Abstract
The nucleosome is the fundamental repeating unit of eukaryotic chromatin. Here, we assessed the interplay between DNA sequence and ATP-dependent chromatin-remodeling factors (remodelers) in the nucleosomal organization of a eukaryotic genome. We compared the genome-wide distribution of Drosophila NURD, (P)BAP, INO80, and ISWI, representing the four major remodeler families. Each remodeler has a unique set of genomic targets and generates distinct chromatin signatures. Remodeler loci have characteristic DNA sequence features, predicted to influence nucleosome formation. Strikingly, remodelers counteract DNA sequence-driven nucleosome distribution in two distinct ways. NURD, (P)BAP, and INO80 increase histone density at their target sequences, which intrinsically disfavor positioned nucleosome formation. In contrast, ISWI promotes open chromatin at sites that are propitious for precise nucleosome placement. Remodelers influence nucleosome organization genome-wide, reflecting their high genomic density and the propagation of nucleosome redistribution beyond remodeler binding sites. In transcriptionally silent early embryos, nucleosome organization correlates with intrinsic histone-DNA sequence preferences. Following differential expression of the genome, however, this relationship diminishes and eventually disappears. We conclude that the cellular nucleosome landscape is the result of the balance between DNA sequence-driven nucleosome placement and active nucleosome repositioning by remodelers and the transcription machinery.
INTRODUCTION
Chromatin plays a crucial role in all processes involving the eukaryotic genome. The nucleosome, comprising 147 bp of DNA wrapped tightly in ∼1.7 left-handed superhelical turns around a core histone octamer of H2A, H2B, H3, and H4, is the fundamental unit of eukaryotic chromatin (29, 36). Nucleosomes form regular arrays in which the average spacing between nucleosomes normally varies, in a species- and cell type-specific manner, between 10 and 50 bp (25, 45, 54). A major consequence of packaging genomic DNA into chromatin is that nucleosomes can impede access of DNA-binding proteins to their target sequences (17, 22, 25, 56). There is no basal transcription on chromatin templates in vitro, generating an absolute requirement for factors mediating nucleosome ejection or repositioning. In specific contexts, however, nucleosomes can also play positive roles in transcription (31, 56). Thus, nucleosome dynamics provides a powerful and pervasive level of gene regulation by modulating the presentation of target DNA elements.
A number of non-mutually exclusive factors determine nucleosome distribution. (i) Intrinsic DNA sequence properties can either promote or counteract the tight wrapping of DNA around the histone octamer during nucleosome formation (9, 49–50, 54). (ii) A fixed barrier, created by a DNA binding protein or paused RNA polymerase, can have a “knock-on” effect on the positioning of more distant nucleosomes within an array. This is referred to as statistical nucleosome positioning (30, 64). (iii) Processes such as transcription involve the dynamic removal and reassembly of nucleosomes (12, 22, 25, 31, 56–57). (iv) The DNA-binding energy of some transcription factors is sufficient to exclude histones from binding (27, 63). Finally, ATP-dependent chromatin remodeling complexes (remodelers) are highly abundant chromatin-associated molecular motors that mediate the assembly, sliding, restructuring, or ejection of nucleosomes (3, 6–8, 11, 16, 23, 35, 51, 65).
The relative importance of these various mechanisms for the in vivo nucleosome organization of the genome has been the subject of debate (25, 50, 53–54, 64–65). On one side of the spectrum, a genomic code for nucleosome positioning has been proposed as the major determinant of in vivo nucleosome organization (28, 49). On the other side, dynamic utilization of chromatin within cells, combined with statistical positioning, is viewed as the dominant factor in the cellular organization of nucleosomes (64). Here, we explored the interplay between intrinsic histone-DNA sequence preferences and the cellular enzymatic machinery dedicated to nucleosome mobilization.
There are four major families of remodelers, each named after its central ATPase: SWI/SNF, ISWI, CHD/MI2, and INO80 (6, 23). Remodeler complexes of distinct classes are also characterized by unique sets of associated proteins, which provide a plethora of DNA- and histone-binding domains. In addition to the ATPases, noncatalytic subunits can determine the function of remodelers. Remodelers do not act in a generic, interchangeable manner; rather, each performs unique biological functions (6, 23, 27). Here, we assessed the relationship between remodeler action and intrinsic histone-DNA sequence preferences in chromatin organization. We found that distinct remodelers are distributed differentially and generate distinct chromatin signatures. Our results suggest that there is a class-specific antagonism between remodeler activity and DNA sequence-driven nucleosome placement.
MATERIALS AND METHODS
Cell culture, RNAi procedures, and expression analysis.
Drosophila S2 cells were cultured in Schneider's medium (21720-024; Invitrogen) and treated with double-stranded RNA (dsRNA) for 4 days as described previously (59). Double-stranded RNA was synthesized using an Ambion Megascript T7 kit according to the manufacturer's protocol. RNA interference (RNAi) knockdown experiments with BRM, SAYP, OSA, ISWI, MI2, and MEP1 were performed exactly as we described previously (4, 39, 46). For INO80 and BAP111 knockdowns, the primers INO80 (5′-CCCCCGTGCCATGGCGGAGC-3′ and 5′-GTGCGACGCCGCCTCTTGCG-3′) and BAP111 (5′-ATGGCCCTGCCAAGCAACTAC-3′ and 5′-CATATCCACGTCGGTCTTCAC-3′), flanked by the T7 promoter sequence 5′-TTAATACGACTCACTATAGGGAGA-3′, were used to synthesize dsRNA. dsRNA against green fluorescent protein (GFP) was synthesized with the primers 5′-CAAGAGTGCCATGCCCGAAGGT-3′ and 5′-TGTGGTCACGCTTTTCGTTGGG-3′ and was used for the mock knockdowns. The efficiency of RNAi knockdown was tested by immunoblotting of cell extracts with specific antibodies. Immunoblotting experiments were performed using standard procedures (39).
