 8 wk-old cerulein-treated KCY mice were almost as abundant as CPCs in vehicle-treated PKCY mice of the same age (Fig. 6G). Stated otherwise, cerulein pancreatitis had nearly the same effect on CPC number as the addition of a single floxed p53 allele. Similar changes were observed in PKCY PanIN and PDAC mice treated with cerulein (Fig. 6C, F; Supp. Fig. 5E–F), as well as control CY animals treated with cerulein (Fig. 6A, D), resulting in a significant increase in CPC number across all groups following cerulein treatment (p=0.014 by 2-way ANOVA; Fig. 6G). CPCs from cerulein-treated KCY mice exhibited a nearly hundred-fold enrichment of CD24+CD44+ cells compared to the source pancreas, as had been observed for PanIN CPCs, although this did not result in an increase in clonogenic growth for KCY-derived CPCs in the pancreatosphere assay (Supp. Fig. 4B–C).
Figure 6. Inflammation augments EMT and dissemination.
(A–F) Fluorescent images of 8 wk-old control Pdx1-Cre; RosaYFP (CY; A, D), KrasG12D; Pdx1-Cre; RosaYFP (KCY; B, E), and PanIN (C, F) pancreata 3d after injection with vehicle (A–C) or cerulein (D–F) to induce acute pancreatitis (n=3 for each condition). YFP+Zeb1+ cells present in PanIN mice or observed following cerulein treatment of CY and KCY mice are shown (C–F, insets). (G) Quantification of CPC number after 3d of treatment with vehicle or cerulein for mice with the indicated genotypes (n=3 for each group; p<0.05 comparing the two groups of KCY mice by Student's t-test; p=0.014 for the effect of cerulein versus control across all groups by two-way ANOVA). (H–M) Images of 10-wk-old control (H, K) and PanIN pancreata (I–J, L–M) following 7d treatment with vehicle (DMSO; H–J) or dexamethasone (Dex; K–M) and analyzed 24h after the last injection. YFP+Zeb1+ cells are seen in vehicle-treated controls (J, inset) but not in Dex-treated animals (M, inset). (N) Quantification of CPC number in vehicle- and Dex-treated PanIN mice (p=0.029, n=6 for each group). See also Figure S6.
To confirm that inflammation promotes EMT and bloodstream entry, we employed a second paradigm of pancreatitis and performed pancreatic duct ligation (PDL) on 8–10 wk-old PKCY PanIN mice. One week after PDL, the portion of the pancreas distal to the ligation was enlarged and nodular compared to the proximal portion from the same mouse or sham-treated PanIN mice (Supp. Fig. 6A, D). This resulted in inflammation, more advanced PanINs, and a loss of epithelial markers (Supp. Fig. 6B–C, E–F), as well as an increase in CPCs (Supp. Fig. 6G; p=0.042, n=7). Thus, both chemical and surgical methods for inducing pancreatitis result in an increase in EMT and CPC number.
Finally, we sought to determine whether inflammation is necessary for EMT and bloodstream entry. We treated 10 wk-old PKCY mice with dexamethasone (Dex), a potent anti-inflammatory drug that has no effect on pancreatic histology in control animals (Fig. 6H, K). As expected, vehicle-treated mice at this age had PanIN lesions with an inflammatory stroma and evidence of EMT but no tumors (Fig. 6I–J). Daily treatment with Dex (10 mg/kg for 7d) resulted in a marked reduction in inflammation (Fig. 6L–M; n=6 each group). Remarkably, PanINs and ADMIs were almost undetectable in these pancreata (Fig. 6K–M), and this change in histology was associated with a significant drop in CPC number compared to vehicle-treated controls (Fig. 6N). Importantly, neither cerulein nor Dex treatment of PanIN-derived epithelial cells in vitro had any effect on morphology, proliferation, or expression of epithelial or mesenchymal markers (Supp. Fig. 6H–N).
