Abstract
Replacing palpating fingers with an ultrasound (US) probe has resulted in an epidemic of thyroid nodules. Despite the high prevalence of thyroid nodules in the general population, thyroid malignancy is rare. Although no imaging modality can accurately predict the nature of every nodule, high-resolution US is the most sensitive, easily available and cost-effective diagnostic test available to detect thyroid nodules, measure their dimensions and identify their structure. The presence of calcifications, irregular spiculated outline, hypoechogenicity in a solid nodule, chaotic intranodular vascularity and an elongated shape are well-known US features of malignancy in thyroid nodules. Cervical lymph node metastasis and extrathyroidal extension of a thyroid nodule are highly specific for malignancy but seen infrequently. Spongiform nodules, purely or predominantly cystic nodules, nodules with well-defined hypoechoic halo and echogenic as well as isoechoic nodules are usually benign. None of the US characteristics have 100% accuracy in detecting or excluding malignancy. Fine-needle biopsy is currently the best triage test for pre-operative evaluation of a thyroid nodule. There is no significant difference in the risk for malignancy between palpable and non-palpable nodules and size is not a reliable indicator for their malignant potential. The best tool for risk stratification for malignancy in thyroid nodules is US and guided biopsy of nodules with suspicious imaging features. This is especially relevant in patients with multinodular goitre.
Keywords: Thyroid nodule, ultrasound, risk stratification, thyroid biopsy
Introduction
Over the last 2 decades, replacing the palpating fingers with an ultrasound (US) probe has resulted in an epidemic of thyroid nodules. Sub-centimetre thyroid nodules are not usually detected at palpation. In comparison, high-resolution US accurately demonstrates nodules as small as 1–2 mm. Hence, the prevalence of thyroid nodules in the general population goes up from 8% to 76% when evaluated with US instead of clinical examination[1–3]. Even at autopsy, the prevalence of thyroid nodules is high with multiple thyroid nodules seen in 37.3% and solitary nodules found in 12.2% of random autopsies[4]. Thyroid nodules are ubiquitous but thyroid malignancy is rare with just 1 of 20 clinically detected nodules being malignant. This corresponds to approximately 2 to 4 cases per 100,000 people per year, constituting only 1% of all cancers and 0.5% of all cancer deaths[5]. This justifies against the use of screening US for thyroid nodules in the general population. Controversy exists in many areas of management of thyroid nodules, including the most cost-effective approach in their diagnostic evaluation. Practice guidelines from several expert groups such as the American Association of Clinical Endocrinologists, the American Thyroid Association and the Society of Radiologists in Ultrasound attempt to address them. However, there is still a lack of consensus on certain key areas.
Evaluation of thyroid nodules
A thyroid nodule is defined as a discrete lesion within the thyroid gland that is radiologically distinct from the surrounding thyroid parenchyma[6] (Fig. 1). Pathologically, they are classifiable into 5 types with distinct histologic features: hyperplasic, neoplastic, colloid, cystic and thyroid nodules[7]. Fundamental to their evaluation is differentiating medical from surgical disease and identifying the odd malignant one. Clinical information may often give a clue to this. Nodules increasing in size are suspicious for malignancy, but lesions with rapid increase in size over a few hours are likely to be haemorrhagic. Haemorrhagic changes are more commonly encountered in malignant than benign nodules[8]. A benign multinodular goitre (MNG) grows in size over the years but malignancy typically grows in weeks. Rapid growth during levothyroxine therapy is especially suggestive of cancer[2]. Abruptly appearing large nodules (>3 cm) over a short duration of 2 months or less have a high probability to be lymphoma, metastasis or anaplastic carcinoma[9]. Even thyroiditis can cause rapid increase in size but the ancillary findings usually enable the differentiation. Symptoms from mass effect such as airway compression, hoarseness, and dysphagia are more often seen with MNG. However, if these symptoms are seen in the absence of MNG, invasive forms of thyroid carcinoma are likely. Most of the well-differentiated thyroid carcinomas (DTC) are smaller and are unlikely to cause alarming symptoms.
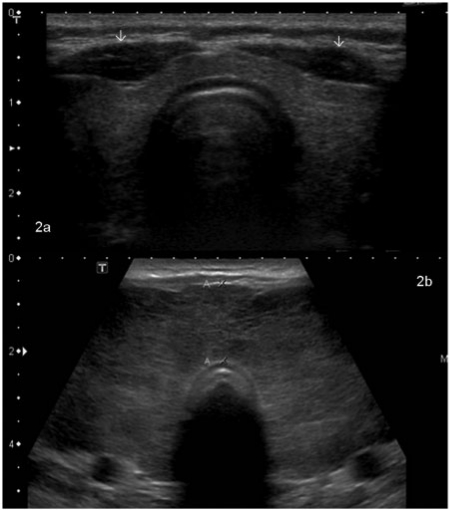
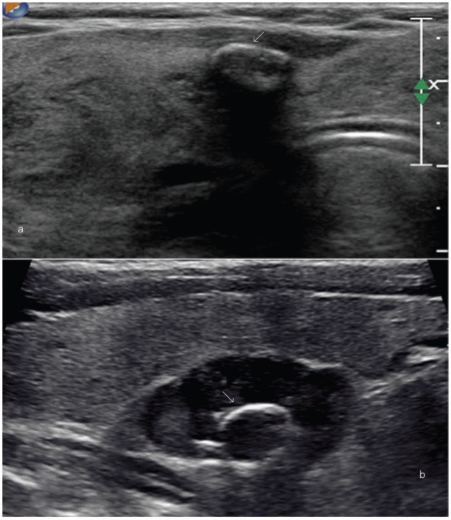
Figure 1.
Any lesion within the thyroid gland that is radiologically distinct from the surrounding thyroid parenchyma qualifies as a thyroid nodule. (a) A large nodule (arrow) in the right lobe that was palpable at clinical examination. High-resolution ultrasound can detect much smaller nodules. (b,c) Longitudinal and axial images of the same lesion (shown by arrows) that measures 1×2 mm in size.
