Abstract
Magnetic resonance (MR)-high-intensity focused ultrasound (HIFU) is an innovative, noninvasive tumour ablation technique. MR imaging and focused ultrasound are combined allowing real-time anatomic guidance and temperature mapping during treatment. Recently, the volumetric ablation approach has been introduced in order to reduce treatment length and provide more homogeneous tumour ablation. After successful treatment of uterine fibroids, MR-HIFU is currently being investigated for the treatment of malignant tumours. Palliative treatment of painful bone metastases is already applied in clinical practice. Several issues need to be further investigated for successful cancer treatment with MR-HIFU, including patient selection criteria, definition of treatment margins and optimal transducer technology.
Keywords: Magnetic resonance imaging (MRI), high-intensity focused ultrasound (HIFU), cancer treatment, tumour ablation, MR-guided focused ultrasound
Introduction
Magnetic resonance (MR)-guided high-intensity focused ultrasound (HIFU) is a noninvasive treatment modality that combines real-time MR imaging for tumour targeting and focused ultrasound beams for thermal ablation[1]. The ultrasound beams originate from multiple piezoelectric transducer elements (phased arrays) integrated in an MR-compatible tabletop (Fig. 1). The phased array design facilitates precise targeting and focus shaping, important for the treatment of large and complex tumour volumes[1]. The highly focused ultrasound beams are propagated through tissue layers as high-frequency pressure waves and have the ability to pass tissue without being absorbed. In the focal point of the convergent ultrasound beams, the acoustic energy is converted to thermal energy, leading to localized thermal tissue ablation. Increase in tissue temperature beyond 57–60°C leads to denaturation of cell proteins sufficient to cause coagulative necrosis[2,3].
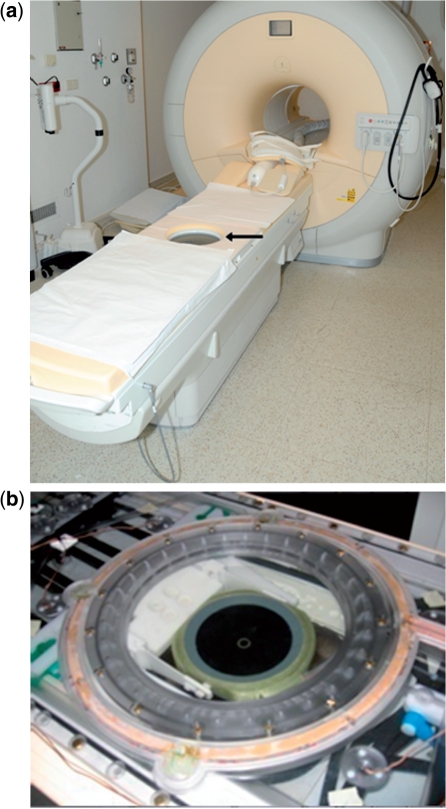
Figure 1.
(a) Philips Healthcare clinical HIFU platform (Sonalleve) integrated into a 1.5-T Philips Achieva MR scanner. The arrow indicates the HIFU transducer embedded in the MR tabletop. (b) A close-up of the transducer is provided. The transducer consists of 256 piezoelectric elements (phased arrays).
As a noninvasive tumour ablation technique, MR-HIFU has the ability to locally treat malignancies without damaging healthy surrounding tissue in cancer patients. It is therefore expected that MR-HIFU will revolutionize cancer treatment in the next decade[1,4]. In this article we provide an overview of the development of MR-HIFU for noninvasive tissue ablation, different MR-HIFU techniques and potential applications of MR-HIFU for treatment of breast and bone malignancies.