Discussion
Invasive behavior precedes frank tumorigenesis
Using in vivo lineage tracing, we found that EMT, migration of epithelially-derived cells into the stroma, bloodstream entry, and seeding of the liver occur at a stage of pancreatic adenocarcinoma progression previously thought to be pre-invasive based on standard histological examination. The relevance of these findings to patients is supported by our detection of delaminated Pdx1+ cells adjacent to PanIN lesions in sections of human pancreata. Thus, our data support a model for pancreatic cancer progression in which the seeding of distant organs occurs before, and in parallel to, tumor formation at the primary site. Such an interpretation is especially applicable to PDAC, since the vast majority of patients with pancreatic cancer have metastatic disease at the time of diagnosis. More than 75% of patients who undergo surgical resection of small pancreatic tumors with clear surgical margins and no evidence of metastasis die from metastatic disease within five years (Neoptolemos et al., 2004), a finding that is consistent with early spread. Moreover, metastatic PDAC has been documented in a cohort of patients that underwent pancreatectomy for chronic pancreatitis in whom histologic analysis of the resected pancreas revealed only PanIN lesions (Sakorafas and Sarr, 2003).
Recent genetic studies examining low-passage cell lines or micro-dissected primary tumors and matched metastases have concluded that metastasis is a late event in human PDAC (Campbell et al., 2010; Yachida et al., 2010). In these studies, a large proportion of mutations were shared among primary and metastatic lesions, leading to the conclusion that metastasis constituted a terminal event in the disease process. However, mathematical modeling of such phylogenetic relationships relies on assumptions about proliferation and mutation rates at stages of metastatic progression (e.g. micro-metastasis) that are not measured easily. Indeed, the notion that colonization occurs early in PDAC is supported by the observation that proliferation is significantly lower in metastatic lesions (Okimura et al., 2009) compared to the primary tumor (Yachida et al., 2010), and yet their sizes at the time of diagnosis are similar.
Our in vivo studies do not provide direct evidence that CPCs from PanIN mice (and the corresponding liver-seeding cells) ultimately give rise to metastases. Nevertheless, several lines of evidence indicate that at least some of these cells may be capable of doing so. First, CPCs found in the blood of PanIN-bearing mice exhibit increased survival and self-renewal properties in vitro, suggesting that they may be able to persist for long periods of time in a foreign environment such as the liver. Second, most PanIN-derived CPCs exhibit a YFP+E-cad− cell surface phenotype, which our transplantation experiments showed was associated with enhanced tumor-initiating capacity. Finally, a wealth of clinical and experimental data from other systems is consistent with early spread (Weinberg, 2008). Additional experiments will be needed to prove that cells which enter the circulation prior to the development of frank malignancy have metastatic potential. Nevertheless, the finding that delaminating cells are indistinguishable from surrounding stromal cells by routine histology indicates that current histological criteria to diagnose invasive PDAC may be inadequate. Our data also suggest that the location of sampling within the peripheral blood may influence the detection rate of circulating cells.
EMT, MET, and the acquisition of stem cell characteristics
Lineage tracing enabled us to distinguish pancreatic cells that had acquired mesenchymal characteristics from those that retained an epithelial phenotype. The majority of labeled cells that delaminated from the epithelium (i.e. locally-invasive cells) expressed Zeb1, indicating that they had undergone an EMT. This strong correlation between invasive behavior and the acquisition of mesenchymal characteristics in vivo suggests that EMT is not merely an epiphenomenon but rather represents a critical hurdle that cells must clear to escape from their epithelial neighbors. At present, the signals that initiate EMT in advanced PanINs and ADMIs in vivo remain to be determined.
When PDAC cells were separated according to EMT status (i.e., YFP+E-cad+ or YFP+E-cad−) and transplanted orthotopically into the pancreas, the resultant tumors were similar with respect to their epithelial and mesenchymal composition. This result demonstrates that an epithelial or mesenchymal phenotype is not a stable property of a malignant cell and provides direct evidence for a mesenchymal-to-epithelial transition (MET) in vivo. We also noted that orthotopically-transplanted YFP+E-cad− cells from 8–10 wk-old PKCY PanIN mice gave rise to tumors with a much shorter latency than the same number of YFP+E-cad+ cells. One interpretation of this result is that cells that have undergone EMT at the PanIN stage are more tumorigenic; alternatively, the YFP+E-cad− population may be enriched for a subset of cells with greater or more rapid tumor-initiating properties. In either case, our findings highlight the striking degree of plasticity that exists between epithelial and mesenchymal states during tumor progression in vivo.
