Abstract
Biomechanical factors play an important role in the health of diarthrodial joints. Altered joint loading — associated to obesity, malalignment, trauma, or joint instability — is a critical risk factor for joint degeneration, whereas exercise and weight loss have generally been shown to promote beneficial effects for osteoarthritic joints. The mechanisms by which mechanical stress alters the physiology or pathophysiology of articular cartilage or other joint tissues likely involve complex interactions with genetic and molecular influences, particularly local or systemic inflammation secondary to injury or obesity. Chondrocytes perceive physical signals from their environment using a variety of mechanisms, including ion channels, integrin-mediated connections to the extracellular matrix that involve membrane, cytoskeletal, and intracellular deformation. An improved understanding of the biophysical and molecular pathways involved in chondrocyte mechanotransduction can provide insight into the development of novel therapeutic approaches for osteoarthritis.
Keywords: biomechanics, arthritis, obese, adipokines, joint loading, mechanical stress
Introduction
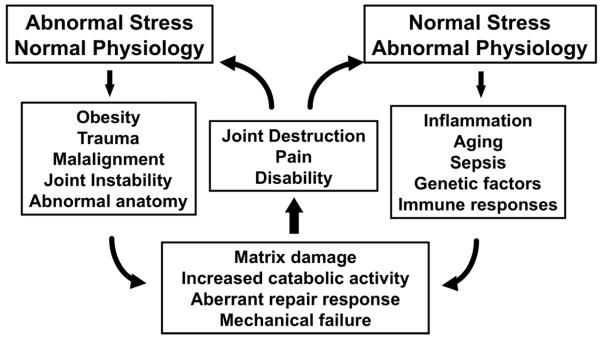
Osteoarthritis is a painful and debilitating disease of the synovial joints, affecting an estimated 12-15% of the population 25-74 years of age [1]. The prevalence of this disease increases significantly with age, with radiographic evidence observed in over 70% of the population over age 65. Considering the extensive impact and consequences of this disease, its etiology is not fully understood. Indeed, osteoarthritis likely represents a family of diseases that have a similar endpoint, but with multifactorial etiopathogenesis involving genetic, molecular, and environmental influences, particularly biomechanical stress. In fact, in many cases, biomechanical factors appear to play a critical role in the events that lead to the initiation and progression of osteoarthritis (Figure 1).
Figure 1. The role of mechanical loading in osteoarthritis.
Biomechanical factors have been implicated in many hypotheses on the etiology and pathogenesis of osteoarthritis, either due to abnormal loading acting on normal physiology, or normal loading acting in the presence of abnormal physiology. It is important to note that many of these risk factors (e.g., obesity) involve both biomechanical and physiologic influences. Adapted from [100], with permission.
Osteoarthritis is distinctively characterized by progressive degenerative changes in the morphology, composition, and mechanical properties of the articular cartilage, subchondral bone, synovium, and other joint tissues. Under normal physiological conditions, the components of the cartilage extracellular matrix are in a state of slow turnover, retained in a homeostatic balance between the catabolic and anabolic events of the chondrocytes. These activities are controlled through the processing of both genetic and environmental information, which includes the action of soluble mediators (e.g., growth factors and cytokines), local matrix composition, and biophysical factors, including mechanical or osmotic stresses. In particular, the progressive degeneration of articular cartilage indicates that the normal balance of metabolic activities of the chondrocytes has been severely disrupted. Indeed, many studies have shown that the mechanical stress environment of the joint is an important factor that influences (and presumably regulates) the activity of the chondrocytes in vivo. In this regard, knowledge of the role of specific mechanical phenomena in regulating chondrocyte metabolism under inflammatory conditions would provide new insights into the etiopathogenesis of osteoarthritis. Furthermore, growing evidence indicates that pro-inflammatory cytokines and mediators, and their interactions with mechanical stress, may be in part responsible for the catabolic events that occur in osteoarthritic cartilage.
