Abstract
Survival, quality-adjusted survival and mortality are important and related measures of outcome in cancer care. The impact of imaging on these outcomes can be ascertained from observational and modelling studies, frequently performed to evaluate cost-effectiveness. Examples where incorporation of imaging into cancer care can be shown to improve survival include breast cancer screening, characterization of solitary pulmonary nodules, staging of non-small cell lung cancer, treatment response assessment in Hodgkin lymphoma, postoperative surveillance of colorectal cancer and selective internal radiation therapy of colorectal liver metastases. Modelling suggests the greatest opportunities for improvements in survival through imaging detection of cancer may lie in the investigation of mildly symptomatic patients. For applications where the improvements in survival are more modest, use of imaging frequently has additional demonstrable benefits including reductions in health care expenditure.
Keywords: Survival, mortality, clinical effectiveness, decision modelling
Introduction
The effectiveness of cancer imaging is frequently expressed in terms of diagnostic performance parameters such as sensitivity and specificity. However, to be truly of value, cancer imaging should also impact on ultimate health outcomes such as survival and mortality[1]. In themselves, diagnostic tests such as imaging cannot improve the health of patients. Any improvements in survival can only arise through diagnostic imaging appropriately directing patients to an effective treatment.
With increasing concerns about the costs and, in the case of examinations that entail ionizing radiation and/or contrast material, the risks of imaging, there is an onus on imagers to demonstrate that inclusion of imaging in clinical management can bring health benefits to patients with cancer. The article presents evidence to show that the incorporation of imaging into clinical management can improve the survival and/or mortality of patients with cancer.
Survival, quality-adjusted survival and mortality
Although clearly important, the survival of patients does not provide a complete picture of the outcomes of cancer care. Despite overall benefits, there may also be negative effects of cancer management that impact on the quality of life experienced during cancer survivorship. To capture these effects, the concept of quality-adjusted survival has been proposed in which the survival time is multiplied by a factor that represents the quality of life during the period of survival ranging between 0 (i.e. death) and 1 (complete health). These utility values are usually derived from validated quality-of-life questionnaires completed by appropriate patients.
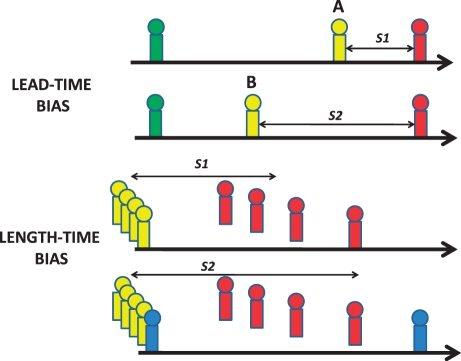
When assessing the impact of cancer imaging on health, in certain situations such as the early detection of cancer or tumour recurrence, it is more appropriate to measure mortality rather than survival. This is because of the potential for survival data to be affected by lead-time bias (Fig. 1). Lead-time bias can occur when imaging detects a patient with cancer or recurrence earlier but subsequent treatment has no impact and time of death is unchanged. The patient appears to have survived longer simply because the diagnosis has been made earlier and there will be added anxiety as the patient must live with knowledge of the disease for longer. Length-time bias (below) can occur when incidental tumours that would not lead to death of the patient are detected by imaging resulting in an apparent increase in survival. However, for both sorts of bias, overall mortality is unchanged.
Figure 1.
Representation of health states in lead-time and length-time biases. Green, undetected tumour; yellow, detected tumour; red, dead; blue, incidental non-fatal tumour. Lead-time bias (above) can occur when imaging detects a tumour or recurrence at time B rather than time A but subsequent treatment does not alter the time of death. The apparent survival time is increased from S1 to S2. Length-time bias (below) can occur when an incidental non-fatal tumour is detected by imaging resulting in an apparent increase in survival from S1 to S2.
