Abstract
The commonest urogenital tumours in childhood are Wilms tumour of the kidney and rhabdomyosarcoma in the pelvis. We review these tumours along with other primary renal tumours and less common ovarian and testicular tumours in childhood. Current clinical concepts, relevant staging investigations and imaging features are described.
Keywords: Urogenital, tumour, child, Wilms, rhabdomyosarcoma, renal, ovary, testis
Introduction
The commonest urogenital tumours in childhood are Wilms tumour of the kidney and rhabdomyosarcoma in the pelvis. We review these tumours along with other primary renal tumours and less common ovarian and testicular tumours in childhood. Current clinical concepts, relevant staging investigations and imaging features are described.
Renal tumours
Malignant renal tumours comprise 6% of childhood cancer. Of these, 90% are Wilms tumours. Whilst imaging cannot differentiate between types of paediatric renal tumour, it plays a crucial role in screening, diagnostic work-up, assessing response to treatment and follow-up.
Nephroblastomatosis complex
Fetal nephrogenesis is complete by 36 weeks gestation. A focus of persistent fetal metanephric blastema beyond this point is called a nephrogenic rest. Multifocal or diffuse nephrogenic rests are called nephroblastomatosis (NBS). NBS commonly regresses in the first few months of life but there is a malignant potential for degeneration into Wilms tumour. Foci of NBS are found in 1% of all neonatal autopsies, 35% of kidneys with unilateral Wilms tumour and in nearly all cases of bilateral Wilms tumours[1]. The risk of an individual lesion progressing is unknown but it is small. NBS is divided into an intralobar type (occurring within the renal lobe) and the more common perilobar type (arising in the cortex around the renal lobe). They have different appearance, associations and malignant potential. The commonest presentation of NBS is during the evaluation of a Wilms tumour or screening at-risk populations. When it is massive or confluent, it may present as a palpable mass. Most patients with nephroblastomatosis are under 2 years of age.
Imaging nephroblastomatosis (NBS)
Foci of NBS are usually smaller than Wilms tumours and are less likely to be spherical. There are no prospective trials on the imaging differentiation of Wilms tumours and NBS but a retrospective study of 29 patients found that homogeneity on all modalities was the most reliable feature in the latter[2]. An area of NBS that grows larger, rounder or becomes heterogeneous is suspicious for malignant degeneration.
Ultrasound
Focal nephrogenic rests may be iso- or hypoechoic and resemble normal cortex in a nodular, ovoid or plaque-like configuration. Diffuse NBS may appear as a thick, homogeneous and hypoechoic peripheral rind on ultrasound (US) or a diffusely enlarged kidney with loss of corticomedullary differentiation that may be difficult to distinguish from leukaemia/lymphoma, except that children with NBS are usually under 2 years of age.
Early studies suggested that US is less sensitive than computed tomography (CT) for detecting NBS although current high resolution scanning has not been assessed[2].
CT
It is essential to give contrast for CT or magnetic resonance imaging (MRI) as this greatly increases the conspicuity of NBS. Appearances include focal ovoid or plaque-like areas of iso- or hypoattenuation with decreased enhancement or a diffuse rind of low attenuation encasing normally enhancing but distorted parenchyma.
MRI
NBS may be isointense or hypointense on T1-weighted images and does not enhance. The appearance on T2-weighted images is variable (hypo-, iso- or hyperintense) but importantly, it appears homogeneous. It is thought that NBS that is dark on T2-weighted images is sclerotic or regressing unlike hyperplastic NBS (with malignant potential), which, like Wilms tumour, is bright on T2-weighted images[3].
Wilms tumour (nephroblastoma)
Wilms tumour comprises more than 95% of paediatric renal tumours and is the 5th commonest paediatric malignancy.
Presentation
Patients typically present before the age of 5 years with a peak incidence of 3 years. Males and females are equally affected with the highest incidence seen in the black population of the United States and Africa. Clinical presentation is most commonly with an abdominal mass. Haematuria, especially following minor trauma, is another typical presentation. Other presentations include abdominal pain, fever and hypertension. It is occasionally detected on surveillance of patients with associated syndromes. Microscopic haematuria is found in 25% at presentation. Bilateral Wilms tumour occurs in 4–13% of cases with two-thirds of these being synchronous and one-third metachronous. In bilateral cases, one mass is usually dominant. Bilateral cases almost exclusively occur in patients with nephroblastomatosis, congenital malformation or predisposing syndromes. The management implications of bilateral tumours are complicated by the inherent risk of renal failure and preoperative chemotherapy and nephron-sparing surgery may be considered.
Staging
The (post-surgical) staging of Wilms tumour according to the North American National Wilms Tumor Study Group is summarized in Table 1.
Table 1.
North American National Wilms Tumor Study Group staging system for renal tumours
| Stage | Description |
|---|---|
| Stage I | Tumour confined to the kidney without capsular or vascular invasion |
| Stage II | Tumour beyond renal capsule, vessel infiltration |
| Stage III | Positive lymph nodes in the abdomen or pelvis, peritoneal invasion or residual tumour at surgical margins or intraoperative tumour rupture |
| Stage IV | Metastatic disease outside the abdomen or pelvis |
| Stage V | Bilateral tumours at original diagnosis |
Histopathology
Histology is termed favourable or unfavourable. Favourable histology is determined by the presence of all 3 fetal cell lines (epithelial, stromal and blastemal). These tumours have an excellent prognosis with an overall survival in excess of 90%. Unfavourable histology, seen in 6% of Wilms tumours, is determined by the presence of anaplastic elements (hyperchromatic cells with large nuclei). This occurs in a slightly older age group with a peak at 5 years and increased incidence in African and black Americans.
Molecular genetics
Many chromosomes and genes with prognostic significance have recently been identified and are playing an increasing role in treatment stratification. Loss of heterozygosity at chromosomes 1 p and 16q are adverse prognostic factors, as is high telomerase expression. A WT gene mutation has been identified in 10–15% of sporadic cases of Wilms tumour.
Imaging
The aim of initial work-up is to identify locoregional extension, metastases and involvement of the contralateral kidney. Preliminary diagnosis of all paediatric abdominal masses is with abdominal US including colour Doppler. This is readily available, non-invasive and non-ionizing and gives the best information regarding vascular invasion and movement of the mass relative to other organs. Further imaging with CT or MRI is usually considered necessary to confirm the renal origin, local and distant extent and assess the contralateral kidney. However, some European centres rely on US alone for preoperative assessment without any apparent detriment to patient care. MRI is preferred to CT as it is non-ionizing, but many centres use CT due to speed and availability. Chest imaging is traditionally with anteroposterior and lateral chest radiographs but this is gradually being superseded by more sensitive CT chest. The implications of CT-only lesions are discussed later. Newer techniques showing promise include positron emission tomography (PET)/CT and functional MRI with diffusion-weighted imaging. Technetium 99 m-labelled dimercaptosuccinic acid (DMSA) scanning may also be used in the work-up for surgery in bilateral tumours or single functioning kidneys.
US
The primary tumour is usually demonstrated as a large (mean diameter 11 cm, smaller in those on surveillance), spherical and at least partly intrarenal mass. Echogenicity may be equal to or greater than normal kidney. The mass has a smooth, well-defined margin, a fibrous pseudocapsule and is usually heterogeneous, containing hypoechoic areas of haemorrhage or necrosis. Native renal tissue can be difficult to identify and is characteristically stretched around the periphery of the tumour.
The contralateral kidney is assessed for synchronous disease (4–12% have contralateral Wilms tumour or NBS) and the renal vein and inferior vena cava (IVC) are interrogated for tumour thrombus (identified in 4–10%). US has been shown to be the most effective modality for detecting IVC thrombus[4]. The liver is imaged for metastases, which are found in 2% at presentation, and local lymph nodes are also assessed. Imaging cannot differentiate reactive from involved nodes, for which lymph node biopsy at surgery is mandatory.
CT/MRI
CT and MRI are used for further delineation of the primary tumour, exclusion of adenopathy, liver metastases, vascular involvement and signs of rupture and for examination of the other kidney (Figs. 1 and 2). Tumour measurements are usually given in 3 planes although there is no current consensus regarding the technique used.
Figure 1.
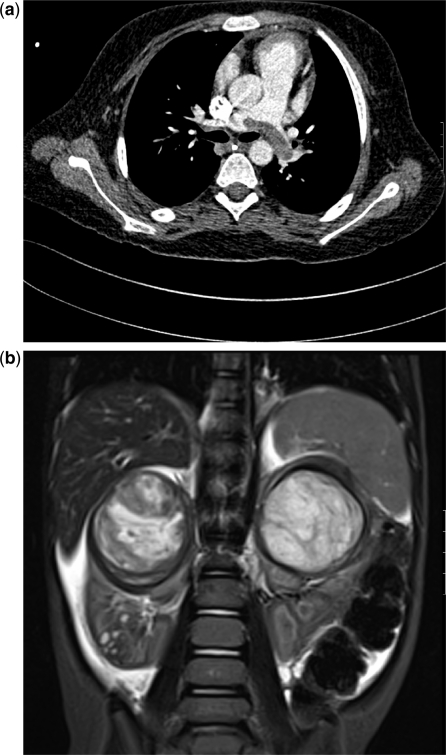
Wilms tumours in different patients. (a) This 5-year-old girl survived a severe syncopal episode 2 weeks prior to presenting with an abdominal mass. There was a small tumour thrombus protruding into the IVC from a large less renal mass. Presumably the saddle embolus in the pulmonary arteries had been in the IVC and detached itself, resulting in a near death episode. (b) Coronal T2-weighted images showing bilateral Wilms tumours in the upper poles and some generalized ascites. Small hyperintense areas in the lower pole of the right kidney were thought to be foci of nephroblastomatosis.
Figure 2.
Wilms tumour in a 2-year-old boy. (a) The right renal mass appeared mostly cystic on US and T2-weighted MRI but in (b) a solid enhancing component was clearly visible on the post-gadolinium-enhanced T1 images.