The best initial laboratory test of thyroid function in a patient with thyroid nodule is serum thyroid stimulating hormone (TSH). If a patient is not euthyroid, the diagnosis points towards a benign functional disorder, such as Hashimoto thyroiditis or a toxic nodule. Hence, TSH assay may be followed by measurement of free thyroxine and tri-iodothyronine if the TSH value is low; or the measurement of anti-thyroid peroxidase antibodies (TPOAb) if the TSH value is high. A hyperthyroid state with low TSH suggests a toxic nodule; hypothyroid status with increased TPOAb indicates Hashimoto thyroiditis. A single, non-stimulated calcitonin measurement can be used in the initial workup of thyroid nodules as a marker for tumours of parafollicular cell origin[3].
US imaging and documentation
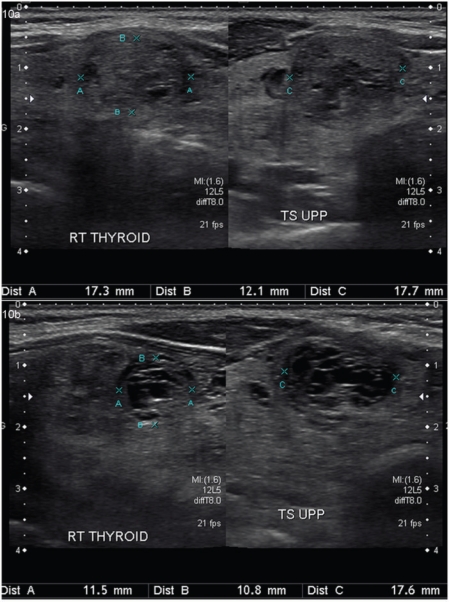
High-resolution US is the most sensitive test available to detect thyroid lesions, measure their dimensions, identify their structure, and evaluate diffuse changes in the gland[3]. We scan the patient in a supine position with the neck hyperextended. A high-resolution probe of 10–15 MHz is used. In the first stage, the shape of the gland and the size of both lobes (3 dimensions) and the anterior-posterior thickness of the isthmus are measured and the continuity of the thyroid capsule is confirmed. Lobes up to 2 cm in thickness are considered normal and greater than 2.5 cm is deemed enlarged. The upper limit of normal for the isthmus is 1 cm. The normal thyroid gland is uniformly echogenic relative to the strap muscles of the neck (Fig. 2).Thyroid nodules are identified and their various gray scale and Doppler interrogation characteristics are assessed for the risk of malignancy. The authors prefer to document all nodules more than 1 cm in diameter and smaller nodules with suspicious US features on a thyroid diagram representing their relative position within the gland. This gives better orientation for the referring clinician as well as the for the radiologist doing the biopsy, and makes future comparisons at follow-up US more reliable and practical. The neck is then screened for lymph nodes. Images of the nodules are stored in both axial and longitudinal planes and the diameter is recorded along all 3 axes, preferably on a single screen shot.
Figure 2.
Transverse grayscale mode image of a normal thyroid gland that is uniformly echogenic relative to overlying strap musculature (arrows) (a). In comparison, (b) shows a thyroid gland with heterogeneous parenchymal echotexture and diffuse enlargement consistent with thyroiditis. This patient had Graves disease.
Tissue harmonic imaging, three-dimensional US and compound imaging can add value to routine US evaluation[10]. In early studies, real-time US elastography has shown sensitivity of 88%, specificity of 90%, a positive predictive value of 81%, and a negative predictive value of 93% in differentiating benign from malignant nodules, independent of nodule size[11]. Contrast-enhanced US (CEUS) has shown differences in the enhancement pattern of benign and malignant nodules[12]. However, currently the use of CEUS is restricted to definition of the size and limits of necrotic zones after US-guided ablation procedures.
US characteristics of thyroid nodules: malignant versus benign
There is considerable overlap between the appearance of benign and malignant nodules and no single imaging feature can be considered pathognomonic. However, the simultaneous presence of 2 or more suspicious sonographic findings increases the risk of thyroid malignancy[13,14]. The following discussion comments on the relative value of the various US features of thyroid nodules in suspecting thyroid malignancy.
Calcifications
The patterns of calcifications seen in the thyroid are: microcalcifications, rim calcifications and coarse calcifications (Figs. 3–6). Compared with a non-calcified predominantly solid nodule, the presence of microcalcifications increases the cancer risk three-fold, and coarse macro-calcifications increase cancer risk two-fold[15]. As a single US feature, microcalcifications have the highest accuracy (76%), specificity (44–95%) and positive predictive value (77.9%) for detecting malignancy in a thyroid nodule. The major drawback is the low sensitivity (26.1–59.1%)[4,15–17]. In comparison, coarse calcifications are less specific for malignancy as they are more frequently seen in benign nodules of multinodular goitre than in malignancy (Fig. 3). However, when macro-calcification is found in a solitary nodule, the risk of malignancy is as high as 75%[18]. The limitation of this US feature that needs to be kept in mind while interpreting images is the low sensitivity with coarse calcifications noted in 9.7% of malignant nodules compared with 3.8% of benign nodules[17].
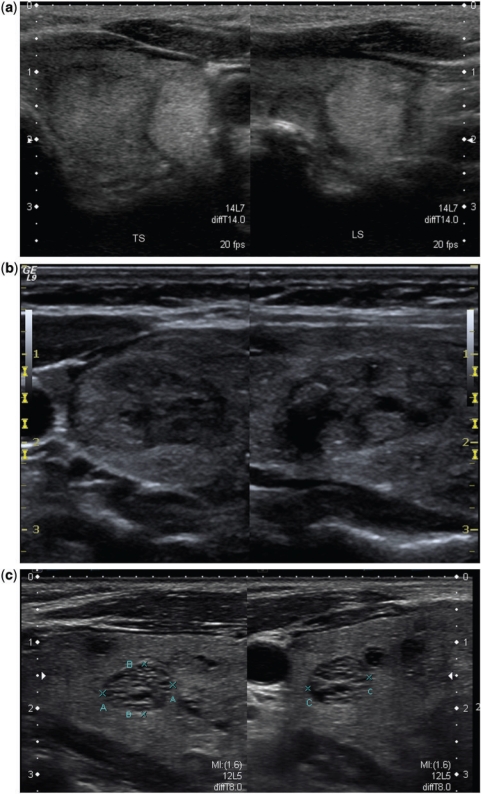
Figure 3.
This grayscale image of the left lobe of the thyroid along its long axis in a patient with multinodular goitre shows a nodule with coarse chunky calcification (arrow). At histology, this was found to be benign. However, coarse calcifications when present in a solitary nodule are highly suspicious for malignancy.
