Abstract
The purpose of this study was to systemically review the available literature regarding the diagnostic performance of positron emission tomography (PET) using 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) in patients with thymic epithelial tumors. We reviewed 13 studies that evaluated the diagnostic role of thymic epithelial tumors with [18F]FDG-PET. [18F]FDG-PET is a useful radiological modality for differentiating between thymomas and thymic carcinoma. However, [18F]FDG-PET may not be useful for differentiating low-risk thymoma and high-risk thymoma. One paper reported that [18F]FDG-PET has a predictive significance for treatment and prognosis in thymic epithelial tumors. Two papers reported that the degree of [18F]FDG uptake in thymic epithelial tumors is based on glucose metabolism. [18F]FDG-PET may have a further use for radiological differential diagnosis of thymomas and thymic carcinomas.
Keywords: Thymic epithelial tumor, [18F]FDG-PET, systemic review, differential diagnosis
Introduction
Thymic epithelial tumors are broadly classified into thymoma and thymic carcinoma and are the most common primary neoplasms of the anterior mediastinum. Tumors of the thymus are a heterogeneous group of tumors, ranging from relatively benign thymomas to highly aggressive carcinomas. The World Health Organization (WHO) published a new histologic classification of thymic epithelial tumors, dividing them into 3 subgroups: low-risk thymomas (types A, AB and B1), high-risk thymomas (types B2 and B3) and thymic carcinomas[1,2]. Several studies have documented that positron emission tomography (PET) using 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) is increasingly important for the imaging technique in the diagnosis, grading malignancy, staging and assessment of response to therapy in patients with thymic epithelial tumors[3–16]. According to these reports, [18F]FDG-PET is effective in differentiating thymic carcinoma from other entities within the thymus. However, these published reports consisted of clinical trials with small sample size, and we cannot conclude on the diagnostic performance of [18F]FDG-PET in thymic epithelial tumors from these results.
The purpose of this study is to systematically review the available literature regarding the diagnostic performance of [18F]FDG-PET in patients with thymic epithelial tumors, which may contribute to the development of guidelines for the usefulness of PET.
Materials and methods
Search strategy
We addressed the performance of [18F]FDG-PET as a diagnostic test for differentiating thymoma from thymic carcinoma and for the grade of malignancy in thymic epithelial tumors. We performed a systematic search of the MEDLINE and PubMed databases to identify all clinical trials regarding the relationship between [18F]FDG-PET and thymic epithelial tumors. The search strategy included articles published between January 1995 and August 2011 using the following keywords: “PET” or “positron emission tomography”; “positron emission tomography/computer tomography” or “PET/CT”; “[18F]FDG” or “fluorodeoxyglucose”; “thymic epithelial tumor”, “thymoma”, “thymic carcinoma” or “thymic”. The search did not restrict the type of publication or periodical. We did not include preliminary results published as abstracts or meeting proceedings. We selected all published reports that clearly described the diagnostic performance of [18F]FDG-PET in patients with thymic epithelial tumors. The search was restricted to material published in English.
Study selection
The inclusion criteria were as follows: [18F]FDG-PET was used to identify or characterize thymic epithelial tumors; [18F]FDG was used as tracer; scanner apparatus was [18F]FDG-PET for use on humans; sample size with at least 10 participants in each study. Criteria for exclusion were insufficient information to construct 2×2 contingency tables, and duplicate studies on the same patients. Two reviewers independently selected studies for possible inclusion by checking titles and abstracts. The final decision regarding inclusion was based on the full article. Disagreement was resolved in a consensus meeting.
Results
Characteristics of the published reports
Based on our research criteria, we identified 13 studies that evaluated the diagnostic role of thymic epithelial tumors with [18F]FDG-PET[3–15]. The characteristics of the studies are presented in Table 1. The total number of patients in a study ranged from 10 to 49 (median, 18 patients). Reported age ranged from 19 to 85 years, and the population of male patients ranged from 24% to 70%. Most studies comprised both thymoma (n = 231) and thymic carcinoma (n = 86). Mean tumor size range from 47 to 79 mm. Four studies were analyzed according to the Masaoka classification (non-invasive thymoma, invasive thymoma and thymic carcinoma), and 9 studies used a simplified WHO classification (low-risk thymoma, high-risk thymoma and thymic carcinoma). In 10 of 13 studies, measurement of [18F]FDG uptake was performed by maximal standardized uptake value (SUVmax).
