Abstract
In this article, we trace the chronology of developments in breast imaging technologies that are used for diagnosis and staging of breast cancer, including mammography, ultrasonography, magnetic resonance imaging, computed tomography, and positron emission tomography. We explore factors that affected clinical acceptance and utilization of these technologies from discovery to clinical use, including milestones in peer-reviewed publication, US Food and Drug Administration approval, reimbursement by payers, and adoption into clinical guidelines. The factors driving utilization of new imaging technologies are mainly driven by regulatory approval and reimbursement by payers rather than evidence that they provide benefits to patients. Comparative effectiveness research can serve as a useful tool to investigate whether these imaging modalities provide information that improves patient outcomes in real-world settings.
Keywords: Breast cancer, Centers for Medicare and Medicaid Services, comparative effectiveness research, computed tomography, diagnostic imaging, US Food and Drug Administration, magnetic resonance imaging, mammography, positron emission tomography, ultrasonography
Introduction
Despite advances in diagnosis and treatment, breast cancer remains a significant issue in women's health. In 2010, an estimated 207,090 women were diagnosed with breast cancer and 39,840 died of the disease[1]. Although death rates have dropped in recent years, breast cancer is still one of the leading causes of death for women[1]. Imaging is a key technology used to diagnose and assess the extent of breast cancer[2]. Although technological upgrades in advanced imaging are constantly improving the ability to detect breast tumors[3–5], some of these advances have been associated with increased mastectomy rates[6–9] and delays in beginning breast cancer treatment[10,11]. Clinical use of imaging has increased greatly in recent years[12] and several analyses have found imaging to be one of the most rapidly increasing Medicare services, which has had a major impact on total health care costs[13–16]. A 2010 study found that 2-year costs for all types of cancer increased at a mean annual rate of 1.8% to 4.6% from 1999 to 2006; for diagnostic imaging specifically, however, the mean 2-year imaging costs per patient increased from 5.1% to 10.3% per year in that time[13].
Unlike regulations for new drug therapies, the US Food and Drug Administration (FDA) does not require great amounts of evidence of effectiveness for new or modified imaging technologies, resulting in little motivation by device manufacturers to conduct studies documenting the long-term effects of imaging on outcomes. Comparative effectiveness research (CER) can be used to investigate whether advanced imaging technologies improve patient outcomes and decrease health care costs in diverse community settings. The Advancing Innovative Comparative Effectiveness Research (ADVICE) project engages a multi-disciplinary and cross-institutional network of stakeholders to conduct comparative effectiveness studies in western Washington State. In order to understand key gaps in evidence pertaining to the relationship between breast cancer imaging and patient outcomes that could benefit from future CER studies, we investigated the timeline of the development of several diagnostic breast imaging technologies for this article, including dates of (1) initial appearance in peer-reviewed scientific literature; (2) FDA approval; (3) Centers for Medicare and Medicaid Services (CMS) coverage; and (4) adoption into National Comprehensive Cancer Network (NCCN) guidelines. We limited the scope of this work to 5 imaging applications – mammography, ultrasonography, magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET) (now largely performed as combined PET/CT) – that are used in the peridiagnostic period between the detection of breast cancer and the start of treatment. Many of these techniques are also used during screening, treatment monitoring, restaging, and surveillance, but these uses are not the focus of this review. We instead chose to study the peridiagnostic time period because several recent studies have questioned whether use of advanced imaging for breast cancer staging is associated with improved outcomes[5,17,18]. We excluded technologies that are used to detect metastases in other areas of the body, such as bone scans and MRI scans of the brain. We also report the milestones of technologies that were offshoots of the 5 main modalities, such as computer-assisted imaging, breast coils, and contrast enhancement.
Methods
We performed literature searches for mammography, ultrasonography, MRI, CT, PET, and the key technological subtypes related to each in PubMed to find the first appearance in the scientific literature for use of each technology for breast tumor imaging. We restricted our search to articles published in English and excluded studies that only reported in vitro imaging. We also searched the Premarket Approval (PMA) and Premarket Notification (510(k)) databases[19,20] on the FDA website with each technology's product code (the categorization scheme used to determine regulatory requirements) to find the dates of FDA approval or clearance of these devices. To find the first evidence of reimbursement for the technologies by the CMS, we searched their website (http://www.cms.gov) for National Coverage Determinations (NCDs) pertaining to each technology. We also investigated publicly available technology assessments from private insurers, including the Blue Cross Blue Shield Association Technology Evaluation Center[21], Aetna Clinical Policy Bulletins[22], and Group Health Clinical Reviews from Group Health Cooperative of Puget Sound[23].
To assess the first appearance of device recommendations in national guidelines, we surveyed archives available from the National Comprehensive Cancer Network (NCCN), which represents 21 comprehensive cancer centers in the United States and is one of the most influential breast cancer diagnosis and treatment guidelines. The NCCN has been publishing guidelines for breast cancer staging, treatment, and surveillance since 1996[24] and archives of guidelines from 1996 to 2011 (excluding 1998 when guidelines were not published) were available for this article[25–39].
Results
Table 1 summarizes the dates of appearance in the peer-reviewed breast cancer literature, FDA approval, CMS reimbursement, and appearance in the NCCN guidelines of the major imaging modalities and their subsidiary technologies. Each modality is described in detail below.
Table 1.
Milestones of major breast imaging technologies used for diagnosis and staging of breast cancer
| Modality | Appearance in peer-reviewed breast cancer literature | FDA approval/ clearancea | CMS coverage decision for breast cancer | Appearance in NCCN guidelinesb |
|---|---|---|---|---|
| Mammography | 1960 | Pre-1976 MDA | 1978 | 1996 |
| FFDM | 1996 | 2000 | 2000 | None |
| Mammography CAD | 1987 | 1998 | 2000 | None |
| Ultrasonography | 1951 | Pre-1976 MDA | 1966 | 1997 |
| MRI | 1973 | 1984 | 1985 | 2001 |
| Contrast-enhanced MRI | 1986 | 1988 | 2000 | None |
| MRI breast coils | 1985 | 1992 | 2012 | 2002 |
| MRS | 1988 | 2008 | None | None |
| CT | 1976 | Pre-1976 MDA | 1985 | 2001 |
| Breast-specific CT | 1978 | None | None | None |
| PET | 1984 | Pre-1976 MDA | 2002c | 2003 |
| FDG tracer | 1991 | 1994 | 2002c | 2010 |
| PET/CT | 2003 | 2000 | 2009d | 2009 |
Abbreviations: FDA, US Food and Drug Administration; CMS, Centers for Medicare and Medicaid Services; NCCN, National Comprehensive Cancer Network; MDA, Medical Device Amendments of 1976; FFDM, full field digital mammography; CAD, computer-aided detection; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; CT, computed tomography; PET, positron emission tomography; FDG, fluorodeoxyglucose; PET/CT, positron emission tomography/computed tomography.
aFDA approval/clearance not necessarily for breast cancer indications.
bGuidelines first published by NCCN in 1996.
cCovered only for metastatic breast cancer.
dPET/CT was not specifically mentioned in a coverage determination prior to 2009, but was probably covered under the same conditions as PET.