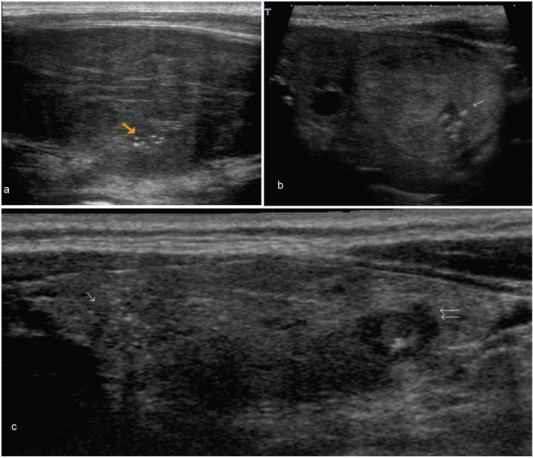
Figure 4.
These images show microcalcifications in various thyroid pathologies. Although one of the most specific US features of malignancy, microcalcifications may also be seen in benign conditions. (a) An enlarged heterogeneous thyroid gland with biopsy proven benign microcalcifications (arrow), in this patient with Hashimoto thyroiditis. (b) The long axis view of a well-defined, isoechoic left lobe thyroid nodule with hypoechoic rim shows a cluster of microcalcifications (arrow). At histology, this was diagnosed as a follicular adenoma. (c) The long axis view of the right lobe of the thyroid shows an ill-defined hypoechoic area with several microcalcifications at the cranial third (single arrow) and an irregular hypoechoic nodule with few microcalcifications at the lower third (double arrow). Surgery confirmed multifocal papillary thyroid carcinoma.
Figure 5.
An invasive papillary carcinoma. (a) The transverse grayscale image shows a hypoechoic nodule with irregular outlines infiltrating the overlying strap muscle. Both coarse and microcalcifications are seen in this nodule. At colour Doppler interrogation (b) there is chaotic vascularity in the entire nodule.
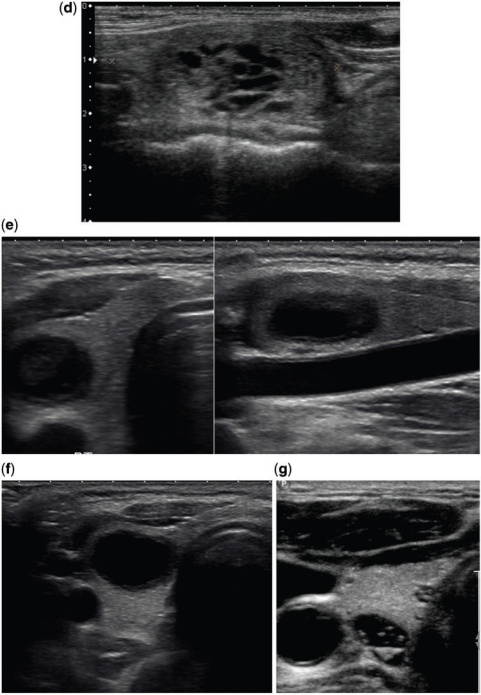
Figure 6.
Examples of eggshell calcifications. (a) A bulky thyroid gland in a patient with multinodular goitre shows a small nodule with partial rim calcification (arrow). (b) There is a well-defined hypoechoic solid nodule with eggshell calcification (arrow) within. This was a follicular adenoma at surgical excision.
Microcalcifications are seen at US as tiny hyperechoic foci of around 1 mm with or without acoustic shadowing or as simple fine acoustic shadows (Fig. 4). Pathologically they correspond to clusters of psammoma bodies[16]. Traditionally a hallmark of papillary thyroid carcinoma (PTC), microcalcifications are also seen in benign lesions such as hyperplastic nodular goitre, Graves disease or lymphocytic thyroiditis. Inspissated colloid in benign thyroid lesions may mimic microcalcifications in thyroid malignancies, but the former can be distinguished from the latter if the comet tail or reverberation artefacts are appropriately demonstrated (Fig. 7g)[19]. The person performing the US scan needs to give due attention to this technical aspect to avoid misinterpretation. Coarse calcification manifests as echogenic focus with posterior shadowing. It may appear as spicules, fragmented plates, or granular deposits. They represent dystrophic amorphous deposits of calcium in fibrous tissue or necrotic material. When seen in a solitary nodule, some groups recommend surgery, regardless of the result of fine-needle biopsy (FNB)[18]. Coarse calcifications may coexist with microcalcifications in papillary cancers, (Fig. 5) but they are also the most common type of calcification seen in medullary thyroid carcinomas[20]. Eggshell-like rim calcification (Fig. 6) is infrequently seen and it may be misinterpreted as calcified lymph nodes. Although formerly considered a feature of benignity, eggshell calcifications have been reported in thyroid cancers, mostly in papillary carcinoma and rarely in follicular carcinoma[21].
Figure 7.
Various histology proven benign thyroid nodules. (a) An isoechoic and an echogenic nodule. The isoechoic nodule is seen better in the transverse section (TS) and the echogenic one in the longitudinal section (LS). Besides the echotexture that favours benignity in both these nodules, they also show a well-defined hypoechoic halo that strongly favours benignity. (b) This mixed echotexture solid nodule shows a discrete hypoechoic rim along its entire circumference. A complete uniform halo around a nodule is highly suggestive of benignity, with a specificity of 95%. (c) The well-defined nodule here shows an aggregation of innumerable tiny cystic spaces giving it a spongiform appearance. Such nodules are usually benign and do not need biopsy. (d) Here the isoechoic nodule has multiple cystic spaces occupying more than two-thirds of its volume with a well-defined hypoechoic halo. Such microcystic components in more than 50% of the nodule volume are 99.7% specific for identification of a benign nodule[6]. (e,f) Predominantly cystic and purely cystic thyroid nodules. Thyroid cancer is not common in predominantly cystic nodules and purely cystic nodules are almost never malignant. (g) This transverse image of the right thyroid lobe shows a cyst with multiple echogenic foci with posterior ring-down artefacts. This appearance is diagnostic of a colloid cyst.