On CT, Wilms tumour is generally spherical and intrarenal. It may be iso- or hypoattenuating compared with normal renal tissue and shows decreased enhancement. The tumour tends to be heterogeneous, containing areas of haemorrhage and necrosis. The claw or beak sign describes normal renal tissue stretched around the tumour. Dystrophic calcification is seen in 9%. Wilms tumour typically forms a pseudocapsule. Evidence of perinephric spread is seen in 20% with a thickened capsule or fat infiltration.
On MRI, Wilms tumour is heterogeneous in 80% and may be hypointense or isointense on T1-weighted images with decreased enhancement post gadolinium. The appearance on T2-weighted images is variable with iso- or hyperintense signal, which may be hypointense following treatment. Calcification and fat are seen in less than 15%.
In centres using preoperative chemotherapy, further preoperative imaging with US and or MRI may be performed to aid surgical planning. Most tumours undergo a change in size or necrosis. Increase in size occurs in 5% which may be a poor prognostic factor.
New imaging techniques: PET/CT and functional MRI
FDG-PET/CT
PET/CT with fluorodeoxyglucose (FDG) has an established role in imaging lymphoma and sarcoma in the paediatric patient. FDG uptake has been described in Wilms tumour but the role of PET/CT is not yet established in this disease. In a study by Misch et al.[5] of 12 patients with Wilms tumour, PET was found to be advantageous over conventional imaging in the detection of residual disease following treatment, in staging of relapse (by helping to distinguish scar tissue from active disease) and also in the detection of unfavourable/anaplastic histology.
Quantitative MRI
Diffusion-weighted imaging in MRI has an established role in neuroradiology and is now increasingly used in body MRI with minimal increase in imaging time. Apparent diffusion coefficient (ADC) maps have been shown to correlate with cellularity and have potential uses in guiding biopsies away from areas of necrosis and predicting early response to therapy[6].
Pulmonary metastases
Up to 10% of patients with Wilms tumour have lung metastases at presentation. The chest radiograph has traditionally been used for the assessment of lung metastases. CT is now widely used as it more sensitive but its role in staging is still being established. Uncertainty exists regarding the management of small volume pulmonary lesions which are visible on CT only.
The differential diagnosis for small pulmonary lesions is broad and there is significant morbidity associated with the treatment of pulmonary disease. In the National Wilms Tumor Study (NWTS)-5 trial, 25% of solitary and 31% of multiple nodules on CT were not malignant[7]. On the other hand, small lesions detectable on CT have been shown to affect outcome and this has led some centres to upstage tumours to stage 4 with subsequent change of treatment. The United Kingdom Children's Cancer Study Group (UKCCSG) WT2 trial demonstrated a higher pulmonary relapse rate in patients with otherwise stage 1 disease and CT-only lung disease compared with other stage 1 patients[8].
Clearly a consensus approach for the treatment of CT-only lung disease is required to allow meaningful review of the outcome data. The international collaborative groups aim to rationalize the approach. The current International Society of Paediatric Oncology (SIOP) Wilms study recommends CT for lung disease and treatment of nodules greater than or equal to 10 mm. The AREN0533 arm of the current Children's Oncology Group (COG) trial uses central staging and a response-based approach to pulmonary lesions whereby only those that do not respond to 6 weeks of chemotherapy and are biopsy positive will be treated with whole lung radiotherapy.
Associated syndromes and surveillance
Most Wilms tumours occur sporadically in otherwise healthy children. A minority occur in patients with congenital abnormalities such as cryptorchidism or horseshoe kidney or in certain predisposing genetic syndromes. These conditions include Denys Drash, WAGR syndrome (Wilms tumour, aniridia, genital anomalies and mental retardation), Beckwith–Wiederman syndrome, sporadic aniridia, Perlman syndrome and hemihypertrophy.
Screening of high-risk groups is undertaken in the United Kingdom and parts of Europe and North America but the risks and benefits of screening are unknown as there are no randomized controlled trials and the numbers involved are small. Several small studies have shown potential benefits with earlier diagnosis of smaller and lower stage tumours enabling less toxic treatment. The potential difficulties include false-positive results and the burden of anxiety for families. The Wilms Tumour Surveillance Working Group 2005 have produced a guideline recommending screening of children with conditions with a greater than 5% risk of Wilms tumour up to the age of 5 or 7 years (depending on the condition)[9].
Pretreatment biopsy
The role of pretreatment biopsy remains debated. The UKCCSG recommends pretreatment biopsy in all suspected cases of Wilms tumour in children over 6 months. The goal of biopsy is to identify the small group of paediatric renal tumours that are not Wilms tumours. In the UKCCSG Wilms tumour study 3, 12% of biopsies demonstrated an alternative renal malignancy. Also preoperative biopsy allows a clinicopathologic assessment of response to treatment[10].
The commonest complication is bleeding (20%) with rarer complications including serious bleeding, rupture and needle tract seeding. The primary argument against biopsy is the risk of seeding. In the 2009 COG study, a preoperative biopsy automatically upstages the tumour to stage 3 in line with intraoperative spill. Currently COG recommend biopsy only in unresectable disease and SIOP recommend biopsy only in cases of unusual clinical (e.g. >6 years of age or urosepsis) or atypical imaging findings (e.g. voluminous lymph nodes or calcification).
Treatment
Long-term survival of localized disease (stage I–III) is over 90% and of metastatic disease (stage IIII) greater than 70%. The favourable survival has led to a shift in management towards stratified treatment with the aim of reducing toxicity to low-risk patients whilst intensifying treatment in the high-risk group. Stage and histopathologically subtype are well-established prognostic factors but more recently patient age, tumour weight and molecular genetic factors are recognized and are increasingly used to stratify treatment.
Surgery, chemotherapy and radiotherapy are used with diverse protocols around the world. The main dichotomy is between NWTS incorporating COG in the United States and SIOP in Europe. The North American approach advocates immediate surgery followed by adjuvant chemotherapy according to surgical staging and histology. In Europe preoperative chemotherapy is used to downstage the tumour prior to surgery.
There is ongoing debate regarding the use of neoadjuvant chemotherapy but there is no significant difference in overall survival between the 2 groups. The advantages of preoperative chemotherapy are: (1) reducing tumour size facilitates surgery with reduced risk of intraoperative tumour rupture and tumour spillage; (2) downstaging the tumour potentiates the use of less toxic chemotherapy and possibly minimally invasive surgery. Due to preoperative downstaging, the incidence of stage 3 disease is 15% in the United Kingdom and Europe compared with 30% in the United States[11].
The advantages of primary nephrectomy are to ensure histological diagnosis prior to treatment thus avoiding unnecessary treatment of benign disease or under treatment of other tumours such as malignant rhabdoid tumours or renal peripheral neuroectomdermal tumours (PNETs). Preoperative chemotherapy is given in certain circumstances in the United States such as bilateral Wilms tumour, IVC or right atrial involvement and those tumours considered too big to resect safely. The current COG study also gives neoadjuvant chemotherapy to patients with predisposing conditions to promote nephron-sparing surgery.
The standard surgical approach is radical nephrectomy with a transverse, transperitoneal approach and lymph node sampling. Nephron-sparing surgery may be used in certain circumstances such as bilateral disease, underlying renal abnormality, predisposing genetic syndromes and uncomplicated unilateral cases. SIOP 2001 lists exclusion criteria for nephron-sparing surgery that are based mainly on imaging findings such as capsular invasion, renal or IVC involvement, metastases or lymphadenopathy.
Follow-up imaging
Relapse is infrequent and in 90% of cases it occurs within the first 4 years after treatment[12]. Recurrence occurs more commonly in the lung (58%) than the abdomen (29%)[13]. As relapse is curable, accurate and timely diagnosis is essential. However, the excellent long-term outcome for Wilms tumour survivors necessitates a minimally invasive/minimally harmful approach to follow-up. There is no consensus on frequency of follow-up. SIOP 2001 uses chest radiograph and abdominal US for 5 years follow-up. There is more reliance on repeated CT for follow-up in COG protocols. Imaging is more frequent in metastatic disease, partial nephrectomy and NBS.
Other renal tumours
Other renal tumours are rare but clear cell sarcoma, malignant rhabdoid tumour and renal cell carcinoma account for a disproportionate number of relapses and death.
Clear cell sarcoma
Clear cell sarcoma of the kidney, formerly called bone metastasizing renal tumour of childhood comprises 4% of all paediatric renal neoplasms. It affects a similar age group to Wilms tumour. There is a wider range of metastases in clear cell sarcoma with a predilection for bone metastases at presentation. Brain, liver, lymph nodes and lung may also be affected. There are no distinguishing imaging features but the presence of lytic bone metastases at presentation in a renal mass that is otherwise typical for Wilms tumour should suggest the diagnosis.
Due to the nature of the metastatic spread, staging includes bone scintigraphy, MRI brain and CT chest. This is an aggressive tumour with a poor prognosis. Long-term survival is around 60–70%.
Malignant rhabdoid tumour
Previously considered a sarcomatous subtype of Wilms tumour but now reclassified as a separate entity, malignant rhabdoid tumour comprises 1–2% of paediatric renal tumours. It is the most aggressive with a bleak prognosis (survival is often less than 1 year from diagnosis). It occurs exclusively in children, in a slightly younger age group than Wilms tumours. The median age at presentation is 13 months.
The imaging features of a solid heterogeneous mass are indistinguishable from Wilms tumour. It often involves the hilum and renal vein involvement is common. A peripheral fluid sign/subcapsular fluid has been described at CT but is seldom seen and is not pathognomic.
Rhabdoid tumour metastasizes to lungs, liver and brain and it is also associated with primary lesions of the central nervous system, usually midline posterior fossa PNETs. Brain MRI brain is therefore recommended following tissue diagnosis.
