Abstract
Multifunctional magnetic resonance imaging (MRI) techniques are increasingly being used to address bottlenecks in prostate cancer patient management. These techniques yield qualitative, semi-quantitative and fully quantitative biomarkers that reflect on the underlying biological status of a tumour. If these techniques are to have a role in patient management, then standard methods of data acquisition, analysis and reporting have to be developed. Effective communication by the use of scoring systems, structured reporting and a graphical interface that matches prostate anatomy are key elements. Practical guidelines for integrating multiparametric MRI into clinical practice are presented.
Keywords:
Introduction
It is clear that the prostate cancer imaging landscape has changed radically in recent years. There is now an increased opportunity to perform prostate gland magnetic resonance imaging (MRI) using sequences that yield functional information. With the advent of faster sequences performed on high-performance, high field strength MRI scanners, it is possible to combine morphological and multiple functional prostatic imaging into a more comprehensive evaluation, with only a small additional time penalty. In addition to morphological T2-weighted sequences, the major functional techniques used for the prostate are diffusion-weighted MRI[1], dynamic contrast-enhanced MRI[2] and proton MR spectroscopic imaging[3]. These techniques can yield quantitative information that reflects on the biological properties of prostatic tissues. These techniques have the potential to provide unique information that can be used for tumour detection in the treated and untreated gland, for predicting future tumour behaviour and for monitoring and predicting the likelihood of response to treatment. It is now widely recognized that the multiparametric MRI approach for evaluating the prostate goes beyond what can be achieved using any single functional MRI technique.
This article does not discuss in detail the methods of data acquisition or analyse the individual techniques; details regarding these can be found in many recently published review articles[1–3]. The potential roles that can be played by the different MRI techniques depend on clinical requirements, which change during the prostate cancer patient's journey (Table 1). Since the limitations of each technique are often non-overlapping, it is recommended that multiple functional MRI sequences are used for making diagnoses and therapeutic decisions. This article focuses on the practical methods for integrating multiparametric MRI into clinical practice providing guidance on communicating functional MRI information to urologists and radiotherapists so that they may be used for individual patient decision making.
Table 1.
The prostate cancer patient journey and contribution of MRI in patient care
| Clinical journey begins here | → Suspect cancer | → Stage known cancer |
→ Treatment of initial disease |
→ Monitoring effectiveness of therapy | → Surveillance of treated disease | → Suspect relapse |
→ Treatment of relapsed disease |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial observation (active surveillance) | Curative intent |
Palliative | Local salvage | Palliative | ||||||||
| Surgery | Ablative therapies (HIFU, PDT, cryotherapy brachytherapy) | External beam radiotherapy to prostate±pelvic nodes | ||||||||||
| Clinical scenario | Raised PSA with negative TRUS biopsy or biopsies | Cancer presence confirmed by biopsy | Small volume. Low aggressiveness | Organ confinement. No tumour at prostatic apex. No metastases | Organ confined disease. No metastases | Usually includes neoadjuvant hormonal therapy | Usually hormonal therapy ± radiotherapy | Usually after focal therapies | Rare to use imaging in this role (serum PSA surveillance) | Significant rise in serum PSA | Disease is localized and salvage is possible | Disease is not localized and salvage is impossible |
| Clinical (C) or research (R) requirements | Define tumour location and size for targeted biopsy (C) | TNM stage (C). Define dominant lesion (C). Define lesion aggressiveness (C/R). Therapy planning (C) | Confirm organ confinement (C). Document size and location (C). Depict lesion aggressiveness (C/R) | Detect adverse features (C). Target pelvic nodal dissection (C) | Define dominant lesion location and size (C/R) | Confirm confinement to pelvis (C). Nodal mapping (C/R) | Define extent of nodal and distant metastases (C). Requirements for local palliation (C) | Treatment verification (R). Define volume and extent of residual disease (R) | Detect active disease in the absence of significant increase in PSA (R) | Identify site and volume of recurrence (C) | Define extent of local disease and absence of metastases (C) | Define extent of relapsed disease and complications (C). Requirements for local palliation (C) |
| Contribution made by MRI techniquesa | ||||||||||||
| Morphology | +++ | +++ | +++ | +++ | +++ | +++ | ++ | +++ | ++ | +++ | +++ | +++ |
| MRI biopsy | + | 0 | + | 0 | 0 | 0 | 0 | + | + | ++ | ++ | 0 |
| MRSI | +++ | + | ++ | ++ | + | + | + | 0 | +++ | ++ | + | 0 |
| DW-MRI | +++ | ++ | +++ | ++ | ++ | ++ | + | + | ++ | +++ | ++ | 0 |
| DCE-MRI | +++ | + | +++ | ++ | + | + | 0 | +++ | +++ | +++ | +++ | 0 |
aThese are author's opinions are based on literature reviews and personal experiences, and recommendations are partly dependent on subjective assessments of ease of imaging data acquisition, analysis and interpretations. 0, no requirement; +, possible requirement; ++, probably indicated; +++, definite indication.