Development of MR-HIFU for noninvasive tissue ablation
The concept of thermal tumour ablation with focused ultrasound beams was initially proposed more than 50 years ago[5]. Much preclinical work was started[4,6,7], but translation to treatment of clinical patients remained unattainable due to technical problems. The most important technical challenges were the lack of image guidance for soft tissue visualization and the inability to monitor temperature accurately during treatment[7]. The introduction of MR imaging in combination with HIFU in the 1990s, made controlled thermal ablation possible and was therefore crucial for the translation to clinical practice[8,9]. In addition to excellent soft tissue visualization, MR imaging facilitates thermal mapping within the targeted tumour in a quantitative manner[1]. The first clinical study with MR-HIFU was the treatment of patients with breast fibroadenomas[10]. This feasibility study was followed by important improvements in thermometry and transducer technology[7].
The first commercially available MR-HIFU system (ExAblate 2000, InSightec, Haifa, Israel) was approved by the US FDA in 2004 for the treatment of benign uterine fibroids[6]. Since then, multiple studies have demonstrated the safety and clinical effectiveness of MR-HIFU for treatment of patients with symptomatic uterine fibroids[11–14].
MR-HIFU technique
MR-HIFU as performed by the InSightec ExAblate 2000 system consists of iterative sonications within the targeted volume, producing small cigar-shaped treatment cells, with each sonication followed by a cooling period. To completely ablate the targeted volume, overlapping small individual lesions must be produced[15]. In this method, only a limited proportion of the applied energy is used for the actual ablation, since a large part of the energy is lost through heat diffusion. Therefore, single point ablation remains inefficient and time consuming. Another important disadvantage of single point ablation is inhomogeneous heat distribution[15].
In order to increase treatment efficiency, the Sonalleve MR-HIFU system (Philips Healthcare, Vantaa, Finland) was designed (Fig. 1). This system uses a volumetric ablation technique. In volumetric ablation, the focal spot is continuously moving outwards and in a spiral wise fashion resulting in larger and more homogeneously ablated volumes than in single point ablation[15]. The treatment cell volume can be chosen by the operator: 4, 8, 12 or 16 mm treatment cell diameters (with corresponding volumes of 0.1, 0.6, 2.3, and 5.4 ml, respectively). Another improvement of the Sonalleve system is the real-time temperature monitoring by closed loop feedback, resulting in automatic temperature control while sonicating[16]. If the measured temperature profile reaches a certain user-predefined threshold, the sonication is automatically stopped, preventing under- or overtreatment[17].
MR-HIFU for oncologic applications
Radical tumour removal is critical for curative cancer treatment. To compete with oncologic surgery, MR-HIFU thermal ablation should secure 100% complete tumour necrosis. This remains one of the biggest and most important challenges for MR-HIFU thermal ablation. Several factors are important to attain complete tumour destruction, including optimal imaging technology, treatment protocols for different tumour types and organs, use of treatment margins, and optimal patient selection[18,19]. In order to identify oncologic patients likely to benefit from MR-HIFU treatment in a curative setting, treat-and-resect studies are required in which the efficacy of the MR-HIFU treatment is histopathologically confirmed.