Renal cell carcinoma
Renal cell carcinoma (RCC) is the commonest renal malignancy in adults but it is rare in childhood comprising less than 1% of paediatric renal tumours. There is a high incidence of personal or family history of associated disorders (e.g. von Hippel–Lindau and tuberous sclerosis (TS)) and it is described as a secondary malignancy following chemotherapy and radiotherapy (especially following neuroblastoma). RCC tends to affect an older age group than Wilms tumour with a mean age at presentation of 9 years. Presentation is non-specific with a mass or flank pain or less commonly with haematuria.
On imaging, RCC is indistinguishable from Wilms tumour but it is usually smaller at presentation (average size 4 cm). Calcification, which may be ring-like, is more common (seen in 25%) (Fig. 3). On CT, RCC may be hyperattenuating and it shows decreased enhancement relative to normal renal tissue. Metastases are to lymph nodes, renal vein, bones and brain. Twenty percent have metastases at diagnosis.
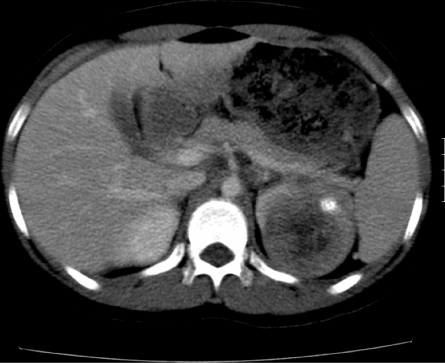
Figure 3.
Renal cell carcinoma. Contrast-enhanced CT scan shows an ill-defined calcified mass in the left kidney in an adolescent girl. The calcification and age of the patient are more in favour of an RCC than a Wilms tumour (which is only rarely calcified at presentation).
Generally the childhood form has a better prognosis than adult RCC. Unlike in adults, locoregional lymph node involvement does not adversely affect prognosis[14].
Renal medullary carcinoma
This highly malignant tumour of epithelial origin develops in the renal medulla and infiltrates the cortex. It is seen almost exclusively in adolescents and young adults with sickle trait or Hb sickle cell disease.
On imaging it is typically centrally located, encasing the renal pelvis and causing caliectasis. Like other paediatric renal tumours it is heterogeneous and hypovascular. Intratumoral or subcapsular haemorrhage is common, as is renal vein thrombus or encasement. Metastasis is rapid to lymph nodes and liver. The response to chemotherapy and radiotherapy is poor and there is a poor prognosis.
Congenital mesoblastic nephroma
Congenital mesoblastic nephroma (CMN) has similar imaging features to Wilms tumour and was previously considered to be an infantile form but it now recognized as a separate entity with different histology (spindle cells) and an excellent prognosis. It is the commonest solid tumour of the newborn with a mean age at presentation of 3 months, although it has been described in older children and adults. It comprises 72% of renal tumours under 3 months and 16% of renal tumours under 6 months[15].
Presentation is with an abdominal mass, typically in a neonate and occasionally on antenatal US. On imaging, CMN cannot reliably be distinguished from Wilms tumour although it tends to be more solid as haemorrhage and necrosis are relatively uncommon. Invasion of vessels or the renal pelvis is rare.
CMN is almost always unilateral. Local infiltration of the capsule is common and local recurrence may occur. It rarely metastasizes but spread to brain, bone and lung has been reported[16]. Cellular variant, older age and positive surgical margins appear to increase the risk of recurrence and metastasis.
Treatment is usually with nephrectomy using wide resection margins. Chemotherapy is used in recurrence. Prognosis following complete resection is excellent.
Multilocular cystic renal tumour
Multilocular cystic renal tumour has a bimodal age distribution occurring in boys under the age of 4 years and women over the age of 40 years. On histology it may be completely differentiated, in which case it is termed cystic nephroma (more common in women) or if it contains blastemal elements it is termed cystic partially differentiated nephroblastoma. The latter type is more common in childhood.
Presentation is non-specific with a painless mass or haematuria. On imaging it is a well-defined intrarenal mass with multiple, non-communicating cystic spaces separated by soft tissue septa that may involve the whole kidney or a small portion. US is better than CT for the differentiation of septa. The septa enhance on CT and MRI and may contain curvilinear calcification. The pelvicalyceal system may be distorted or displaced. Treatment is with nephrectomy. Although this is a benign tumour, it may recur if incompletely excised.
Renal angiomyolipoma
Renal angiomyolipomas are hamartomas occurring in patients with TS. They are present in up to 80% of children with TS and are commonly bilateral. Diagnosis is usually incidental or on screening but patients may present with pain or anaemia secondary to haemorrhage or renal failure.
On imaging, they contain a variable amount of fat and have a characteristic blood supply with tortuous vessels and aneurysms. They appear hyperechoic on US and of low attenuation on CT with identifiable areas of fat. On MRI they appear as high signal on T1- and T2-weighted images and demonstrate fat suppression.
These are benign lesions but the major complication is life-threatening retroperitoneal haemorrhage. For this reason, patients with TS are screened with US and lesions larger than 4 cm may be selectively embolized.
Leukaemia
Acute lymphoblastic leukaemia (ALL) is the commonest malignancy of childhood typically presenting at 3–5 years. Kidneys may be involved during active disease or act as a sanctuary site during bone marrow remission. Renal involvement is usually occult but may cause pain, haematuria, hypertension or renal failure.
The typical appearance on imaging is of bilaterally enlarged kidneys that are iso- or slightly hypoechoic on US and show normal or slightly reduced attenuation on CT with minimal enhancement. Occasionally focal or multiple hypoechoic or hypoattenuating masses are seen.
Lymphoma
The kidney does not normally contain lymphoid tissue therefore renal lymphoma is more likely due to haematogenous spread or retroperitoneal extension than a primary lesion. Renal involvement is a late manifestation of lymphoma, which is generally associated with widespread disease elsewhere. Renal involvement is more common in non-Hodgkin lymphoma especially B-cell Burkitt lymphoma. It tends to be asymptomatic and is picked up incidentally at imaging or autopsy.
Several patterns are seen at imaging. The lesions cannot be distinguished from other renal tumours but the presence of hepatosplenomegaly or lymphadenopathy may be suggestive. The commonest appearance is of multifocal, homogeneous masses but diffuse enlargement of the kidneys may also be seen. Focal lesions are subtle on imaging and may be occult. Hypo-, iso- and hyperechoic subcortical masses have been described at US. On CT they appear as multiple round masses which enhance less than normal renal tissue. At MRI they are slightly hypointense on T1-weighted images with increased conspicuity following gadolinium and hyperintense on T2-weighted images.
Rhabdomyosarcoma
Epidemiology
Rhabdomyosarcoma (RMS) is the third commonest extracranial solid tumour in children after neuroblastoma and Wilms tumour[17] comprising 4% of paediatric malignancies[18]. There is a bimodal age distribution with almost two-thirds presenting under the age of 6 years and a second smaller peak in mid-adolescence[19].
RMS arises from embryonal mesenchyme with the potential to differentiate into skeletal muscle. It can arise anywhere in the body but in children it most commonly affects the head and neck (26%) and genitourinary tract (22%)[17]. Genitourinary RMS (GURMS) is divided into 2 broad groups based on prognosis and treatment strategy. These are bladder or prostate RMS (BPRMS) and other sites (non-BPRMS), which include paratesticular, perineum, vulva, vagina and uterus. The former have a worse prognosis.
Most cases are sporadic but there is an association with certain familial cancer syndromes such as the Li–Fraumeni syndrome, Beckwith–Wiederman syndrome and Gorlin syndrome[20]. Neurofibromatosis type 1 is also prevalent in RMS cohorts, especially embryonal RMS (ERMS) and GURMS[21].
Histology/pathology
RMS is one of the small round blue cell tumours of childhood. It is a fast-growing, high-grade, primitive malignant mesenchymal tumour containing certain elements that are found in skeletal muscle. The following are essential for diagnosis: myofibrils, cross striations and positive immunohistochemistry staining for markers of muscle differentiation, e.g. desmin and myoD1.
There are 3 main histological variants: embryonal (80%), alveolar (15–20%) and pleomorphic (not typically found in the genitourinary tract of children). Botryoid and spindle cell are variants of ERMS. Botryoid RMS is associated with a younger age group and a genitourinary site. It has a predilection for hollow organs such as the bladder and vagina. Spindle cell RMS is most common in a paratesticular location and has an excellent prognosis. Other ERMS have an intermediate prognosis. Alveolar RMS (ARMS) is typically found in older children and is associated with a poorer outcome[22]. Both ARMS and ERMS may show rhabdoid tumour-like features or anaplasia. Sclerosing RMS is also described although it is not clear whether this is a separate subtype[23].
Genetics
RMS variants have different genetic profiles. ARMS is associated with 2 reciprocal translocations that generate fusions of the PAX 3 and PAX 7 genes with FKHR (also known as FOX01). No consistent translocation is described in ERMS but it is associated with loss of heterozygosity at 11p15. Also involved in RMS prognosis are EGFR, ErbB-2 and expression of the AURKA gene[24]. As in neuroblastoma, high copy number and overexpression of MycN confers a poor prognosis in RMS[25].
Clinical presentation and spread
Presentation depends on site, patient age and the presence of metastases. Most symptoms are due to compression by the primary tumour or associated lymph nodes. There are no classic paraneoplastic syndromes. Fifteen percent of patients have metastases at presentation (Group IV). These patients may complain of non-specific symptoms such as fatigue, weight loss or low blood counts.
Bladder and prostate RMS typically present with urinary obstruction. Other presentations include a pelvic mass and constipation and urethral strangury. Bladder RMS is typically polypoid and intraluminal occurring in or near trigone. The mean age at presentation is 4.5 years[26]. Lymph node involvement is seen in 20%[27]. Prostate RMS tends to be more locally invasive. It can spread laterally into periurethral tissues and posteriorly into perivesical tissues or bladder base, hence the term bladder/prostate RMS. It may also spread superiorly and anteriorly into the retropubic space of Retzius. The mean age at presentation is 5.1 years[26]. In infants or older boys, lung, bone marrow and bone metastases often occur early. BPRMS is often so large that it is not possible to determine the exact site of origin.
