Abstract
The poor outcome in symptomatic lung cancer patients and the much better prognosis when lung cancer is diagnosed and treated at early asymptomatic stages call for screening. As lung cancer predominantly affects smokers and individuals exposed to other carcinogens, screening programs need not include the whole population but only these risk groups. Every screening program will tend to better identify the more indolent tumours that grow slowly enough to be detected by screening before symptoms develop, whereas aggressive fast-growing tumours may present as interval cancers despite screening (length-time bias). Some malignant tumours detected with screening may never cause the person’s death due to competing causes for death, particularly in heavy smokers, such as cardiovascular disease or other cancers (overdiagnosis bias). If a cancer is still lethal despite detection through screening, the affected individual may live longer with the diagnosis of cancer but not longer altogether (lead-time bias). It is likely that this will have a negative effect on that individual’s quality of life. Participation in screening programs may have beneficial as well as adverse effects on smoking habits; in the worst case it may encourage people to continue smoking. Trials assessing chest radiography or sputum microscopy have not demonstrated a reduction in lung cancer mortality through screening, probably because the tests were not sensitive enough. computed tomography promises better sensitivity. Other modern tests such as fibre optic bronchoscopy, analysis of molecular markers or genetic testing in serum, sputum or exhaled air are not yet ready for clinical practice.
Keywords: Lung cancer, early detection, screening, cigarette smoking, overdiagnosis bias, length-time bias, lead-time bias
Introduction
Lung cancer represents a spectrum of biologically different tumours[1–9]. These include very aggressive entities with rapid growth (tumour volume doubling within weeks) and spread of metastases within weeks or months such as small cell lung cancer (SCLC) and some subgroups of non-small cell lung cancer (NSCLC; Fig. 1) such as poorly differentiated large cell lung cancer. On the other hand, other tumours take years to double their volume and develop lymphatic or haematogenous metastases, e.g. bronchioloalveolar carcinoma and other types of well-differentiated adenocarcinoma. Nevertheless, lung cancer is the leading cause of death from malignancy worldwide with an estimated 1.3 million deaths per year.
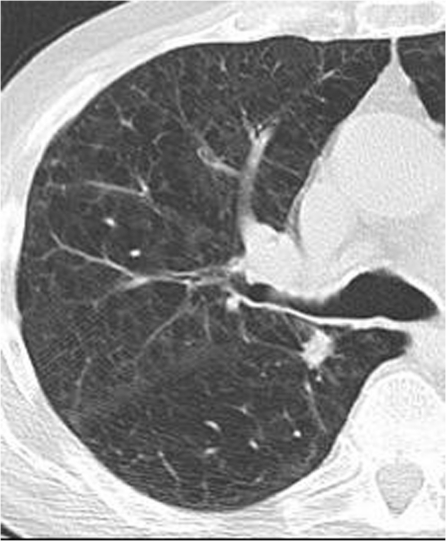
Figure 1.
Asymptomatic male smoker with a 12-mm ill-defined nodule posterior to the right upper lobe bronchus in the apical segment of the right lower lobe representing stage IA NSCLC.
Despite extensive research and improvement in surgical, oncologic and radiation therapy during the last decades, its prognosis remains dismal with an overall 5-year survival of <15%. This is predominantly due to the fact that, in most patients, the diagnosis is made at advanced stages either with infiltration of adjacent structures or with lymphatic or distant metastases. However, if the diagnosis is made at an early stage with no metastases, 5-year survival in NSCLC may be >65% and with very small lesions as high as >80%. For SCLC, the difference in cure rates at early versus advanced tumour stages, unfortunately, is much less pronounced. However, SCLC currently accounts for only approximately 20% of lung cancer cases. Therefore, hope for improved cure of lung cancer patients was based on approaches to detect small and usually asymptomatic early stages non-small cell lung cancer using diagnostic tests.
Risk groups for lung cancer
This appears particularly promising as screening would not need to include the entire population but only subsets at an increased risk for lung cancer. Contrary to other malignant tumours such as breast cancer or colorectal cancer in which no individual risk factors can be identified in most patients, lung cancer is almost exclusively limited to individuals with previous exposure to certain carcinogens. The most common risk factor is a history of cigarette smoking. It is estimated that at least 85% of lung cancer patients are active or former smokers. Even after smoking cessation, the risk of developing lung cancer decreases slowly. Therefore, currently in the United States, the majority of lung cancer patients are ex-smokers. In non-smoking lung cancer patients, exposure through passive smoking (inhalation of smoke from other smokers) may contribute to the development of the tumour. Other carcinogens include asbestos, uranium, nickel, cobalt, chrome, etc. for which exposure may occur at specific workplaces. A combination of different risk factors may multiply the individual risk of developing lung cancer, e.g. smoking workers exposed to asbestos have a markedly higher risk than smokers with the same exposure to cigarettes alone.