The effects of mechanical loading on cartilage in vivo
Under physiologic conditions, joints of the body are subjected to millions of cycles of loading resulting in forces of up to ten times body weight passing through the joints. Under normal circumstances, such loads have no adverse effects on the cartilage or other joint tissues, and furthermore, clinical data indicate that a variety of exercises are safe and effective for individuals with osteoarthritis [2]. In fact, exercise for osteoarthritis has been strongly recommended in treatment guidelines by both the American College of Rheumatology and EULAR. Furthermore, obesity is potentially the primary risk factor that for joint disease that is directly modifiable. In particular, obesity is a strong risk factor for osteoarthritis incidence, progression, and disability [3]. While these effects have been assumed to be related to altered gait and increased joint stresses secondary to increased body weight [4], there is increasing evidence of the presence of systemic inflammation due to obesity [5, 6] that may interact with biomechanical factors to promote the progressive degradation of joint cartilage in other joints such as the hand [7]. The benefits of exercise in an arthritic population include not only weight loss, but also decreased systemic inflammation, increased muscle strength, flexibility, energy, a sense of well-being, better coping abilities, quality of sleep, decreased blood pressure, and fewer heart attacks [8].
Clinical and animal studies of altered joint loading have provided strong evidence that “abnormal” loads can lead to alterations in the composition, structure, metabolism, and mechanical properties of articular cartilage and other joint tissues. Abnormal loading may be caused by a variety of factors such as obesity, immobilization, joint instability, overuse, or trauma. For example, obesity is strongly associated with osteoarthritis [9], and a decrease of 5 kg of body weight has been shown to decrease the risk of osteoarthritis by over 50% [10]. Altered joint loading due to instability or injury of the joint is now well known to be a significant risk factor for the onset and progression of osteoarthritis [11]. Impact loads cause significant damage to the articular cartilage, including splitting of the ECM, increased cellular activity, increased tissue hydration, and remodeling of the subchondral bone [12]. These characteristics are generally consistent with the early stages of osteoarthritis, suggesting that hyperphysiologic stresses may be an important factor in the etiopathogenesis of this disease. Joint instability, induced by ligament transection [13, 14] or meniscectomy [15, 16], also induce profound and repeatable changes in joint tissues which mimic changes seen in early human osteoarthritis, including increased hydration, collagen disruption, and matrix turnover accompanied by decreased tissue stiffness in tension, compression and shear (e.g., [17-23]). Articular cartilage and synovial fluid from these models of osteoarthritis shows significant increases in various biomarkers (reviewed in [24]) that are correlated with histologic damage in the joint [25]. Inflammatory mediators and cytokines seem to play an important role in these altered loading models of osteoarthritis, although the precise relationships between biomechanical factors and inflammation are not fully understood. Nonetheless, previous studies have shown decreased osteoarthritis severity in animal models through the administration of nitric oxide synthase inhibitors or interleukin-1 receptor antagonists [26-28].
Importantly, similar models have been developed and validated in small animals such as mice [29] and rats [30]. In particular, destabilization of the medial meniscus (termed “DMM” in mice and “MMT” in rats) provides a fairly reproducible model of knee osteoarthritis in mice that progresses to moderate-to-severe levels within weeks post-surgery [29]. The DMM model has sufficient sensitivity to show disease modification, as observed with the ADAMTS-5 knock out mouse [31], suggesting that instability-induced osteoarthritis could be potentially ameliorated using pharmacologic inhibitors of the aggrecanase pathways.
Obesity, Mechanical Loading, and Osteoarthritis
Obesity (or factors associated with obesity) is a primary risk factor for osteoarthritis [32]. Within the past two decades, the prevalence of obesity has risen dramatically such that two-thirds of adults in the United States are now overweight or obese [33], with similar increases in the prevalence of obesity in Europe [34] and worldwide [35]. Significant evidence shows that obesity alters gait and joint biomechanics, although not necessarily to increase the overall magnitude of joint loads or torques [36-39]. A systematic review of this topic showed that at self-selected speed, obese individuals walk slower, with shorter and wider steps, and have longer stance durations compared with normal-weight individuals [40]. While these altered biomechanics can affect the load-bearing regions of the articular cartilage, the role of such changes as risks factors for osteoarthritis are not fully understood.
The effects of obesity on the joint historically have been dismissed as simply increased “wear- and-tear”; however, there is increasing evidence of multifactorial, systemic links between obesity and osteoarthritis [41]. For example, only a small reduction in body weight (5 kg), and in particular body fat (2.4%), is associated with significant improvements in lowering the risk or progression of osteoarthritis [10, 42]. Furthermore, obesity is associated with increased osteoarthritis of the hand [7], which is not a “weight-bearing” joint per se.