Table 1.
Characteristics of the 13 studies included
| Study | Year | No. of patients | Sex (male/ female) | Mean age, years (range) | Histology (thymoma/ thymic carcinoma) | Mean tumor size (mm) | [18F]FDG dose (MBq) | Measurement of [18F]FDG uptake | Analysis according to Masaoka or WHO classification |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al.[3] | 1995 | 10 | 6/4 | 47 (30–66) | 10/0 | (–) | 370 | TLR | Non-invasive thymoma, and invasive thymoma |
| Kubota et al.[4] | 1996 | 10 | 7/3 | 62 (35–83) | 7/3 | (–) | 180 | DUR | Non-invasive thymoma, invasive thymoma, and thymic carcinoma |
| Sasaki et al.[5] | 1999 | 31 | 19/12 | 58 (19–85) | 17/14 | 68 | 226 | SUV | Non-invasive thymoma, invasive thymoma, and thymic carcinoma |
| Sung et al.[6] | 2006 | 33 | 15/18 | 55 (34–68) | 17/16 | 54 | 370 | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| El-Bawab et al.[7] | 2007 | 17 | 4/13 | 40 (25–72) | 14/3 | (–) | 370 | SUV | Non-invasive thymoma, invasive thymoma, and thymic carcinoma |
| Inoue A et al.[11] | 2009 | 46 | 29/17 | 58 (31–75) | 35/11 | (–) | 370 | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Luzzi et al.[10] | 2009 | 13 | (–) | (–) | 7/6 | 59 | 355 | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Kumar et al.[8] | 2009 | 18 | 14/4 | 38 (19–58) | 14/5 | 62 | 370 | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Shibata et al.[9] | 2009 | 39 | 16/23 | 56 (28–77) | 36/3 | 52 | 4.6 (MBq/kg) | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Kaira et al.[12] | 2010 | 49 | 23/26 | 64 (32–80) | 38/11 | 60 | 200–250 | T/M ratio | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Nakajo et al.[13] | 2010 | 11 | 5/6 | 55 (41–71) | 10/1 | 58 | 3.7 (MBq/kg) | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Igai et al.[14] | 2010 | 13 | 6/7 | 59 (36–78) | 8/5 | 47 | 3.5 (MBq/kg) | SUV | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
| Terzi et al.[15] | 2011 | 26 | 14/12 | 56 (34–85) | 18/8 | 79 | 330–400 | SUV and T/M ratio | Low-risk thymoma, high-risk thymoma, and thymic carcinoma |
DUR, differential uptake ratio, radioactivity concentration in the region of interest (Bq/mm3)/injected dose (Bq)/weight of patients (g); SUV, standardized uptake value; TLR, tumor to lung ratio; T/M ratio is the ratio of the peak SUV of the tumor to the mean SUV of the mediastinum.