MR-HIFU for breast cancer
After the study on fibroadenomas by Hynynen et al.[10] a case report on the first MR-HIFU treatment of a patient with biopsy-proven invasive breast cancer was published by Huber et al.[17]. Only a limited tumour volume was treated, and analyzed microscopically after consecutive breast conserving surgery. Histological analysis showed some lethal but mostly sublethal tumour damage within the treated volume. In 2003, Gianfelice et al.[18] reported the first prospective study of MR-HIFU for breast cancer. In this treat-and-resect study, 12 patients diagnosed with invasive breast cancer underwent MR-HIFU treatment followed by resection. Histopathological analysis revealed a mean tumour necrosis rate of 88%. Tumour margins were viable in all cases, indicating the need for larger (>5 mm) safety margins. Similar results were obtained in another study by the same study group in a treat-and-resect study in 17 patients with invasive breast cancer[19]. No residual tumour was found in only 4 patients and less than 10% residual tumour was found in 9 patients. In the remaining 4 patients, the residual tumour rate ranged between 30 and 75%. In addition, this study showed that dynamic contrast-enhanced MR imaging (DCE-MRI) is a reliable method for the prediction of residual tumour presence after MR-HIFU treatment. In 2005, Zippel et al.[20] described the results of a small study of 10 patients with breast cancer treated with MR-HIFU 1 week prior to lumpectomy. Complete 100% necrosis was seen in 2 patients (20%) only. It was thereafter concluded that several problems need to be addressed before MR-HIFU can be implemented for breast cancer treatment in clinical practice. In 2006, Khiat et al.[21] assessed the effect of post-treatment delay of DCE-MRI imaging on the evaluation of response after MR-HIFU treatment of breast cancer. They recommended a 7-day interval to determine treatment efficacy, since inflammatory changes, oedema, fibrosis and necrosis can resemble malignant processes on imaging. Furusawa et al.[22] presented improved results of MR-HIFU for breast cancer in another treat-and-resect study of 30 breast cancer patients. On histopathological analysis they found 97% tumour necrosis on average and even 100% tumour necrosis in 15 patients. In 2007, the same study group published the results of MR-HIFU treatment for breast cancer in 21 patients with invasive and noninvasive ductal carcinoma[23]. After treatment, patients received 3-monthly breast ultrasound and MR imaging. Mean follow-up was 14 months. In most patients (95%) no recurrence was found.
In general, it can be concluded that these results are rather disappointing and will be insufficient to support clinical application of breast MR-HIFU. The main disadvantages of previously conducted studies included: (1) no uniform patient selection criteria; (2) no defined treatment margins; (3) no dedicated breast MR-HIFU system. Therefore, a prototype of a dedicated breast MR-HIFU system has been developed by Philips Healthcare and is currently installed in the University Medical Center Utrecht, The Netherlands.
MR-HIFU for bone metastases
MR-HIFU has recently been proposed as a safe and viable alternative technique for treatment of patients with painful bone metastases refractory to radiotherapy[24–26]. Bone pain is a common event in patients with skeletal metastases, leading to immobility and decreased quality of life in cancer patients. In addition to pain medication, palliative radiotherapy is the standard of care for patients with uncomplicated localized metastatic bone pain[27]. Palliative radiotherapy is effective in around 60–70% of patients, and only around 30% of responding patients are totally pain free[28]. However, radiotherapy is not effective in all patients and the effect is often temporary as pain relapse occurs in around 50% of patients[29]. Reirradiation is the current treatment for those patients, yielding approximately the same efficacy as initial radiotherapy[30–33].
Since bone absorbs approximately 50 times more acoustic energy than soft tissue, it can easily be heated with MR-HIFU[34,35]. Applying acoustic energy with MR-HIFU on the bone surface will result in bone cortex heating, indirectly ablating the adjacent periosteum and tumour tissue. The periosteum is considered to be a major source of pain in patients with bone metastases and ablating the source of pain with MR-HIFU may produce fast and long-lasting pain relief[36]. To date, three prospective cohort studies of MR-HIFU for painful bone metastases have been published; an overview of these studies is provided in Table 1. The first experience of MR-HIFU for treatment of metastatic bone pain was reported by Catane et al.[24] in 2007. They describe the use of MR-HIFU ablation for treatment of 14 painful metastatic bone lesions in 13 patients. One patient was unable to tolerate sonication-related pain. No other adverse events were reported. At the 3-month follow-up, improvement in both pain score and dosage of pain-reducing medication was found in all patients. Unfortunately, response rate was not quantified. Another feasibility study followed in 2008, reported by Gianfelice et al.[25]. In this study, 11 patients with metastatic bone pain were treated by MR-HIFU ablation. One patient reported complete pain relief 3 days after treatment and in the remaining patients pain relief was seen at around 14 days after treatment. Pain relief increased during follow-up with an overall response rate of 100% at 3 months. Overall and complete response rates at 1 and 3 months are provided in Table 1. No treatment-related adverse events were recorded. In the same year, a multicentre study on 31 patients with painful bone metastases was published by Liberman et al.[26]. All patients except two tolerated the MR-HIFU treatment and no device-related severe adverse events were recorded. In over 50% of patients, substantial pain relief was seen 3 days after treatment. Overall response rate at 3 months was 72%, and 36% of patients obtained complete response.