Obesity, Biomechanics, and Inflammation
In addition to alterations in joint mechanics, obesity has been associated with a state of low-grade chronic systemic inflammation [5]. Adipose tissue is a source of local and systemic inflammation and excess adiposity has been implicated in a number of diseases, such as type 2 diabetes, cardiovascular disease, and cancer, [43-45]. A number of studies have shown that pro-inflammatory cytokines associated with adipose tissue (i.e., adipokines), such as interleukin 1 (IL-1), IL-6, tumor necrosis factor alpha (TNF-α), leptin, adiponectin, etc. have a strong effect influence on cartilage biology [6]. These adipose-derived cytokines, or “adipokines,” are believed to play important roles in a number of diseases related to the metabolic syndrome. In obese but otherwise healthy women, weight loss of 3 kg due to dietary restriction causes a marked decrease in serum IL-6 levels [46]. In fact, IL-6 has been proposed as a critical factor in the link between inflammation, obesity, stress, and coronary heart disease. [47]. Additionally, psychosocial stressors have been shown to further increase plasma IL-6 levels [48]. These intriguing findings suggest that the link between obesity and osteoarthritis may not be solely biomechanical, but may in fact reflect the influence of biomechanical, metabolic, and psychosocial factors on the joint in the presence of systemic inflammation.
While obesity is clearly a risk factor for clinical osteoarthritis, only a few animal models have been reported for studying the relationship between obesity and osteoarthritis (reviewed in [49]). These generally have involved rodent models that are either diet-induced with high-fat feeding or are overweight/obese inbred strains that spontaneously develop osteoarthritis. High-fat diet-induced obese mouse models, especially the C57BL/6J strain, have been extensively studied for their utility as animal models of human obesity [50]. When fed a low-fat diet, C57BL/6J mice remain lean and display normal insulin levels and blood pressure. However, when fed a high-fat diet, C57BL/6J mice develop central adiposity, hyperinsulinemia, hyperglycemia, and hypertension—many of the features of human obesity and metabolic syndrome [50]. C57BL/6J mice fed a high-fat diet also develop osteoarthritis-like changes at an earlier age and to a more severe degree than C57BL/6J mice fed a lower-fat control diet [51-54]. Within these models, the incidence of osteoarthritis is similar between males and females [51], and lard-based fat supplements induce osteoarthritis more severely than vegetable-based fat supplements [52, 53]. High-fat feeding also induces osteoarthritis in rats [52, 53]. Two inbred strains of spontaneous osteoarthritis — the STR/Ort mouse and the Hartley guinea pig — are heavy compared to other mouse and guinea pig strains, respectively, and therefore may be considered obese animal models of osteoarthritis [53]. Interestingly, a recent study has shown via quantitative trait analyses that STR/ort mice possess three chromosomal locations associated with body weight or fatty acid metabolism [55]. In other studies, body weight restriction in the Hartley guinea pig by limiting food greatly reduced the severity of osteoarthritis in the knee joints [56].
These findings suggest that in these animal models of osteoarthritis body weight and/or body fat is a mediating factor in the development of spontaneous osteoarthritis of the knee. However, recent studies in obese mice have shown that leptin-impaired mice, which become morbidly obese, do not exhibit knee osteoarthritis [57]. Furthermore, mice fed an extremely high-fat diet (60% fat by kcal) exhibit knee osteoarthritis that is partially ameliorated by exercise, in the absence of any weight loss [58]. These findings suggest that adiposity (or weight) in and of itself, may not be a risk factor for joint degeneration, but rather, that interactions among joint loading, diet, and local or systemic inflammation may be responsible for the initiation and progression of osteoarthritis [59].
Overall, these in vivo studies emphasize the relationship between mechanical loading and the health of the joint, and suggest that the normal mechanical regulation of chondrocyte activity may be detrimentally influenced by the presence of pro-inflammatory mediators and cytokines in the joint. Taken together, they suggest that a critical level and manner of joint loading is required to regulate the normal homeostatic balance of cartilage anabolism and catabolism. The interactions of biomechanical and inflammatory mechanisms involved in the progression of cartilage degeneration are not known. Currently, there is significant evidence to implicate the role of various cytokines, specifically IL-1, leptin, and TNF-α, in these processes [60]. Taken together, the collective disuse and exercise studies suggest is that degenerative changes are associated with hyperphysiologic magnitudes of loading or by alterations in the normal loading pattern of the joint (e.g., loading of normally unloaded regions of cartilage).