Diagnostic comparison of [18F]FDG uptake
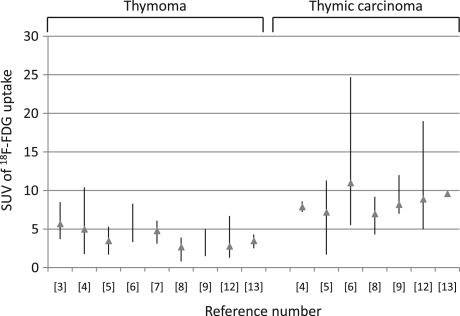
[18F]FDG accumulation according to the Masaoka and WHO classifications is presented in Tables 2 and 3, respectively. Seven of 13 studies compared the measurement of [18F]FDG uptake between thymoma and thymic carcinoma[5,6,8,9,12,14,15], and these 7 studies demonstrated that [18F]FDG uptake in thymic carcinoma was significantly higher than in thymoma. [18F]FDG-PET images are shown in Fig. 1[16]. In the analysis of the 8 studies according to the WHO classification (Table 3), the median values for the mean SUV in low-risk thymoma, high-risk thymoma and thymic carcinoma were 3.2 (range, 2.6–4.0), 5.0 (range, 2.1–14.1) and 9.2 (range, 7.0–17.1), respectively. Seven of these 9 studies showed a statistically significant higher [18F]FDG uptake in high-risk thymoma or thymic carcinoma than in low-risk thymoma[6,8–13,15], and 4 studies revealed that [18F]FDG uptake was significantly higher in thymic carcinoma than in high-risk thymoma[6,8,9,12]. Although 2 studies described the usefulness of [18F]FDG-PET for differentiating between low-risk thymoma and high-risk thymoma[12,15], 2 studies reported that no statistically significant difference was found among these groups[6,8]. Fig. 2 shows the distribution of [18F]FDG uptake on PET in patients with thymic epithelial tumors. Three of 13 studies investigated the relationship between [18F]FDG uptake and the grading of the WHO classification and between [18F]FDG uptake and the grading of the Masaoka classification, demonstrating a statistically significant correlation[10,12,15].
Table 2.
Comparison of [18F]FDG uptake according to the Masaoka classification
| Study | Thymoma vs thymic carcinoma |
Statistical analysis | ||
|---|---|---|---|---|
| Non-invasive thymoma | Invasive thymoma | Thymic carcinoma | ||
| Liu et al.[3] | 5.7 ± 1.7 | NA | ||
| Kubota et al.[4] | 2.30 ± 0.40 | 8.62 ± 2.31* | 7.85 ± 0.69 | *P < 0.005 compared with non-invasive thymoma. Not compared with thymic carcinoma |
| Sasaki et al.[5] | 3.0 ± 1.0 | 3.8± 1.3 | 7.2 ± 2.9* | *P < 0.01 compared with non-invasive thymoma and invasive thymoma |
| El-Bawab et al.[7] | 4.75 ± 0.88 | NA | ||
NA, not available.
Table 3.
Comparison of [18F]FDG uptake according to the WHO classification
| Study | Thymoma vs thymic carcinoma |
Statistical analysis | ||
|---|---|---|---|---|
| Low-risk thymoma | High-risk thymoma | Thymic carcinoma | ||
| Sung et al.[6] | 4.0 ± 0.42 | 5.6 ± 1.90** | 10.5 ± 4.68* | *P < 0.001 compared with thymoma. **No difference was observed between low-risk and high-risk thymoma |
| Inoue et al.[11] | Early SUVmax 3.2 (1.1–5.3); delayed SUVmax 3.4 (1.8–6.4) | Early SUVmax 6.0 (2.2–12.9)*; Delayed SUVmax 7.4 (3.7–16.3)** | *P < 0.001 compared with low-risk thymoma. **P = 0.001 compared with low-risk thymoma | |
| Luzzi et al.[10] | 3.3 ± 0.5 | 13.5 ± 7.0* | *P < 0.01 compared with low-risk thymoma | |
| Kumar et al.[8] | 3.0 (1.7–3.9) | 2.1(0.8–2.8) | 7.0 (4.3–9.2) * | *P < 0.01 compared with thymoma |
| Shibata et al.[9] | Type A/AB, B1, B2 and B3; 3.2 ± 0.7, 4.8 ± 2.0, 3.7 ± 1.2, 5.0 ± 1.4 | 9.2 ± 2.4* | *P = 0.048, P = 0.007, P = 0.001, and P = 0.001 compared with type A/AB, B1, B2 and B3, respectively | |
| Kaira et al.[12] | 2.6 ± 0.9 | 4.3 ± 1.6** | 8.9 ± 3.6* | *P < 0.01 compared with high-risk thymoma. **P < 0.01 compared with low-risk thymoma |
| Nakajo et al.[13] | 3.05 ± 0.55 | 5.24 ± 2.44* | *P = 0.008 compared with low-risk thymoma | |
| Igai et al.[14] | 3.43 ± 2.19 | 8.15 ± 7.88* | *P = 0.0084 compared with thymoma | |
| Terzi et al.[15] | SUVmax 4.0 ± 1.7; T/M ratio 2.0 ± 0.5 | SUVmax 14.1 ± 8.3*; T/M ratio 7.8 ± 5.2** | SUVmax17.1 ± 8.5***; T/M ratio 9.6 ± 5.5*** | *P = 0.005 and **P < 0.001 compared with low-risk thymoma. ***No difference was observed between low-risk and high-risk thymoma |
T/M ratio is the ratio of the peak SUV of the tumor to the mean SUV of the mediastinum.