Vaginal and vulval RMS is almost invariably botryoid and occurs in very young girls with a median age of 21 months[28]. Presentation is with bleeding, an introital mass or micturition problems. It rarely spreads to local lymph nodes[29].
Cervical and uterine RMS is less common than vulvovaginal RMS. It typically occurs in older girls with a mean age at presentation of 15 years[28]. Presenting symptoms include bleeding and discharge. Lymphadenopathy is uncommon but it should be excluded on imaging[30].
Paratesticular RMS arises from the mesenchyme of the spermatic cord, epidydimis and tunics. Spermatic cord involvement is usually distal and may invade the testis. It is the commonest extra-testicular scrotal malignancy in childhood and occurs in prepubertal and postpubertal boys. The typical presentation is with a painless unilateral scrotal or inguinal mass. It may be associated with a hydrocoele[31]. Lymphadenopathy is rare under 10 years but para-aortic and para-caval lymph node involvement is seen in 50% of older boys[32]. Lung and bone metastases are present in 10–25%. The histology in the first 2 decades is usually ERMS (classic or spindle).
Imaging
Imaging plays an essential role in the diagnosis of genitourinary RMS. It is used to assess tumour size, spread and relation to or involvement of adjacent structures as well as spread to regional nodes and distant metastases to bone and lung.
US
US is usually the first-line investigation for any abdominal, pelvic or scrotal mass in a child. RMS has a non-specific appearance but typically appears as a well-defined, slightly hypoechoic, inhomogeneous mass that can show significant increased flow[23] and rarely demonstrates calcification.
CT/MRI of primary tumour
CT or MRI may be used for staging the primary tumour. The choice may depend on local availability and expertise. MRI has superior soft tissue resolution and importantly does not use ionizing radiation. It has previously been reported that CT is better than MRI for the detection of abdominal lymph nodes but this is no longer the case with new MRI techniques.
CT of the abdomen and pelvis should be performed with oral and intravenous contrast due to the high incidence of retroperitoneal lymph node involvement. There was a trend away from routine lymph node dissection towards CT in detection of nodal involvement. However, this resulted in higher relapse rates such that the COG protocols are now reverting back to ipsilateral retroperitoneal lymph node sampling in all boys over 10 years regardless of imaging findings[33].
The MRI appearance of RMS is non-specific with intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images (Fig. 4). The tumours usually demonstrate strong enhancement. The report should include local extent, vascular involvement (more than 180 degrees contact of a vessel with the tumour is considered positive) and locoregional lymph node involvement. Imaging of the pelvis should include coronal and sagittal sequences. T1-weighted sequences are particularly important in bladder tumours as urine in the bladder may obscure bladder wall involvement on T2-weighted images. Layering of contrast in the bladder may also obscure bladder wall enhancement on T1-weighted images, however, so cystoscopy and or biopsy are also required.
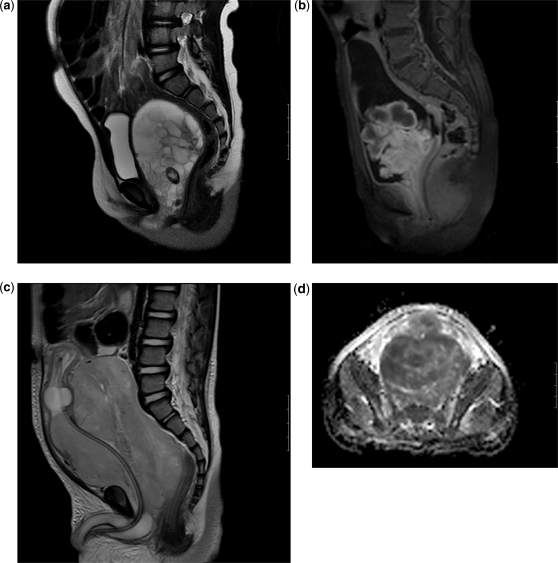
Figure 4.
Rhabdomyosarcoma in different patients. (a) Sagittal T2-weighted image shows a large multicystic vaginal tumour in an infant girl protruding towards the introitus. (b) Sagittal T1-weighted images after gadolinium enhancement showing a lobulated intravesical mass in a 3-year-old girl. (c) Sagittal T2-weighted MRI in a 3-year-old boy shows a very large solid prostatic mass displacing a catheterized bladder superiorly. A fluid-filled balloon catheter is seen in a displaced and compressed bladder. (d) ADC map of the same prostatic tumour shows restricted diffusion, indicating high cellularity (and likely malignancy). In addition to demonstrating the extent of the pelvic primary tumour, it is important that the scanning (all modalities) is sufficient to assess also for locoregional lymphadenopathy.
CT chest is mandatory for all patients to assess for pulmonary metastases. Pulmonary metastases may be seen on CT with a negative chest radiograph[34]. The European Paediatric Soft Tissue Sarcoma Group (EpSSG) protocol 2005 includes imaging guidelines. The criteria for positive lung metastases are 1 nodule ≥10 mm, 2 or more well-defined nodules measuring 5–10 mm or 5 or more well-defined nodules <5 mm[35].
Bone scan
99mTc methylene diphosphonate bone scan is used routinely to detect or exclude bone involvement at diagnosis. Plain film and cross-sectional imaging may be used additionally in isolated bone uptake[36].
Newer techniques
FDG-PET/CT
Studies of PET in childhood RMS have demonstrated a sensitivity of 77–100% for primary tumour, 62–77% for regional or distant disease and a specificity of 83–95%[37-39]. The sensitivity of PET alone is equivalent to conventional imaging for primary tumours, superior for bone and lymph node involvement and inferior for lung metastases[38]. McCarville et al.[40] reported early experience of PET/CT in 61 paediatric sarcoma patients including 28 RMS. They found it to be beneficial in localizing RMS of unknown primary and in localizing unusual sites of metastasis or recurrence.
A retrospective study of 19 paediatric sarcoma patients (7 with RMS) comparing FDG-PET/CT with conventional imaging found that FDG-PET/CT was sensitive for local relapse and complementary to conventional imaging in the detection of distant metastases[39]. A further retrospective study of 43 childhood sarcomas (including 5 cases of RMS) found PET/CT had a greater sensitivity than PET alone for the detection of distant metastases but was not superior to CT. The sensitivity for lung metastases greater than 0.5 cm and for lymph nodes <1 cm diameter was significantly better than CT, however[41].
Further studies are needed to see if additional information from PET/CT helps to change management in order to justify the additional radiation involved. The potential role in the detection of recurrence is promising as current protocols emphasize the importance of early detection to help outcome in this poor prognostic group.
Whole-body MRI
Small series have provided promising results for whole-body MRI in bone marrow assessment[42] but further studies are required.
Staging
A number of different staging systems have been used by various collaborative paediatric oncology groups. Risk group classification is now used to determine treatment protocols based on a combination of a post-surgical staging system and a TNM staging system.
The most commonly used post-surgical staging is that developed by the International Rhabdomyosarcoma Staging Group (IRSG) shown in Table 2. A pretreatment, site-modified TNM staging system was also developed (Table 3) that attempted to standardize the staging system in the United States with those in other parts of the world[43]. This system takes site and size into account but does not take the extent of surgery into consideration. Both stage and group have been shown to be highly predictive of outcome and were correlated[43,44].
Table 2.
Post-surgical clinical group staging system for rhabdomyosarcoma:
| Group | Extent of disease and surgical result |
|---|---|
| I | Localized disease, completely resected (regional lymph nodes not involved) |
| Ia | Confined to muscle or organ of origin |
| Ib | Contiguous involvement: infiltration outside the muscle or organ of origin, as through facial planes |
| II | Total gross resection with evidence of regional spread |
| IIa | Grossly resected tumour with microscopic residual disease |
| IIb | Regional disease with involved nodes, completely resected with no microscopic residual |
| IIc | Regional disease with involved nodes, grossly resected, but with evidence of microscopic residual and/or histological involvement of the most distal regional node (from the primary site) in the dissection |
| III | Incomplete resection with gross residual disease |
| IIIa | After biopsy only |
| IIIb | After gross or major resection of the primary (>50%) |
| IV | Distant metastatic disease (lung, liver, bones, bone marrow, brain, lymph nodes) |
Table 3.
TNM staging system of rhabdomyosarcoma
| Stage | Description |
|---|---|
| Stage I | Disease is localized and involves the orbit, the head, and neck region (excluding parameningeal sites), or the non-bladder-prostate genitourinary region |
| Stage II | Includes any localized disease of any unfavourable primary site not included in the Stage I category. The primary tumour must be less than or equal to 5 cm in diameter |
| Stage III | The criteria are the same as in Stage II except the primary tumour is larger than 5 cm in diameter and/or it involves regional lymph nodes |
| Stage IV | Like Group IV, Stage IV implies metastatic disease at diagnosis |
The COG in the United States and more recently the EpSSG have combined the 2 approaches for treatment based on clinical risk group. Children are assigned to 1 of 3 risk classifications (Table 3) based on stage, group and histology. Prognostic factors are incorporated into the strategy including location, size and post-surgical group.
Treatment
Collaborative studies have led to dramatically improved survival over the last 40 years. A new understanding of molecular biology has allowed risk stratification and development of new therapeutic targets. Relapsed and refractory cases remain the most challenging to treat.
Treatment involves surgery, chemotherapy and radiotherapy with enrolment into clinical trials where possible. Treatment in the United States is currently based on protocols of the soft tissue sarcoma (STS) committee within COG. In Europe, EpSSG protocols are used. All groups use a multidisciplinary approach with consideration of long-term survival and acute and long-term toxicities. All patients have chemotherapy and some form of local control, which can be surgery or radiotherapy or a combination of the 2.
Whether primary resection is possible should be decided by an experienced paediatric surgeon[29]. Organ preservation is considered where feasible. The goal of treatment in BPRMS is survival with an intact and functional bladder[45].
Paratesticular RMS requires radical inguinal orchidectomy with ipsilateral retroperitoneal lymph node dissection for all clinically or radiologically enlarged nodes in boys under 10 years but also for all boys over 10 years regardless of clinical or imaging findings. A significant number in this age group have nodal disease without enlarged nodes on imaging[46].