Margins and shape
Most thyroid nodules, both malignant and benign, have ovoid-to-round shape and a well-defined smooth margin. Iso- or hyperechoic nodules may show a hypoechoic halo representing a pseudocapsule of fibrous connective tissue or compressed thyroid tissue and vessels (Fig. 7a). A complete, uniform halo around a nodule (Fig. 7b) is highly suggestive of benignity, with a specificity of 95%[22]. Such a nodule is 12 times more likely to be benign than malignant. Even if the halo is incomplete, it is 4 times more likely to be benign[23]. However, 15–30% of malignant nodules may also show a halo limiting the utility of this sign[23].
The nodule margins are described as well-defined regular, well-defined irregular or ill-defined[4]. Irregular margins (Fig. 4b and 8) may be microlobulated or spiculated in appearance; in ill-defined nodules, the demarcation between the tumour and the surrounding normal glandular parenchyma is indistinct. The irregular spiculated or microlobulated margin is suggestive of malignancy; an ill-defined margin can be seen in both benign and malignant nodules[17]. There is considerable ambiguity in the interpretation of the nodule margin. The distinction between irregular and ill-defined margins is the subject of some debate. It has the highest interobserver variability among all the routinely evaluated sonographic features of thyroid nodule[24]. Some of this ambiguity may be unarguably attributed to the type of US probe used. With high-resolution probes, a previously described blurred margin could actually be a spiculated margin with sharp demarcation or a poorly defined margin where the tumour cannot be discriminated from the normal parenchyma. Despite the subjective variations in margin interpretation, an irregular margin is a useful marker of malignancy with high accuracy, specificity and positive predictive value of 73.4%, 83–91.8%, 60–81.3%, respectively[4,17,25]. The sensitivity is once again low at 48.3–55%[17,25]. As expected, in studies that defined lack of a smooth outline or halo as irregular margin, this parameter had lower positive predictive value (30%) and higher sensitivity (77.5%)[13,26].
Figure 8.
Images from a patient with multifocal papillary thyroid carcinoma are shown here. (a) A malignant nodule in the right lobe; (b) another one in the left lobe. Both nodules show irregular spiculated outlines, are solid and hypoechoic with few microcalcifications. The nodule in (b) is taller than wide.
The centrifugal tendency in tumour growth does not necessarily occur at a uniform rate in all dimensions. Hence malignant nodules can be taller than they are wide (Fig. 8b) Although this has only limited mention in the literature, with accuracy, specificity and sensitivity in the range of 67%, 92% and 40% respectively, it is another valuable marker for detecting the likelihood of malignancy in a thyroid nodule[17].
Vascularity
Several imaging tools in oncology are based on assessment of the difference in vascular signatures between malignant and benign tumours. The early descriptions on the patterns of vascularity in thyroid nodules (Fig. 9) as seen at US Doppler imaging, identified 3 forms: type I, complete absence of flow signal within the nodule; type II, exclusive perinodular flow signals; and type III, intranodular flow with multiple vascular poles chaotically arranged, with or without significant perinodular vessels[27]. Type III was considered highly suggestive of malignancy. However, since this original description from the early 1990s, US technology has developed immensely and newer machines allow blood flow imaging of vessels with very small diameter (<100 µm) and slow flow rates of only a few millimetres per second. Hence, the current generation of scanners tend to show some degree of internal vascularity in most of the solid thyroid nodules, dissipating the initial enthusiasm with Doppler interrogation of thyroid lesions. Despite extensive overlap in the colour Doppler appearance of benign and malignant thyroid nodules, a predominantly peripheral flow pattern is considered as a feature of a benign nodule, and a malignant nodule tends to have a predominantly central chaotic blood flow. The specificity, sensitivity and positive predictive value of internal hypervascularity in detecting a malignant nodule is 81%, 74% and 24%, respectively[4,13]. Studies have shown that solid hypervascular thyroid nodules have a high likelihood of malignancy (nearly 42%)[28]. The assumption of a completely avascular nodule being very unlikely to be malignant is challenged by certain large series that failed to demonstrate vascularity in more than 20% of malignant nodules[13,20].
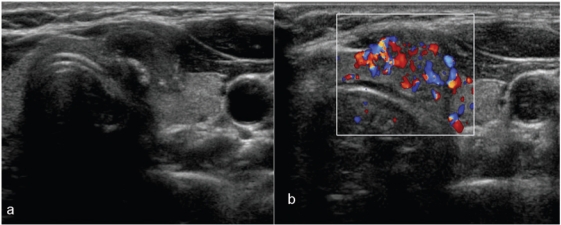
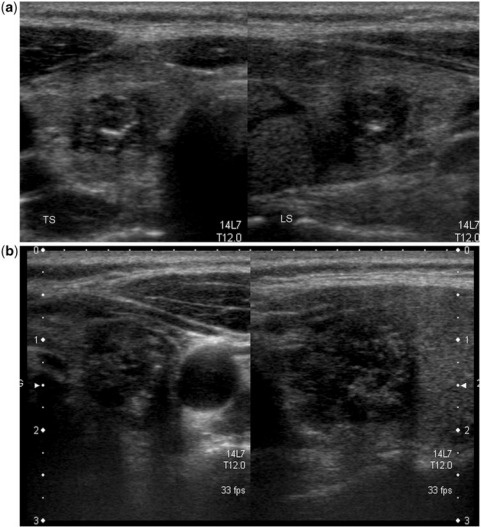
Figure 9.
Patterns of vascularity in thyroid nodules. The nodule in (a) with markedly chaotic central and peripheral vascularity is suspicious for malignancy (also note the internal microcalcification); the peripheral vascularity in the isoechoic nodule in (b) is a feature of benignity. On greyscale imaging, the well-defined nodule in (c) with a hypoechoic halo and honeycomb appearance is almost certain to be a benign lesion. However, at colour Doppler imaging (d) the nodule shows both central and peripheral vascularity. The greyscale and colour Doppler imaging features of this nodule are contradictory. At biopsy, it was a benign nodule.
Echotexture
The texture of a nodule is a reflection of its internal architecture and contents. Nodules may be purely cystic (Fig. 7f), purely solid, predominantly solid (<50% cystic), predominantly cystic (>50% cystic) (Fig. 7e) or spongiform in appearance (Fig. 7c).