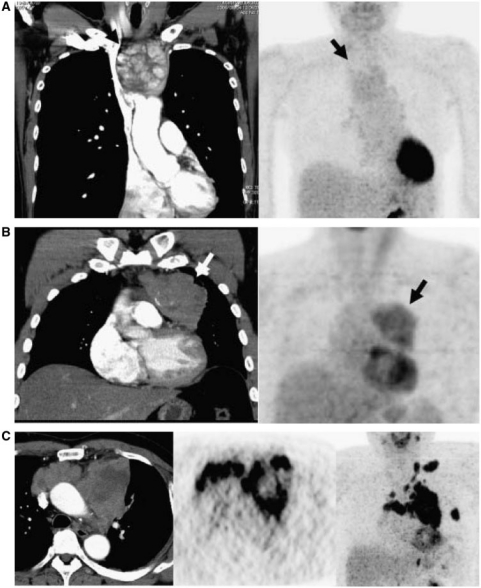
Figure 1.
(a) Low-risk thymoma (type A and Masaoka stage I), (b) high-risk thymoma (type B2 and Masaoka stage II), and (c) thymic carcinoma (Masaoka stage IV).
Figure 2.
Distribution of [18F]FDG uptake on PET in patients with thymic epithelial tumors according to each study included in this review.
To differentiate thymic carcinoma from thymoma, the sensitivity, specificity and SUV cutoff values were 84.9%, 92.3% and 5.0 in Ref.[5], 100%, 92% and 6.3 in Ref.[9], and 63.6%, 91.4% and 6.2 in Ref.[11]. The results for SUV values between low-risk thymoma and high-risk thymoma were 78.3% and 91.3%, respectively, when 4.5 was used as a cutoff[11].
The role of therapeutic monitoring and outcome
One study reported that high uptake of [18F]FDG is significantly associated with poor prognosis[12]. This study included 11 thymic carcinomas among 49 thymic epithelial tumors, and the 11 patients with thymic carcinoma had a significantly high [18F]FDG uptake compared with thymomas. However, it remains unclear whether [18F]FDG uptake is associated with outcome in patients with thymoma.
In monitoring treatment by [18F]FDG-PET, one study documented that [18F]FDG-PET was useful for monitoring response after treatment (chemotherapy or radiation) in inoperable thymic epithelial tumors. Of these 11 patients with inoperable thymic epithelial tumors (3 thymomas and 6 thymic carcinomas), 8 patients received [18F]FDG-PET before and after treatment. In patients with any response (n = 5), [18F]FDG uptake after treatment was significantly lower than at baseline. In patients without any response (n = 3), however, [18F]FDG uptake after treatment was significantly higher than at baseline.
The relationship between [18F]FDG uptake and relevant molecular abnormalities
Two studies investigated the relationship between glucose transporter 1 (Glut1) expression and [18F]FDG uptake in thymic epithelial tumors, and [18F]FDG uptake within tumor cells has been reported to be closely correlated with Glut1 expression[12,13]. These studies demonstrated that the degree of [18F]FDG uptake in thymic epithelial tumors is closely related to the amount of Glut1, hexokinase II and hypoxic markers.
An in vitro study using a thymic cancer cell line[12], the uptake of [18F]FDG was markedly decreased by the inhibition of Glut1 or hypoxic inducible factor-1alpha (HIF-1α), whereas Glut1 upregulation by the induction of HIF-1α increased the [18F]FDG uptake. The results of this study indicate that cellular uptake of [18F]FDG is mediated by Glut1 and that the expression of Glut1 protein is regulated by HIF-1α.