In the PKCY model, cells with a CD24+CD44+ phenotype – a population possessing tumor-initiating properties by xenograft assay (Li et al., 2007) – were significantly enriched in the circulation compared to the pancreas. Accordingly, circulating cells exhibited increased survival and self-renewal in low-attachment conditions. Thus, our findings provide in vivo support for the notion that EMT is associated with the initiation of a stem cell program (Mani et al., 2008) and indicate that acquisition of a CD24+CD44+ phenotype facilitates entry into the circulation and/or survival within the bloodstream.
The development of technologies to identify circulating tumor cells (CTCs) from patients represents an enormous advance in metastasis research (Pantel et al., 2008). CTC number correlates with clinical outcome and response to chemotherapeutics in many cancers (Cristofanilli et al., 2004), and isolated disseminated cells can be interrogated for molecular changes that are associated with an increased risk of death (Stoecklein et al., 2008). CTC biology thus has both clinical utility and the potential to advance our understanding of the metastatic cascade. However, standard techniques for isolating CTCs rely heavily upon the use of epithelial markers, particularly EpCAM, for detection (Pantel et al., 2008). The presence of EpCAM-negative YFP+ CPCs in our studies raises several possibilities: 1) standard methods that employ epithelial epitopes may not capture all CTCs; 2) cells may enter the circulation after undergoing an “incomplete” EMT, in which residual expression of EpCAM is maintained but not detected by flow cytometry; or 3) CTCs may enter the bloodstream with a mesenchymal phenotype and subsequently “revert” to an epithelial phenotype through a process of MET.
Inflammation promotes EMT and dissemination
Inflammation has a well-established role in promoting tumor progression (Grivennikov et al., 2010). Using two models of pancreatitis, we have demonstrated that inflammation induces EMT in CY and KCY animals and results in an increase in CPC number across all genotypes tested. Although mice bearing a single mutant Kras allele seemed to have the greatest increase in CPC number following cerulein treatment, even non-mutant pancreatic cells underwent EMT and entered the circulation in the setting of inflammation. This latter observation is quite surprising, as hematogenous spread has not been thought to occur in adult animals in the absence of a tumor. Phenotypically normal cells injected into the bloodstream can seed distant organs and persist for long periods of time until stimulated to grow (Podsypanina et al., 2008), and in both mice and humans, chronic pancreatic inflammation is strongly associated with pancreatic cancer (Grover and Syngal, 2010; Guerra et al., 2007). Our study suggests that inflammation may promote cancer progression through two independent mechanisms: by facilitating changes in the microenvironment at the primary site of neoplasia and by facilitating invasion and dissemination by increasing cellular access to the circulation.
There is likely to be heterogeneity among pancreatic epithelial cells at the PanIN stage, and it is possible that bloodstream entry prior to the development of a recognizable tumor is facilitated by loss of the second allele of p53 in PKCY mice. However, the increase of CPCs in KCY and CY mice (which bear no cancer-promoting mutations) following experimental pancreatitis suggests that loss of this tumor suppressor gene is not required for bloodstream entry. Interestingly, although pancreatitis augmented CPC number in KCY animals, these cells did not have the same clonal growth properties exhibited by PKCY CPCs (compare Supp. Fig 4C with Fig. 4F), supporting the notion that p53 loss enhances the survival and/or self-renewal of circulating cells.
Finally, we have demonstrated that the inflammatory stroma is necessary for EMT and dissemination. Treatment of 10 wk-old PKCY mice with dexamathasone for 1 wk resulted in an almost complete elimination of PanIN lesions in the pancreas and loss of YFP+ cells from the blood, underscoring a critical requirement for the inflammatory stroma in the maintenance of premalignant PanIN lesions. Similar regression of murine PanIN disease was also seen after treatment with the cyclooxygenase-inhibitor sulindac (Guerra et al., 2011). Our findings have implications for the management of individuals at high risk for pancreatic cancer, including patients with hereditary pancreatitis or kindreds with inherited pancreatic cancer. If dissemination and seeding of pancreatic epithelial cells precedes the detection of a tumor in humans, as it does in the mouse model, a window of opportunity may exist for prophylactic therapy in high risk patients. Indeed, anti-inflammatory drugs have proven moderately successful at reducing mortality due to several cancers, including PDAC (Rothwell et al., 2011).