Table 1.
Overview of studies on MR-HIFU for painful bone metastases
| Study | Patients | Primary tumour (n) | Location (n) | Average duration, min (range) | Average no. of sonications (range) | No. of patients with 3-month follow-up (%) | Pain responsea |
|---|---|---|---|---|---|---|---|
| Catane et al. (2007)[24] | 13 (14 lesions) | Breast (4) | Ilium (10) | 80 (22–158) | 29 (11–39) | 11/13 (85) | 3 days after treatment: improvement in most cases |
| Prostate (2) | Ischium (1) | ||||||
| Lung (1) | Sacrum (1) | 3 months after treatment: improvement in both pain score and dosage of pain-reducing medication | |||||
| Renal (1) | Humerus (1) | ||||||
| Colorectal (1) | Femur (1) | ||||||
| Other (4) | |||||||
| Gianfelice et al. (2008)[25] | 11 (12 lesions) | Breast (5) | Ilium (7) | 28–103 | 12–18 | 11/11 (100) | 3 days after treatment: OR=73% and CR=9% |
| Renal (4) | Ischium (1) | ||||||
| Lung (1) | Scapula (2) | 1 month after treatment: OR=91% and CR=27% | |||||
| Liver (1) | Clavicula (1) | 3 months after treatment: OR=100% and CR=45% | |||||
| Liberman et al. (2008)[26] | 31 (32 lesions) | Breast (11) | Ilium (18) | 66 (22–162) | 17.3 (8–32) | 25/31 (81) | 3 months after treatment: OR=72% and CR=36% |
| Renal (6) | Ischium (4) | ||||||
| Prostate (5) | Sacrum (4) | ||||||
| Colorectal (2) | Femur (1) | ||||||
| Lung (1) | Scapula (2) | ||||||
| Other (6) | Humerus (1) | ||||||
| Clavicula (1) |
Abbreviations: OR, overall response; CR, complete response.
aThe listed response rates are derived from the articles using the Update of the International Consensus on Palliative Radiotherapy Endpoints for Future Clinical Trials in Bone Metastases[37]. OR is defined as pain score of 0 without analgesic increase; CR is defined as pain reduction ≥2 without analgesic increase or analgesic reduction ≥25% without pain increase.
In all studies, the InSightec ExAblate 2000 system was used and patients were generally treated only once. Lesions in the spine, ribs and skull were not treated as this is regarded as unsafe at the moment because of the close proximity of the spinal cord, brain and lungs, respectively. Interestingly, in all studies lesions were mostly located in the iliac bones. Pain relief seems to occur relatively fast following treatment, as opposed to radiotherapy which may take up to 4 weeks[29]. Lytic as well as osteoblastic lesions were treated in all studies. Although data are limited, Liberman et al.[26] found that MR-HIFU is equally effective in achieving pain palliation in both lesion types.
Overall, these initial studies indicate that MR-HIFU is a safe and effective treatment although all studies were published by the same study group. To define the exact nature and extent of possible adverse events and the effectiveness of the treatment, more extensive research is needed. In the near future, a prospective study on MR-HIFU for pain palliation in radio refractory bone metastases will start in the University Medical Center Utrecht, where patients are already being treated in a clinical setting (Fig. 2). Treatments are performed by means of the Philips Sonalleve system, which has recently obtained CE labelling for this specific application.
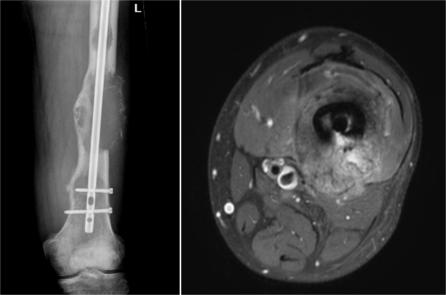
Figure 2.