The role of [11C]methionine- or [11C]acetate-PET
One of 12 studies examined the diagnostic significance of [11C]methionine (MET)-PET in thymic epithelial tumors [5] and another study[9] examined [11C]acetate (AC)-PET. MET-PET was not significantly different among thymic carcinoma, invasive thymoma and non-invasive thymoma; MET uptake in thymic tumors correlated with [18F]FDG uptake. Currently, AC-PET can be used to predict the histologic type of thymoma. AC uptake in type A/AB thymomas was significantly higher than in type B1, B2, B3 thymomas and thymic carcinoma. This is contradictory to the results of [18F]FDG uptake in thymic epithelial tumors. However, SUV by AC-PET was not significantly different between thymomas and thymic carcinomas.
Discussion
This is the first review to evaluate the clinical significance of [18F]FDG-PET in patients with thymic epithelial tumors. Our study found that [18F]FDG-PET is useful for differentiating between thymomas and thymic carcinomas. Previous studies reported that thymic carcinoma can be differentiated from thymoma at a diagnostic specificity of more than 90% if thymic tumors indicate an SUV value of 5.0 or more in [18F]FDG-PET[5,9,11]. Moreover, the results in 4 papers confirmed that [18F]FDG-PET is useful for differentiating between high-risk thymoma and thymic carcinoma. One paper documented that the SUV values for low-risk thymoma and high-risk thymoma had a sensitivity of 78.3% and specificity of 91.3% using a cutoff value of 4.5 for SUV[11], but 2 papers found that [18F]FDG-PET could not differentiate high-risk thymoma from low-risk thymoma. Since the SUV value overlaps between low-risk thymoma and high-risk thymoma, [18F]FDG-PET may not be a useful diagnostic modality for differentiating these groups. Because previous studies had small sample size, a large-scale study is warranted for assessing whether [18F]FDG-PET could be useful for distinguishing tumor subgroups in thymomas.
[18F]FDG-PET imaging of response to chemotherapy or radiation has been reported to be useful for patients with thoracic tumors[17,18]. A recent study suggests that [18F]FDG-PET is useful for monitoring response and outcome after treatment in unresectable thymic epithelial tumors[19]. Although this study consists of only 12 patients who received chemotherapy or radiation because of advanced or metastatic disease, [18F]FDG uptake after treatment in 6 patients with any response was significantly lower than at baseline (P = 0.0017). Moreover, the overall survival after treatment tended to be longer in patients with partial metabolic response compared with those with non-partial metabolic response. As these are preliminary data, further investigation is warranted.
Determination of malignant lesions with [18F]FDG-PET is based on glucose metabolism[20,21]. The overexpression of Glut1 has been shown to be closely related to [18F]FDG uptake in human cancer[22,23]. Glut1 is thought to be a possible intrinsic marker of hypoxia, and the expression of Glut1 has been found to be regulated by hypoxia in an HIF-1α-dependent way[24,25]. HIF-1α is considered to support tumor growth by the induction of angiogenesis via the expression of vascular endothelial growth factor (VEGF) and by high and anaerobic metabolic mechanisms[26]. Two papers have reported that the degree of [18F]FDG uptake in thymic epithelial tumors is closely correlated with the amount of Glut1 and hypoxic markers[12,13]. One of these studies demonstrated that upregulation of Glut1 and HIF-1α was closely associated with [18F]FDG uptake into thymic cancer cells[12]. Biologically, the expression of Glut1 plays a crucial role in the accumulation of [18F]FDG within tumor cells.
MET-PET has been used to measure amino acid metabolism in vivo, and therefore a high MET uptake in the tumor cells is thought to reflect an increase in either the transport mechanism of amino acids or protein synthesis[27]. However, MET uptake was not found to differ between thymic carcinoma and thymoma. Recent studies have documented that AC is a useful PET tracer for the detection of slow-growing tumors that cannot be identified using [18F]FDG-PET, such as prostate cancer and well-differentiated adenocarcinoma of the lung[28,29]. The AC uptake in type A/AB thymoma was significantly higher than that in other histological types. Although AC-PET cannot predict the invasiveness of thymomas assessed by tumor stage, AC-PET has been reported to be useful for predicting the histological type of thymomas[9]. However, we cannot differentiate thymoma from thymic carcinoma using AC-PET. Thus, AC-PET may not be appropriate for differentiating the histological type of thymic epithelial tumors. Compared with MET- or AC-PET tracer, nowadays, [18F]FDG is a better PET tracer for differentiating between thymoma and thymic carcinoma.