In vitro studies of the effects of mechanical loading on cartilage metabolism
In vitro explant models of mechanical loading provide systems in which the biomechanical and biochemical environments of the chondrocytes can be better controlled as compared to the in vivo situation. Considerable research effort has been directed toward understanding the processes by which biophysical signals are converted to a biochemical signal by the chondrocyte population. Clarification of the specific biophysical factors and biological signaling mechanisms in normal and inflamed cartilage would provide a better understanding of cartilage physiology and may also yield new insights on the pathogenesis of osteoarthritis. This information could potentially reveal new targets for the prevention or treatment of disease.
Explant models of cartilage loading have been utilized in a number of different loading configurations, including unconfined compression, indentation, tension, and osmotic and hydrostatic pressure. The general consensus of these studies is that static compression suppresses matrix biosynthesis, and cyclic and intermittent loading stimulate chondrocyte metabolism (e.g., [61-65]). These responses have been reported over a wide range of loading magnitudes, and exhibit a stress-dose dependency (reviewed in [66]). Excessive loading (e.g., high magnitude, long duration) seems to have a deleterious effect, resulting in cell death, tissue disruption and swelling [67, 68]. Injurious compression (high loading rate) can cause damage to the collagen fibril network and increased tissue swelling [69]. Increase proteoglycan release and NO were also observed, suggesting that mechanical stress alone can stimulate cell death as well as a range of biomechanical and biochemical alterations to the matrix. Interestingly, mechanical compression can induce a dose-dependent increase in the production and release of a variety of osteoarthritis biomarkers, including proteoglycan epitopes such as 3B3, 5D4, and cartilage oligomeric matrix protein [70].
Importantly, mechanical stress has been shown to influence directly the inflammatory cascade of chondrocytes to induce or inhibit the production of pro-inflammatory mediators such as nitric oxide (NO) or prostaglandin E2 (PGE2), depending on culture configuration and specific loading regimen. For example, chondrocytes in monolayer exhibit increased production of NO in a manner that can be inhibited by hydrostatic pressure [71], whereas cyclic stretch in monolayer can produce an anti-inflammatory response against the effects of IL-1 [72]. Mechanical compression of cartilage explants induces production of NO and PGE2 [73, 74], although chondrocytes embedded in an agarose matrix show an opposite effect [75, 76] suggesting that interactions with the native extracellular matrix may influence this response.
Mechanisms of mechanical signal transduction in cartilage
In response to loading of the cartilage extracellular matrix, chondrocytes are exposed to a diverse array of biophysical signals that vary with time and location in the tissue, consisting of stress, strain, fluid flow, fluid pressure, electrokinetic effects, and changes in the tissue fixed charge density, in addition to changes in the shape and volume of the chondrocytes [77-81]. Determining the relative contribution of each factor has been difficult, but several studies have pursued a “reductionist” approach in an attempt to determine the biophysical mechanisms involved in the chondrocytes’ response to mechanical stress [82]. There is growing evidence that chondrocytes sense these physical signals through the integrated actions of ion channels, integrin-mediated connections to the extracellular matrix, and intracellular or membrane deformation [83 , 84-86]. In this regard, the pericellular matrix, which completely encapsulates all chondrocytes, is likely to serve as a transducer of the physical signals in the cells’ environment [87]. Thus, mechanical alterations that occur in the microenvironment of the chondrocytes with aging or degeneration my further alter the cells’ response to loading [80]. Furthermore, The cartilage extracellular matrix is inherently negatively charged due to the large concentration of the anionic proteoglycan aggrecan that attracts cations to counterbalance the fixed charge. While the tissue components are intrinsically incompressible [88], compression of cartilage results in pressurization and exudation of the interstitial water, increasing the apparent concentration of proteoglycans, and thus the local fixed-charge density [89]. Thus, chondrocytes can experience fluctuations in extracellular osmolarity, resulting in the activation of intracellular signaling cascades and acute volume change, followed by active volume regulation, which involves cytoskeletal F-actin restructuring as well as solute transport and extracellular Ca++ influx, which is amplified by release from intracellular stores [90-95]. One novel potential candidate involved in chondrocyte osmo-mechanotransduction, is the Ca++-permeable, nonspecific cation channel TRPV4, of the vanilloid subfamily of transient receptor potential (TRP) channels, which has recently been shown to control the mechano-osmotic transduction cascade in chondrocytes [96, 97]. An understanding of the precise sequence of events by which mechanical signals are converted to intracellular signal will hopefully provide new insights into the development of pharmacologic and physical therapies that can modify the course of osteoarthritis.