Mammography
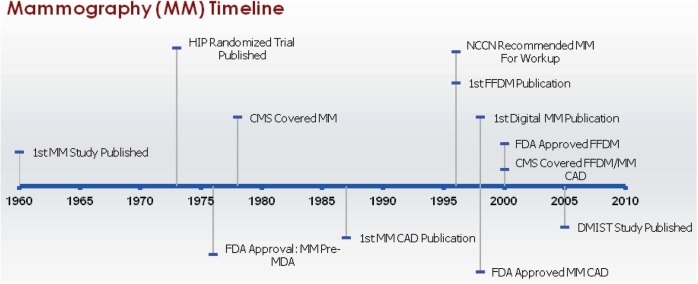
Diagnostic mammography is typically the first imaging test used in women who have positive screening mammograms or symptoms that might be indicative of breast cancer, such as palpable breast lumps, nipple discharge or retraction, or skin changes on the breast[40]. Diagnostic mammography is distinguished from screening mammography in that more views are taken, usually at different magnification levels and with varying compression techniques, and often includes online review of the images and physical examination. Although we attempted to differentiate between the two types of mammography where feasible and provide information on mammography used for diagnostic purposes, we were unable to completely distinguish screening from diagnostic mammography for the milestones reported for this article (Fig. 1).
Figure 1.
Milestones in mammography. Abbreviations: MM, mammography; HIP, Health Insurance Plan of Greater New York; FDA, US Food and Drug Administration; MDA, Medical Device Amendments of 1976; CMS, Centers for Medicare and Medicaid Services; CAD, computer-aided detection; NCCN, National Comprehensive Cancer Network; FFDM, full field digital mammography; DMIST, Digital Mammographic Imaging Screening Trial.
Use of x-rays to examine breast tissue was reported as early as 1913 by A. Salomon[41], but the first peer-reviewed report of use of this technology to diagnose breast cancer was from R.L. Egan in 1960[42]. A randomized control trial conducted by the Health Insurance Plan (HIP) of Greater New York, published in 1973, showed a 33% reduction in breast cancer mortality for women screened with mammography and helped this modality achieve widespread clinical acceptance[43]. Full field digital mammography (FFDM) utilizes digital detectors rather than x-ray film to improve some of the inherent weaknesses of film-based radiology, such as lack of contrast range. The first reference in the scientific literature to the design of FFDM was by M.B. Williams in 1996[44] and a randomized controlled trial showing that the overall accuracy of FFDM and film mammography were the same, with improvements with FFDM in some subgroups, was published in 2005 by the ACRIN group, which conducted the Digital Mammographic Imaging Screening Trial (DMIST)[45]. Computer-aided detection (CAD; sometimes referred to as computer aided diagnosis) uses image processing and computer vision techniques to augment a human reader's ability to detect breast lesions. The first paper to discuss this application for breast tumors was published by Chan in 1987[46] and the first commercial CAD mammography systems appeared in 1998[47].
Because mammography was used clinically prior to the Medical Device Amendments (MDA) of 1976, analog (film screen) mammography did not receive FDA premarket approval or 510(k) clearance. CAD interpretation of mammography received FDA approval in 1998[48]. In January 2000, the FDA approved FFDM for screening and diagnosis of breast cancer for the same clinical indications as traditional film-based mammography[19].
CMS has covered diagnostic mammography since May 1978 for patients with distinct signs and symptoms, a history of breast cancer, or are asymptomatic but for whom physicians deem mammography appropriate[49]. Digital mammography and mammography CAD for both screening and diagnostic mammography have been reimbursed by CMS since 2001 as a result of the Benefits Improvement and Protection Act (BIPA) of 2000[50]. Most private insurers now reimburse FFDM, but the evaluation of mammography CAD is mixed. For example, a recent report from the Blue Cross Blue Shield Association stated that the evidence for mammography CAD for diagnostic purposes was insufficient[51]; an Aetna Clinical Policy Bulletin stated CAD was “a medically necessary adjunct to mammography”[52].
Guidelines published by the NCCN have recommended that diagnostic mammography be used for local breast staging for breast cancer (determining the extent of disease in the breast) since 1996[25–39]. FFDM and mammography CAD have never received specific mention in NCCN guidelines.
Ultrasonography
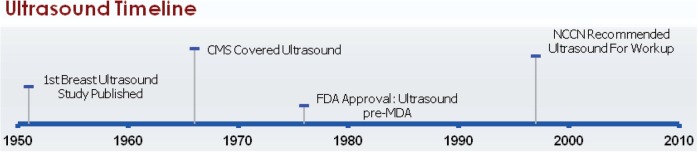
The earliest reports of the use of ultrasound technology to examine breast tissue were reported in 1951, when it was used to visualize benign and malignant tumors and to determine the extent of metastases (Fig. 2)[53,54]. In the late 1970s, concern about the level of radiation from mammography led to an interest in utilization of ultrasonography to screen for breast cancer. However, ultrasonography generally has a number of limitations compared with mammography, including lower detection of microcalcifications, inability to differentiate benign from malignant solid lesions, and poor imaging of masses smaller than 1 cm in diameter, and was determined to be impractical as a substitute for screening mammography[55].
Figure 2.
Milestones in ultrasound imaging of the breast. Abbreviations: CMS, Centers for Medicare and Medicaid Services; FDA, US Food and Drug Administration; MDA, Medical Device Amendments of 1976.
Breast ultrasonography is currently used to supplement mammograms for screening[56], to distinguish benign cysts from solid masses[57], for image-guided interventions such as large-core breast and fine-needle aspiration biopsies[55,58], and for local breast staging. It is increasingly used for locoregional staging as well, for determining the status of locoregional lymph nodes[59]. Ultrasonography is also the primary method of imaging palpable breast masses in women under 30 years old, since the density of the breast tissue in younger women rules out mammography[58]. Ultrasonography is recommended for surveillance in women who have had mastectomies[60–62] and breast conservation therapy[63,64].
Conventional ultrasound devices were on the market prior to the MDA of 1976 and therefore most were excluded from original FDA approval. (The exception is the HDI 3000 US System, which was approved under the PMA process in 1996 as an adjunct to mammography and physical breast examination[65].) CMS has covered breast ultrasonography since 1966[66].
Since 1997, the NCCN has indicated that ultrasonography can be used as part of the work-up for women with clinical indications of all stages of breast cancer[26–39].
MRI
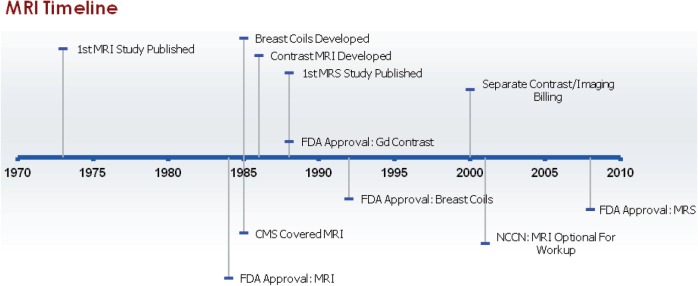
Use of MRI for examining malignant breast tissue was first reported by R. Damadian and colleagues in 1973[67], but the usefulness of MRI in relation to breast cancer was not appreciated until contrast agents, which enhanced the appearance of tumors compared with normal tissue, were introduced in 1986 (Fig. 3)[68]. The quality of breast MRI was also enhanced by the introduction of breast imaging coils, first reported in 1985[69]. Magnetic resonance spectroscopy (MRS) uses modified MRI hardware and software to characterize the chemical composition of breast tumors since some proton MRS studies showed that breast cancers exhibit increased levels of choline compared with normal tissue, but remains largely investigational[70]. This technology was first reported in the peer-reviewed literature for use with breast tumors in 1988[71], although in vitro studies were published prior to this[67].