Adverse effects from screening
Detection of cancer at an asymptomatic stage may not always and automatically translate into a benefit for the patient. A small asymptomatic lung tumour detected with a diagnostic test may still represent metastatic disease. Up to 20% of stage 1 (T1, 2, N0, M0) NSCLC patients after curative resection later develop recurrence mostly at distant sites, which indicates that distant micrometastases were present before surgical removal of the primary tumour. To our current knowledge, this subset of patients does not benefit from early detection and surgery.
In contrast, detection of the lung tumour in an asymptomatic patient with metastatic disease probably does not affect the prognosis but turns a presumably healthy individual into a cancer patient with potential negative effects on that individual’s quality of life. Furthermore, if such a patient is followed, the fact that the disease was detected earlier than in an individual with identical disease without screening means that survival from the moment of diagnosis will be longer than without screening, erroneously suggesting that screening has improved the time of survival (e.g. longer median survival, higher 5-year survival rate). This effect is called lead-time bias with the lead time representing the time between diagnosis through the screening test and the time at which the individual would have presented with symptoms.
Similar to individuals with metastatic disease at diagnosis, there is another group of patients in which diagnosis of an asymptomatic tumour may not be beneficial to the patient. If the diagnostic test detects an early tumour which in the remaining life-time would not have caused symptoms, the person becomes a cancer patient with the adverse effects of diagnostic and therapeutic procedures as well as the diagnosis itself, without any benefit to the person. This is true of every individual screening programme, as there will always be a proportion of individuals who die with but not from the disease, for example from a traffic accident shortly after the diagnosis is made.
As mentioned above, some lung cancers may grow very slowly and metastasize very late. Well-differentiated adenocarcinoma and in particular bronchioloalveolar carcinoma may sometimes require several years to double their volume. If, for example, a tumour is detected at 1 cm diameter and has a volume doubling time of 3 years, it will take 12 years for this tumour to reach a diameter of 2.5 cm and 21 years to grow to a diameter of 5 cm, at which stage it may still not have spread. If the person dies before this date, the tumour did not have any impact on that individual’s health. This effect of screening is called overdiagnosis bias.
Every diagnostic screening test performed at regular intervals is more likely to detect this type of slow-growing indolent tumour as an asymptomatic lesion, whereas very aggressive fast growing tumours with early metastases are more likely to present as interval tumours between two screening tests. This propensity of every screening test to detect an unrealistically high proportion of more benign lesions is called length-time bias. Median survival, 5-year survival, overall survival, cure rate, rate of metastases, etc. will, therefore, always be favourable in a screening programme, irrespective of the test applied. One has to consider these effects when interpreting classic outcome surrogate markers in screening programmes.
Differences between examinations in symptomatic patients and screening asymptomatic individuals
In general, in a screening programme any test is applied to a large proportion of individuals who do not have the disease to detect a small proportion of patients with the disease. Only in this subgroup will there be potential benefit from the test, whereas the side effects such as the consumption of resources, potential further procedures to differentiate between disease and insignificant changes in uncertain findings, radiation exposure, etc. occurs in all screenees. For this reason the diagnostic performance of a test needs to be much better in a screening setting than when applied to a symptomatic patient.
If the sensitivity (proportion of true positive (TP) diagnoses from all patients with the disease (TP + false negative (FN)) as well as the specificity (proportion of true negative (TN) diagnoses from all patients without the disease (TN + false positive (FP)) is 90% respectively in a group of 1000 symptomatic patients with a prevalence of the disease of 50% (50% of patients with the symptoms have the disease), this will result in the following figures:
1000 individuals with symptoms
500 individuals have the disease
500 individuals do not have the disease
450 individuals with the disease correctly identified
50 FN diagnoses
50 FP diagnoses
Therefore, the positive predictive value (TP/TP+FP) is 450/450+50=90%, i.e. 90% of patients in whom the diagnosis is suggested by computed tomography (CT) do have lung cancer.
If however, the disease is rare in the population in a screening setting (e.g. disease present in 1 of 100 screened subjects=prevalence of 1%), the same diagnostic test will result in these figures:
1000 individuals screened
10 individuals have the disease
990 individuals do not have the disease
9 individuals with the disease correctly identified
1 FN diagnosis
90 FP diagnoses
In this setting the positive predictive value (TP/TP+FP) is 9/9+90=9%, i.e. only 9% of patients in whom the diagnosis is suggested by CT do have lung cancer. In other words: 91% of individuals in whom a diagnosis of lung cancer is suggested by CT do not actually have lung cancer. This means that the test performs quite well in a setting of symptomatic patients in missing the diagnosis in only 10% (50/500) of affected patients and wrongly suggesting the diagnosis in 10% (50/500) of cases in which the diagnosis is made.