Experimental Procedures
Mouse strains and experimental treatment
The behavior of mutant strains bearing various allele combinations of Pdx1-Cre, KrasG12D, p16/p19fl, and p53fl has been described previously (Aguirre et al., 2003; Bardeesy et al., 2006; Hingorani et al., 2003). To perform lineage tracing, we introduced a RosaYFP reporter allele into these mutant backgrounds to generate a panel of compound mutant strains: Pdx1-Cre; RosaYFP (“CY”), Pdx1-Cre; KrasG12D; RosaYFP (“KCY”), Pdx1-Cre; KrasG12D; p16/p19fl/+; RosaYFP (“IKCY”) and Pdx1-Cre; KrasG12D; p53fl/+; RosaYFP (“PKCY”). All experiments involving the KCY model employed mice between 8–10 wks of age. For studies involving mice harboring only PanIN lesions by histology (“PanIN” mice), PKCY animals were sacrificed at 8–10 wks of age based on prior observations regarding tumor progression (Bardeesy et al., 2006); no PKCY mice (out of 18 examined) had evidence of carcinoma at this timepoint (see Supplemental Information for details of histological analysis by a pancreatic pathologist (A.M.)).
For PDAC mice, animals were examined three times per week for evidence of morbidity and sacrificed when they exhibited limited physical activity, depressed response to toe pinch, dehydration, and/or abdominal enlargement from ascites. More than 90% of IKCY and PKCY mice were 34–38 wks of age or 16–20 wks of age, respectively, at the time of sacrifice.
Experimental pancreatitis was elicited with cerulein as described (Siveke et al., 2008) and specimens were obtained after 3d of treatment. 3–4 CY, KCY, PanIN PKCY and PDAC PKCY were used for cerulein and vehicle (PBS) treated cohorts. Orthotopic transplantations were performed on NOD/SCID mice by injecting 1×105 sorted pancreas cells as previously described (Mohammad et al., 1998). PKCY PanIN mice aged 10 wks were treated with seven daily injections of dexamethasone or vehicle (DMSO) as described (Stairs et al., 2011) and analyzed 24h later.
Cell Staining
For experiments involving flow cytometry and sorting of pancreatic cells, at least 25% of the tissue was saved for histologic analysis and was processed and stained as described in Supp. Experimental Procedures.
Pancreatosphere assay
YFP+ blood or pancreatic cells from the same mouse were sorted into ultra-low attachment 96 well plates (Corning) at 1 cell per well, confirmed by microscopy. Cells were grown as previously described (Rovira et al., 2010) and assayed at 5 days for clonal growth of fluorescent cells (defined as clusters of cells >3 cell widths in diameter) or presence of live YFP+ cells (singlets or doublets). 3–4 mice were analyzed for each category.
Additional information can be found in the Supplementary Information.