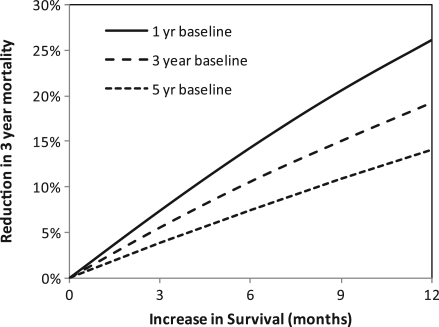
The relationship between survival and mortality data can be readily determined by use of the declining exponential approximation to life expectancy[2]. Fig. 2 illustrates this association by displaying the relationship between an increase in survival (x-axis) and the corresponding decrease in 3-year mortality (y-axis) calculated in the absence of the biases described above. The decrease in mortality for a given increase in survival is also affected by the initial baseline survival from which the increased survival is gained. Therefore there are three curves reflecting baseline survivals of 1, 3 and 5 years, respectively. These curves show, for example, that a 10% reduction in 3-year mortality can be achieved with a modest increase in survival of between 4 and 8 months.
Figure 2.
Relationship between increase in survival and reduction in 3-year mortality derived using the declining exponential approximation to life expectancy[2].
Demonstrating the impact of imaging on survival and mortality
The impact of imaging on survival and mortality can be demonstrated in two ways, observational studies and modelling. In both cases, the information is frequently derived as part of an assessment of cost-effectiveness. Such studies often demonstrate reductions in health care expenditure in addition to improved survival[3].
Observational studies ideally are controlled trials in which patients are randomized to either clinical management without imaging or to management incorporating the imaging modality in question. However, such trials can be problematic for a number of reasons. First, because the impact of imaging on health is small relative to the impact of treatment, the effects of imaging are difficult to observe. Thus, a randomized controlled trial assessing the impact of imaging on survival is likely to be very expensive and take a long time to accomplish, some protocols requiring in excess of 4 years to complete[4]. As diagnostic imaging technologies are evolving very rapidly and unpredictably, such prolonged studies are at risk of being outdated by their time of completion. Randomized trials that aim to determine the impact of imaging on survival can also be associated with ethical difficulties resulting from the requirement to randomize some patients to a study arm that omits a diagnostic imaging method already shown to be more accurate.
Modelling studies represent an alternative to randomized controlled trials. These studies use decision trees to represent clinical management with and without imaging. Data relevant to the population of interest, the natural history of the tumour, the diagnostic performance of imaging and the effectiveness of treatments can be obtained from published studies and entered into a model to estimate the impact of imaging on ultimate health states. This method is illustrated by the example given below.
Modelling the impact of imaging surveillance for colorectal cancer
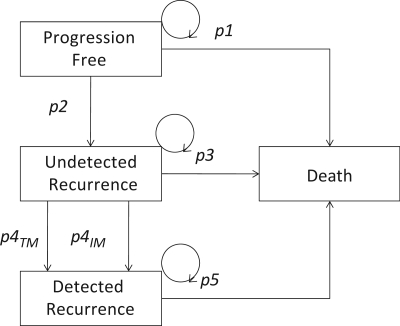
In the United Kingdom, 37,000 patients are diagnosed with colorectal cancer each year of whom 27,750 (75%) can be expected to enter a surveillance program. The purpose of including liver imaging in surveillance strategies is to identify patients with isolated hepatic metastases as hepatectomy for liver metastases can be associated with an improved 5-year survival of 33%[5]. The impact of imaging surveillance for colorectal cancer can be estimated using a Markov model which is a recursive model that estimates the probability of individuals moving between specified health states within a given time period. For surveillance of patients with colorectal cancer, the model (Fig. 3) may comprise four possible health states: progression free, undetected recurrence, detected recurrence and death. The transition from undetected recurrence to detected recurrence could follow either measurement of serum tumour markers (carcinoembryonic antigen (CEA)) or imaging with computed tomography (CT). The strategies envisaged in this illustration are: (a) no follow-up at all, (b) follow-up with 3-monthly estimations of serum CEA for 3 years, (c) follow-up with 3-monthly estimations of serum CEA and yearly CT for 3 years (as recommended in the 2005 guidelines of the American Society for Clinical Oncology[6]). The cycle time is 3 months and the time line for the analysis is 6 years.
Figure 3.
Markov models for the surveillance of patients with colorectal cancer. p1–p5 represent the probabilities of transition between health states. p4TM and p43IM represent separate probabilities for detection of recurrence by serum tumour markers and imaging, respectively.
The probabilities for transition between health states can be derived from previous published reports[8,9] as shown in Table 1 and the proportion of patients in each health state at the end of the time are shown in Table 2. Both surveillance strategies are associated with reduced mortality and prolonged survival whilst CEA and imaging together produce the greatest benefit.