Biological basis for observations
Dynamic contrast-enhanced (DCE)-MRI using small molecular weight gadolinium chelates enables non-invasive imaging characterization of prostatic vascularity. Established clinical roles for the prostate gland include lesion detection and localization, tumour staging and the detection of suspected tumour recurrence[2] (Table 2). Diffusion-weighted (DW)-MRI uniquely displays information about the extent and direction of random water motion in tissues. DW-MRI provides information on extracellular space tortuosity, tissue cellularity, glandular formation, the integrity of cellular membranes and perfusion. Clinical data indicate a number of potential roles in prostate cancer including lesion localization and characterization, the latter via the determination of the aggressiveness of the lesion[1]. DW-MRI images at high b-value (≥800 s/mm2) should always be interpreted with morphological and apparent diffusion coefficient (ADC) images. Proton MR spectroscopic imaging (MRSI) of the prostate depicts relative metabolite levels within tissues. Normal prostatic glandular tissues show high citrate levels, whereas aggressive prostate cancer is characterized by relatively higher levels of choline compared with citrate. Studies to date suggest that MRSI might provide information that could be used to increase staging accuracy for less experienced readers and thereby reduce interobserver variability. MRSI also allows the non-invasive assessment of tumour aggressiveness and maybe helpful for directing biopsies and focal therapies[3–5]. The ability of MRSI to aid in tumour localization appears to be limited particularly for low-grade, low-volume tumours[6]. Two basic strategies have been explored for MRI-guided prostate gland biopsy: (1) co-registration of acquired diagnostic MRI images to transrectal ultrasound (TRUS) biopsy devices, and (2) stereotactic needle interventions within conventional diagnostic scanners using careful patient positioning or with the aid of simple manipulators[7]. Such techniques can be used for needle-based interventions for prostate cancer, including biopsy, brachytherapy, and thermal/laser ablation[7–10].
Table 2.
MRI techniques and their use in prostate cancer patients
| Technique | Basis of usage | Indications | Authors' opinions on indicationa |
|---|---|---|---|
| Morphology on T2-weighted MRI | Depiction of the tumour extent | At almost every stage of the patient journey (not routinely used for very early stage cancers or for very advanced disease) | +++ |
| MRI biopsy | To obtain histologic material targeting a lesion/area. Rarely to direct focal treatments to a specified region | Not routinely indicated. Used when cancer is suspected, TRUS biopsies are negative and MRI depicts suspicious lesion(s) | + |
| Proton MRSI | For assessing lesion aggressiveness (complementary information to DW-MRI) | For lesion characterization. Lesion depicted on T2-weighted DCE or DW-MRI and suspected to contain high grade elements (Gleason 4 or 5) | +++ |
| For depicting and confirming the location of the primary prostate cancer | ++ | ||
| PSA relapse following external beam radiotherapy when is bone scan is negative and in whom salvage therapy is being considered (DW-MRI and DCE-MRI – probably outperform MRSI for this indication) | + | ||
| DW-MRI | For depicting the intraprostatic tumour extent (complementary information to T2-weighted MRI and DCE-MRI and should be used together) | For depicting and confirming the location of the primary prostate cancer | ++ |
| PSA relapse when bone scan is negative and salvage therapy is being considered | +++ | ||
| DCE-MRI | For depicting the intraprostatic tumour extent (complementary information to DW-MRI and T2-weighted MRI and should be used together) | For depicting and confirming the location of the primary prostate cancer | ++ |
| For monitoring response to hormonal therapy | + | ||
| For the assessment of the effectiveness of focal therapies (e.g. PDT, HIFU) | +++ | ||
| PSA relapse when bone scan is negative and salvage therapy is being considered | +++ |
aThese author's opinions are based on literature reviews and personal experiences, and recommendations are partly dependent on subjective assessments of ease of imaging data acquisition, analysis and interpretations. 0, no requirement; +, possible requirement; ++, probably indicated; +++, definite indication.
Clinical value of multiparametric MRI
An important area where the use of multifunctional MRI is proving to be of clinical value is for the localization of the site of the dominant intraprostatic cancer mass[11]. The importance of locating significant intraprostatic focal disease lies in 2 clinical areas. First, in men with persistently raised serum prostate-specific antigen (PSA) levels and in whom there have been multiple negative biopsies or positive but low-grade low-volume tumour with discordant PSA kinetics; it is estimated that up to 30–50% of these men have undiagnosed, clinically significant cancers[12]. These undiagnosed cancers need to be located and evaluated histologically before therapy can be instituted[4,12–15]. Second, the use and future success of local ablative treatments such as high intensity focused ultrasound (HIFU) is dependent on the accurate identification of the dominant intraprostatic lesion, also known as the index lesion[16,17]. This is because the largest cancer focus determines patient prognosis (overall Gleason score, total tumour volume, extracapsular tumour extension and seminal vesicle invasion are almost invariably determined by the index lesion), and secondary lesions do not contribute to the overall clinical outcome (satellite lesions tend to be small and well differentiated)[18,19].