Solid composition of a nodule is the US feature with highest sensitivity for malignancy (69.0–75.0%). However, it has a low positive predictive value in that a solid nodule has only a 15.6–27.0% chance of being malignant. The specificity is reasonable at 52–56%[15,28]. However, most benign nodules, as well as malignant nodules, are predominantly solid. Thus, in practice, it is not very useful for differentiating benign from malignant nodules.
Decrease in echogenicity increases the risk for malignancy. Most of the hyperechoic and isoechoic nodules are benign. The incidence of malignancy is only 4% among solid hyperechoic lesions; it increases to 26% for isoechoic lesions[23]. If the solid components of a nodule are hypoechoic to the strap muscles, it may be called markedly hypoechoic and if the echogenicity is just less than the surrounding parenchyma, it is considered hypoechoic. Malignant nodules, both carcinoma and lymphoma, typically appear solid and hypoechoic when compared with normal thyroid parenchyma. The specificity, positive predictive value and diagnostic accuracy of a markedly hypoechoic nodule for malignancy is around 92%, 68% and 71%, respectively. The sensitivity is again low in a range of 26–41%[4,17,25]. Approximately 30–55% of benign nodules are also hypoechoic[24,29] thereby decreasing the usefulness of this US marker.
A pure cystic nodule, although rare (<2% of all nodules), is highly unlikely to be malignant[30]. Nodules that are nearly completely cystic are usually benign nodules that have undergone cystic or haemorrhagic degeneration and are virtually never cancers[23]. Nevertheless, a cystic component may be seen in 13–26% of thyroid cancers and 6% of papillary carcinoma of the thyroid may be predominantly cystic[15,31]. A spongiform appearance in more than 50% of the nodule volume is 99.7% specific for benignity with a negative predictive value of 98.5% for malignancy[6]. Temporal evolution of the nodule echotexture from solid to cystic (Fig. 10) as detected at serial imaging is also common among benign nodules.
Figure 10.
Temporal evolution of a benign thyroid nodule. (a) A solid nodule with few cystic spaces and hypoechoic halo is demonstrated in the longitudinal and transverse planes. (b) The follow-up US images of the same nodule obtained after 1 year shows an increase in the cystic degeneration of the nodule with a slight decrease in its size.
Size and multiplicity
Nodule size is not predictive of malignancy[15]. In patients with a thyroid cancer and multiple thyroid nodules, the cancer is often unifocal but not always present in the largest nodule. Hence, cytologic sampling of lesions with suspicious US features rather than of a larger or clinically dominant nodule is recommended. Lack of any statistically significant relationship between nodule size and likelihood of thyroid cancer has been proven and the same holds true for multiplicity of nodules. In the presence of one or more thyroid nodules larger than 10 mm in diameter, the likelihood of thyroid cancer per patient is independent of the number of nodules[30]. The overall incidence of cancer in patients with thyroid nodules selected for fine-needle aspiration (FNA) is approximately 9.2–13.0%, no matter how many nodules are present at US[15]. This contradicts the commonly held belief that the presence of multiple nodules decreases the likelihood of thyroid cancer. In patients with multiple nodules, the cancer rate per nodule decreases, but the decrease is approximately proportional to the number of nodules so that the overall rate of cancer per patient is the same as that in patients with a solitary nodule.
Extrathyroid extension and lymph node metastasis
Extrathyroid extension into the adjacent viscera and metastases to lymph nodes are highly specific signs of thyroid malignancy. At surgical pathology, extracapsular extension is seen in more than a third of thyroid cancers[13]. Extrathyroidal extension is a key risk factor for both lymph node and distant metastases. As a rule of thumb, papillary thyroid carcinoma (PTC) preferentially spreads to lymph nodes, follicular thyroid carcinoma (FTC) to distant organs, and medullary thyroid carcinoma (MTC) to both lymph nodes and distant organs. Fifty percent of PTC has nodal metastasis at presentation and in some 20% of cases nodal metastasis is the sole or initial manifestation[32,33]. Lymph node metastasis is also a risk factor for distant metastasis[34].
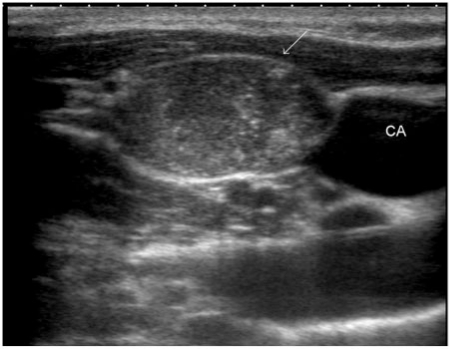
US features suspicious for lymph node metastases (Fig. 11) include loss of its reniform shape, increase in size, replacement of fatty hilum, irregular margins, heterogeneous echotexture or an echotexture replicating the thyroid gland, the presence of calcifications and cystic areas, and vascularity throughout the lymph node instead of normal central hilar vessels[23,35]. Nodal metastases are usually solid, but up to 40% tend to cavitate and some are entirely cystic (Fig. 12). A cystic nodal metastasis from an occult primary in the thyroid can be misleading, especially if it is solitary, when it mimics benign lesions such as infected branchial cleft cyst[33]. In the authors' practice, for every cystic nodal mass in the neck, metastasis from PTC is the first differential diagnosis. The next differential diagnosis to be considered in such a context, is oropharyngeal carcinoma associated with the human papilloma virus as they have a particular tendency to cause cystic changes in their lymph node metastasis[36].
Figure 11.
Metastasis from papillary thyroid carcinoma into a right lateral neck node (arrow). Note the nodal echotexture that almost replicates the echotexture of the thyroid gland as well as the presence of microcalcifications within the node (CA, carotid artery).
Figure 12.
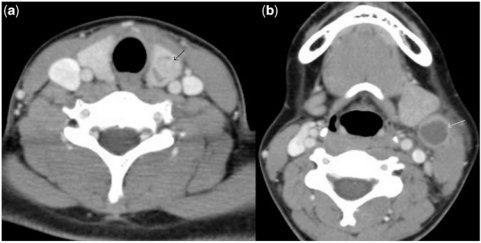
In these contrast-enhanced axial computed tomography images of the neck, there is a nodule (black arrow) in the left lobe of the thyroid (a) and a cystic structure (white arrow) in the lateral neck (b). At histology, the former was confirmed as papillary thyroid carcinoma of thyroid and the latter as cystic metastasis into a cervical lymph node.