Table 1.
Input assumptions for the Markov model
| Probability values | Value | Source |
|---|---|---|
| Progression free to death (p1) | 0.0153 | Murray et al.[7] |
| Progression free to undetected recurrence (p2) | 0.023 | Murray et al.[7] |
| Undetected recurrence to death (p3) | 0.106 | Murray et al.[7] |
| Detection of recurrence by CEA (p4CEA) | 0.604 | Park et al.[8] |
| Detection of recurrence by CT (p4CT) | 0.790 | Park et al.[8] |
| Detected recurrence to death (p5) | 0.059 | Park et al.[8] |
Table 2.
Summary of outputs from the Markov model
| No follow-up | 3-monthly CEA | 3-monthly CEA and yearly CT | |
|---|---|---|---|
| Health states at 5 years (%) | |||
| Progression free | 45.8 | 45.8 | 45.8 |
| Undetected recurrence | 10.7 | 6.5 | 6.2 |
| Alive with detected recurrence | 0 | 8.4 | 8.9 |
| Death | 43.5 | 39.2 | 39.0 |
| Median survival (months)a | 72.8 | 83.6 | 84.1 |
| Impact of surveillance | |||
| Patients detected with recurrence/1000 | 197 | 212 | |
| Reduction in mortality (%) | 9.9 | 10.3 | |
| Risk difference (cf. no follow-up) | −0.043 | −0.045 | |
| Average survival gain (months)a | 10.8 | 11.3 | |
aEstimated using the declining exponential approximation to life expectancy[2].
Improvements in survival and/or mortality as a result of cancer imaging
The major roles of imaging in the management of patients with cancer are diagnosis, staging, response evaluation and detection of recurrence. Imaging can also be used to guide cancer treatment. Examples in which imaging improves survival and/or mortality are given for each of these roles.
Cancer diagnosis
A well recognized example of improvements in mortality afforded by imaging for cancer detection is the use of screening mammography for detection of breast cancer. Based on randomized controlled trials, the reported reductions in mortality from bi-annual screening are 39% for women aged 50–79 years and 13% for women aged 40–49[9]. Annual screening has been estimated to reduce mortality further (44–46% for ages 50–79 years and 36% for ages 40–49 years)[9]. Modelling studies have assessed the impact of image-based screening on breast cancer mortality of BRCA1 gene mutation carriers[10]. Compared with clinical surveillance, annual mammography from aged 25 years would reduce mortality by 16.4%, annual magnetic resonance imaging (MRI) by 17.8% and annual combined imaging by 22.3%.
Other cancer screening applications of imaging that have been shown through modelling studies to affect mortality include low-dose CT for lung cancer and CT colonography. For lung cancer screening, although the results of randomized trials are awaited, a non-randomized comparison of screened and unscreened cohorts has indicated 36–64% reductions in lung cancer mortality from low-dose CT[11]. These figures correspond closely to the 42.5–45.6% reductions in mortality determined in a modelling study[12]. For CT colonography, Hassan et al.[13] modelled the outcomes for a cohort of 100,000 with comparison against optical colonoscopy. Compared with no screening, CT colonography was associated with a total gain in life expectancy of 9835 years through detection of colorectal cancer. When combined with gains in life expectancy of 1994 years and 298 years for detection of aortic aneurysms and extracolonic cancers, respectively, the total gain in life expectancy exceeded that from optical colonoscopy.
In the context of cancer diagnosis, imaging is also used to determine whether a detected abnormality is benign or malignant. One possible approach to diagnosis is surgical excision or biopsy, often with image guidance. In this situation imaging diagnosis can prolong survival if the mortality risk of surgery exceeds the reduction in survival that could result from a delay in diagnosis due to a false-negative imaging test. This situation can be illustrated by modelling strategies for the management of thyroid nodules. Based on a reported operative mortality for thyroidectomy of 0.2% and a loss of life expectancy of 14.6 years for a delayed diagnosis of thyroid cancer[14,15], for a typical prevalence of malignancy of 5%, strategies that use ultrasound-guided biopsy or methoxyisobutylisonitrile (MIBI) scintigraphy and biopsy to select patients for surgery would increase mean survival by 0.20 and 0.29 months, respectively, compared with surgery for all nodules[16].