Conclusion
This review found that [18F]FDG-PET is a useful radiological modality for differentiating between thymomas and thymic carcinoma. Some papers have reported that thymic carcinoma can be differentiated from thymoma at a diagnostic specificity of more than 90% using an SUV cutoff value more than 5.0. However, [18F]FDG-PET may not be useful for distinguishing these groups, because [18F]FDG accumulation overlaps in low-risk thymoma and high-risk thymoma. [18F]FDG-PET may have an important role in predicting response to treatment and prognosis in thymic epithelial tumors. [18F]FDG-PET may have an additional use for radiological differential diagnosis between thymoma and thymic carcinoma.
Conflict of interest
None of the authors has any financial or personal relationships with other people or organizations that could inappropriately influence our work.
References
- 1.Rosai J, Sobin LH. International histological classification of tumours. 2nd ed. New York: Springer; 1999. Histological typing of tumours of the thymus; pp. 1–59. [Google Scholar]
- 2.Travis WD, Brambillia E, Muller-Hermelink HK, et al. Pathology and genetics of tumors of the lung, pleura, thymus and heart. Lyon: IARC Press; 2004. WHO classification of tumors. [Google Scholar]
- 3.Liu RS, Yoho SH, Huang MH, et al. Use of fluorine-18 fluorodexyglucose positron emission tomography in the detection of thymoma: a preliminary report. Eur J Nucl Med. 1995;22:1402–7. doi: 10.1007/BF01791148. [DOI] [PubMed] [Google Scholar]
- 4.Kubota K, Yamada S, Kondo T, et al. PET imaging of primary mediastinal tumours. Br J Cancer. 1996;73:882–6. doi: 10.1038/bjc.1996.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki M, Kuwabara Y, Ichiya Y, et al. Differential diagnosis of thymic tumors using a combination of 11C-methionine PET and FDG-PET. J Nucl Med. 1999;40:1595–601. [PubMed] [Google Scholar]
- 6.Sung YM, Lee KS, Kim BT, et al. 18F-FDG-PET/CT of thymic epithelial tumors: usefulness of distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–34. [PubMed] [Google Scholar]
- 7.El-Bawab H, Al-Sugair AA, Rafay M, et al. Role of fluorine-18 fluorodexyglucose positron emission tomography in thymic pathology. Eur J Cardiothorac Surg. 2007;31:731–6. doi: 10.1016/j.ejcts.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Regmi SK, Dutta R, et al. Characterization of thymic masses using 18FDG-PET-CT. Ann Nucl Med. 2009;23:569–77. doi: 10.1007/s12149-009-0283-z. [DOI] [PubMed] [Google Scholar]
- 9.Shibata H, Nomori H, Uno K, et al. 18F-fluorodexyglucose and 11C-acetate positron emission tomography are useful modalities for diagnosing the histologic type of thymoma. Cancer. 2009;115:2531–8. doi: 10.1002/cncr.24278. [DOI] [PubMed] [Google Scholar]
- 10.Luzzi K, Campione A, Gorla A, et al. Role of fluorine-fluorodeoxyglucose positron emission tomography/computed tomography in preoperative assessment of anterior mediastinal masses. Eur J Cardiothorac Surg. 2009;36:475–9. doi: 10.1016/j.ejcts.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 11.Inoue A, Tomiyama N, Tatsumi M, et al. 18F-FDG-PET for the evaluation of thymic epithelial tumors: correlation with the World Health Organization classification in addition to dual-time-point imaging. Eur J Nucl Med Mol Imaging. 2009;36:1219–25. doi: 10.1007/s00259-009-1082-4. [DOI] [PubMed] [Google Scholar]
- 12.Kaira K, Endo M, Abe M, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol. 2010;28:3746–53. doi: 10.1200/JCO.2009.27.4662. [DOI] [PubMed] [Google Scholar]