A few studies have evaluated the value of 1H-MRSI in patients with elevated PSA levels and previous negative biopsies to localize peripheral zone tumours and have shown that combining T2-weighted MRI with 1H-MRSI can potentially help to direct biopsies towards suspicious sites so as to increase the cancer detection rates and therefore limit the total number of biopsies needed to make a histologic diagnosis[4,13,20–23]. Futterer et al.[24] have taken the multiparametric approach further by showing that it was only when 1H-MRSI and morphological data were combined with DCE-MRI that lesion localization in the prostate was optimal. Mazaheri et al.[25] suggested combining DW-MRI and 1H-MRSI for lesion localization but only for the peripheral zone of the prostate gland. Lesion localization is particularly problematic in the transition zone because benign prostatic hyperplasia shares many of the MRI characteristics of cancers (nodule formation, mass effect and hypervascularization)[26]. A number of studies have recently reported that multiparametric imaging can help guide prostate biopsies in men with persistently raised serum PSA levels and previous negative TRUS biopsies[4,12,13,15]. For example, Hambrock et al.[15] combined DCE-MRI with DW-MRI, and reported that 59% of 68 patients could be diagnosed to have an underlying cancer using this approach. Of the 40 patients with identified tumours, 37 (93%) were considered highly likely to harbour clinically significant disease.
Relaying multiparametric MRI information to clinical colleagues
In order for multifunctional MRI data to inform on patient management, multiparametric data need to be communicated to oncologists/urologists in a simple but meaningful way. This will ensure that patients are treated effectively and in the most appropriate way. This is best done using structured reporting systems via a graphical interface that matches prostate anatomy on T2-weighted MRI[27] (Fig. 1). Narrative reports should also relay prostate dimensions/volume, a formal assessment of TNM staging including the location and the probability of extra-prostatic disease and other pertinent and incidental findings. Such narrative reports should be accompanied by a radiologist's overall score (from 1 to 5) that represents the radiologist's personal view on the likelihood of significant malignancy being present at the patient level; this score should be a synthesis derived from radiologic scores for sectors and/or lesions from each imaging technique, also taking into account clinical and clinical data including patient history and symptoms, serum PSA, digital rectal examination (DRE) findings, concomitant medications (particularly anti-androgens) and time since TRUS biopsy.
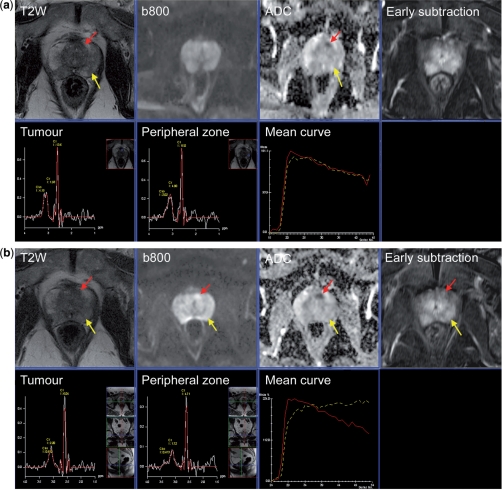
Figure 1.
Communicating multifunctional MRI data at diagnosis and results of therapy monitoring. (a) A 67-year-old male with raised serum PSA (5.3 ng/ml). MRI scan before TRUS biopsy shows diffuse low signal intensity change in the peripheral zone bilaterally (yellow arrow) without focal features (T2-weighted score 3/5). The diffusion sequences show bilateral abnormalities on the high b-value image (b800 s/mm2) and ADC map (score 2/5) with no focal features. DCE-MRI early subtraction image shows diffuse enhancement with no focal features with washout curve (yellow line) (score 3/5). The MRSI image from the peripheral zone is normal (score 1/5). A small 6-mm tumour (red arrow) behind the anterior fibromuscular stroma is barely visible on T2-weighted images (2/5). The diffusion sequences are consistent with a focal tumour with an ADC value of 835 µm2/s (score 4/5). DCE-MRI shows the focal mass lesion which has a washout pattern (score 5/5). The MRSI is normal (1/5). Results of TRUS biopsy were small foci of Gleason 3 + 3 from the left side with prostatitis. It is clear that TRUS cannot sample the anterior gland tumour. The patient went onto an active surveillance program and received antibiotics for prostatitis. (b) Re-evaluation MRI after 1 year. The T2-weighted image again shows diffuse low signal intensity change in the peripheral zone bilaterally (yellow arrow) without focal features (T2-weighted score 3/5). The diffusion sequences show a bilateral abnormality on the b800 image and ADC map (score 2/5) with no focal features. DCE-MRI early subtraction image shows diffuse enhancement with no focal features but no washout is observed (yellow line) (score 2/5). The MRSI image from the peripheral zone remains normal (score 1/5). The anterior gland tumour has increased in size to 10 mm (T2-weighted score 3/5). The diffusion sequences show an enlarging tumour with an ADC value of 583 µm2/s (score 4/5). DCE-MRI shows a focal mass lesion with washout pattern (score 5/5). The MRSI remains normal (1/5). (c) Pictorial report of first year follow-up study (b) used to present the multifunctional MRI findings prior to template biopsy. Template biopsy of the left anterior lesion contained a 5-mm core of Gleason 3 + 4 cancer. Bilateral peripheral zone tumour foci (Gleason 3 + 3) were also seen. The patient opted for HIFU therapy of the anterior gland tumour. (d) Re-evaluation MRI after androgen deprivation therapy prior to HIFU therapy. The T2-weighted image shows prostate gland shrinkage. The T2-weighted image continues to show an abnormality at the site of the anterior gland lesion (score 3/5) which is not well seen on the DW image or ADC map (ADC 1355 µm2/s) (score 3/5). DCE-MRI show a plateau type curve at the location of the anterior tumour with a focal, asymmetric lesion still present (score 4/5) type. The peripheral zone shows slow washin only (score 1/5). There is marked metabolic atrophy on MRSI at both locations consistent with glandular atrophy induced by hormonal therapy.