Alternatively, a watch-and-wait strategy can be adopted in which repeated clinical examination or less intensive imaging is used to look for growth prior to surgical excision. In this situation, more intensive imaging to characterize the lesion further can result in improved survival. A study modelling the outcomes associated with a range of strategies for investigation of solitary pulmonary nodules illustrates this situation[17]. The improvements in quality-adjusted survival compared with the watch-and-wait strategy are shown for various pre-test probabilities of malignancy in Table 3.
Table 3.
Average improvements in quality-adjusted survival from use of intensive imaging for investigation of solitary pulmonary nodules (from Gould et al.[17])
| Probability of malignancy | Improvements in quality-adjusted survival (months) |
|
|---|---|---|
| CT strategies | CT and PET strategies | |
| Low (26%) | 4.17 | 4.37–4.47 |
| Intermediate (55%) | 9.00–9.26 | 9.56 |
| High (79%) | 13.50 | 13.93 |
Staging
The opportunities to improve survival through use of imaging for staging patients with cancer comprise (a) avoidance of surgical mortality in patients with undetected advanced disease in whom surgery would be futile and (b) appropriate downstaging of patients so that curative treatment which, would have been withheld on the basis of less accurate staging, can now be offered. These outcomes have underpinned the use of fluorodeoxyglucose (FDG)-positron emission tomography (PET) for staging cancer patients, for example those with non-small cell lung cancer as demonstrated by the modelling studies within the lung cancer guidance of the UK's National Institute of Health and Clinical Excellence (NICE)[18]. For patients being considered for surgery, avoidance of futile thoracotomies through the use of FDG-PET increased quality-adjusted survival per patient by 0.44 months when compared with thoracotomy and 0.23 months when compared with the use of mediastinoscopy prior to surgery. The use of FDG-PET prior to radical radiotherapy increased average quality-adjusted survival by 0.52 months, primarily by identifying patients who were suitable for curative surgery.
Response assessment
The presence of residual active tumour following cancer therapy has a significant impact on subsequent management. In the absence of a diagnostic test that can identify residual disease on completion of treatment, there are two potential management pathways: surveillance based on a combination of clinical symptoms, tumour markers and/or imaging to identify active tumour on account of disease progression, or use of consolidation treatment for all patients. Surveillance exposes patients with residual tumour to a potential loss of life associated with an unnecessary delay in commencement of consolidation treatment, whereas consolidation treatment for all would subject some patients to the potential mortality associated with further unnecessary treatment. Effective imaging that could accurately identify residual disease immediately on completion of therapy could therefore potentially improve survival.
This situation is illustrated by a modelling study performed by the Health Technology Board of Scotland. The study estimates the increase in survival that would occur by use of FDG-PET to identify patients with residual disease following treatment of Hodgkin lymphoma in comparison with surveillance for all patients and consolidation for all[19]. Important increases in survival (0.4–2.0 years) are seen as summarized in Table 4.
Table 4.
Increases in survival by use of FDG-PET on completion of therapy for Hodgkin lymphoma (from Bradbury et al.[19])
| Scenario | Increase in survival compared with surveillance for all patients (years) | Increase in survival compared with consolidation for all patients (years) |
|---|---|---|
| Female 20 years | 1.2 | 0.7 |
| Female 40 years | 1.6 | 0.6 |
| Female 40 years | 0.7 | 1.6 |
| Male 20 years | 1.5 | 0.6 |
| Male 40 years | 1.3 | 0.9 |
| Male 60 years | 0.4 | 2.0 |
Detection of recurrence
To illustrate the improvements in survival that can be achieved by use of imaging for detection of recurrence of cancer, we can return to the postoperative surveillance of patients with colorectal cancer as described in the model outlined above. A systematic review undertaken by the Cochrane Collaboration based on eight reported studies concluded that colorectal cancer surveillance incorporating imaging was associated with reduced mortality (odds ratio 0.64; 95% confidence interval 0.49–0.85, risk difference −0.09; 95% confidence interval −0.14 to −0.03)[20]. Although the overall conclusions are similar to those obtained from the model developed earlier, the magnitude of the benefits of imaging in the observational studies included in the review was greater.