FNB of thyroid nodules
FNB is the most accurate and cost-effective method for evaluating thyroid nodules. Compared with surgery performed on the basis of clinical findings alone, FNB reduces the number of thyroidectomies by approximately 50%, roughly doubles the surgical confirmation of carcinoma, and reduces the overall cost of medical care by 25%[37]. FNB with US guidance is recommended over that guided by palpation[2,4,6,7,38,39] for the following reasons:
Around 20–48% of patients with one palpable thyroid nodule are found to have additional nodules on US investigation.
The prevalence of cancer in palpable and non-palpable thyroid nodules is similar at 5.4–7.7% and 5.0–6.5%, respectively.
The US characteristics that suggest malignancy are equally applicable to and identified in both palpable and non-palpable nodules.
The false-negative rate with palpation-guided FNB (p-FNB) (1–3%) was higher than that with US-guided FNB (0.6%).
US FNB also improves the cytologic diagnostic accuracy, sensitivity, and positive predictive value in comparison with p-FNB thereby decreasing the non-diagnostic yields.
Although FNB is currently the best test for the pre-operative evaluation of thyroid nodules, even in centres with substantial experience, the rate of non-diagnostic yield is as high as 15–20%[15]. Non-diagnostic specimens usually result from cystic nodules that yield few or no follicular cells, benign or malignant sclerotic lesions, nodules with a thick or calcified capsule, abscesses and hypervascular or necrotic lesions. After an initial non-diagnostic cytology result, repeat FNB with US guidance tends to yield a diagnostic cytology specimen in 75% of solid nodules and 50% of cystic nodules[40]. As the reported malignancy rate in an earlier non-diagnostic aspirate is 2–12%[4], such biopsies must be repeated[6] preferably with on-site cytologic evaluation. Despite good technique and repeated biopsy, at least 5% of nodules do not yield a diagnosis at FNB. Most non-diagnostic solid nodules are surgically excised, but some, on the basis of clearly favourable clinical and US findings, may be followed up with close clinical and US surveillance[6].
Which nodule to biopsy?
In the presence of multiple nodules, it is often difficult to decide which nodule to biopsy. If the dominant nodule is biopsied, one-third of the malignancies would be missed[15]. Again, PTC can often be multifocal (up to 87%)[41]. Thus, FNB interrogation of the dominant nodule alone is clearly inadequate. Besides, no single sonographic feature can accurately predict malignancy, although some are better than others. The suggestions on thyroid nodule biopsy emerging from a consensus conference of the Society of Radiologists in Ultrasound[15], a statement released by the American Thyroid Association[6] and practice guidelines of the American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association[3] are enumerated in Tables 1, 2 and 3, respectively. Clearly, there is a lack of consensus between various scientific societies for those small nodules that are less than 1 cm but still have a similar risk of harbouring malignancy as larger ones. The problem is compounded by the fact that most of the thyroid nodules seen in the general population are less than 1 cm in size, and a significant number of individuals who die from other causes have thyroid cancers incidentally identified at autopsy that apparently did not adversely affect their health[1]. Now the question is whether early detection of a malignant nodule while it is less than 1 cm has any definite advantage on the survival or quality of life when compared with later detection after it has grown to more than 1 cm. Although FTCs that are <2 cm in diameter have not been shown to be associated with metastatic disease[42] even sub-centimetre PTCs (also called as papillary thyroid microcarcinoma (PTMC)) can have nodal as well as distant metastasis[43]. Despite PTMC being the most often encountered malignancy in sub-centimetre nodules and their potential for metastasis, cancer-related death reported in patients with PTMC is only 0.34%[43]. Interestingly, despite the increased prevalence of PTMC, and their aggressive management, thyroid cancer-related mortality has not shown any change over the years[44]. It has been reported that in patients with stage 1 DTC (i.e. any T, any N, M0 in patients less than 45 years of age and T1, N0, M0 in the rest of the age groups) none of the therapies such as total/near total thyroidectomy, radioactive iodine treatment and L-T4 suppressive therapy make any impact on the final outcome[45]. Not surprisingly, most PTMCs are stage 1 disease. In another study, Ito et al.[46] compared clinical outcomes of 162 untreated patients with biopsy proven PTMC against 626 patients with the same initial tumour characteristics who were treated surgically. They concluded that papillary microcarcinomas do not frequently become clinically apparent, and that patients can choose observation while their tumours are not progressing, although they are pathologically multifocal and involve lymph nodes with high incidence. From such evidence of PTMC having a generally benign clinical course, the use of increasingly sophisticated diagnostic procedures and aggressive treatment may appear unnecessary; however, the scientific perception and patient perception on this matter seems to often differ[43]. Against such background information, in the opinion of the authors, a radiologist in practice would have to adopt a reasonable path taking cognizance of the concerns of the referring clinician and the patient, instead of stubbornly adhering to nodule size cut off or indiscriminately needling every visible nodule. Knowledge of the various US characteristics and their specificity and accuracy to detect malignancy should guide such judgement call. In our experience, FNB of more than 3–4 nodules is generally not very agreeable to any patient. Hence, for sub-centimetre nodules FNB may be attempted only in the presence of strong US suspicion for malignancy. In the presence of multiple nodules >1 cm, the American Thyroid Association recommendation of preferentially needling those with suspicious US features and if none of them have suspicious features needling the largest is a reasonable common sense approach. Although the American Association of Clinical Endocrinologists guidelines emphasize sonographic characteristics rather than nodule size and challenge the wisdom of only needling nodules more than 1 cm, when they state that 'it is rarely necessary to biopsy more than 2 nodules when they are selected on the basis of previously described (US) criteria' it is understood that even they do not suggest indiscriminate sampling of innumerable nodules. An appropriate interpretation of the various guidelines with individual discretion is essential.
Table 1.
Recommendations on thyroid nodule biopsy based on the Society of Radiologists: Ultrasound Consensus Conference Statement[15]
| Management recommendations for thyroid nodules 1 cm or larger in diameter | |
|---|---|
| Solitary nodule | |
| Microcalcifications | Strongly consider US-guided FNA if >1 cm |
| Solid or coarse calcification | Strongly consider US-guided FNA if >1.5 cm |
| Mixed cystic and solid or almost entirely cystic with solid mural component | Consider US-guided FNA if >2 cm |
| None of the above but substantial growth since prior US | Consider US-guided FNA |
| Almost completely cystic and without significant growth or features above | US-guided FNA probably not necessary |
| Multiple nodules | Consider US-guided FNA of one or more nodules, prioritizing selection based on criteria for solid nodules in order listed above |
Table 2.