Summary
Biomechanical loading is clearly necessary for the maintenance of cartilage homeostasis, as evidence by the rapid loss of proteoglycans in joints that are immobilized or in disuse [59, 98]. However, abnormal, altered, or injurious loading is associated with inflammatory and metabolic imbalances that may eventually lead to osteoarthritis [11, 99]. In the absence of a full understanding of the role of biomechanical factors in etiopathogenesis of osteoarthritis, few treatment options are available. Pharmacologic treatment is palliative and often based on analgesics or non-steroidal anti-inflammatory drugs, agents that may provide some symptomatic relief but are not disease-modifying and pose special hazards to older populations due to vascular side effects. Rest, through joint protection techniques and avoidance of vigorous activity, is often recommended to minimize joint stress and maintain mobility. Nonetheless, recent studies indicate that exercise may play many important beneficial roles in an overall therapeutic regimen for osteoarthritis. These include decreased pain, increased mobility, and increased muscle strength. Clearly there is an urgent need to develop more effective clinical modalities for the treatment or prevention of osteoarthritis, particularly for the significant obese population. Before we can develop preventive or therapeutic treatments for osteoarthritis, it is necessary to understand the mechanisms and influence of mechanical factors in the regulation of homeostasis and degeneration of articular cartilage. Identification of these mechanisms will hopefully lead to new treatments that exploit optimal mechanical and biochemical manipulations for the prevention of disease.
Practice points.
Biomechanical loading is an important factor that affects joint physiology in both health and disease.
Altered joint loading, associated to obesity, malalignment, trauma, or joint instability, are important risk factors for osteoarthritis.
Exercise and weight loss generally have beneficial effects for osteoarthritic joints.
Research Agenda.
An improved understanding of the biophysical and molecular pathways involved in the transduction of mechanical signals by joint tissues can provide insight into the development of novel therapeutic approaches for osteoarthritis.
Obesity remains a primary risk factor for osteoarthritis, yet the mechanisms by which increased body weight or adiposity increase the risk for joint disease are not fully understood and may involve both biomechanical and metabolic factors.
Acknowledgments
The author would like to thanks Drs. Virginia Kraus, Lori Setton, and Tim Griffin for many insightful discussions. Supported in part by the Arthritis Foundation and National Institutes of Health Grants AR50245, AR48852, AG15768, and AR48182.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Praemer A, Furner S, Rice DP. Musculoskeletal Conditions in the United States. American Academy of Orthopaedic Surgeons; Rosement, IL: 1999. [Google Scholar]
- [2].Ytterberg SR, Mahowald ML, Krug HE. Exercise for arthritis. Baillieres Clinical Rheumatology. 1994;8:161–89. doi: 10.1016/s0950-3579(05)80230-4. [DOI] [PubMed] [Google Scholar]
- [3].Messier S, Loeser R. Exercise and weight loss in obese older adults with knee osteoarthritis: a preliminary study. J Am Geriatrics Soc. 2000;48 doi: 10.1111/j.1532-5415.2000.tb04781.x. al. e. [DOI] [PubMed] [Google Scholar]
- [4].Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994;26:1446–52. [PubMed] [Google Scholar]
- [5].Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17:953–66. doi: 10.1016/s0899-9007(01)00672-4. [DOI] [PubMed] [Google Scholar]
- [6].Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. Jama. 1999;282:2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- [7].Felson DT, Chaisson CE. Understanding the relationship between body weight and osteoarthritis. Baillieres Clin Rheumatol. 1997;11:671–81. doi: 10.1016/s0950-3579(97)80003-9. [DOI] [PubMed] [Google Scholar]
- [8].Neuberger GB, Press AN, Lindsley HB, Hinton R, Cagle PE, Carlson K, et al. Effects of exercise on fatigue, aerobic fitness, and disease activity measures in persons with rheumatoid arthritis. Research in Nursing & Health. 1997;20:195–204. doi: 10.1002/(sici)1098-240x(199706)20:3<195::aid-nur3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [9].Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10:161–6. [PubMed] [Google Scholar]
- [10].Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–9. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- [11].Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. Journal of Rheumatology - Supplement. 1995 Feb;43:13–5. 1995. [PubMed] [Google Scholar]