It is generally agreed that scoring systems similar to that used successfully by breast radiologists (for x-ray mammography and MRI) need to be developed and prospectively validated for prostate MRI. These scoring systems should clearly indicate the probability of cancer being present and its extent. Such scoring systems will have the effect of improving the utility of multiparametric MRI providing the basis for future validation in prospective studies. Such scoring needs to be assigned for each region of interest (prostatic sectors) and for each individual lesion identified.
Reviews of the literature show that Likert-like 5-grade scoring systems are often used to evaluate multiparametric MRI of the prostate[4,28–32]. In keeping with this, a recent consensus meeting of prostate cancer experts used the UCLA-RAND appropriateness method and recommended that a 5-point scale be used for the scoring of prostatic sectors[27]:
Score 1: clinically significant disease is highly unlikely to be present
Score 2: clinically significant cancer is unlikely to be present
Score 3: the presence of clinically significant cancer is equivocal
Score 4: clinically significant cancer is likely to be present
Score 5: clinically significant disease is highly likely to be present
The definition of clinically significant prostate cancer varies among published reports and depends on local practice. One definition often used is tumour volume ≥0.5 ml and/or Gleason pattern 4 or 5 and/or extracapsular disease[33]. Definitions of prostatic sectors are detailed below after discussions of scoring systems for individual MRI sequences.
Scoring systems for multiparametric MRI assessments
Spectroscopy
There are no generally accepted criteria for assignments of scores for MRSI[3]. A commonly used system was developed by Jung et al.[32] who devised a standardized scoring system for spectral evaluation of peripheral zone spectral data, and the combined central gland data were added later[34]. This scoring system which uses a visual classification system and a threshold metabolite approach (the (choline + creatine)/citrate integral ratio) has gained wide agreement amongst radiologists and is recommended for use (Fig. 2). The accuracy of the scoring system improves if at least 3 adjacent voxels show similar findings provided that all metabolite peaks are greater than 5 times the standard deviation of noise level[5]. In addition to using the 5-point scoring system, readers interpreting the images can designate spectra as unusable if marked lipid contamination or misalignment of metabolite resonance peaks is present. The 5-point scale of Jung et al.[32] has been found to be reasonably accurate and to have excellent interobserver agreement (κ = 0.80) in differentiation of benign from malignant tissue.
Figure 2.
MRSI curve shape assessments. Representative spectra acquired at 3 T (no endorectal coil) with scores 1–5 (from left to right). Choline (Cho) + creatinine (Cr) to citrate (Ci) ratios of the individual spectra are given above each spectrum. The irregular line of each spectrum is the acquired data. The smooth lines are the corresponding fitted data from which the C + C/C ratio is calculated.
T2-weighted imaging
Since there are no generally agreed scoring systems for T2-weighted MRI, the scheme presented in Table 3 can serve as a starting point for interested readers to begin to perform systematic reporting of individual lesions identified on multifunctional prostate MRI. These assessment guidelines should be not be used in isolation but rather should be supplemented by personal experience. Of course image appearances on other sequences including the presence of blood depicted on T1-weighted images will influence image interpretations on T2-weighted sequences.
Table 3.