MR-HIFU for palliative treatment of bone metastases. Radiograph (left) and MR image (right) of the left femur of a patient with a symptomatic bone metastasis secondary to renal cell carcinoma. The expansive lytic lesion was resistant to radiotherapy and therefore treated with MR-HIFU.
Conclusion
MR-HIFU is a unique noninvasive tumour ablation technique because of the combination of focused ultrasound with real-time MR guidance. Recently, the volumetric ablation technique has been proposed to enhance treatment efficacy. Volumetric ablation results in larger and more homogeneously ablated volumes than single point ablation. The first applications of volumetric MR-HIFU for treatment of cancer patients will be in the area of breast cancer treatment and palliative treatment of bone metastases.
References
- 1.Jolesz FA. MRI-guided focused ultrasound surgery. Annu Rev Med. 2009;60:417–30. doi: 10.1146/annurev.med.60.041707.170303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jolesz FA, Hynynen K, McDannold N, Tempany C. MR imaging-controlled focused ultrasound ablation: a noninvasive image-guided surgery. Magn Reson Imaging Clin North Am. 2005;13:545–60. doi: 10.1016/j.mric.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 4.Bradley WG., Jr MR-guided focused ultrasound: a potentially disruptive technology. J Am Coll Radiol. 2009;6:510–13. doi: 10.1016/j.jacr.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol. 1942;26:179–93. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50:221–9. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Jolesz FA, McDannold N. Current status and future potential of MRI-guided focused ultrasound surgery. J Magn Reson Imaging. 2008;27:391–9. doi: 10.1002/jmri.21261. [DOI] [PubMed] [Google Scholar]
- 8.Cline HE, Schenck JF, Hynynen K, Watkins RD, Souza SP, Jolesz FA. MR-guided focused ultrasound surgery. J Comput Assist Tomogr. 1992;16:956–65. doi: 10.1097/00004728-199211000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI-guided noninvasive ultrasound surgery. Med Phys. 1993;20:107–15. doi: 10.1118/1.597093. [DOI] [PubMed] [Google Scholar]
- 10.Hynynen K, Pomeroy O, Smith DN, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219:176–85. doi: 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 11.Fennessy FM, Tempany CM, McDannold NJ, et al. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery – results of different treatment protocols. Radiology. 2007;243:885–93. doi: 10.1148/radiol.2433060267. [DOI] [PubMed] [Google Scholar]
- 12.Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34:584–9. doi: 10.1002/uog.7455. [DOI] [PubMed] [Google Scholar]
- 13.LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment: report of 80 patients. AJR Am J Roentgenol. 2010;194:274–80. doi: 10.2214/AJR.09.2842. [DOI] [PubMed] [Google Scholar]
- 14.Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol. 2007;110:279–87. doi: 10.1097/01.AOG.0000275283.39475.f6. [DOI] [PubMed] [Google Scholar]
- 15.Kohler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36:3521–35. doi: 10.1118/1.3152112. [DOI] [PubMed] [Google Scholar]
- 16.Enholm JK, Kohler MO, Quesson B, Mougenot C, Moonen CT, Sokka SD. Improved volumetric MR-HIFU ablation by robust binary feedback control. IEEE Trans Biomed Eng. 2010;57:103–13. doi: 10.1109/TBME.2009.2034636. [DOI] [PubMed] [Google Scholar]
- 17.Huber PE, Jenne JW, Rastert R, et al. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res. 2001;61:8441–7. [PubMed] [Google Scholar]
- 18.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness – initial experience. Radiology. 2003;227:849–55. doi: 10.1148/radiol.2281012163. [DOI] [PubMed] [Google Scholar]