- [12].Radin EL, Martin RB, Burr DB, Caterson B, Boyd RD, Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. Journal of Orthopaedic Research. 1984;2:221–34. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- [13].Gilbertson EMM. Development of periarticular osteophytes in experimentally induced osteoarthrosis in the dog. Annals of Rheumatic Diseases. 1975;34:12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pond MJ, Nuki G. Experimentally induced osteoarthritis in the dog. Annals of Rheumatic Diseases. 1973;32:387–88. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. Journal of Orthopaedic Research. 1983;1:4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- [16].Moskowitz RW, Davis W, Sammarco J. Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis & Rheumatism. 1973;16:397–405. doi: 10.1002/art.1780160317. [DOI] [PubMed] [Google Scholar]
- [17].Altman RD, Tenenbaum J, Latta L, Riskin W, Blanco LN, Howell DS. Biomechanical and biochemical properties of dog cartilage in experimentally induced osteoarthritis. Annals of Rheumatic Diseases. 1984;43:83–90. doi: 10.1136/ard.43.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Elliott DM, Guilak F, Vail TP, Wang JY, Setton LA. Tensile properties of articular cartilage are altered by meniscectomy in a canine model of osteoarthritis. Journal of Orthopaedic Research. 1999;17:503–8. doi: 10.1002/jor.1100170407. [DOI] [PubMed] [Google Scholar]
- [19].Eyre DR, McDevitt CA, Billingham ME, Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochemical Journal. 1980;188:823–37. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McDevitt CA, Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. Journal of Bone and Joint Surgery. 1976;58B:94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- [21].Ratcliffe A, Billingham ME, Saed-Nejad F, Muir H, Hardingham TE. Increased release of matrix components from articular cartilage in experimental canine osteoarthritis. Journal of Orthopaedic Research. 1992;10:350–8. doi: 10.1002/jor.1100100307. [DOI] [PubMed] [Google Scholar]
- [22].Sandy JD, Adams ME, Billingham ME, Plaas A, Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis & Rheumatism. 1984;27:388–97. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- [23].Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. Journal of Orthopaedic Research. 1994;12:451–63. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- [24].Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, et al. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. Journal of Orthopaedic Research. 2000;18:269–80. doi: 10.1002/jor.1100180216. [DOI] [PubMed] [Google Scholar]
- [25].Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. J Orthop Res. 2002;20:92–100. doi: 10.1016/S0736-0266(01)00066-3. [DOI] [PubMed] [Google Scholar]
- [26].Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis & Rheumatism. 1996;39:1535–44. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- [27].Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. American Journal of Pathology. 1999;154:1159–69. doi: 10.1016/S0002-9440(10)65368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pelletier JP, Jovanovic DV, Lascau-Coman V, Fernandes JC, Manning PT, Connor JR, et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis & Rheumatism. 2000;43:1290–9. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [29].Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [30].Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48:3452–63. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- [31].Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–8. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- [32].Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- [33].Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- [34].Doak CM, Wijnhoven TM, Schokker DF, Visscher TL, Seidell JC. Age standardization in mapping adult overweight and obesity trends in the WHO European Region. Obes Rev. 2011 doi: 10.1111/j.1467-789X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- [35].Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- [36].Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–8. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- [37].Handrigan GA, Corbeil P, Simoneau M, Teasdale N. Balance control is altered in obese individuals. J Biomech. 2010;43:383–4. doi: 10.1016/j.jbiomech.2009.08.041. author reply 5-6. [DOI] [PubMed] [Google Scholar]
- [38].DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. J Biomech. 2003;36:1355–62. doi: 10.1016/s0021-9290(03)00119-2. [DOI] [PubMed] [Google Scholar]
- [39].Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:822–8. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- [40].Runhaar J, Koes BW, Clockaerts S, Bierma-Zeinstra SM. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev. 2011 doi: 10.1111/j.1467-789X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- [41].Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol. 2011;7:65–8. doi: 10.1038/nrrheum.2010.123. [DOI] [PubMed] [Google Scholar]