- 13.Nakajo M, Kajiya Y, Tani A, et al. 18FDG-PET for grading malignancy in thymic epithelial tumors: significant differences in 18FDG uptake and expression of glucose transporter-1 and hexokinase II between low and high-risk tumors: preliminary study. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.08.010. Aug 30. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Igai H, Matsuura N, Tarumi S, et al. Usefulness of [F]fluoro-2-deoxy-D-glucose positron emission tomography for predicting the World Health Organization malignancy grade of thymic epithelial tumors. Eur J Cardiothorac Surg. 2011;40:143–5. doi: 10.1016/j.ejcts.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Terzi A, Bertolaccini L, Rizzardi G, et al. Usefulness of 18-FDG-PET/CT in the pre-treatment evaluation of thymic epithelial neoplasms. Lung Cancer. 2011 doi: 10.1016/j.lungcan.2011.02.018. March 23. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Endo M, Nakagawa K, Ohde Y, et al. Utility of 18F-FDG for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;61:350–5. doi: 10.1016/j.lungcan.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 18.Ceresoli GL, Chiti A, Zucali PA, et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F]fluorodeoxyglucose. J Clin Oncol. 2006;24:4587–93. doi: 10.1200/JCO.2006.06.8999. [DOI] [PubMed] [Google Scholar]
- 19.Kaira K, Murakami H, Miura S, et al. 18F-FDG uptake on help predict outcome and response after treatment in unresectable thymic epithelial tumors. Ann Nucl Med. 2011;25:247–53. doi: 10.1007/s12149-010-0455-x. [DOI] [PubMed] [Google Scholar]
- 20.Brock CS, Meikle SR, Price P. Dose fluorine-18 fluorodexyglucose metabolic imaging of tumor benefit oncology? Eur J Nucl Med. 1997;24:691–705. doi: 10.1007/s002590050108. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman JM, Waskin HA, Schifter T, et al. The use of FDG-PET in differentiating infectious from malignant central nervous system lesions in patients with AIDS. J Nucl Med. 1992;33:838. [PubMed] [Google Scholar]
- 22.Higashi K, Ueda Y, Sakurai A, et al. Correlation of Glut-1 glucose transporter expression with [18F] FDG uptake in non-small cell lung cancer. Eur J Nucl Med. 2000;27:1778–85. doi: 10.1007/s002590000367. [DOI] [PubMed] [Google Scholar]
- 23.Chung JH, Cho KJ, Lee SS, et al. Over expression of Glut 1 in lymphoid follicles correlates with false-positive 18F-FDG-PET results in lung cancer staging. J Nucl Med. 2004;45:999–1003. [PubMed] [Google Scholar]
- 24.Vleugel MM, Greijer AE, Shvarts, et al. Differential prognostic impact of hypoxia induced and diffuse HIF-1 alpha expression in invasive breast cancer. J Clin Pathol. 2005;58:172–7. doi: 10.1136/jcp.2004.019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elson DA, Ryan HE, Snow JW, et al. Coordinate up-regulation of hypoxia inducible factor (HIF)-1a and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing 1. Cancer Res. 2000;60:6189–95. [PubMed] [Google Scholar]
- 26.Ryan HE, Polni M, McNulty W, et al. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–15. [PubMed] [Google Scholar]
- 27.Ishiwata K, Enomoto K, Sasaki T, et al. A feasibility study on L-[1-carbon-11]tyrosine and L-[methyl-carbon-11]methionine to assess liver protein synthesis by PET. J Nucl Med. 1996;37:279–85. [PubMed] [Google Scholar]
- 28.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–21. [PubMed] [Google Scholar]
- 29.Nomori H, Kosaka N, Watanabe K, et al. 11C-acetate positron emission tomography imaging for lung adenocarcinoma 1 to 3 cm in size with ground-glass opacity images on computed tomography. Ann Thorac Surg. 2005;80:2020–5. doi: 10.1016/j.athoracsur.2005.06.003. [DOI] [PubMed] [Google Scholar]