Figure 3.
Milestones in MRI of the breast. Abbreviations: MRI, magnetic resonance imaging; FDA, US Food and Drug Administration; CMS, Centers for Medicare and Medicaid Services; MRS, magnetic resonance spectroscopy; Gd, gadolinium; NCCN, National Comprehensive Cancer Network.
The FDA approved MRI scanners, the first imaging devices to be approved through the PMA process as class III devices, in 1984[19,65]. In 1991, MRI was reclassified for use as a supplement to mammography to diagnose breast cancer[65]. Gadolinium-based contrast agents (GBCAs), currently the only FDA-approved contrast agents for evaluation of the breast, received FDA clearance in 1988[72]. Breast coils for MRI were cleared through the 510(k) process in 1992[20]. MRS received FDA 510(k) clearance in 2008[73].
CMS began reimbursing for breast MRI, as long as the MRI unit had received FDA premarket approval, in November 1985[74]. Although no NCD was given specifically for contrast MRI, legislation in 2000 directed the Health Care Financing Administration to create separate billing codes for contrast agents and imaging procedures, which allowed CMS to pay for these separately. Currently, no additional reimbursement is made for imaging with dedicated breast coils, but as a result of the Medicare Improvements for Patients and Providers Act of 2008 (MIPPA), facilities will be required to use breast coils in order to receive Medicare reimbursements for breast MRIs beginning in 2012. Although many private insurers require preauthorization for diagnostic breast MRI, most reimburse for it in at least some cases[75]. Currently MRS is not reimbursed by CMS[76] and private insurers view the test as experimental[77].
NCCN guidelines stated that body MRI, specifically abdominal MRI, might be used for symptomatic areas for systemic staging of women with metastatic or recurrent breast cancer beginning in 1996[26]. However, breast MRI, which is distinct from MRI for body imaging, was not addressed until 2001, when it was mentioned as optional for women with clinical stages I, IIA, and IIB breast cancers, largely for local breast staging[29]. In 2002, the guidelines specified that a dedicated breast coil should be used, although the MRI was still listed as optional and only in patients with equivocal results from other tests who were considering breast conserving therapy[30]. Beginning in 2004, the option of breast MRI was extended to women with clinically identified stage III cancer[32]. In 2005, language was added to the guidelines to emphasize that whether to perform breast conservation therapy or mastectomy should not be decided solely on the basis of results from the MRI[33]. Further caveats were added in 2007, such as that MRI should not replace diagnostic mammography or ultrasonography and that the MRI should be performed and read by an expert breast imaging team[35]. Beginning in 2008, guidelines for inflammatory breast cancer were included and these listed breast MRI as optional[36–39]. From 2009 to 2011, the guidelines also included the stipulation that no data had indicated that use of MRI to choose local therapy improved outcomes (e.g. recurrence or survival) and false-positive findings from MRI were common[37–39]. MRS has not been mentioned in the NCCN guidelines.
CT
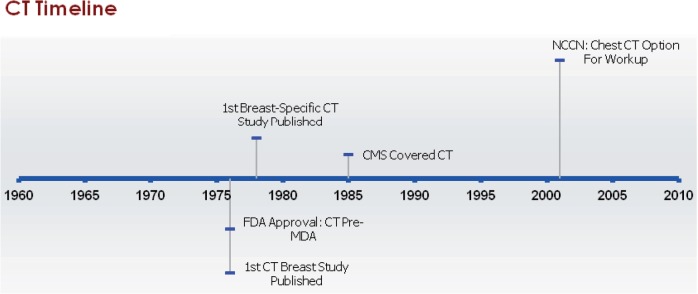
The algorithms that enable reconstruction of cross-sectional imaging in CT were introduced by D.E. Kuhl and R.Q. Edwards in 1962 (Fig. 4)[78]. The first peer-reviewed documentation of the use of CT technology in the diagnosis of breast lesions was reported by D.F. Reese et al. in 1976[79] and the first use of a dedicated breast CT scanner was reported in 1978 by C.H. Chang and colleagues[80]. Although CT initially sparked interest for detecting malignancies, concerns about its specificity, radiation dose, and exposure to iodinated contrast agents that can cause allergic reactions prevented its adoption into regular use for diagnosis and staging of breast cancer patients[81–83].
Figure 4.
Milestones in computed tomography (CT) imaging of the breast. Abbreviations: CT, computed tomography; FDA, US Food and Drug Administration; MDA, Medical Device Amendments of 1976; CMS, Centers for Medicare and Medicaid Services; NCCN, National Comprehensive Cancer Network.
Commercial CT systems existed before the 1976 MDA and therefore CT has no date of initial FDA approval. To date, a breast-specific CT device has not been cleared by the FDA, although there has been some recent renewed enthusiasm for this device[84]. CT has been covered by CMS since November 1985 for a variety of indications, but the coverage decision did not specify whether breast cancer staging was among them[85].
NCCN guidelines for CT relate to its use for systemic staging of breast cancer. Although NCCN guidelines recommended abdominal CT for stage III breast cancers beginning in 1996, chest CT was not mentioned until 2001, when the guidelines stated that consideration could be given to using chest CT in women with stage II cancer with at least 4 positive nodes[25–29]. In 2002, the guidelines also stated that chest CT could be considered for women with stage IV or recurrent breast cancers[30]. Beginning in 2003, the guidelines recommended use of chest CT for stage III breast cancer and also indicated that CT should be used for treatment planning when radiation therapy was to be delivered to the mammary lymph node field[31–33]. From 2006 to 2011, chest CT was still a consideration for women with stages I or II cancer with at least 4 positive nodes and was recommended for treatment planning, but, for work-up of clinical stages III, IV, and recurrent cancers, the guidelines stated that chest imaging should be done and did not specifically mention CT[34–39]. Beginning in 2008, guidelines for inflammatory breast cancer were included and these recommended chest CT to evaluate for the presence of distant metastases[36–39].
Fluorodeoxyglucose(FDG)-PET and FDG-PET/CT
Because tumors have increased glucose utilization rates compared with non-cancerous tissue, FDG-PET has potential to be an effective imaging test in oncology[86]. However, PET scans are not widely used for diagnosis and staging breast cancer because they are expensive and can have poor sensitivity for detection of small tumors and ductal carcinoma in situ (DCIS) compared with other imaging techniques[87–89].