Recommendations on thyroid nodule biopsy based on revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer[6]
| Nodule: sonographic feature or clinical feature | Recommended threshold size of nodule for FNA cytology |
|---|---|
| High-risk history | |
| Nodule WITH suspicious sonographic features | >5 mm |
| Nodule WITHOUT suspicious sonographic features | >5 mm |
| Abnormal cervical lymph nodes | All |
| Microcalcifications present in nodule | ≥1 cm |
| Solid nodule | |
| AND hypoechoic | >1 cm |
| AND iso- or hyperechoic | >1–1.5 cm |
| Mixed cystic–solid nodule | |
| WITH any suspicious US features | >1.5–2 cm |
| WITHOUT suspicious US features | >2 cm |
| Spongiform nodule | >2 cm |
| Purely cystic nodule | Not indicated |
Table 3.
Recommendations on thyroid nodule biopsy based on the American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules[3]
| FNB is recommended for nodule/nodules |
|---|
| Of diameter larger than 1.0 cm that is solid and hypoechoic on US |
| Of any size with US findings suggestive of extracapsular growth or metastatic cervical lymph nodes |
| Of any size with a patient history of neck irradiation in childhood or adolescence; PTC, MTC, or MEN 2 in first-degree relatives; previous thyroid surgery for cancer; increased calcitonin levels in the absence of interfering factors |
| Of diameter smaller than 10 mm along with US findings associated with malignancy; the coexistence of 2 or more suspicious US criteria greatly increases the risk of thyroid cancer |
| FNB is not recommended for nodule/nodules hot on scintigraphy |
| For solid cystic nodules, FNA the solid areas and aspirate cystic areas for cytology |
| In the presence of suspicious lymph nodes, FNA the node as well as the thyroid nodule |
Technique of needle biopsy
Placement of a needle(s) into a suspected abnormal lesion or organ for the purpose of obtaining tissue or cells for diagnosis is defined as percutaneous needle biopsy (PNB). PNB done with the use of a thin, hollow needle (22 gauge and smaller) is called as FNB. As details of the technique are well described in the literature[4,47], we only allude to certain issues that are infrequently addressed. A pre-procedure evaluation of coagulation profile is not routinely required. Patients receiving warfarin anticoagulation or with known or suspected liver disease should have their international normalized ratio measured and corrected if more than 2. For patients on heparin, activated partial thromboplastin time needs to be monitored and preferably kept below 1.5 times the normal. Platelet count is not routinely required and aspirin as well as plavix need not be stopped before a thyroid FNB. If platelets are known to be below 50,000/mm3, then transfusion is required. For patients on low molecular weight heparin, at least one dose before the procedure should be stopped[48]. When obtaining consent, one should not forget to inform the patient of the possibility of a non-diagnostic aspiration (up to 20%) that may even mandate a repeat procedure. In our practice, patient tolerance and comfort during the procedure is much improved by the use of local anaesthetic. We recommend the use of a 27 G needle for subcutaneous injection of local anaesthetic. A 23 G needle is routinely used for the needle biopsy. Thinner needles 25 G may be useful in highly vascular nodules that tend to give a haemorrhagic sample. A thicker 18/19 G needle may be required to aspirate thick colloid material. FNB of the thyroid nodules may be done with aspiration or non-aspiration techniques. The former involves moving the needle back and forth within the lesion with gentle suction using a syringe attached to its hub and as the material starts appearing at the hub, the suction is released and the needle removed. The non-aspiration technique depends on obtaining the aspirate through capillary action. When performed under US guidance, the sample adequacy and diagnostic yield of both techniques are comparable[49]. The non-aspiration technique may be preferentially used if repeated haemorrhagic aspirates are being obtained. Availability of an on-site cytotechnician greatly improves the outcome of the thyroid biopsy although it may be a logistic constraint in several set ups.
Conclusion
Thyroid nodules are extremely common and most are benign, which makes the detection of a malignant nodule an onerous task. The presence of calcifications (microcalcifications are more specific), an irregular spiculated outline with no halo, hypoechogenicity in a solid nodule, chaotic intranodular vascularity, a taller than wide shape, cervical lymph node metastasis and extracapsular extension of a thyroid nodule are the more specific US features of malignancy. However, their value is limited by their low sensitivity for detecting malignancy. A radiologist is expected to use such knowledge in the risk stratification of thyroid nodules and biopsy the most appropriate ones.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–40. doi: 10.1001/archinte.154.16.1838. [DOI] [PubMed] [Google Scholar]
- 2.Hegedus L. The thyroid nodule. N Eng J Med. 2004;351:1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 3.Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16(Suppl 1):1–43. doi: 10.4158/10024.GL. [DOI] [PubMed] [Google Scholar]
- 4.Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955;15:1270–80. doi: 10.1210/jcem-15-10-1270. [DOI] [PubMed] [Google Scholar]
- 5.Wong CKM, Wheeler MH. Thyroid nodules: rational management. World J Surg. 2000;24:934–41. doi: 10.1007/s002680010175. [DOI] [PubMed] [Google Scholar]
- 6.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 7.Salabe GB. Pathogenesis of thyroid nodules: histologic classification? Biomed Pharmacother. 2001;55:39–53. doi: 10.1016/s0753-3322(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 8.McHenry CR, Slusarczyk SJ, Khiyami A. Recommendations for management of cystic thyroid disease. Surgery. 1999;126:1167–72. doi: 10.1067/msy.2099.101423. [DOI] [PubMed] [Google Scholar]
- 9.King AD, Ahuja AT, King W, et al. The role of US in the diagnosis of a large, rapidly growing, thyroid mass. Postgrad Med J. 1997;73:412–14. doi: 10.1136/pgmj.73.861.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szopinski KT, Wysocki M, Pajk AM, et al. Tissue harmonic imaging of thyroid nodules: initial experience. J US Med. 2003;22:5–12. doi: 10.7863/jum.2003.22.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Hong Y, Liu X, Li Z, Zhang X, Chen M, Luo Z. Real-time US elastography in the differential diagnosis of benign and malignant thyroid nodules. J US Med. 2009;28:861–7. doi: 10.7863/jum.2009.28.7.861. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Jiang YX, Liu JB, et al. Utility of contrast- enhanced US for evaluation of thyroid nodules. Thyroid. 2010;20:51–7. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 13.Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of US and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–6. doi: 10.1210/jc.87.5.1941. [DOI] [PubMed] [Google Scholar]