In a screening setting, the test also misses the cancer in only 10% (1/10) of patients with the disease, but wrongly suggests the diagnosis in >90% (90/99) of positive tests. This has a significant impact on the cost/benefit ratio of the test, including the concerns of individuals in whom the diagnosis is incorrectly made, the number of unnecessary further tests and maybe invasive procedures.
Effects of screening for lung cancer on smoking habits
It is absolutely clear, that the best way to prevent death from lung cancer is for a person to never smoke and if started, to give up smoking immediately. However, unfortunately, the success rates of all smoking prevention and cessation programs are poor. If a screening program is to be instituted, effects on smoking habits need to be assessed. Both positive and negative effects may be possible. If the invitation to a screening test could increase awareness of the serious health effects of smoking, potentially supported by demonstrating the extent of smoking-related non-tumorous changes (e.g. emphysema, atherosclerotic disease), the test in combination with a smoking cessation program could prove doubly beneficial. If, however, the screened person (erroneously) believes, that there is no need to stop smoking as the test “will identify any cancer in time to cure it and there is, therefore, no need to stop smoking” the test could increase the likelihood of dying from lung cancer instead of decreasing it.
Screening with techniques other than CT
Chest radiography and sputum cytology were extensively analysed for their potential as screening instruments in smokers in the 1970s. However, results were disappointing in that more tumours were diagnosed and resected, but mortality from lung cancer did not decrease in the screening groups compared with groups with no screening. The explanation is probably that both tests are not sensitive enough to detect early cancers.
In the 1990s, CT was shown to be much more sensitive for small pulmonary nodules, which is the most common presentation of NSCLC. Unfortunately, standard chest CT is associated with a relatively high level of radiation exposure, which in a screening setting is particularly problematic and important as it affects a large proportion of individuals without lung cancer. It has, however, been shown that the sensitivity and specificity of CT for small lung cancers did not decrease significantly with major reduction of radiation exposure.
Recently non-radiological tests have been proposed to screen for lung cancer. Fibre optic bronchoscopy, particularly when using fluorescence or autofluorescence, is able to identify early cancer or even precancerous lesions, however, there are several problems which as yet prevent its widespread use in a population at risk. It is semi-invasive, is not generally accepted by asymptomatic individuals, is not widely available, and is too expensive and time consuming. It may, however, be useful to identify and even treat early lesions if the affected individuals are diagnosed with another test (see below). Laboratory tests using modern techniques may allow the diagnosis of lung cancer to be made non-invasively in the future. Different approaches for testing for specific molecular markers in sputum, serum or exhaled air, assessing genetic anomalies in sputum or blood cells and other tests are under investigation. At the time of this writing, however, none of these has proven applicable in clinical routine.
References
- 1.Austin JHM, Romney BM, Goldsmith LS. Missed bronchogenic carcinoma: radiographic findings in 27 patients with a potentially resectable lesion evident in retrospect. Radiology. 1992;182:115. doi: 10.1148/radiology.182.1.1727272. [DOI] [PubMed] [Google Scholar]
- 2.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol. 2010;37:202. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diederich S. Screening for early lung cancer with low-dose spiral computed tomography. Lancet. 2003;362:588. doi: 10.1016/S0140-6736(03)14205-5. [DOI] [PubMed] [Google Scholar]
- 4.Diederich S, Lenzen H, Windmann R, et al. Low-dose CT of pulmonary nodules: experimental and clinical studies. Radiology. 1999;213:289. doi: 10.1148/radiology.213.1.r99oc29289. [DOI] [PubMed] [Google Scholar]
- 5.Diederich S, Semik M, Lentschig MG, et al. Helical CT of pulmonary nodules in patients with extrathoracic malignancy: CT-surgical correlation. AJR. 1998;172:353. doi: 10.2214/ajr.172.2.9930781. [DOI] [PubMed] [Google Scholar]
- 6.Goldstraw P, Crowley J on behalf of the IASLC International Staging Project. The IASCL international staging project on lung cancer. J Thor Oncol. 2006;1:281. doi: 10.1097/01243894-200605000-00002. [DOI] [Google Scholar]
- 7.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 8.Patz EF, Jr, Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 9.Sobin LH, Gospodarowicz MK, Wittekind Ch. TNM classification of malignant tumours. 7th edition. John Wiley; [Google Scholar]