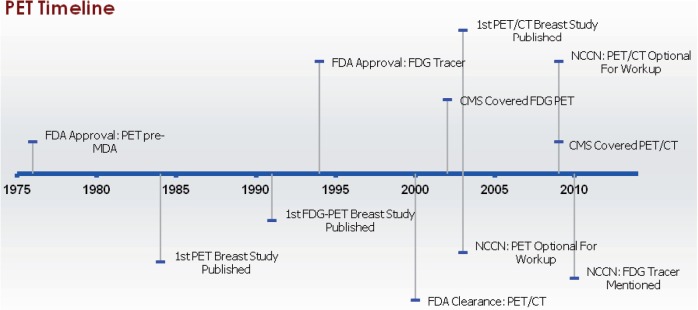
As with CT technology, prototype PET systems appeared shortly after D.E. Kuhl and R.Q. Edwards introduced reconstruction algorithms for emission CT in 1962 (Fig. 5)[90]. The first commercially available PET system, the ECAT II, was developed by E&G Ortec in 1976[90]. Studies evaluating PET in breast cancer patients first appeared in 1984[91–93]. and the first study to evaluate the use of FDG as a tracer for use of PET on breast cancer patients was reported in 1991[94]. D.W. Townsend and colleagues developed the first combined PET/CT prototype in 1998[95]. Its first use in breast cancer patients was reported in 2003[96]. By 2006, all scanners purchased in the United States were combined PET/CT[88]. Since the ECAT II system was introduced before implementation of the 1976 MDA, it was marketed without FDA premarket approval or 510(k) clearance. PET may be used with a number of different radiotracers, but the only FDA-approved tracer for breast imaging is FDG, which received initial FDA approval in 1994 for the identification of regions associated with epileptic seizures[97]. In 1999, the FDA Center for Drug Evaluation and Research (rather than the Center for Devices and Radiological Health, which approved PET scanners) approved FDG for use as a radiopharmaceutical[97] and in 2000 it was approved for oncologic purposes[98]. Also in 2000, the first PET/CT device received 510(k) clearance[99].
Figure 5.
Milestones in PET imaging of the breast. Abbreviations: PET, positron emission tomography; FDA, US Food and Drug Administration; MDA, Medical Device Amendments of 1976; FDG, fluorodeoxyglucose; PET/CT, positron emission tomography/computed tomography; CMS, Centers for Medicare and Medicaid Services; NCCN, National Comprehensive Cancer Network.
PET scans were reimbursed by CMS for breast cancer patients in 2002 as an adjunct to other imaging modalities for (1) staging breast cancer patients with distant metastases, (2) restaging patients with locoregional recurrence or metastases, and (3) monitoring tumor response to treatment for women with locally advanced and metastatic breast cancer if a change in therapy was considered[100]. Use of PET for diagnosis of breast cancer and staging of axillary lymph nodes for breast cancer patients was not covered by CMS[100]. Although no changes were made to the reimbursement decisions on the use of PET for breast cancer, CMS issued another NCD in 2009 that made changes to PET with regard to other types of cancer and this indicated that PET/CT scanners were covered in the same manner as PET-only machines[101]. Private insurers use similar criteria as CMS for decisions about reimbursement for PET scans in breast cancer patients[102].
Use of PET scans in the NCCN guidelines relates to systemic and locoregional staging. Use of PET scans was first incorporated into NCCN guidelines in 2003, when they were described as an optional imaging procedure for stage IV metastatic or recurring breast cancer; however, the guidelines recommended that PET scans not replace more established imaging methods[31]. This remained consistent until 2007[32–35] but in 2008, PET or PET/CT scans were not mentioned in the guidelines[36]. The 2009–2011 NCCN guidelines specified that PET (and, for the first time, PET/CT was specifically mentioned) were not indicated for (1) detecting primary breast cancer, (2) staging of the primary tumor, axilla, or metastatic disease in patients with early-stage disease, or (3) post-treatment disease surveillance[37–39]. However, the guidelines indicated that PET/CT scans may be considered as an adjunct to other imaging modalities for initial evaluation of recurrent or metastatic disease, locally advanced, or inflammatory breast cancer when the results of other imaging tests were unclear, but even then, the guidelines stated that biopsies would probably be more informative than PET or PET/CT scans[37–39]. FDG was specified in the guidelines beginning in 2010[38].
Discussion
In this article, we tracked the chronology of development of imaging modalities that are commonly used for diagnosis and staging of breast cancer. Although peer-reviewed papers documenting the ability of these technologies to image breast tumors appeared as early as the 1950s, FDA approval or clearance and CMS reimbursement often took place years later. We found that 4 of these technologies (mammography, ultrasonography, CT, and PET) were widely used before 1976 and never received initial FDA approval. Mammography and ultrasonography have been reimbursed by CMS for more than 30 years and are commonly used for diagnosis and staging of breast cancer[39]. Although CT is covered by CMS for some indications, breast cancer is not specified as one and its diagnostic use for breast cancer has never been strongly advocated in NCCN guidelines. MRI received FDA approval and CMS reimbursement coverage in the mid-1980s, but NCCN guidelines have been very cautious about its use for diagnosis and staging. For women with non-metastatic breast cancer, PET and PET/CT have not been covered by CMS or recommended by NCCN guidelines but, nonetheless, anecdotal evidence indicates their use is not uncommon.
The factors driving acceptance and clinical use of new imaging technologies are complex. FDA regulatory approval is crucial, but without CMS reimbursement, technologies are unlikely to be widely used. For example, MRI was approved by the FDA in 1984, reimbursed by CMS 1 year later, and utilization rates began soaring immediately[103]. In contrast, PET was commercially available from 1976 but its use did not extend beyond the research setting until CMS began reimbursing for numerous cancer indications in 2001[104]. However, CMS reimbursement does not automatically translate to increased use in the clinic; for example, CT has been FDA-approved since 1976 and reimbursed by CMS since 1985, but since early studies did not show convincing evidence of benefit compared with other techniques, few clinicians have used CT for detection and locoregional assessment of breast lesions[13].
The FDA requirements for approval of imaging technologies are not very stringent, resulting in few studies documenting long-term effects on outcomes such as disease recurrence or survival that would be required for novel drug therapies. The evidence of effectiveness of diagnostic breast imaging technologies varies. Diagnostic mammography, for example, has generally been used without controversy in patients with suspicious breast lesions because several studies have shown it has fairly high sensitivity and specificity, ranging from 78% to 93% and from 87% to 98%, respectively[40,105–109], and because its use as a screening tool has been shown to reduce breast cancer mortality[110]. Similarly, use of ultrasonography for diagnostic breast imaging is generally not controversial because it is not invasive, does not entail exposure to radiation, and evidence indicates that its use is advantageous compared with other imaging modalities in some situations such as differentiating benign cysts from solid masses[57] or examining dense breast tissue in younger women[58]. On the other hand, evidence for long-term benefits stemming from use of MRI, CT, and PET or PET/CT in the peridiagnostic period is lacking (although some evidence has shown that use of MRI for screening high-risk women leads to reduced diagnoses of advanced-stage breast cancer compared with women screened conventionally)[111]. While diagnostic MRI has great potential to detect otherwise occult cancers, whether these findings actually lead to reductions in re-excision, recurrence, and mortality rates has not been shown[17,18,112,113]. Furthermore, the high rate of false-positive MRI scans may lead to harmful consequences such as greater rates of (sometimes unnecessary) mastectomies[6–9] and delays in beginning treatment[10,11]. Similarly, a review published by the Agency for Healthcare Research and Quality (AHRQ) in 2006 concluded that the negative predictive value of PET, at 64.8%, was too low to indicate routine use for ruling out breast cancer after detection of abnormalities[114]. Similarly, the 2011 NCCN guidelines cited evidence that PET scanning has a high false-negative rate for detection of lesions less than 1 cm, low sensitivity for detection of metastases, and a high rate of false-positive scans, and recommended that PET or PET/CT not be used for staging breast cancer in most cases[39]. CT has not been widely used for breast imaging, likely because small studies indicated that it was not as sensitive and specific compared with other modalities[115–118] and could entail substantially greater radiation exposure[83].