Image-guided therapy
Whilst the survival benefits of diagnostic imaging in cancer are indirect through direction of patients to effective treatments, image-guided therapies are intended to produce a direct therapeutic benefit. Therefore, the impact of image-guided therapy on survival should be demonstrable using the randomized trial methodology applicable to other therapeutic manoeuvres. To date, such trials of image-guided therapies have been performed infrequently. One exception is a randomized trial assessing the use of yttrium-labelled microspheres for selective internal radiation therapy (SIRT) of colorectal liver metastases[21]. Survival following SIRT combined with fluorouracil and leucovorin chemotherapy (18.6 months) was significantly longer than following chemotherapy alone (3.6 months, p < 0.0005).
Maximizing the survival benefit through imaging detection of cancer
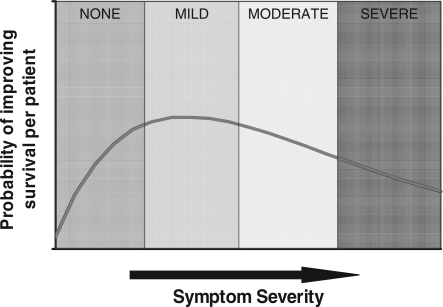
Imaging for suspected cancer can be used when patients are asymptomatic (i.e. screening) or when patients have symptoms suggesting a possibility of malignancy. The probability of detecting malignancy is least when patients are asymptomatic but through early detection, the opportunities to improve survival are greater. As patients become more symptomatic, the likelihood of detecting malignancy increases. However, if a malignancy is present, more marked symptoms are likely to be associated with more advanced disease and hence a reduced opportunity for improving survival. These relationships are summarized in Fig. 4. If these two probabilities are combined, the resulting curve represents the hypothetical probability of improving survival per patient imaged (Fig. 4). The combined relationship suggests that the greatest opportunity for improving survival occurs when patients have mild symptoms.
Figure 4.
If the likelihood of malignancy increases but the probability of improving survival decreases with symptom severity (above), the combined effect suggests the maximum likelihood of improving survival per patient examination would occur with mild symptoms (below).
Exploiting this relationship between symptom severity and survival benefit would have implications for how imaging services should be structured. Because patients with mild symptoms most commonly present to general practitioners, the focus on diagnostic cancer imaging would need to be its use in primary care. A recent study concluded that there is strong evidence that stage at diagnosis and delay in accessing care explain some of the differences in cancer survival between England and other countries[22]. The authors identified improvements in general practitioner access to radiography, ultrasound and CT and MRI scans as an important contributor to improving survival rates for cancer patients.
Balancing survival and mortality against costs and patient-related outcomes
Although the aim of this paper has been to demonstrate improvements in survival or mortality resulting from inclusion of imaging with cancer care, these benefits need to be considered in the context of imaging costs and patient-related outcomes. In many cases the improvements in survival or mortality resulting from use of imaging are also associated with a reduction in the overall cost of health care through avoidance of futile treatments that would have been selected on the basis of less accurate methods[3]. However, in other clinical scenarios, the improvements in health outcomes achievable through imaging are constrained by costs. An example of the latter is the postoperative surveillance of patients with colorectal cancer where MRI and FDG-PET are more sensitive than CT for detection of tumour recurrence[23]. This increased sensitivity offers an opportunity for even greater reductions in mortality. However, this potential benefit needs to be offset by the greater cost and lower availability of these techniques. The mortality benefits achievable through more intense surveillance also need to be balanced against patient-related outcomes such as the anxiety associated with false-positive examinations[23]. On the other hand, deployment of more accurate imaging techniques earlier in the care pathway could reduce the patient inconvenience, cost and anxiety associated with diagnostic algorithms comprising multiple tests applied sequentially.
Summary
Inclusion of imaging in the management of patients with cancer can be associated with improvements in survival and/or mortality. To date, the greatest improvements have been demonstrated for the application of imaging for screening and response evaluation and for image-guided therapy. Modelling suggests the greatest opportunities for improvements in survival through imaging detection of cancer may lie in the investigation of mildly symptomatic patients. For applications where the improvements in survival are more modest, use of imaging frequently has additional demonstrable benefits including reductions in health care expenditure.