Scoring system for T2-weighted images for lesions in the peripheral and transition zonesa
| Score | Peripheral zone criteria | Transition zone criteria |
|---|---|---|
| 1 | Normal peripheral zone high signal intensity | Transition zone containing stromal and glandular hyperplasia/adenoma with no low signal intensity nodules or lenticular shaped lesions |
| 2 | Low signal intensity focus lesion (wedge shaped or linear), ill defined | Round shaped low signal intensity lesion with a smooth capsule. Band like low signal intensity |
| 3 | Intermediate appearances not in categories 1/2 or 3/4 | Intermediate appearances not in categories 1/2 or 3/4 |
| 4 | Low signal (dark gray-black) intensity focus, round shaped, well-defined lesion without extracapsular extension | Lenticular shaped anterior low signal intensity lesion without capsule invasion. Charcoal sign: homogeneous low signal intensity lesion with loss of internal structure and unsharp margins within the transition zone |
| 5 | Low signal intensity mass, round shaped lesion with bulge/irregularity/retraction of the prostate capsule or seminal vesicle invasion | Lenticular or round low signal intensity lesion with bulge/irregularity/retraction of the anterior prostate capsule. Irregular, infiltrating mass destroying transition zone architecture, invading adjacent peripheral zone/SV/bladder |
aSubtract 1 from the score if there is biopsy related haemorrhage in the region of suspected abnormality.
DW-MRI
For qualitative assessments both high b-value DW-MR images (800–1000 s/mm2) and ADC maps are useful using the guidance given in Table 4; these should be evaluated in combination with T2-weighted images for the anatomic detail. It should be noted that some normal prostatic tissue may reveal high signal intensity on high b-value DW images because of T2 shine through effects. This problem can be overcome by imaging at very high b-values (>1000 s/mm2); but image quality is less due to decreased signal-to-noise ratio.
Table 4.
Scoring system for diffusion images for lesions in the peripheral and transition zones
| Score | Criteria |
|---|---|
| 1 | No reduction in ADC compared with normal glandular tissue. No increase in signal on any high b-value image |
| 2 | Diffuse, hyperintensity on high b-value images with diffuse low ADC; no focal features, linear, triangular or geographic features allowed |
| 3 | Intermediate appearances not in categories 1/2 or 3/4 |
| 4 | Focal area(s) of reduced ADC but isointense signal intensity on high b-value images |
| 5 | Focal area/mass of hyperintensity on the high b-value images with corresponding reduced ADC |
DCE-MRI
In general, prostate cancer in the peripheral zone tends to enhance earlier, faster, to a greater extent and shows earlier contrast washout compared with healthy prostate tissue. Benign proliferative hyperplasia of the transition zone can show similar enhancement characteristics to cancers. Analysis of DCE-MRI is done using visual assessments of curve shapes and on the morphological appearances of enhancing lesions in the peripheral zone.
Score +1, +2 or +3 for type 1 (slow rising), 2 (fast upslope and plateau with less than 10% washout) and type 3 (early peak and more than 10% washout from the peak).
Score +1 for focal enhancing lesion with curve shape 2 or 3
Score +1 of lesion is asymmetric with curve shape 2 or 3
The DCE-MRI criteria of transition zone tumours in the presence of benign prostatic hyperplasia (BPH) are as yet undefined and radiologists are advised that the above peripheral zone criteria likely overestimate the probability of cancer being present and to use them with caution.
Prostatic sectors
The clinical case for sector analyses includes improved precision in communicating the location of potential abnormalities, thus potentially enabling few but more targeted biopsies. The numbers of areas/sectors of the prostate that require separate assessments range from 12 to 27 in the literature[27]. In this regard, the position of the boundary that separates the anterior from the posterior sectors requires special consideration. There are good radiologic reasons for radiologists to use the peripheral zone–transition zone boundary as the dividing line because it is easily identified and the peripheral zone is usually easier to evaluate for the presence of cancer. The hypertrophied transition zone is often heterogeneous, with stromal, glandular and mixed BPH being present in varying amounts. Stromal hyperplasia can have low ADC values and may be hypervascular[26]. Thus detecting transition zone cancers is harder to do. Morphological appearances and lesion aggressiveness also differ for tumours arising in the transition zone[35,36]. In addition, image artefacts are often more commonly seen in the peripheral zone because of its proximity to the air-filled rectum.
On the other hand, in order to be able to cross correlate between TRUS biopsy (where both the peripheral and posterior transition zones are sampled), then the anterior–posterior border needs to be adjusted. Readers should note that the separate identification of the peripheral and transition zones is difficult to do by histopathologic evaluations of core biopsy specimens. One suggestion has been to place the anterior–posterior line 17 mm from the prostate's posterior surface (i.e. the biopsy core length as it passes obliquely through the gland)[27]. A 10-core extended biopsy scheme would be expected to sample the 10 posterior sectors. No consensus method for segmenting the prostate into anterior and posterior sectors has yet emerged.