Although regulatory and reimbursement structures in the United States are generally distinct from other countries, advanced diagnostic breast imaging technologies are commonly used internationally, as shown by recent publications from international research centers investigating the usefulness of diagnostic breast advanced imaging[18,119]. One of these, a recent randomized controlled trial conducted at 45 clinical centers in the United Kingdom, showed no difference in re-excision rates in women randomized to receive MRI versus women assigned to receive usual care (clinical, radiologic (mammogram and ultrasound scan), and pathologic assessment)[18]. The other, a randomized controlled trial conducted in The Netherlands, found an increase in re-excision rates in women who were randomized to receive pre-operative MRI compared with women who received routine medical care[119]. Although utilization rates of advanced breast imaging technologies in non-US health systems are likely driven by different factors than those in the United States, research on the most appropriate use of these modalities is lacking and investigations on these evidence gaps would be useful worldwide.
Although randomized controlled trials are considered the gold standard in identifying best medical practices, these are impractical for determining the best uses of advanced imaging, including imaging for staging and work-up of breast cancer, for several reasons. Imaging technologies are constantly changing so by the time clinical trials are completed, technologies are often obsolete and the results are no longer applicable to clinical care[120]. Also, although no long-term studies have shown improvements in breast cancer recurrence or survival rates as a result of receipt of advanced imaging, many clinicians and patients believe that the added information derived from imaging must lead to better care[121], so withholding imaging from breast cancer patients could be viewed as unethical. The situation is also complicated by the array of tests and treatments available to breast cancer patients and differentiating the role on outcomes that imaging has compared with other variables is difficult[122].
Because many of these imaging technologies are widely used with little evidence that they affect long-term outcomes in breast cancer patients, CER is as an ideal method to investigate whether advanced imaging technologies improve patient outcomes and/or decrease costs in real-world settings. Unlike randomized controlled trials that restrict variation as much as possible, CER studies examine as many alternative diagnostic tests and therapies as the data allow and often have much larger numbers of subjects to study because strict inclusion and exclusion criteria are not factors. The wide variety of patients that can be studied allows CER results to be generalizable to entire communities rather than specific patient populations. Two key imaging societies for breast imaging, the American College of Radiology Imaging Network (ACRIN) and the Society for Nuclear Medicine (SNM), have embraced CER[123,124]. Although CER was not used to evaluate these breast imaging technologies prior to FDA approval, recent publications have reported use of this research to examine diagnostic breast imaging[18,125] and CER is a vital component in determining international guidelines[126]. An integral part of CER is involvement of stakeholders in the identification and prioritization of research topics to ensure that the research studies are relevant[127]. For CER pertaining to diagnostic breast imaging, the key stakeholders from the community would likely be payers, breast oncologists and other health care providers, and breast cancer patients or survivors[127].
Historically, breast imaging technologies have followed varying paths from appearing in the peer-reviewed literature to being recommended for use in clinical guidelines. Some, such as ultrasonography and mammography, were discovered decades before their use was appreciated. Others, such as MRI, were reported in the literature, approved by the FDA, reimbursed by CMS, and mentioned as optional imaging modalities in clinical guidelines in a fairly short period. This article demonstrates that some of these breast imaging technologies reached widespread use with very little data that they improved breast cancer outcomes, such as reducing recurrence rates or increasing survival time, or decreased costs. By tracing the dates of discovery, FDA clearance or approval, reimbursement, and adoption into clinical guidelines of each diagnostic imaging technology, we show that these milestones were often achieved without evidence of positive effects on long-term outcomes in breast cancer patients. The fact that these historic milestones occurred with little evidence that they improved long-term outcomes demonstrates that CER is much needed for diagnostic breast cancer imaging research. In addition, CER is an important tool to study the many emerging diagnostic imaging technologies that have not yet come into widespread use so that modalities that improve long-term outcomes can be favored over those that do not. In order to ensure that only the most effective and cost-efficient imaging technologies reach or remain in clinical practice, CER will play a critical role in evaluation of future diagnostic technologies.
Acknowledgments
This work was supported by the ADVICE grant (1RC2CA148433-01) from the National Cancer Institute. We would like to thank Dr David Mankoff for providing valuable comments about this paper.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Esserman L. Integration of imaging in the management of breast cancer. J Clin Oncol. 2005;23:1601–2. doi: 10.1200/JCO.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 4.Hollingsworth AB, Stough RG, O'Dell CA, Brekke CE. Breast magnetic resonance imaging for preoperative locoregional staging. Am J Surg. 2008;196:389–97. doi: 10.1016/j.amjsurg.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Lehman CD, DeMartini W, Anderson BO, Edge SB. Indications for breast MRI in the patient with newly diagnosed breast cancer. J Natl Compr Canc Netw. 2009;7:193–201. doi: 10.6004/jnccn.2009.0013. [DOI] [PubMed] [Google Scholar]
- 6.Pettit K, Swatske M, Gao F, et al. The impact of breast MRI on surgical decision-making: are patients at risk for mastectomy? J Surg Oncol. 2009;100:553–8. doi: 10.1002/jso.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katipamula R, Degnim A, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. 2009;27:4082–8. doi: 10.1200/JCO.2008.19.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol. 2008;26:3248–58. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 9.Berg W, Gutierrez L, NessAiver M, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 10.Morrow M, Harris JR. More mastectomies: is this what patients really want? J Clin Oncol. 2009;27:4038–40. doi: 10.1200/JCO.2009.23.0078. [DOI] [PubMed] [Google Scholar]
- 11.Bleicher R, Ciocca R, Egleston B, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180–7. doi: 10.1016/j.jamcollsurg.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith-Bindman R, Miglioretti DL, Larson EB. Rising use of diagnostic medical imaging in a large integrated health system. Health Aff (Millwood) 2008;27:1491–502. doi: 10.1377/hlthaff.27.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinan M, Curtis L, Hammill B, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303:1625–31. doi: 10.1001/jama.2010.460. [DOI] [PubMed] [Google Scholar]
- 14.Sistrom C, Dang P, Weilburg J, Dreyer K, Rosenthal D, Thrall J. Effect of computerized order entry with integrated decision support on the growth of outpatient procedure volumes: seven-year time series analysis. Radiology. 2009;251:147–55. doi: 10.1148/radiol.2511081174. [DOI] [PubMed] [Google Scholar]
- 15.Levin D, Rao V, Parker L. Physician orders contribute to high-tech imaging slowdown. Health Aff (Millwood) 2010;29:189–95. doi: 10.1377/hlthaff.2009.0528. [DOI] [PubMed] [Google Scholar]
- 16.US Government Accountability Office. Medicare Part B: rapid spending growth and shift to physician offices indicate need for CMS to consider additional management practices. http://www.gao.gov/products/GAO-08-452. Accessed June 22, 2011.
- 17.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin. 2009;59:290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 18.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563–71. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. PMA premarket approval database. 2010. [Google Scholar]
- 20.US Food and Drug Administration (FDA) 510(k) premarket notification. May 5, 2010. [Google Scholar]
- 21.Blue Cross and Blue Shield Association Technology Evaluation Center (TEC), Evidence-based Practice Center. http://www.ahrq.gov/clinic/epc/bcbsatec.htm. Accessed June 21, 2011.