- 19.Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused ultrasound surgery of breast cancer: correlation of dynamic contrast-enhanced MRI with histopathologic findings. Breast Cancer Res Treat. 2003;82:93–101. doi: 10.1023/B:BREA.0000003956.11376.5b. [DOI] [PubMed] [Google Scholar]
- 20.Zippel DB, Papa MZ. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer. 2005;12:32–8. doi: 10.2325/jbcs.12.32. [DOI] [PubMed] [Google Scholar]
- 21.Khiat A, Gianfelice D, Amara M, Boulanger Y. Influence of post-treatment delay on the evaluation of the response to focused ultrasound surgery of breast cancer by dynamic contrast enhanced MRI. Br J Radiol. 2006;79:308–14. doi: 10.1259/bjr/23046051. [DOI] [PubMed] [Google Scholar]
- 22.Furusawa H, Namba K, Thomsen S, et al. Magnetic resonance-guided focused ultrasound surgery of breast cancer: reliability and effectiveness. J Am Coll Surg. 2006;203:54–63. doi: 10.1016/j.jamcollsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Furusawa H, Namba K, Nakahara H, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS) Breast Cancer. 2007;14:55–8. doi: 10.2325/jbcs.14.55. [DOI] [PubMed] [Google Scholar]
- 24.Catane R, Beck A, Inbar Y, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases – preliminary clinical experience. Ann Oncol. 2007;18:163–7. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 25.Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging–guided focused ultrasound. Radiology. 2008;249:355–63. doi: 10.1148/radiol.2491071523. [DOI] [PubMed] [Google Scholar]
- 26.Liberman B, Gianfelice D, Inbar Y, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16:140–6. doi: 10.1245/s10434-008-0011-2. [DOI] [PubMed] [Google Scholar]
- 27.Meeuse JJ, van der Linden YM, van Teinhoven G, Gans RO, Leer JW, Reyners AK. Efficacy of radiotherapy for painful bone metastases during the last 12 weeks of life: results from the Dutch Bone Metastasis Study. Cancer. 2010;116:2716–25. doi: 10.1002/cncr.25062. [DOI] [PubMed] [Google Scholar]
- 28.Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy – a systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;(2):CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–9. doi: 10.1016/S0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 30.Jeremic B, Shibamoto Y, Igrutinovic I. Single 4 Gy re-irradiation for painful bone metastasis following single fraction radiotherapy. Radiother Oncol. 1999;52:123–7. doi: 10.1016/S0167-8140(99)00108-5. [DOI] [PubMed] [Google Scholar]
- 31.Mithal NP, Needham PR, Hoskin PJ. Retreatment with radiotherapy for painful bone metastases. Int J Radiat Oncol Biol Phys. 1994;29:1011–14. doi: 10.1016/0360-3016(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 32.Uppelschoten JM, Wanders SL, de Jong JM. Single-dose radiotherapy (6 Gy): palliation in painful bone metastases. Radiother Oncol. 1995;36:198–202. doi: 10.1016/0167-8140(95)01587-7. [DOI] [PubMed] [Google Scholar]
- 33.van der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys. 2004;59:528–37. doi: 10.1016/j.ijrobp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Smith NB, Temkin JM, Shapiro F, Hynynen K. Thermal effects of focused ultrasound energy on bone tissue. Ultrasound Med Biol. 2001;27:1427–33. doi: 10.1016/S0301-5629(01)00454-9. [DOI] [PubMed] [Google Scholar]
- 35.Hynynen K, Darkazanli A, Unger E, Schenck JF. MRI-guided noninvasive ultrasound surgery. Med Phys. 1993;20:107–15. doi: 10.1118/1.597093. [DOI] [PubMed] [Google Scholar]
- 36.Lipton A. Management of bone metastases in breast cancer. Curr Treat Options Oncol. 2005;6:161–71. doi: 10.1007/s11864-005-0023-0. [DOI] [PubMed] [Google Scholar]
- 37.Chow E, Hoskin P, Mitera G, et al. Update of the International Consensus on Palliative Radiotherapy Endpoints for Future Clinical Trials in Bone Metastases. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.02.008. doi:10.1016/j.ijrobp.2011.02.008. [DOI] [PubMed] [Google Scholar]