- [42].Toda Y, Toda T, Takemura S, Wada T, Morimoto T, Ogawa R. Change in body fat, but not body weight or metabolic correlates of obesity, is related to symptomatic relief of obese patients with knee osteoarthritis after a weight control program. J Rheumatol. 1998;25:2181–6. [PubMed] [Google Scholar]
- [43].Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17:145–56. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- [44].Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- [45].Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wellnitz B, Boehm BO, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2006-0195. [DOI] [PubMed] [Google Scholar]
- [46].Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. Journal of Clinical Endocrinology & Metabolism. 2000;85:3338–42. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- [47].Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- [48].Zhou D, Kusnecov W, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–30. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- [49].Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- [50].Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–8. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [51].Silberberg M, Silberberg R. Effects of a high fat diet on the joints of aging mice. AMA Arch Pathol. 1950;50:828–46. [PubMed] [Google Scholar]
- [52].Silberberg M, Silberberg R. Osteoarthrosis in mice fed diets enriched with animal or vegetable fat. Arch Pathol. 1960;70:385–90. [PubMed] [Google Scholar]
- [53].Sokoloff L, Mickelsen O, Silverstein E, Jay GE, Jr., Yamamoto RS. Experimental obesity and osteoarthritis. Am J Physiol. 1960;198:765–70. doi: 10.1152/ajplegacy.1960.198.4.765. [DOI] [PubMed] [Google Scholar]
- [54].Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jaeger K, Selent C, Jaehme W, Mahr S, Goebel U, Ibrahim S, et al. The genetics of osteoarthritis in STR/ort mice. Osteoarthritis Cartilage. 2008;16:607–14. doi: 10.1016/j.joca.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [56].Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 1991;34:1180–4. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- [57].Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60:2935–44. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high fat diet in mice: Effects of short-term exercise. Arthritis Rheum. 2011 doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- [60].van den Berg WB. Lessons for joint destruction from animal models. Curr Opin Rheumatol. 1997;9:221–8. doi: 10.1097/00002281-199705000-00008. [DOI] [PubMed] [Google Scholar]
- [61].Bonassar LJ, Grodzinsky AJ, Srinivasan A, Davila SG, Trippel SB. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Archives of Biochemistry & Biophysics. 2000;379:57–63. doi: 10.1006/abbi.2000.1820. [DOI] [PubMed] [Google Scholar]
- [62].Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. Journal of Orthopaedic Research. 1988;6:777–92. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- [63].Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis & Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- [64].Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. Journal of Orthopaedic Research. 1989;7:619–36. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- [65].Torzilli PA, Grigiene R. Continuous cyclic load reduces proteoglycan release from articular cartilage. Osteoarthritis & Cartilage. 1998;6:260–8. doi: 10.1053/joca.1998.0119. [DOI] [PubMed] [Google Scholar]
- [66].Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic orthopaedic biomechanics. 2nd ed Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- [67].Milentijevic D, Helfet DL, Torzilli PA. Influence of stress magnitude on water loss and chondrocyte viability in impacted articular cartilage. J Biomech Eng. 2003;125:594–601. doi: 10.1115/1.1610021. [DOI] [PubMed] [Google Scholar]
- [68].Torzilli PA, Grigiene R, Borrelli J, Jr., Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433–41. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- [69].Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, et al. Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Archives of Biochemistry & Biophysics. 2000;381:205–12. doi: 10.1006/abbi.2000.1988. [DOI] [PubMed] [Google Scholar]
- [70].Piscoya JL, Fermor B, Kraus VB, Stabler TV, Guilak F. The influence of mechanical compression on the induction of osteoarthritis-related biomarkers in articular cartilage explants. Osteoarthritis Cartilage. 2005;13:1092–9. doi: 10.1016/j.joca.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [71].Lee MS, Trindade MC, Ikenoue T, Schurman DJ, Goodman SB, Smith RL. Intermittent hydrostatic pressure inhibits shear stress-induced nitric oxide release in human osteoarthritic chondrocytes in vitro. J Rheumatol. 2003;30:326–8. [PubMed] [Google Scholar]