- 22.Aetna Inc. Aetna Medical clinical policy bulletins. http://www.aetna.com/healthcare-professionals/policies-guidelines/medical_clinical_policy_bulletins.html. Accessed June 21, 2011.
- 23.Group Health Clinical Review. https://provider.ghc.org/open/referralsAndClinicalReview/criteria/index.jhtml. Accessed June 21, 2011.
- 24.Wood EH. The National Comprehensive Cancer Network (NCCN) J Med Libr Assoc. 2004;92:382–3. [Google Scholar]
- 25.National Comprehensive Cancer Network. NCCN breast cancer practice guidelines. 1996. [Google Scholar]
- 26.National Comprehensive Cancer Network. Update of the NCCN guidelines for treatment of breast cancer. 1997. [Google Scholar]
- 27.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 1999. [DOI] [PubMed] [Google Scholar]
- 28.National Comprehensive Cancer Network. Breast cancer screening and diagnosis v. 1. National Comprehensive Cancer Network; 2000. [DOI] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2001. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2002. [DOI] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2003. [DOI] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2004. [PubMed] [Google Scholar]
- 33.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2005. [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2006. [DOI] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network. Breast cancer v. 1. National Comprehensive Cancer Network; 2007. [DOI] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. Breast cancer screening and diagnosis v. 1. National Comprehensive Cancer Network; 2008. [DOI] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network. Breast cancer screening and diagnosis v. 1. National Comprehensive Cancer Network; 2009. [DOI] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network. Breast cancer screening and diagnosis v. 1. NCCN clinical practice guidelines in oncology; 2010. http://www.nccn.org. Accessed January 20, 2010. [DOI] [PubMed] [Google Scholar]
- 39.National Comprehensive Cancer Network. Breast cancer. March 25, 2011. [Google Scholar]
- 40.Barlow WE, Lehman CD, Zheng Y, et al. Performance of diagnostic mammography for women with signs or symptoms of breast cancer. J Natl Cancer Inst. 2002;94:1151–9. doi: 10.1093/jnci/94.15.1151. [DOI] [PubMed] [Google Scholar]
- 41.Gold RH, Bassett LW, Widoff BE. Highlights from the history of mammography. Radiographics. 1990;10:1111–31. doi: 10.1148/radiographics.10.6.2259767. [DOI] [PubMed] [Google Scholar]
- 42.Egan RL. Experience with mammography in a tumor institution. Evaluation of 1,000 studies. Radiology. 1960;75:894–900. doi: 10.1148/75.6.894. [DOI] [PubMed] [Google Scholar]
- 43.Strax P, Venet L, Shapiro S. Value of mammography in reduction of mortality from breast cancer in mass screening. Am J Roentgenol Radium Ther Nucl Med. 1973;117:686–9. doi: 10.2214/ajr.117.3.686. [DOI] [PubMed] [Google Scholar]
- 44.Williams MB, Fajardo LL. Digital mammography: performance considerations and current detector designs. Acad Radiol. 1996;3:429–37. doi: 10.1016/S1076-6332(05)80680-4. [DOI] [PubMed] [Google Scholar]
- 45.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 46.Chan HP, Vyborny CJ, MacMahon H, Metz CE, Doi K, Sickles EA. Digital mammography. ROC studies of the effects of pixel size and unsharp-mask filtering on the detection of subtle microcalcifications. Invest Radiol. 1987;22:581–9. doi: 10.1097/00004424-198707000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Feig SA, Yaffe MJ. Digital mammography. Radiographics. 1998;18:893–901. doi: 10.1148/radiographics.18.4.9672974. [DOI] [PubMed] [Google Scholar]
- 48.Fenton JJ, Foote SB, Green P, Baldwin LM. Diffusion of computer-aided mammography after mandated Medicare coverage. Arch Intern Med. 2010;170:987–9. doi: 10.1001/archinternmed.2010.104. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Medicare and Medicaid Services (CMS) National Coverage Decision for Mammograms (220.4) 1978. [PubMed] [Google Scholar]
- 50.Centers for Medicare and Medicaid Services (CMS) Medicare, Medicaid, and SCHIP Benefits Improvement and Protection Act of 2000. Ruling No. 01–01: http://www.cms.gov/Rulings/downloads/CMSR0101.pdf. Accessed June 21, 2011.
- 51.Blue Cross Blue Shield Association. Computer-aided detection with full-field digital mammography. 2006. [PubMed] [Google Scholar]
- 52.Aetna Inc. Clinical policy bulletin: mammography. Clinical Policy Bulletins; http://www.aetna.com/cpb/medical/data/500_599/0584.html. Accessed June 22, 2011. [Google Scholar]
- 53.Howry DH, Stott DA, Bliss WR. The ultrasonic visualization of carcinoma of the breast and other soft-tissue structures. Cancer. 1954;7:354–8. doi: 10.1002/1097-0142(195403)7:2<354::AID-CNCR2820070220>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Wild J, Neal D. Use of high-frequency ultrasonic waves for detecting changes of texture in living tissues. Lancet. 1951;1:655–7. doi: 10.1016/s0140-6736(51)92403-8. [DOI] [PubMed] [Google Scholar]
- 55.Bassett L, Kimme-Smith C. Breast sonography. AJR Am J Roentgenol. 1991;156:449–55. doi: 10.2214/ajr.156.3.1899737. [DOI] [PubMed] [Google Scholar]
- 56.Nothacker M, Duda V, Hahn M, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335. doi: 10.1186/1471-2407-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kerlikowske K, Smith-Bindman R, Ljung BM, Grady D. Evaluation of abnormal mammography results and palpable breast abnormalities. Ann Intern Med. 2003;139:274–84. doi: 10.7326/0003-4819-139-4-200308190-00010. [DOI] [PubMed] [Google Scholar]
- 58.Mehta TS. Current uses of ultrasound in the evaluation of the breast. Radiol Clin North Am. 2003;41:841–56. doi: 10.1016/S0033-8389(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 59.Krishnamurthy S. Current applications and future prospects of fine-needle aspiration biopsy of locoregional lymph nodes in the management of breast cancer. Cancer Cytopathol. 2009;117:451–62. doi: 10.1002/cncy.20055. [DOI] [PubMed] [Google Scholar]
- 60.Gunther-Tritsch K, Ohlinger R, Bojahr B. Diagnostic value of palpation and ultrasonography for diagnosing breast cancer recurrence after mastectomy–a comparison. Ultraschall Med. 2009;30:577–584. doi: 10.1055/s-0028-1109701. [DOI] [PubMed] [Google Scholar]
- 61.Rissanen TJ, Makarainen HP, Mattila SI, Lindholm EL, Heikkinen MI, Kiviniemi HO. Breast cancer recurrence after mastectomy: diagnosis with mammography and US. Radiology. 1993;188:463–7. doi: 10.1148/radiology.188.2.8327698. [DOI] [PubMed] [Google Scholar]
- 62.Yilmaz MH, Esen G, Ayarcan Y, et al. The role of US and MR imaging in detecting local chest wall tumor recurrence after mastectomy. Diagn Interv Radiol. 2007;13:13–18. [PubMed] [Google Scholar]
- 63.Riebe E, Gunther K, Schulz K, et al. Recurrent disease after breast preserving therapy (BPT) and radiation therapy for breast cancer–diagnostic yield of palpation, mammography and ultrasonography. Ultraschall Med. 2007;28:394–400. doi: 10.1055/s-2007-963019. [DOI] [PubMed] [Google Scholar]