- 14.Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–51. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 15.Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in US consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 16.Triggiani V, Guastamacchia E, Licchelli B, Tafaro E. Microcalcifications and psammomma bodies in thyroid tumours. Thyroid. 2008;18:1017–18. doi: 10.1089/thy.2008.0082. [DOI] [PubMed] [Google Scholar]
- 17.Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation: Multicenter retrospective study. Radiology. 2008;247:762–70. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 18.Khoo ML, Freeman JL, Witterick IJ, et al. Underexpression of p27/Kip in thyroid papillary microcarcinomas with gross metastatic disease. Arch Otolaryngol Head Neck Surg. 2002;128:253–7. doi: 10.1001/archotol.128.3.253. [DOI] [PubMed] [Google Scholar]
- 19.Jun P, Chow LC, Jeffrey RB. The sonography features of papillary thyroid carcinomas: pictorial essay. US Q. 2005;21:39–45. [PubMed] [Google Scholar]
- 20.Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US Features of thyroid malignancy: pearls and pitfalls. Radiographics. 2007;27:847–60. doi: 10.1148/rg.273065038. discussion 861–5. [DOI] [PubMed] [Google Scholar]
- 21.Taki S, Terahata S, Yamashita R, et al. Thyroid calcifications; sonographic patterns and incidence of cancer. Clin Imaging. 2004;28:368–71. doi: 10.1016/S0899-7071(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 22.Lu C, Chang TC, Hsiao YL, Kuo MS. Ultrasonographic findings of papillary thyroid carcinoma and their relation to pathologic changes. J Formos Med Assoc. 1994;93:933–8. [PubMed] [Google Scholar]
- 23.Ahuja AT. The thyroid and parathyroid. In: Ahuja AT, Evans R, editors. Practical head and neck ultrasound. London: Greenwich Medical Media; 2000. pp. 37–64. [Google Scholar]
- 24.Wienke JR, Chong WK, Fielding JR, et al. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J US Med. 2003;22:1027–31. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 25.Kim EK, Park CS, Chung WY, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–91. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 26.Cappelli C, Castellano M, Pirola I, et al. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100:29–35. doi: 10.1093/qjmed/hcl121. [DOI] [PubMed] [Google Scholar]
- 27.Lagalla R, Cariso G, Midiri M, Cardinale AE. Echo-Doppler couleru et pathologie thyroidienne. J Echograph Med Ultrasons. 1992;13:44–7. [Google Scholar]
- 28.Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in the prediction of malignancy of thyroid nodules? J US Med. 2003;22:127–31. doi: 10.7863/jum.2003.22.2.127. [DOI] [PubMed] [Google Scholar]
- 29.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J US Med. 2004;23:1455–64. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 30.Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–17. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 31.Chan BK, Desser TS, McDougall IR, et al. Common and uncommon sonographic features of papillary thyroid carcinoma. J US Med. 2003;22:1083–90. doi: 10.7863/jum.2003.22.10.1083. [DOI] [PubMed] [Google Scholar]
- 32.Wunderbaldinger P, Harisinghani MG, Hahn PF, et al. Cystic lymph node metastases in papillary thyroid carcinoma. Am J Roentgenol. 2002;178:693–7. doi: 10.2214/ajr.178.3.1780693. [DOI] [PubMed] [Google Scholar]
- 33.Anil G. Cystic nodal metastasis from papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:1035–7. doi: 10.1001/archoto.2011.166-a. [DOI] [PubMed] [Google Scholar]
- 34.Machens A, Holzhausen HJ, Lautenschläger C, Thanh PN, Dralle H. Enhancement of lymph node metastasis and distant metastasis of thyroid carcinoma. Cancer. 2003;98:712–9. doi: 10.1002/cncr.11581. [DOI] [PubMed] [Google Scholar]
- 35.Ahuja AT, Chow L, Chick W, King W, Metreweli C. Metastatic cervical nodes in papillary carcinoma of the thyroid: US and histological correlation. Clin Radiol. 1995;50:229–31. doi: 10.1016/S0009-9260(05)83475-0. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–90. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 37.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–9. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 38.Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 39.Izquierdo R, Arekat MR, Knudson PE, et al. Comparison of palpation-guided versus US-guided fine-needle aspiration biopsies of thyroid nodules in an outpatient endocrinology practice. Endocr Pract. 2006;12:609–14. doi: 10.4158/EP.12.6.609. [DOI] [PubMed] [Google Scholar]
- 40.Alexander EK, Heering JP, Benson CB, et al. Assessment of nondiagnostic US-guided fine needle aspiration of thyroid nodules. J Clin Endocrinol Metab. 2002;87:4924–7. doi: 10.1210/jc.2002-020865. [DOI] [PubMed] [Google Scholar]
- 41.Trisha M, Shattuck BS, William H, et al. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. 2005;352:2406–12. doi: 10.1056/NEJMoa044190. [DOI] [PubMed] [Google Scholar]
- 42.Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103:2269–73. doi: 10.1002/cncr.21055. [DOI] [PubMed] [Google Scholar]
- 43.Roti E, degli Uberti EC, Bondanelli M, Braverman LE. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159:659–73. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 44.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 45.Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–42. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 46.Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–7. doi: 10.1089/105072503321669875. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 48.Kim MJ, Kim EK, Park SI, et al. US-guided fine-needle aspiration of thyroid nodules: indications, techniques, results. Radiographics. 2008;28:1869–86. doi: 10.1148/rg.287085033. [DOI] [PubMed] [Google Scholar]
- 49.Malloy PC, Grassi CJ, Kundu S, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20:S240–49. doi: 10.1016/j.jvir.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 50.Tublin ME, Martin JA, Rollin LJ, Pealer K, Kurs-Lasky M, Ohori NP. US-guided fine-needle aspiration versus fine-needle capillary sampling biopsy of thyroid nodules: does technique matter? J US Med. 2007;26:1697–701. doi: 10.7863/jum.2007.26.12.1697. [DOI] [PubMed] [Google Scholar]