The main cause of treatment failure is failure of local control. The dilemma for all groups is to balance the extent of primary treatment to avoid local recurrence and the maintenance of organ function (e.g. bladder, vagina, testis, ovary, sexual function). Bladder preservation is possible in 80% but complications include urinary incontinence in one-third, urinary tract infection and decreased renal function[47].
Radiotherapy forms an important part of treatment in most RMS[21]. The main difference between the US and European approach is the use of radiotherapy, which was previously routine in the United States and used less in Europe. The approaches are now converging. The current EpSSG protocol uses radiotherapy for ARMS, IRSG Group 2 with high-risk factors and all Group 3 unless there is a favourable site or histology[23]. The long-term morbidity of pelvic radiotherapy is significant. This may be reduced with newer techniques such as intensity-modulated radiation therapy (IMRT), brachytherapy and proton beam therapy[21].
Prognosis
The following prognostic factors are identified in the current EpSSG protocol[48]: histology (alveolar worse than embryonal), post-surgical status (IRS Group), tumour site (non-BP GU) is favourable, node involvement, tumour size (>5 cm is unfavourable) and patient age (unfavourable if ≥10 years).
Currently, the reported cure rate for RMS is approximately 70%. The high-risk and relapsed group continue to have a poor prognosis. Three-year disease-free survival is <30% in the high-risk group and even lower for those with tumour The COG in the United States and more recently the EpSSG have combined the 2 approaches for treatment based on clinical risk group. Children are assigned to 1 of 3 risk classifications (Table 3) based on stage, group and histology. Prognostic factors are incorporated into the strategy including location, size and post-surgical group[49]. BPRMS have the worst prognosis of GURMS. The 5-year overall survival rates for all RMS from IRS IV are: 88% low risk, 70% intermediate risk and 25 % for those with metastases[50].
Complications of treatment
Complications of pelvic radiotherapy include growth delay, bowel obstruction, gonadal dysfunction and secondary malignancy. Half of female survivors of pelvic RMS suffer with long-term endocrine, gynaecologic, genitourinary, gastrointestinal or musculoskeletal complications. There is a 6-fold increase in secondary malignancy such as acute myelocytic leukaemia, melanoma and sarcoma[51]. Of the 62% of BPRMS survivors who retain their bladder, only 40% self-report normal function[47].
Gynaecologic Neoplasms
Gynaecologic neoplasms are uncommon in children. The commonest paediatric gynaecologic tumours are ovarian germ cell tumours (GCTs) and rhabdomyosarcoma (RMS) of the vagina, uterus and cervix (discussed above). The UK National Registry of Childhood tumours recorded 163 gynaecologic tumours in girls aged up to 14 years between 1991 and 2000[52]. Of these, 126 were ovarian of which 112 were GCT. Other ovarian tumours included 6 carcinoma, 6 lymphoma, 1 neuroblastoma and 1 mesothelioma. Of the non-ovarian genital tumours, there were 19 RMS, 12 GCT, 5 carcinoma and 1 other sarcoma. Adult type malignancies such as squamous cell carcinoma of the cervix are also rarely seen in adolescent patients[53].
Ovarian tumours
Ovarian tumours account for approximately 1% of paediatric malignancies[54]. Ovarian tumours may occur at any age but the incidence increases at 8–9 years and peaks at 19 years[55]. They are most common in the second decade. Approximately 80% of ovarian tumours in childhood are benign including epithelial cysts and teratomas[56].
The gonad has 3 cell types with malignant potential: germ cells, sex cord/stromal cells and coelomic epithelium. In contrast to adult ovarian tumours, two-thirds of paediatric ovarian neoplasms are GCT with tumours of epithelial and stromal origin occurring less frequently[57].
Presentation is usually related to the primary tumour with abdominal pain or an abdominal or pelvic mass[58]. Children with peritoneal deposits (usually immature teratoma) may present with abdominal distension and ascites. Up to one-third present with an acute surgical abdomen[59], often related to torsion or rupture. Other presentations include fever and constipation. More unusual presentations may be due to secondary hormonal effects or paraneoplastic syndromes, e.g. hypercalcaemia in ovarian dysgerminoma[60].
GCT of the ovary
GCTs originate from the primordial germ cells of the urogenital ridge and can arise either in the gonads or along normal or aberrant migrational pathways. GCTs comprise two-thirds of paediatric ovarian tumours[57]. Approximately 25% of these are malignant[56]. The peak incidence is around 12 years[61] and they are rare under 5 years of age. In decreasing order of frequency, the ovarian GCTs seen in childhood are mature teratoma, dysgerminoma, endodermal sinus tumour (also known as yolk sac carcinoma), immature teratoma, mixed GCT and embryonal carcinoma[57]. Other rare types of GCT include gonadoblastoma, polyembryoma and choriocarcinoma.
Teratomas contain well-differentiated tissues of the 3 germ cell layers (endoderm, ectoderm and mesoderm). They are classified as mature, immature or malignant. Mature teratomas contain mature elements such as hair, skin and teeth and are benign. Immature teratomas contain varying degrees of immature fetal tissue and have malignant potential. They are graded 1–3 by the amount of immature tissue present[57]. Malignant teratomas contain at least one malignant germ cell component or malignant somatic component.
Mature teratomas are the commonest ovarian GCT. They are most common in the second decade and 10% are bilateral[62]. The median age of presentation for immature teratomas is 10 years[63]. Spread beyond the ovary is seen in 41%[64] at presentation. Metastases may be to lymph nodes, liver, peritoneum and rarely lung.
Dysgerminoma is the commonest malignant ovarian GCT of childhood and adolescence[65]. It may present at any age, being most frequent in adolescence with a peak at 19 years. Most present with stage 1 disease but it can spread via lymphatics to the para-aortic region as well as to liver, lungs and subdiaphragmatic lymph nodes[66]. Twenty percent are bilateral.
Endodermal sinus tumour or yolk sac tumour is the most aggressive ovarian GCT in childhood. The median age of presentation is 19 years. Most have raised alpha-fetoprotein (AFP). Most (75%) present with stage 1 disease but spread is rapid to lymph nodes, peritoneum, liver and lung[57]. All patients require chemotherapy regardless of stage due to the aggressive nature of the tumour.
Imaging
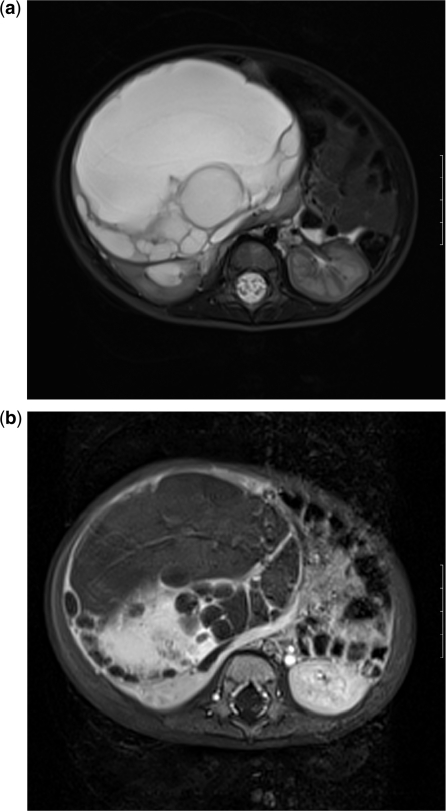
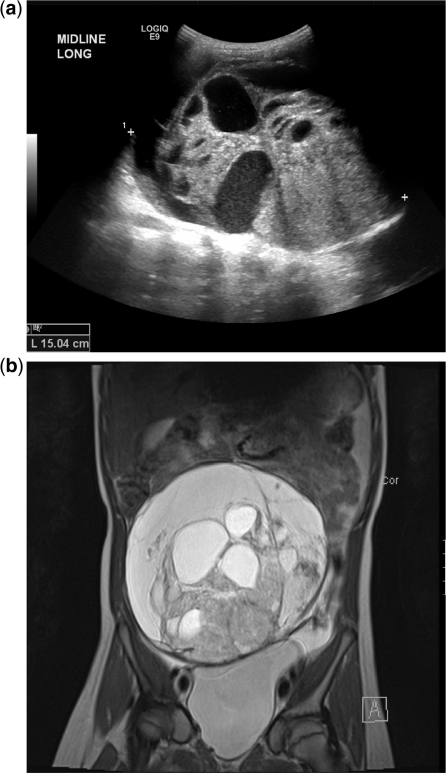
Preoperative imaging is useful for surgical planning to delineate local extent and spread to lymph nodes, peritoneum, omentum, liver or lungs. Imaging cannot reliably differentiate benign from malignant disease. Trans-abdominal US of the pelvis with a full bladder is the first-line investigation (Fig. 5a). This should be followed, where necessary, with MRI (Fig. 5b), which avoids the radiation associated with CT scanning and provides excellent tissue contrast.
Figure 6.
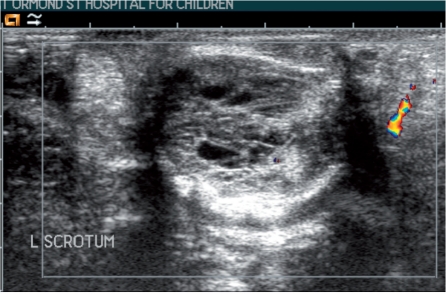
US of a left testicular mass in a 4-year-old boy shows a heterogeneous cystic lesion, proven at orchidectomy to be a mature teratoma.
Figure 5.
Ovarian germ cell tumour. (a) US shows a heterogeneous solid and cystic mass in an 8-year-old girl. (b) Coronal T2-weighted MRI shows the mass is a large cyst with numerous internal smaller cysts and solid elements lying superior to the bladder. Only one normal ovary was identifiable.