Other comments regarding data communication
There is some debate concerning the number of individual lesions that require specific identification and whether or not a definite identification of the index prostatic lesion is required, given that prostate cancer is a multifocal disease. There is histologic data that shows that most of the total cancer volume (up to 90%) can be attributed to the dominant focus and that 80% of the small foci have tumour volumes less than 0.5 ml[16]. Furthermore, there is increasing evidence that the risk of cancer progression is positively related to the largest index lesions and to high histologic grade components (Gleason >7)[18,19]. Because index tumour volume and the presence of Gleason 7 tumour are related, it is recommended that it should be specifically identified and where possible with its dimension(s) and/or volume. Where possible, its functional characteristics should also be noted including ADC value, choline + creatine/citrate ratio, and enhancement curve shapes using the scoring system defined above (Fig. 1).
There is uncertainty about how to weight sequence results for a given clinical situation. In other words, do the results from one sequence outweigh results from other sequences in a particular clinical scenario such as lesion localization or when determining lesion aggressiveness. Many investigators have noted that for lesion localization, the performance of DW-MRI and DCE-MRI is superior to T2-weighted MRI and 1H-MRSI. In contradistinction, when assessing lesion aggressiveness in terms of predicting underlying Gleason score, the performance of DW-MRI and 1H-MRSI is superior to T2-weighted MRI and DCE-MRI[4,37–43]. Discordant results between these sequences for a given lesion are likely to represent differences in the biological evolution of cancer characteristics. Thus, it has been noted that results of functional MRI tests are often discordant particularly for lesions found in patients undergoing active surveillance (early stage disease or small-volume tumour) and that concordant high scoring results are most often found in larger lesions with higher Gleason scores. The optimal way of dealing with discordant results has not been formulated, and it is recommended that the current strategy is to record the scores of individual sequences separately and to act on the sequence yielding the highest score as clinically indicated.
Challenges for multiparametric MRI
It is possible to acquire spatially matched multiparametric MRI data in potentially every prostate patient at a given time point. Currently, co-localization and integration of the information provided between these multidimensional datasets represents a major challenge. If multiparametric data is acquired several times during a period of observation or treatment, then there is an added level of complexity brought on by changing morphological and functional features and patient repositioning (Fig. 1). Sophisticated, user-friendly software workspaces need to be developed urgently in order to be able to integrate/cross correlate data analysis procedures to allow for disease characterization and to follow changes in response to therapy. These computer platforms need to also incorporate bioinformatics approaches, so that imaging findings can be correlated with findings from patient metadata including clinical findings, serum PSA levels, prostate cancer antigen 3 (PCA3) findings, histology including immunohistochemistry and ultimately with gene and protein expression profiles. Ultimately, multispectral analyses should be able to generate probability biomaps of biologically important characteristics or to infer underlying molecular gene expression patterns of tissues and tumours. Such composite biomaps incorporating functional imaging would be invaluable in lesion detection and characterization, for biopsy and therapy planning, for assessing the effects of therapies and prognosis prediction.
These computer workplaces need to have methods for dealing with entirely missing MRI sequence datasets (not obtained, corruption, artefacts) and partly corrupted data, which may be regional (often affecting the posterior gland due to rectal gas or rectal movements). These computer platforms need to also incorporate the differing relative sensitivity of each imaging sequence for a given clinical scenario; the relative weighting of each sequence will change as the evidence base grows. Thus, it is now increasingly recognized that in the setting of lesion localization, the performance of DW-MRI and DCE-MRI are superior to T2-weighted MRI and 1H-MRSI. In contradistinction, lesion aggressiveness in terms of the underlying Gleason score is best assessed with DW-MRI and 1H-MRSI[4,37–43] compared with T2-weighted MRI and DCE-MRI.
If multiparametric MRI is to evolve successfully into a clinically useful tool for the evaluation of the prostate, then there will need to be agreements on the standards for measurement, analysis and display for the whole spectrum of promising functional MRI techniques. The current general lack of such standards is a major impediment to the development and maturation of physiologic MRI in the prostate. Consensus by the prostate MRI community would attract research funding to support the development of these techniques for a number of clinical purposes, and would allow imaging equipment manufacturers and software companies to focus their research and development resources on improving measurement and analysis methods[27].
Prostate MRI biomarker combinations need to be validated in the context of well-designed, clinically relevant trials, particularly those that are associated with tissue sampling and in studies that incorporate accepted clinical efficacy end points (for clinical validation), where there is minimization of patient selection bias. In these efforts, it should not be forgotten that suitable, tissue equivalent phantoms will be required for quality assurance and quality control purposes. Patient reproducibility and observer variability should be established in as many clinical studies as possible, particularly in multicentre settings in order to establish the robustness of the techniques being evaluated.
Conclusions
In recent years, tremendous experience has been gained in functional MRI techniques and it is becoming increasingly clear that they may be able to address some of the bottlenecks in prostate cancer patient management. The progress made by each technique in the transition to clinical practice varies, but important lessons on their potential uses and limitations are becoming known. If multiparametric MRI is to have a role in the diagnostic pathway of patients with prostate cancer, it will be necessary to develop standard methods of data acquisition, analysis and reporting. One challenge that radiologists are already facing is how to effectively communicate complex multifunctional MRI information to clinicians looking after patients. The use of scoring systems, structured reporting and a graphical interface that matches prostate anatomy are key elements. The guidelines for scoring the multiparametric MRI components discussed can serve as a starting point for such assessments but they will need to be validated and refined by prospective clinical studies.