- [72].Dossumbekova A, Anghelina M, Madhavan S, He L, Quan N, Knobloch T, et al. Biomechanical signals inhibit IKK activity to attenuate NF-kappaB transcription activity in inflamed chondrocytes. Arthritis Rheum. 2007;56:3284–96. doi: 10.1002/art.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729–37. doi: 10.1016/S0736-0266(00)00049-8. [DOI] [PubMed] [Google Scholar]
- [74].Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10:792–8. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- [75].Lee DA, Frean SP, Lees P, Bader DL. Dynamic mechanical compression influences nitric oxide production by articular chondrocytes seeded in agarose. Biochem Biophys Res Commun. 1998;251:580–5. doi: 10.1006/bbrc.1998.9520. [DOI] [PubMed] [Google Scholar]
- [76].Chowdhury TT, Arghandawi S, Brand J, Akanji OO, Bader DL, Salter DM, et al. Dynamic compression counteracts IL-1beta induced inducible nitric oxide synthase and cyclooxygenase-2 expression in chondrocyte/agarose constructs. Arthritis Res Ther. 2008;10:R35. doi: 10.1186/ar2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Trickey WR, Baaijens FP, Laursen TA, Alexopoulos LG, Guilak F. Determination of the Poisson’s ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J Biomech. 2006;39:78–87. doi: 10.1016/j.jbiomech.2004.11.006. [DOI] [PubMed] [Google Scholar]
- [78].Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- [79].Choi JB, Youn I, Cao L, Leddy HA, Gilchrist CL, Setton LA, et al. Zonal changes in the three-dimensional morphology of the chondron under compression: The relationship among cellular, pericellular, and extracellular deformation in articular cartilage. Journal of Biomechanics. 2007 doi: 10.1016/j.jbiomech.2007.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomech. 2005;38:509–17. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- [81].Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- [82].Guilak F, Hung CT. Physical Regulation of Cartilage Metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechanobiology. 3rd ed Lippincott Williams & Wilkins; Philadelphia: 2004. pp. 259–300. [Google Scholar]
- [83].Mobasheri A, Lewis R, Maxwell JE, Hill C, Womack M, Barrett-Jolley R. Characterization of a stretch-activated potassium channel in chondrocytes. J Cell Physiol. 2010;223:511–8. doi: 10.1002/jcp.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ramage L, Nuki G, Salter DM. Signalling cascades in mechanotransduction: cell-matrix interactions and mechanical loading. Scand J Med Sci Sports. 2009;19:457–69. doi: 10.1111/j.1600-0838.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- [85].Kock LM, Schulz RM, van Donkelaar CC, Thummler CB, Bader A, Ito K. RGD-dependent integrins are mechanotransducers in dynamically compressed tissue-engineered cartilage constructs. J Biomech. 2009;42:2177–82. doi: 10.1016/j.jbiomech.2009.05.039. [DOI] [PubMed] [Google Scholar]
- [86].Finan JD, Leddy HA, Guilak F. Osmotic stress alters chromatin condensation and nucleocytoplasmic transport. Biochem Biophys Res Commun. 2011;408:230–5. doi: 10.1016/j.bbrc.2011.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- [88].Bachrach NM, Mow VC, Guilak F. Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J Biomech. 1998;31:445–51. doi: 10.1016/s0021-9290(98)00035-9. [DOI] [PubMed] [Google Scholar]
- [89].Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–58. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- [90].Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. J Biomech. 2001;34:1527–35. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- [91].Erickson GR, Northrup DL, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarthritis Cartilage. 2003;11:187–97. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- [92].Bush PG, Hall AC. Regulatory volume decrease (RVD) by isolated and in situ bovine articular chondrocytes. J Cell Physiol. 2001;187:304–14. doi: 10.1002/jcp.1077. [DOI] [PubMed] [Google Scholar]
- [93].Mobasheri A, Mobasheri R, Francis MJ, Trujillo E, de la Rosa D Alvarez, Martin-Vasallo P. Ion transport in chondrocytes: membrane transporters involved in intracellular ion homeostasis and the regulation of cell volume, free [Ca2+] and pH. Histol Histopathol. 1998;13:893–910. doi: 10.14670/HH-13.893. [DOI] [PubMed] [Google Scholar]
- [94].Yellowley CE, Hancox JC, Donahue HJ. Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J Cell Biochem. 2002;86:290–301. doi: 10.1002/jcb.10217. [DOI] [PubMed] [Google Scholar]
- [95].Chao PH, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291:C718–25. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- [96].Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage. 1997;5:1–16. doi: 10.1016/s1063-4584(97)80027-1. [DOI] [PubMed] [Google Scholar]
- [99].Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- [100].Poole AR, Guilak F, Abramson SB. Etiopathogenesis of osteoarthritis. In: Moskowitz RW, Altman RW, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4th edition Lippincott, Williams, and Wilkins; Philadelphia: 2007. pp. 27–49. [Google Scholar]