- 64.Balu-Maestro C, Bruneton JN, Geoffray A, Chauvel C, Rogopoulos A, Bittman O. Ultrasonographic posttreatment follow-up of breast cancer patients. J Ultrasound Med. 1991;10:1–7. doi: 10.7863/jum.1991.10.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Phillips RA. Email correspondence March 17, 2010. Email correspondence regarding FDA approvals and clearances for diagnostic devices. US Food and Drug Administration (FDA); 2010. [Google Scholar]
- 66.Centers for Medicare and Medicaid Services (CMS) National coverage determination (NCD) for ultrasound diagnostic procedures (220.5) Centers for Medicare and Medicaid Services (CMS); 1966. pp. 100–13. [PubMed] [Google Scholar]
- 67.Damadian R, Zaner K, Hor D, DiMaio T, Minkoff L, Goldsmith M. Nuclear magnetic resonance as a new tool in cancer research: human tumors by NMR. Ann N Y Acad Sci. 1973;222:1048–76. doi: 10.1111/j.1749-6632.1973.tb15323.x. [DOI] [PubMed] [Google Scholar]
- 68.Heywang S, Hahn D, Schmidt H, et al. MR imaging of the breast using gadolinium-DTPA. J Comput Assist Tomogr. 1986;10:199–204. doi: 10.1097/00004728-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Stelling CB, Wang PC, Lieber A, Mattingly SS, Griffen WO, Powell DE. Prototype coil for magnetic resonance imaging of the female breast. Work in progress. Radiology. 1985;154:457–62. doi: 10.1148/radiology.154.2.3966132. [DOI] [PubMed] [Google Scholar]
- 70.Partridge SC. Future applications and innovations of clinical breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:171–6. doi: 10.1097/RMR.0b013e31818a4090. [DOI] [PubMed] [Google Scholar]
- 71.Sijens PE, Wijrdeman HK, Moerland MA, Bakker CJ, Vermeulen JW, Luyten PR. Human breast cancer in vivo: H-1 and P-31 MR spectroscopy at 1.5 T. Radiology. 1988;169:615–20. doi: 10.1148/radiology.169.3.2847230. [DOI] [PubMed] [Google Scholar]
- 72.US Food and Drug Administration (FDA) Questions and answers on gadolinium-based contrast agents. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142889.htm. Accessed June 23, 2011.
- 73.US Food and Drug Administration (FDA) 510(k) summary for the AURORA magnetic resonance diagnostic device. Rockville, MD: FDA; 2008. K073425. [Google Scholar]
- 74.Centers for Medicare and Medicaid Services (CMS) Medicare National Coverage Determinations Manual. Chapter 1, Part 4: CMS; 2010. [Google Scholar]
- 75.Aetna Inc. Clinical policy bulletin: magnetic resonance imaging (MRI) of the breast. Clinical policy bulletins; http://www.aetna.com/cpb/medical/data/100_199/0105.html. Accessed June 23, 2011. [Google Scholar]
- 76.Center for Medicare and Medicaid Services (CMS) National coverage determination (NCD) for magnetic resonance spectroscopy (220.2.1) http://www.cms.gov/medicare-coverage-database/details/ncddetails.aspx?NCDId=287&ncdver=1&DocID=220.2.1&SearchType=Advanced&bc=IAAAABAAAAAA&. Accessed June 23, 2011. [PubMed]
- 77.Aetna Inc. Clinical policy bulletin: magnetic resonance spectroscopy (MRS) Clinical Policy Bulletins; http://www.aetna.com/cpb/medical/data/200_299/0202.html. Accessed June 23, 2011. [Google Scholar]
- 78.Kuhl DE, Edwards RQ. Image separation radioisotope scanning. Radiology. 1963;80:653–62. [Google Scholar]
- 79.Reese D, Carney J, Gisvold J, Karsell P, Kollins S. Computerized reconstructive tomography applied to breast pathology. AJR Am J Roentgenol. 1976;126:406–12. doi: 10.2214/ajr.126.2.406. [DOI] [PubMed] [Google Scholar]
- 80.Chang C, Sibala J, Fritz S, Gallagher J, Dwyer, Templeton A. Computed tomographic evaluation of the breast. AJR Am J Roentgenol. 1978;131:459–64. doi: 10.2214/ajr.131.3.459. [DOI] [PubMed] [Google Scholar]
- 81.Glick S. Breast CT. Annu Rev Biomed Eng. 2007;9:501–26. doi: 10.1146/annurev.bioeng.9.060906.151924. [DOI] [PubMed] [Google Scholar]
- 82.Davidson C, Erdogan A. Contrast media: procedural capacities and potential risks. Rev Cardiovasc Med. 2008;9(Suppl 1):S24–34. [PubMed] [Google Scholar]
- 83.Sechopoulos I, Vedantham S, Suryanarayanan S, D'Orsi C, Karellas A. Monte Carlo and phantom study of the radiation dose to the body from dedicated CT of the breast. Radiology. 2008;247:98–105. doi: 10.1148/radiol.2471071080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindfors K, Boone J, Nelson T, Yang K, Kwan A, Miller D. Dedicated breast CT: initial clinical experience. Radiology. 2008;246:725–33. doi: 10.1148/radiol.2463070410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centers for Medicare and Medicaid Services (CMS) National Coverage Determination (NCD) for Computed Tomography (220.1) Health and Human Services; 2008. [PubMed] [Google Scholar]
- 86.Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess. 2007;11:iii–iv. doi: 10.3310/hta11440. xi-267. [DOI] [PubMed] [Google Scholar]
- 87.Almubarak M, Osman S, Marano G, Abraham J. Role of positron-emission tomography scan in the diagnosis and management of breast cancer. Oncology. 2009;23:255–61. [PubMed] [Google Scholar]
- 88.Podoloff D, Advani R, Allred C, et al. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007;5(Suppl 1):S1–22. quiz S23–22. [PubMed] [Google Scholar]
- 89.Wahl R, Siegel B, Coleman R, Gatsonis C. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22:277–85. doi: 10.1200/JCO.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 90.Nutt R. 1999 ICP Distinguished Scientist Award. The history of positron emission tomography. Mol Imaging Biol. 2002;4:11–26. doi: 10.1016/s1095-0397(00)00051-0. [DOI] [PubMed] [Google Scholar]
- 91.US Oncology, National Electrical Manufacturers Association (NEMA) Medical imaging in cancer care: charting the progress. Arlington, VA: Policy Analysis and Public Affairs; 2006. [Google Scholar]
- 92.Lakhani P, Maidment A, Weinstein S, Kung J, Alavi A. Correlation between quantified breast densities from digital mammography and 18F-FDG PET uptake. Breast J. 2009;15:339–47. doi: 10.1111/j.1524-4741.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 93.Beaney R, Lammertsma A, Jones T, McKenzie C, Halnan K. Positron emission tomography for in-vivo measurement of regional blood flow, oxygen utilisation, and blood volume in patients with breast carcinoma. Lancet. 1984;1:131–4. doi: 10.1016/s0140-6736(84)90063-1. [DOI] [PubMed] [Google Scholar]
- 94.Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991;179:765–70. doi: 10.1148/radiology.179.3.2027989. [DOI] [PubMed] [Google Scholar]
- 95.Townsend DW, Carney JP, Yap JT, Hall NC. PET/CT today and tomorrow. J Nucl Med. 2004;45 (Suppl 1):4S–14S. [PubMed] [Google Scholar]
- 96.Osman MM, Cohade C, Nakamoto Y, Marshall LT, Leal JP, Wahl RL. Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. J Nucl Med. 2003;44:240–3. [PubMed] [Google Scholar]
- 97.US Food and Drug Administration (FDA) Positron emission tomography drug products; safety and effectiveness of certain PET drugs for specific indications. http://www.fda.gov/ohrms/dockets/98fr/031000a.txt. Accessed June 28, 2011.