References
- 1.Tan CH, Wang J, Kundra V. Diffusion weighted imaging in prostate cancer. Eur Radiol. 2011;21:593–603. doi: 10.1007/s00330-010-1960-y. [DOI] [PubMed] [Google Scholar]
- 2.Alonzi R, Padhani AR, Allen C. Dynamic contrast enhanced MRI in prostate cancer. Eur J Radiol. 2007;63:335–50. doi: 10.1016/j.ejrad.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Rajesh A, Futterer JJ, et al. Prostate MRI and 3D MR spectroscopy: how we do it. AJR Am J Roentgenol. 2010;194:1414–26. doi: 10.2214/AJR.10.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villeirs GM, De Meerleer GO, De Visschere PJ, Fonteyne VH, Verbaeys AC, Oosterlinck W. Combined magnetic resonance imaging and spectroscopy in the assessment of high grade prostate carcinoma in patients with elevated PSA: a single-institution experience of 356 patients. Eur J Radiol. 2009;77:340–5. doi: 10.1016/j.ejrad.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Westphalen AC, Coakley FV, Qayyum A, et al. Peripheral zone prostate cancer: accuracy of different interpretative approaches with MR and MR spectroscopic imaging. Radiology. 2008;246:177–84. doi: 10.1148/radiol.2453062042. [DOI] [PubMed] [Google Scholar]
- 6.Weinreb JC, Blume JD, Coakley FV, et al. Prostate cancer: sextant localization at MR imaging and MR spectroscopic imaging before prostatectomy – results of ACRIN prospective multi-institutional clinicopathologic study. Radiology. 2009;251:122–33. doi: 10.1148/radiol.2511080409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pondman KM, Futterer JJ, ten Haken B, et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol. 2008;54:517–527. doi: 10.1016/j.eururo.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011;258:488–495. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindner U, Weersink RA, Haider MA, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–1377. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Roethke M, Anastasiadis AG, Lichy M, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2011 doi: 10.1007/s00345-011-0675-2. [DOI] [PubMed] [Google Scholar]
- 11.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection–histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrentschuk N, Fleshner N. The role of magnetic resonance imaging in targeting prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels. BJU Int. 2009;103:730–733. doi: 10.1111/j.1464-410X.2008.08205.x. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo S, Petracchini M, Della Monica P, et al. Value of endorectal MRI and MRS in patients with elevated prostate-specific antigen levels and previous negative biopsies to localize peripheral zone tumours. Clin Radiol. 2008;63:871–879. doi: 10.1016/j.crad.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed HU, Emberton M. The role of magnetic resonance imaging in targeting prostate cancer in patients with previous negative biopsies and elevated prostate-specific antigen levels. BJU Int. 2009;104:269–270; author reply 270. doi: 10.1111/j.1464-410X.2009.08750_1.x. [DOI] [PubMed] [Google Scholar]
- 15.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–527. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Sartor AO, Hricak H, Wheeler TM, et al. Evaluating localized prostate cancer and identifying candidates for focal therapy. Urology. 2008;72:S12–24. doi: 10.1016/j.urology.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 17.de la Rosette J, Ahmed H, Barentsz J, et al. Focal therapy in prostate cancer-report from a consensus panel. J Endourol. 2010;24:775–780. doi: 10.1089/end.2009.0596. [DOI] [PubMed] [Google Scholar]
- 18.Wise AM, Stamey TA, McNeal JE, Clayton JL. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology. 2002;60:264–269. doi: 10.1016/S0090-4295(02)01728-4. [DOI] [PubMed] [Google Scholar]
- 19.Karavitakis M, Ahmed HU, Abel PD, Hazell S, Winkler MH. Tumor focality in prostate cancer: implications for focal therapy. Nat Rev Clin Oncol. 2011;8:48–55. doi: 10.1038/nrclinonc.2010.190. [DOI] [PubMed] [Google Scholar]
- 20.Costouros NG, Coakley FV, Westphalen AC, et al. Diagnosis of prostate cancer in patients with an elevated prostate-specific antigen level: role of endorectal MRI and MR spectroscopic imaging. Am J Roentgenol. 2007;188:812–816. doi: 10.2214/AJR.06.0165. [DOI] [PubMed] [Google Scholar]
- 21.Amsellem-Ouazana D, Younes P, Conquy S, et al. Negative prostatic biopsies in patients with a high risk of prostate cancer. Is the combination of endorectal MRI and magnetic resonance spectroscopy imaging (MRSI) a useful tool? A preliminary study. Eur Urol. 2005;47:582–586. doi: 10.1016/j.eururo.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Prando A, Kurhanewicz J, Borges AP, Oliveira EM, Jr., Figueiredo E. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005;236:903–910. doi: 10.1148/radiol.2363040615. [DOI] [PubMed] [Google Scholar]