US
Benign ovarian teratomas appear as large, predominantly cystic lesions with echogenic elements that may contain hair, teeth or fat. Mature teratomas may be pure solid lesions however. Malignant tumours tend to appear mixed with more solid elements and thickened irregular walls or septa and increased vascularity.
MRI
Mature teratomas are well demonstrated by MRI. The fat component demonstrates high signal on T1- and T2-weighted images and low signal on fat suppression. Calcification not be visible on MRI but may be seen as signal dropout on all sequences. Fluid components will usually be low or intermediate signal on T1-weighted images and high signal on T2-weighted images and fat fluid levels may be present. Immature teratomas tend to have more solid components, which appear as low or intermediate signal on T1-weighted images, high signal on T2-weighted images and show variable enhancement. Fibrovascular septa tend to show marked contrast enhancement.
CT
CT is considered less desirable than MRI due to the radiation burden involved, particularly in the setting of benign disease. CT may demonstrate different characteristic elements in a mature teratoma, e.g. fat and calcification. In a study evaluating CT characterization of paediatric ovarian masses, only 55% of paediatric teratomas had evidence of fat compared with 94% of adult patients[67]. Solid elements and septa show enhancement and there may be variable amounts of central haemorrhage and necrosis. Classic stippled calcification has been described in dysgerminoma in contrast to the bony calcification seen in teratoma[68].
FDG-PET/CT
As in the other urogenital tumours of childhood, there are early promising results in the use of PET/CT with avid FDG uptake in ovarian neoplasms[69]. The role of PET/CT is not yet fully established however. Its main role currently is to identify residual or recurrent tumour in difficult cases.
Patterns of spread and staging
Ovarian GCTs may spread locally to bladder, rectum, uterus or peritoneum, via the lymphatic system or haematogenously to lung and liver. Peritoneal seeding is more common with immature teratomas. Staging relies on histological findings in the resected tumour and tumour markers. The staging is based on the International Federation of Gynaecology and Obstetrics (FIGO) system for tumours of the female pelvis. This has been modified for the intergroup studies of the COG (Table 5).
Table 4.
COG risk classification for rhabdomyosarcoma
| Risk category | Description |
|---|---|
| Low risk | Patients have embryonal rhabdomyosarcoma at a favourable site (Stage I), at an unfavourable site with complete resection (Group I), or at an unfavourable site with microscopic residual disease (Group II) |
| Intermediate risk | Patients have embryonal rhabdomyosarcoma at an unfavourable site with gross residual disease (Group III), or non-metastatic alveolar rhabdomyosarcoma at any site |
| High risk | Any patient with metastatic disease |
Table 5.
COG staging of ovarian GCTs
| Stage | Characteristics |
|---|---|
| Stage I | Limited to one or both ovaries. Peritoneal washings normal. Tumour markers return to normal after appropriate half-life decline |
| Stage II | Microscopic residual disease or positive lymph nodes. Peritoneal washings normal. Tumour markers either normal or showing evidence of malignancy |
| Stage III | Lymph node involvement. Gross residual disease or biopsy only. Contiguous visceral involvement. Peritoneal washings positive. Tumour markers either normal or showing evidence of malignancy |
| Stage IV | Distant metastases |
Management
Treatment of ovarian GCT is with complete resection followed by adjuvant chemotherapy for malignant tumours. The management of immature teratomas is controversial but the current recommendation is for surgery alone followed by observation. The prognosis is better than in the adult patients with 98% 4-year event-free survival[63].
Raised tumour markers or a predominantly solid tumour are suggestive of malignancy in which case a unilateral salpingo-oopherectomy is indicated via laparotomy. Retroperitoneal nodal masses that persist despite chemotherapy may also be excised. Fertility-sparing surgery (including preservation of the contralateral ovary) is favoured due to the high cure rates. Tube-sparing surgery and a laparoscopic approach may be used for benign, cystic lesions[70,71].
The overall prognosis is good with a 95% survival rate even for stage 3 or 4 tumours[61].
Non-germ cell ovarian tumours
Sex cord stromal tumours (SCSTs) are derived from the sex cord of the fetal gonad. They are rare representing approximately 10% of ovarian malignancies in adolescent girls. This is a heterogeneous group with a range of clinical features and biological behaviours. The 2 main categories are granulosa theca cell and Sertoli-Leydig cell tumours (SLCT). Granulosa theca cell tumours can occur at any age but in the first 2 decades they tend to be the juvenile granulosa cell tumour (JGCT), which is the most common paediatric SCST[72] and often presents early with oestrogen-led precocious puberty. The SLCT are the most aggressive. These may be very large at presentation and can present with androgen-led virilization. Of 70 patients in the Kiel Pediatric Tumour Registry, the mean age at diagnosis was 7 years for JGCT and 14 years for SLCT[73]. Other less frequently occurring SCSTs are fibrothecomas and sclerosing stromal tumours, which have generally benign behaviour.
Epithelial ovarian tumours are the most common ovarian tumours in adults but are rare in childhood comprising approximately 15% of paediatric ovarian masses with a mean age at presentation of 13.9 years[74]. There are 2 main histological subtypes, serous and mucinous, the former being more common. Most are benign cystadenomas but a minority are invasive cystadenomas with a poor prognosis unless diagnosed early.
Secondary tumours
Rarely other tumours may metastasize to the ovary. These include Wilms tumour, Burkitt lymphoma, mucinous adenocarcinoma of the colon and RMS[75]. Ovarian metastatic involvement of neuroblastoma is also reported post mortem but rarely diagnosed in life[76]. Secondary and non-GCT ovarian tumours cannot be reliably distinguished from GCTs on imaging.
Spread may be haematogenous, lymphatic, direct spread or transcoelomic dissemination. Ovarian metastases are more commonly seen in post-menarchal girls possibly due to the rich ovarian vascularization. Ovarian metastases are more likely to be bilateral than primary tumours (56% versus 20%) so the presence of bilateral tumours should raise suspicion of an occult primary tumour elsewhere[75].
Testicular tumours
Primary testicular tumours are rare in childhood. The differential diagnosis of a painless scrotal mass includes leukaemic infiltration, paratesticular rhabdomyosarcoma and a primary testicular tumour.
Epidemiology
Testicular tumours account for 2–3% of solid malignancies in prepubertal boys[57,77]. They may be divided into GCTs and non-GCTs. Most paediatric testicular tumours are of germ cell origin and 75% are malignant[78]. Testicular tumours have a bimodal distribution, largely affecting young children and adolescent boys.
Pathology
In prepubertal boys, GCTs of the testes are mainly either yolk sac tumours or benign teratomas. In adolescent boys, tumours have more varied histologies similar to those seen in adults including embryonal carcinomas, seminomas, teratocarcinoma and malignant GCTs[79].
Presentation
Painless testicular enlargement is the usual presentation. Children with metastatic disease, however, may present with nodal enlargement or malignant ascites. Leydig cell tumours may present with precocious puberty. Serum AFP and human chorionic gonadotrophin levels are often increased in patients with malignant GCTs (up to 90% of cases)[77]. Most testicular tumours are non-metastatic and localized to the scrotum[77]. The tumour may, however, spread to retroperitoneal lymph nodes, lungs and liver or rarely, to bone or brain.
Imaging
US is the technique of choice for the evaluation of any testicular lesion. US can define the cystic or solid nature of a mass and its relationship to the testis. The distinction between intra- and extra-testicular masses is important because most intra-testicular lesions are malignant, whereas most extra-testicular lesions are benign (with the exception of paratesticular rhabdomyosarcoma). At US, CT and MRI, testicular tumours usually appear as discrete solid masses. They may contain calcification or hypoechoic areas due to the presence of haemorrhage or necrosis. Imaging of the chest, abdomen and pelvis are routinely performed to search for metastases. The abdomen and pelvis can be further evaluated by CT or MR. CT is, of course, necessary to evaluate for lung metastases.
Staging
The COG have developed a staging system that accounts for the tumour marker status and surgical approach (Table 6)[57].
Table 6.
COG staging of testicular GCTs
| Stage | Description |
|---|---|
| Stage I | Limited to testis, tumour markers normal after appropriate half-life decline |
| Stage II | Transscrotal orchidectomy, microscopic disease in scrotum or high in spermatic cord (<5 cm from proximal end), retroperitoneal lymph node involvement (<2 cm), increased tumour marker levels after appropriate half-life decline |
| Stage III | Retroperitoneal lymph node involvement (>2 cm), no visceral or extra-abdominal involvement |
| Stage IV | Distant metastases |
Treatment
Stage I disease is cured by radical inguinal orchidectomy alone. Needle biopsy and scrotal incision are contraindicated for testicular tumours due to the risk of tumour seeding. Chemotherapy is reserved for patients with advanced disease (Stage IV) and those whose tumour markers fail to decline after orchidectomy. In patients for whom chemotherapy is indicated, testicular GCTs are generally very chemosensitive[80]. Survival rates exceed 90%.
Secondary tumours
Lymphoma or leukaemia are causes of secondary testicular neoplasms. Leukaemic infiltrates are found at autopsy in 60–90% of children who die from complications of leukaemia, but they are clinically apparent only in less than 10% of children. US may show either a diffusely enlarged testis or a focal hypoechoic lesion. Testicular involvement is usually bilateral, but it may be asymmetric.