Supplementary Material
Highlights
Invading cells exhibit EMT in an autochthonous model of pancreatic cancer
Mutant cells enter the circulation before cancer is found on histology
Circulating pancreatic cells (CPCs) express cancer stem cell-associated markers
Inflammation is necessary and sufficient for EMT, invasion, and dissemination
Acknowledgements
We thank I. Ben-Porath, A. Minn, A. Rustgi, M.C. Simon, L. Chodosh and H. Zong for helpful discussions, M. Emmett, A. Pannikar, and M. Rovira for technical assistance, N. Bardeesy, R. DePinho, S. Konieczny, and D. Melton for mouse strains, and J. Habener and D. Melton for gifts of antibodies. We are grateful to R. Hruban providing confirmation of histological impressions. This work was supported by the NIH (DK088945 and DK007066 to ADR, CA143292 to AM and SDL, CA138907 to GLB, DK083355 and DK083111 to BZS), AGA/FDHN (ADR), National Pancreas Foundation (ADR and MR), AACR and Pancreatic Cancer Action Network (Pathway to Leadership Award to JMB and Career Development Award to BZS), Pennsylvania Department of Health (RHV and BZS) and the Pew Charitable Trusts (BZS). Experiments utilized the Molecular Pathology and Imaging, Molecular Biology, and Cell Culture core facilities within the NIH/Penn Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306), the Abramson Family Cancer Research Institute, and the Diabetes and Endocrine Research Center (P30-DK19525).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, Feng B, Brennan C, Weissleder R, Mahmood U, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, et al. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Greco FA, Hainsworth JD. Introduction: unknown primary cancer. Semin Oncol. 2009;36:6–7. doi: 10.1053/j.seminoncol.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology. 2010;139:1076–1080. 1080, e1071–1072. doi: 10.1053/j.gastro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Brown JR, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19:728–739. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Mani S, Yang J, Hartwell K, Weinberg RA. The evolving portrait of cancer metastasis. Cold Spring Harb Symp Quant Biol. 2005;70:291–297. doi: 10.1101/sqb.2005.70.033. [DOI] [PubMed] [Google Scholar]
- Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hellman S. Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, Metallo J, Kheddoumi N, Singhal SK, Michiels S, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6:e15624. doi: 10.1371/journal.pone.0015624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A, Feldmann G, Suarez-Gauthier A, Rasheed Z, Solomon A, Zou GM, Rubio-Viqueira B, Garcia-Garcia E, Lopez-Rios F, Matsui W, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–314. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Ledford H. Cancer theory faces doubts. Nature. 2011;472:273. doi: 10.1038/472273a. [DOI] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad RM, Dugan MC, Mohamed AN, Almatchy VP, Flake TM, Dergham ST, Shields AF, Al-Katib AA, Vaitkevicius VK, Sarkar FH. Establishment of a human pancreatic tumor xenograft model: potential application for preclinical evaluation of novel therapeutic agents. Pancreas. 1998;16:19–25. doi: 10.1097/00006676-199801000-00004. [DOI] [PubMed] [Google Scholar]
- Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- Okimura A, Hirano H, Nishigami T, Ueyama S, Tachibana S, Fukuda Y, Yamanegi K, Ohyama H, Terada N, Nakasho K. Immunohistochemical analyses of E-cadherin, beta-catenin, CD44s, and CD44v6 expressions, and Ki-67 labeling index in intraductal papillary mucinous neoplasms of the pancreas and associated invasive carcinomas. Med Mol Morphol. 2009;42:222–229. doi: 10.1007/s00795-009-0462-y. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- Park JY, Hong SM, Klimstra DS, Goggins MG, Maitra A, Hruban RH. Pdx1 Expression in Pancreatic Precursor Lesions and Neoplasms. Appl Immunohistochem Mol Morphol. 2011 doi: 10.1097/PAI.0b013e318206d958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dig Liver Dis. 2003;35:482–485. doi: 10.1016/s1590-8658(03)00221-4. [DOI] [PubMed] [Google Scholar]
- Sanger N, Effenberger KE, Riethdorf S, van Haasteren V, Gauwerky J, Wiegratz I, Strebhardt K, Kaufmann M, Pantel K. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer. 2011 doi: 10.1002/ijc.25895. [DOI] [PubMed] [Google Scholar]
- Siveke JT, Lubeseder-Martellato C, Lee M, Mazur PK, Nakhai H, Radtke F, Schmid RM. Notch signaling is required for exocrine regeneration after acute pancreatitis. Gastroenterology. 2008;134:544–555. doi: 10.1053/j.gastro.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, et al. Deletion of p120-Catenin Results in a Tumor Microenvironment with Inflammation and Cancer that Establishes It as a Tumor Suppressor Gene. Cancer Cell. 2011;19:470–483. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein NH, Hosch SB, Bezler M, Stern F, Hartmann CH, Vay C, Siegmund A, Scheunemann P, Schurr P, Knoefel WT, et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell. 2008;13:441–453. doi: 10.1016/j.ccr.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, et al. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer Res. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- Wang F, Sloss C, Zhang X, Lee SW, Cusack JC. Membrane-bound heparin-binding epidermal growth factor like growth factor regulates E-cadherin expression in pancreatic carcinoma cells. Cancer Res. 2007;67:8486–8493. doi: 10.1158/0008-5472.CAN-07-0498. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.