- 23.Franiel T, Stephan C, Erbersdobler A, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding – multiparametric MR imaging for detection and biopsy planning. Radiology. 2011;259:162–172. doi: 10.1148/radiol.10101251. [DOI] [PubMed] [Google Scholar]
- 24.Futterer JJ, Heijmink SW, Scheenen TW, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–458. doi: 10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- 25.Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D 1H MR spectroscopic imaging – correlation with pathologic findings. Radiology. 2008;246:480–488. doi: 10.1148/radiol.2462070368. [DOI] [PubMed] [Google Scholar]
- 26.Oto A, Kayhan A, Jiang Y, et al. Prostate cancer: differentiation of central gland cancer from benign prostatic hyperplasia by using diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology. 2010;257:715–723. doi: 10.1148/radiol.10100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Mueller-Lisse U, Scheidler J, Klein G, Reiser M. Reproducibility of image interpretation in MRI of the prostate: application of the sextant framework by two different radiologists. Eur Radiol. 2005;15:1826–1833. doi: 10.1007/s00330-005-2695-z. [DOI] [PubMed] [Google Scholar]
- 29.Nogueira L, Wang L, Fine SW, et al. Focal treatment or observation of prostate cancer: pretreatment accuracy of transrectal ultrasound biopsy and T2-weighted MRI. Urology. 2010;75:472–477. doi: 10.1016/j.urology.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villers A, Puech P, Mouton D, Leroy X, Ballereau C, Lemaitre L. Dynamic contrast enhanced, pelvic phased array magnetic resonance imaging of localized prostate cancer for predicting tumor volume: correlation with radical prostatectomy findings. J Urol. 2006;176:2432–2437. doi: 10.1016/j.juro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Arumainayagam N, Kumaar S, Ahmed HU, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int. 2010;106:991–997. doi: 10.1111/j.1464-410X.2010.09291.x. [DOI] [PubMed] [Google Scholar]
- 32.Jung JA, Coakley FV, Vigneron DB, et al. Prostate depiction at endorectal MR spectroscopic imaging: investigation of a standardized evaluation system. Radiology. 2004;233:701–708. doi: 10.1148/radiol.2333030672. [DOI] [PubMed] [Google Scholar]
- 33.Bott SR, Ahmed HU, Hindley RG, Abdul-Rahman A, Freeman A, Emberton M. The index lesion and focal therapy: an analysis of the pathological characteristics of prostate cancer. BJU Int. 2010;106:1607–1611. doi: 10.1111/j.1464-410X.2010.09436.x. [DOI] [PubMed] [Google Scholar]
- 34.Futterer JJ, Scheenen TW, Heijmink SW, et al. Standardized threshold approach using three-dimensional proton magnetic resonance spectroscopic imaging in prostate cancer localization of the entire prostate. Invest Radiol. 2007;42:116–122. doi: 10.1097/01.rli.0000251541.03822.bb. [DOI] [PubMed] [Google Scholar]
- 35.King CR, Ferrari M, Brooks JD. Prognostic significance of prostate cancer originating from the transition zone. Urol Oncol. 2009;27:592–597. doi: 10.1016/j.urolonc.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakai I, Harada K, Kurahashi T, Yamanaka K, Hara I, Miyake H. Analysis of differences in clinicopathological features between prostate cancers located in the transition and peripheral zones. Int J Urol. 2006;13:368–372. doi: 10.1111/j.1442-2042.2006.01307.x. [DOI] [PubMed] [Google Scholar]
- 37.Yagci AB, Ozari N, Aybek Z, Duzcan E. The value of diffusion-weighted MRI for prostate cancer detection and localization. Diagn Interv Radiol. 2010 doi: 10.4261/1305-3825.DIR.3399-10.1. [DOI] [PubMed] [Google Scholar]
- 38.Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF., 2nd Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194:W316–322. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimitsu K, Kiyoshima K, Irie H, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging. 2008;27:132–139. doi: 10.1002/jmri.21181. [DOI] [PubMed] [Google Scholar]
- 40.Tamada T, Sone T, Jo Y, et al. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: Comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008;28:720–726. doi: 10.1002/jmri.21503. [DOI] [PubMed] [Google Scholar]
- 41.Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–461. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- 42.Zakian KL, Sircar K, Hricak H, et al. Correlation of proton MR spectroscopic imaging with Gleason score based on step-section pathologic analysis after radical prostatectomy. Radiology. 2005;234:804–814. doi: 10.1148/radiol.2343040363. [DOI] [PubMed] [Google Scholar]
- 43.Vargas HA, Akin O, Franiel T, et al. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775–784. doi: 10.1148/radiol.11102066. [DOI] [PMC free article] [PubMed] [Google Scholar]