References
- 1.Sunshine JH, Applegate KE. Technology assessment for radiologists. Radiology. 2004;230:309–14. doi: 10.1148/radiol.2302031277. [DOI] [PubMed] [Google Scholar]
- 2.Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med. 1982;73:883–8. doi: 10.1016/0002-9343(82)90786-0. [DOI] [PubMed] [Google Scholar]
- 3.Miles KA. Cancer imaging: is it cost-effective? Cancer Imaging. 2004;4:97–103. doi: 10.1102/1470-7330.2004.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunshine JH, McNeil BJ. Rapid method for rigorous assessment of radiologic imaging technologies. Radiology. 1997;202:549–57. doi: 10.1148/radiology.202.2.9015089. [DOI] [PubMed] [Google Scholar]
- 5.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–6. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 6.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512–19. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 7.Murray A, Lourenco T, de Verteuil R, et al. Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2006;10:1–141, iii–iv. doi: 10.3310/hta10450. [DOI] [PubMed] [Google Scholar]
- 8.Park KC, Schwimmer J, Shepherd JE, et al. Decision analysis for the cost-effective management of recurrent colorectal cancer. Ann Surg. 2001;233:310–19. doi: 10.1097/00000658-200103000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenquist CJ, Lindfors KK. Screening mammography beginning at age 40 years: a reappraisal of cost-effectiveness. Cancer. 1998;82:2235–40. doi: 10.1002/(SICI)1097-0142(19980601)82:11<2235::AID-CNCR19>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, McMahon PM, Kong CY, et al. Cost-effectiveness of breast MR imaging and screen-film mammography for screening BRCA1 gene mutation carriers. Radiology. 2010;254:793–800. doi: 10.1148/radiol.09091086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henschke CI, Boffetta P, Gorlova O, Yip R, Delancey JO, Foy M. Assessment of lung-cancer mortality reduction from CT screening. Lung Cancer. 2011;71:328–32. doi: 10.1016/j.lungcan.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Foy M, Yip R, Chen X, Kimmel M, Gorlova OY, Henschke CI. Modeling the mortality reduction due to computed tomography screening for lung cancer. Cancer. 2011;117:2703–8. doi: 10.1002/cncr.25847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan C, Pickhardt PJ, Laghi A, et al. Computed tomographic colonography to screen for colorectal cancer, extracolonic cancer, and aortic aneurysm: model simulation with cost-effectiveness analysis. Arch Intern Med. 2008;168:696–705. doi: 10.1001/archinte.168.7.696. [DOI] [PubMed] [Google Scholar]
- 14.Shrime MG, Goldstein DP, Seaberg RM, et al. Cost-effective management of low-risk papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:1245–53. doi: 10.1001/archotol.133.12.1245. [DOI] [PubMed] [Google Scholar]
- 15.Links TP, van Tol KM, Jager PL, et al. Life expectancy in differentiated thyroid cancer: a novel approach to survival analysis. Endocr Relat Cancer. 2005;12:273–80. doi: 10.1677/erc.1.00892. [DOI] [PubMed] [Google Scholar]
- 16.Wale A, Miles K, Young B, et al. Accuracy and potential cost-effectiveness of 99mTc-methoxyisobutylisonitrile (MIBI) scintigraphy for the assessment of thyroid nodules in the context of the British Thyroid Association (BTA) guidelines. Nuclear Med Commun. 2011;32:435. [Google Scholar]
- 17.Gould MK, Sanders GD, Barnett PG, et al. Cost-effectiveness of alternative management strategies for patients with solitary pulmonary nodules. Ann Intern Med. 2003;138:724–35. doi: 10.7326/0003-4819-138-9-200305060-00009. [DOI] [PubMed] [Google Scholar]
- 18.National Collaborating Centre for Acute Care. Diagnosis and treatment of lung cancer. London: National Collaborating Centre for Acute Care; February 2005. Available from: http://www.rcseng.ac.uk. [Google Scholar]
- 19.Bradbury I, Bonell E, Boynton J, et al. Health technology assessment report 2: positron emission tomography (PET) imaging in cancer management. Glasgow: Health Technology Board for Scotland; 2002. no. 1903961319. [Google Scholar]
- 20.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;1:CD002200. doi: 10.1002/14651858.CD002200.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 22.Foot C, Harrison T. Explaining England's relatively poor rates. London: The King's Fund; 2011. How to improve cancer survival. [Google Scholar]
- 23.Miles K, Burkill G. Colorectal cancer: imaging surveillance following resection of primary tumour. Cancer Imaging. 2007;7(A):S149–50. doi: 10.1102/1470-7330.2007.9011. [DOI] [PMC free article] [PubMed] [Google Scholar]