References
- 1.Beckwith JB. Precursor lesions of Wilms tumor: clinical and biological implications. Med Pediatr Oncol. 1993;21:158–68. doi: 10.1002/mpo.2950210303. [DOI] [PubMed] [Google Scholar]
- 2.Rohrschneider WK, Weirich A, Rieden K, Darge K, Troger J, Graf N. US, CT and MR imaging characteristics of nephroblastomatosis. Pediatr Radiol. 1998;28:435–43. doi: 10.1007/s002470050378. [DOI] [PubMed] [Google Scholar]
- 3.Gylys-Morin V, Hoffer FA, Kozakewich H, Shamberger RC. Wilms tumor and nephroblastomatosis: imaging characteristics at gadolinium-enhanced MR imaging. Radiology. 1993;188:517–21. doi: 10.1148/radiology.188.2.8392214. [DOI] [PubMed] [Google Scholar]
- 4.Ritchey ML, Kelalis PP, Breslow N, Offord KP, Shochat SJ, D'Angio GJ. Intracaval and atrial involvement with nephroblastoma: review of National Wilms Tumor Study-3. J Urol. 1988;140:1113–18. doi: 10.1016/s0022-5347(17)41975-6. [DOI] [PubMed] [Google Scholar]
- 5.Misch D, Steffen IG, Schonberger S, et al. Use of positron emission tomography for staging, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour. Eur J Nucl Med Mol Imaging. 2008;35:1642–50. doi: 10.1007/s00259-008-0819-9. [DOI] [PubMed] [Google Scholar]
- 6.Humphries PD, Sebire NJ, Siegel MJ, Olsen OE. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245:848–54. doi: 10.1148/radiol.2452061535. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich PF, Hamilton TE, Grundy P, Ritchey M, Haase G, Shamberger RC. The value of surgery in directing therapy for patients with Wilms' tumor with pulmonary disease. A report from the National Wilms' Tumor Study Group (National Wilms' Tumor Study 5) J Pediatr Surg. 2006;41:162–7. doi: 10.1016/j.jpedsurg.2005.10.020. discussion 167. [DOI] [PubMed] [Google Scholar]
- 8.Owens CM, Veys PA, Pritchard J, Levitt G, Imeson J, Dicks-Mireaux C. Role of chest computed tomography at diagnosis in the management of Wilms' tumor: a study by the United Kingdom Children's Cancer Study Group. J Clin Oncol. 2002;20:2768–73. doi: 10.1200/JCO.2002.02.147. [DOI] [PubMed] [Google Scholar]
- 9.Owens CM, Brisse HJ, Olsen OE, Begent J, Smets AM. Bilateral disease and new trends in Wilms tumour. Pediatr Radiol. 2008;38:30–9. doi: 10.1007/s00247-007-0681-0. [DOI] [PubMed] [Google Scholar]
- 10.Vujanic GM, Kelsey A, Mitchell C, Shannon RS, Gornall P. The role of biopsy in the diagnosis of renal tumors of childhood: results of the UKCCSG Wilms tumor study 3. Med Pediatr Oncol. 2003;40:18–22. doi: 10.1002/mpo.10216. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard-Jones K. Controversies and advances in the management of Wilms' tumour. Arch Dis Child. 2002;87:241–4. doi: 10.1136/adc.87.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundy P, Perlman E, Rosen NS, et al. Current issues in Wilms tumor management. Curr Probl Cancer. 2005;29:221–60. doi: 10.1016/j.currproblcancer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Grundy P, Breslow N, Green DM, Sharples K, Evans A, D'Angio GJ. Prognostic factors for children with recurrent Wilms' tumor: results from the Second and Third National Wilms' Tumor Study. J Clin Oncol. 1989;7:638–47. doi: 10.1200/JCO.1989.7.5.638. [DOI] [PubMed] [Google Scholar]
- 14.Geller JI, Dome JS. Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer. 2004;101:1575–83. doi: 10.1002/cncr.20548. [DOI] [PubMed] [Google Scholar]
- 15.van den Heuvel-Eibrink MM, Grundy P, et al. Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: a collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr Blood Cancer. 2008;50:1130–4. doi: 10.1002/pbc.21389. [DOI] [PubMed] [Google Scholar]
- 16.Lowe LH, Isuani BH, Heller RM, et al. Pediatric renal masses: Wilms tumor and beyond. Radiographics. 2000;20:1585–603. doi: 10.1148/radiographics.20.6.g00nv051585. [DOI] [PubMed] [Google Scholar]
- 17.Wexler L, Crist W, Helman L. Rhabdomyosarcoma and the undifferentiated sarcomas. In: Pizzo P, Poplack D, editors. Principles and practice of pediatric oncology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2002. pp. 939–71. [Google Scholar]
- 18.Pappo AS, Shapiro DN, Crist WM. Rhabdomyosarcoma. Biology and treatment. Pediatr Clin North Am. 1997;44:953–72. doi: 10.1016/S0031-3955(05)70539-3. [DOI] [PubMed] [Google Scholar]
- 19.Stiller CA, Parkin DM. International variations in the incidence of childhood soft-tissue sarcomas. Paediatr Perinat Epidemiol. 1994;8:107–19. doi: 10.1111/j.1365-3016.1994.tb00439.x. [DOI] [PubMed] [Google Scholar]
- 20.Parham DM, Ellison DA. Rhabdomyosarcomas in adults and children: an update. Arch Pathol Lab Med. 2006;130:1454–65. doi: 10.5858/2006-130-1454-RIAACA. [DOI] [PubMed] [Google Scholar]
- 21.Stehr M. Pediatric urologic rhabdomyosarcoma. Curr Opin Urol. 2009;19:402–6. doi: 10.1097/MOU.0b013e32832c90c2. [DOI] [PubMed] [Google Scholar]
- 22.Castellino SM, McLean TW. Pediatric genitourinary tumors. Curr Opin Oncol. 2007;19:248–53. doi: 10.1097/CCO.0b013e3280ad43ce. [DOI] [PubMed] [Google Scholar]
- 23.Van Rijn RR, Wilde JC, Bras J, Oldenburger F, McHugh KM, Merks JH. Imaging findings in noncraniofacial childhood rhabdomyosarcoma. Pediatr Radiol. 2008;38:617–34. doi: 10.1007/s00247-008-0751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein M, Meller I, Issakov J, Orr-Urtreger A. Novel genes implicated in embryonal, alveolar, and pleomorphic rhabdomyosarcoma: a cytogenetic and molecular analysis of primary tumors. Neoplasia. 2006;8:332–43. doi: 10.1593/neo.05829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson D, Lu YJ, Gordon T, et al. Relationship between MYCN copy number and expression in rhabdomyosarcomas and correlation with adverse prognosis in the alveolar subtype. J Clin Oncol. 2005;23:880–8. doi: 10.1200/JCO.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 26.Hays DM, Raney RB, Jr, Lawrence W, Jr, Soule EH, Gehan EA, Tefft M. Bladder and prostatic tumors in the Intergroup Rhabdomyosarcoma Study (IRS-I): results of therapy. Cancer. 1982;50:1472–82. doi: 10.1002/1097-0142(19821015)50:8<1472::AID-CNCR2820500805>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–22. doi: 10.1002/1097-0142(19930301)71:5<1904::AID-CNCR2820710530>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 28.Martelli H, Oberlin O, Rey A, et al. Conservative treatment for girls with nonmetastatic rhabdomyosarcoma of the genital tract: a report from the Study Committee of the International Society of Pediatric Oncology. J Clin Oncol. 1999;17:2117–22. doi: 10.1200/JCO.1999.17.7.2117. [DOI] [PubMed] [Google Scholar]
- 29.Hayes-Jordan A, Andrassy R. Rhabdomyosarcoma in children. Curr Opin Pediatr. 2009;21:373–8. doi: 10.1097/MOP.0b013e32832b4171. [DOI] [PubMed] [Google Scholar]
- 30.McHugh K. Uncommon pediatric neoplasms. In: Husband J, Reznek R, editors. Imaging in oncology. 3rd ed. Informa Healthcare; 2010. pp. 941–66. [Google Scholar]
- 31.Mak CW, Chou CK, Su CC, Huan SK, Chang JM. Ultrasound diagnosis of paratesticular rhabdomyosarcoma. Br J Radiol. 2004;77:250–2. doi: 10.1259/bjr/20564274. [DOI] [PubMed] [Google Scholar]
- 32.Raney RB, Jr, Tefft M, Lawrence W, Jr, et al. Paratesticular sarcoma in childhood and adolescence. A report from the Intergroup Rhabdomyosarcoma Studies I and II, 1973–1983. Cancer. 1987;60:2337–43. doi: 10.1002/1097-0142(19871101)60:9<2337::AID-CNCR2820600937>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Park K, van Rijn R, McHugh K. The role of radiology in paediatric soft tissue sarcomas. Cancer Imaging. 2008;8:102–15. doi: 10.1102/1470-7330.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHugh K, Boothroyd AE. The role of radiology in childhood rhabdomyosarcoma. Clin Radiol. 1999;54:2–10. doi: 10.1016/S0009-9260(99)91233-3. [DOI] [PubMed] [Google Scholar]
- 35.Brisse H, McHugh K, Scarramuza D. EpSSG RMS and NRSTS therapeutic protocols 57. European Paediatric Soft Tissue Sarcoma Study Group; 2005. RMS and non RMS soft tissue sarcomas. Radiological guidelines. [Google Scholar]
- 36.Brisse HJ. Staging of common paediatric tumours. Pediatr Radiol. 2009;39(Suppl 3):482–90. doi: 10.1007/s00247-009-1193-x. [DOI] [PubMed] [Google Scholar]
- 37.Klem ML, Grewal RK, Wexler LH, Schoder H, Meyers PA, Wolden SL. PET for staging in rhabdomyosarcoma: an evaluation of PET as an adjunct to current staging tools. J Pediatr Hematol Oncol. 2007;29:9–14. doi: 10.1097/MPH.0b013e3180307693. [DOI] [PubMed] [Google Scholar]
- 38.Volker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25:5435–41. doi: 10.1200/JCO.2007.12.2473. [DOI] [PubMed] [Google Scholar]
- 39.Arush MW, Israel O, Postovsky S, et al. Positron emission tomography/computed tomography with 18fluoro-deoxyglucose in the detection of local recurrence and distant metastases of pediatric sarcoma. Pediatr Blood Cancer. 2007;49:901–5. doi: 10.1002/pbc.21150. [DOI] [PubMed] [Google Scholar]