- 98.US Food and Drug Administration (FDA) Center for Drug Evaluation and Research Application Number NDA 20306. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/20306_FLUDEOXYGLUCOSE_APPROV.PDF. Accessed June 28, 2011. [Google Scholar]
- 99.US Food and Drug Administration (FDA) 510(k) summary of safety and effectiveness: POSITRACE combined PET/CT imaging system. August 25, 2010. US FDA; 2000. [Google Scholar]
- 100.Center for Medicare and Medicaid Services (CMS) National coverage determination (NCD) for PET (FDG) for breast cancer (220.6.10) https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=297&ncdver=1&NCAId=71&NcaName=Positron+Emission+Tomography+%28FDG%29+for+Breast+Cancer&IsPopup=y&bc=AAAAAAAABAAA&. Accessed June 28, 2011. [PubMed] [Google Scholar]
- 101. Centers for Medicare and Medicaid Services (CMS). CMS manual system pub 100-03 Medicare National Coverage Determinations: Department of Health and Human Services; 2009.
- 102.Aetna Inc. Clinical policy bulletin: positron emission tomography (PET) Clinical Policy Bulletins; http://www.aetna.com/cpb/medical/data/1_99/0071.html. Accessed June 28, 2011. [Google Scholar]
- 103.Baker LC, Atlas SW, Afendulis CC. Expanded use of imaging technology and the challenge of measuring value. Health Aff (Millwood) 2008;27:1467–78. doi: 10.1377/hlthaff.27.6.1467. [DOI] [PubMed] [Google Scholar]
- 104.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–61. doi: 10.1200/JCO.2007.14.5631. [DOI] [PubMed] [Google Scholar]
- 105.Duijm LE, Guit GL, Zaat JO, Koomen AR, Willebrand D. Sensitivity, specificity and predictive values of breast imaging in the detection of cancer. Br J Cancer. 1997;76:377–81. doi: 10.1038/bjc.1997.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eltahir A, Jibril JA, Squair J, et al. The accuracy of “one-stop” diagnosis for 1,110 patients presenting to a symptomatic breast clinic. J R Coll Surg Edinb. 1999;44:226–30. [PubMed] [Google Scholar]
- 107.Flobbe K, van der Linden ES, Kessels AG, van Engelshoven JM. Diagnostic value of radiological breast imaging in a non-screening population. Int J Cancer. 2001;92:616–18. doi: 10.1002/ijc.1235. [DOI] [PubMed] [Google Scholar]
- 108.Moskowitz M. Breast Imaging. In: Donegan WL, Spratt JS, editors. Cancer of the breast. 4th. Philadelphia, PA: WB Saunders; 1995. pp. 206–239. [Google Scholar]
- 109.Poplack SP, Tosteson AN, Grove MR, Wells WA, Carney PA. Mammography in 53,803 women from the New Hampshire mammography network. Radiology. 2000;217:832–40. doi: 10.1148/radiology.217.3.r00dc33832. [DOI] [PubMed] [Google Scholar]
- 110.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727–37, W237–742. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29:1664–9. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Solin L, Orel S, Hwang W, Harris E, Schnall M. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008;26:386–91. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 113.Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007;16(Suppl 2):S34–44. doi: 10.1016/j.breast.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 114.Bruening W, Launders J, Pinkney N, Kostinsky H, Schoelles K, Turkelson C. Effectiveness of noninvasive diagnostic tests for breast abnormalities. Rockville, MD: Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 115.Uematsu T, Sano M, Homma K, Shiina M, Kobayashi S. Three-dimensional helical CT of the breast: accuracy for measuring extent of breast cancer candidates for breast conserving surgery. Breast Cancer Res Treat. 2001;65:249–57. doi: 10.1023/A:1010641223012. [DOI] [PubMed] [Google Scholar]
- 116.Uematsu T, Sano M, Homma K, Sato N. Comparison between high-resolution helical CT and pathology in breast examination. Acta Radiol. 2002;43:385–90. doi: 10.1034/j.1600-0455.2002.430408.x. [DOI] [PubMed] [Google Scholar]
- 117.Sardanelli F, Calabrese M, Zandrino F, et al. Dynamic helical CT of breast tumors. J Comput Assist Tomogr. 1998;22:398–407. doi: 10.1097/00004728-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto A, Fukushima H, Okamura R, et al. Dynamic helical CT mammography of breast cancer. Radiat Med. 2006;24:35–40. doi: 10.1007/BF02489987. [DOI] [PubMed] [Google Scholar]
- 119.Peters NH, van Esser S, van den Bosch MA, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. Eur J Cancer. 2011;47:879–86. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 120.Pearson SD, Knudsen AB, Scherer RW, Weissberg J, Gazelle GS. Assessing the comparative effectiveness of a diagnostic technology: CT colonography. Health Aff (Millwood) 2008;27:1503–14. doi: 10.1377/hlthaff.27.6.1503. [DOI] [PubMed] [Google Scholar]
- 121.McCaffery KJ, Jansen J. Pre-operative MRI for women with newly diagnosed breast cancer: perspectives on clinician and patient decision-making when evidence is uncertain. Breast. 2010;19:10–12. doi: 10.1016/j.breast.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 122.Pandharipande PV, Gazelle GS. Comparative effectiveness research: what it means for radiology. Radiology. 2009;253:600–5. doi: 10.1148/radiol.2533091286. [DOI] [PubMed] [Google Scholar]
- 123.American College of Radiology. ACR Clinical Research Center Annual Report 2009. http://www.acrin.org/Portals/0/Administrative/ACR-CRC-2010AnnualReport.pdf. Accessed August 3, 2011. [Google Scholar]
- 124.Society of Nuclear Medicine. SNM receives grant from AHRQ for comparative effectiveness research. http://www.snm.org/index.cfm?PageID=9645. Accessed August 3, 2011.
- 125.Berg WA, Madsen KS, Schilling K, et al. Breast cancer: comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology. 2011;258:59–72. doi: 10.1148/radiol.10100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sorenson C, Drummond M, Kanavos P, McGuire A. The National Institute for Health and Clinical Excellence (NICE): How does it work and what are the implications for the US? 2008. http://www.npcnow.org/App_Themes/Public/pdf/Issues/pub_ebm/NICE%20Full%20Report%20Final%206-23-08.pdf Accessed January 3, 2012.
- 127.Whitlock EP, Lopez SA, Chang S, Helfand M, Eder M, Floyd N. AHRQ series paper 3: identifying, selecting, and refining topics for comparative effectiveness systematic reviews: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63:491–501. doi: 10.1016/j.jclinepi.2009.03.008. [DOI] [PubMed] [Google Scholar]