- 40.McCarville MB, Christie R, Daw NC, Spunt SL, Kaste SC. PET/CT in the evaluation of childhood sarcomas. AJR Am J Roentgenol. 2005;184:1293–304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 41.Kleis M, Daldrup-Link H, Matthay K, et al. Diagnostic value of PET/CT for the staging and restaging of pediatric tumors. Eur J Nucl Med Mol Imaging. 2009;36:23–36. doi: 10.1007/s00259-008-0911-1. [DOI] [PubMed] [Google Scholar]
- 42.Goo HW, Choi SH, Ghim T, Moon HN, Seo JJ. Whole-body MRI of paediatric malignant tumours: comparison with conventional oncological imaging methods. Pediatr Radiol. 2005;35:766–73. doi: 10.1007/s00247-005-1459-x. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence W, Jr, Anderson JR, Gehan EA, Maurer H. Pretreatment TNM staging of childhood rhabdomyosarcoma: a report of the Intergroup Rhabdomyosarcoma Study Group. Children's Cancer Study Group. Pediatric Oncology Group. Cancer. 1997;80:1165–70. doi: 10.1002/(SICI)1097-0142(19970915)80:6<1165::AID-CNCR21>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Heyn R, Beltangady M, Hays D, et al. Results of intensive therapy in children with localized alveolar extremity rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1989;7:200–7. doi: 10.1200/JCO.1989.7.2.200. [DOI] [PubMed] [Google Scholar]
- 45.Heyn R, Newton WA, Raney RB, et al. Preservation of the bladder in patients with rhabdomyosarcoma. J Clin Oncol. 1997;15:69–75. doi: 10.1200/JCO.1997.15.1.69. [DOI] [PubMed] [Google Scholar]
- 46.Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol. 2006;24:3844–51. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 47.Raney B, Anderson J, Jenney M, et al. Late effects in 164 patients with rhabdomyosarcoma of the bladder/prostate region: a report from the international workshop. J Urol. 2006;176:2190–4. doi: 10.1016/j.juro.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 48.Bisogno G, Jenney M, Kazanowska B. EpSSG RMS 2005. A protocol for non-metastatic rhabdomyosarcoma. Sarcoma Meeting, Stuttgart 2005. Sarcoma. 2005;9:43–118. [Google Scholar]
- 49.McLean TW, Buckley KS. Pediatric genitourinary tumors. Curr Opin Oncol. 2010;22:268–73. doi: 10.1097/CCO.0b013e32833841a1. [DOI] [PubMed] [Google Scholar]
- 50.Breitfeld PP, Meyer WH. Rhabdomyosarcoma: new windows of opportunity. Oncologist. 2005;10:518–27. doi: 10.1634/theoncologist.10-7-518. [DOI] [PubMed] [Google Scholar]
- 51.Cohen RJ, Curtis RE, Inskip PD, Fraumeni JF., Jr The risk of developing second cancers among survivors of childhood soft tissue sarcoma. Cancer. 2005;103:2391–6. doi: 10.1002/cncr.21040. [DOI] [PubMed] [Google Scholar]
- 52.Stiller C. Epidemiology of childhood tumors. In: Carachi R, Grosfeld J, Azmy A, editors. The surgery of childhood tumours. Berlin: Spinger-Verlag; 2008. pp. 3–15. [Google Scholar]
- 53.Yang L, Fujimoto J, Qiu D, Sakamoto N. Trends in cancer mortality in Japanese adolescents and young adults aged 15–29 years, 1970–2006. Ann Oncol. 2009;20:758–66. doi: 10.1093/annonc/mdn664. [DOI] [PubMed] [Google Scholar]
- 54.Miller RW, Young JL, Jr, Novakovic B. Childhood cancer. Cancer. 1995;75(1 Suppl):395–405. doi: 10.1002/1097-0142(19950101)75:1+<395::AID-CNCR2820751321>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein L, Smith M, Liu L. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program Bethesda, MD; Germ cell, trophoblastic and other gonadal neoplasms. NIH Publication No. 99-4649; 1999, p. 125. [Google Scholar]
- 56.Rescorla F. The surgery of childhood tumours. Berlin: Springer-Verlag; Malignant germ cell tumours; pp. 261–71. [Google Scholar]
- 57.Cushing B, Perlman E, Marina N, Castleberry R. Germ cell tumours. In: Pizzo P, Poplack D, editors. Principles and practice of pediatric oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 58.Panteli C, Curry J, Kiely E, et al. Ovarian germ cell tumours: a 17-year study in a single unit. Eur J Pediatr Surg. 2009;19:96–100. doi: 10.1055/s-0029-1202372. [DOI] [PubMed] [Google Scholar]
- 59.Cronen PW, Nagaraj HS. Ovarian tumors in children. South Med J. 1988;81:464–8. doi: 10.1097/00007611-198804000-00014. [DOI] [PubMed] [Google Scholar]
- 60.Matthew R, Christopher O, Philippa S. Severe malignancy-associated hypercalcemia in dysgerminoma. Pediatr Blood Cancer. 2006;47:621–3. doi: 10.1002/pbc.20476. [DOI] [PubMed] [Google Scholar]
- 61.Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg. 2004;39:424–9. doi: 10.1016/j.jpedsurg.2003.11.027. discussion 429. [DOI] [PubMed] [Google Scholar]
- 62.Comerci JT, Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994;84:22–8. [PubMed] [Google Scholar]
- 63.Cushing B, Giller R, Ablin A, et al. Surgical resection alone is effective treatment for ovarian immature teratoma in children and adolescents: a report of the pediatric oncology group and the children's cancer group. Am J Obstet Gynecol. 1999;181:353–8. doi: 10.1016/S0002-9378(99)70561-2. [DOI] [PubMed] [Google Scholar]
- 64.Gershenson DM, del Junco G, Silva EG, Copeland LJ, Wharton JT, Rutledge FN. Immature teratoma of the ovary. Obstet Gynecol. 1986;68:624–9. [PubMed] [Google Scholar]
- 65.De Palo G, Lattuada A, Kenda R, et al. Germ cell tumors of the ovary: the experience of the National Cancer Institute of Milan. I. Dysgerminoma. Int J Radiat Oncol Biol Phys. 1987;13:853–60. doi: 10.1016/0360-3016(87)90099-x. [DOI] [PubMed] [Google Scholar]
- 66.Krepart G, Smith JP, Rutledge F, Delclos L. The treatment for dysgerminoma of the ovary. Cancer. 1978;41:986–90. doi: 10.1002/1097-0142(197803)41:3<986::AID-CNCR2820410328>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 67.Jabra AA, Fishman EK, Taylor GA. Primary ovarian tumors in the pediatric patient: CT evaluation. Clin Imaging. 1993;17:199–203. doi: 10.1016/0899-7071(93)90110-9. [DOI] [PubMed] [Google Scholar]
- 68.Ratani RS, Cohen HL, Fiore E. Pediatric gynecologic ultrasound. Ultrasound Q. 2004;20:127–39. doi: 10.1097/00013644-200409000-00007. [DOI] [PubMed] [Google Scholar]
- 69.Murphy JJ, Tawfeeq M, Chang B, Nadel H. Early experience with PET/CT scan in the evaluation of pediatric abdominal neoplasms. J Pediatr Surg. 2008;43:2186–92. doi: 10.1016/j.jpedsurg.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 70.De Backer A, Madern GC, Oosterhuis JW, Hakvoort-Cammel FG, Hazebroek FW. Ovarian germ cell tumors in children: a clinical study of 66 patients. Pediatr Blood Cancer. 2006;46:459–64. doi: 10.1002/pbc.20633. [DOI] [PubMed] [Google Scholar]
- 71.Karpelowsky JS, Hei ER, Matthews K. Laparoscopic resection of benign ovarian tumours in children with gonadal preservation. Pediatr Surg Int. 2009;25:251–4. doi: 10.1007/s00383-009-2336-8. [DOI] [PubMed] [Google Scholar]
- 72.Cecchetto G, Ferrari A, Bernini G, et al. Sex cord stromal tumors of the ovary in children: a clinicopathological report from the Italian TREP project. Pediatr Blood Cancer. 2011;56:1062–7. doi: 10.1002/pbc.22918. [DOI] [PubMed] [Google Scholar]
- 73.Schneider DT, Janig U, Calaminus G, Gobel U, Harms D. Ovarian sex cord-stromal tumors–a clinicopathological study of 72 cases from the Kiel Pediatric Tumor Registry. Virchows Arch. 2003;443:549–60. doi: 10.1007/s00428-003-0869-0. [DOI] [PubMed] [Google Scholar]
- 74.Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: a retrospective analysis. J Pediatr Surg. 2003;38:331–5; discussion 335. doi: 10.1053/jpsu.2003.50103. [DOI] [PubMed] [Google Scholar]
- 75.McCarville MB, Hill DA, Miller BE, Pratt CB. Secondary ovarian neoplasms in children: imaging features with histopathologic correlation. Pediatr Radiol. 2001;31:358–64. doi: 10.1007/s002470100436. [DOI] [PubMed] [Google Scholar]
- 76.McHugh K, Pritchard J, Dicks-Mireaux C. Bilateral ovarian involvement at presentation in metastatic (stage 4) neuroblastoma. Pediatr Radiol. 1999;29:741. doi: 10.1007/s002470050686. [DOI] [PubMed] [Google Scholar]
- 77.Skoog SJ. Benign and malignant pediatric scrotal masses. Pediatr Clin North Am. 1997;44:1229–50. doi: 10.1016/S0031-3955(05)70555-1. [DOI] [PubMed] [Google Scholar]
- 78.Green DM. Testicular tumors in infants and children. Semin Surg Oncol. 1986;2:156–62. doi: 10.1002/ssu.2980020308. [DOI] [PubMed] [Google Scholar]
- 79.McLean TW, Castellino SM. Pediatric genitourinary tumors. Curr Opin Oncol. 2008;20:315–20. doi: 10.1097/CCO.0b013e3282f8b053. [DOI] [PubMed] [Google Scholar]
- 80.Castellino SM, Martinez-Borges AR, McLean TW. Pediatric genitourinary tumors. Curr Opin Oncol. 2009;21:278–83. doi: 10.1097/CCO.0b013e328329f201. [DOI] [PMC free article] [PubMed] [Google Scholar]