For microarray analysis of S2 cells, RNA was extracted using the SV total RNA isolation system (Promega) and tested on an Agilent Bioanalyzer (Agilent) as described previously (39). Labeling, hybridization, washes, and staining of microarrays were performed according to Affymetrix specifications. Statistical analysis of the microarray data was performed using R/Bioconductor packages as described previously (39). To separate genes which are consistently “ repressed” or “active” across all 14 replicas, we performed partitioning around medoids (PAM) cluster analysis implemented in R.
Protein purification and mass spectrometry.
Purifications and mass spectrometry analysis of (P)BAP complexes were performed as described previously (4–5). Briefly, Drosophila embryo or S2 cell nuclear extracts (0 to 12 h old) were incubated for 2 h at 4°C with affinity-purified anti-BRM, anti-BAP111, anti-MOR, anti-POLYBROMO, or anti-OSA antibodies coupled to protein A-Sepharose beads (catalog no. 17-0963-03; GE Healthcare). Afterward, beads were washed with HEMG buffer (25 mM HEPES-KOH [pH 7.6], 0.1 mM EDTA, 12.5 mM MgCl2, 10% glycerol) containing 400 mM KCl and 0.1% NP-40. The retained proteins were eluted with 100 mM sodium citrate buffer (pH 2.5), resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and visualized by Coomassie staining. Polypeptides were identified by mass spectrometry on an LTQ-Orbitrap hybrid mass spectrometer (ThermoFischer).
ChIP-chip, FAIRE-chip, and ChIP-qPCR assays.
Log-phase S2 cells were fixed for 10 min with 1% formaldehyde. Fixation was stopped by 125 mM glycine, cells were washed with PBS and resuspended in ice-cold lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl [pH 8.1], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], and 100 ng/ml of leupeptin and aprotinin). Cross-linked chromatin was sonicated to an apparent length of ∼300 to 500 bp (corresponding to ∼200 to 300 bp of free DNA). For Drosophila chromatin, 2 g of embryos or larvae were homogenized and fixed for 15 min in homogenization buffer (30 mM KCl, 15 mM NaCl, 2 mM MgCl2, 7.5 mM HEPES [pH 7.6], 0.5% Triton X-100, and 0.5 mM dithiothreitol) containing 2% formaldehyde. After quenching with 0.125 M glycine, the lysate was filtered through Miracloth. Isolated nuclei were resuspended in lysis buffer and sonicated as described above. Cross-linked chromatin (100 μg) was diluted with 9 volumes of dilution buffer (150 mM NaCl, 20 mM Tris-HCl [pH 8.1], 2 mM EDTA [pH 8.0], 1% Triton X-100, 0.5 mM PMSF, and 100 ng/ml of leupeptin and aprotinin), precleared with 10 μl (bed volume) protein A agarose blocked with salmon sperm DNA (16-157; Upstate), and incubated overnight at 4°C with ∼10 μl of appropriate antibodies, followed by 1 h incubation with 20 μl of preblocked protein A agarose. For mock chromatin immunoprecipitation (ChIP), chromatin was incubated with either 10 μl of preimmune serum (4 replicas) or directly with 20 μl of preblocked protein A agarose (6 replicas). Beads were washed five times with a wash buffer (20 mM Tris-HCl [pH 8.1], 2 mM EDTA [pH 8.0], 0.1% SDS, 1% Triton X-100, 0.5 mM PMSF, and 100 ng/ml of leupeptin and aprotinin) containing 150 mM NaCl and once with the wash buffer containing 500 mM NaCl. DNA was eluted with 250 μl elution buffer (1% SDS, 0.1 M NaHCO3, 500 μg/ml proteinase K) for 2 h at 37°C and overnight at 65°C. DNA was then extracted with a QIAquick PCR purification kit (catalog no. 28106; Qiagen). For formaldehyde-assisted isolation of regulatory elements (FAIRE), 100 μg of cross-linked chromatin was diluted with 9 volumes of dilution buffer, phenol-chloroform extracted and treated with RNase A (1 μg/ml) for 2 h at 37°C (15). Isolated DNA was amplified with REPLI-g (catalog no. 150025; Qiagen), digested with DNase, labeled, and hybridized on Affymetrix Drosophila tiling 2.0R arrays according to the protocols of the manufacturer.
DNA recovered from remodeler ChIP was also analyzed by quantitative PCR (ChIP-qPCR) with SYBR green I, using a Bio-Rad CFX96 real-time system. Oligonucleotide sequences used for qPCR are listed in Table S1 in the supplemental material. In addition, we performed ChIP-qPCR analysis for bxd PRE to validate histone H3 and H2B ChIPs and FAIRE (data not shown), as chromatin structure at this site shows a marked decrease in histone occupancy, which is mirrored by an increase in DNA accessibility (37).
ChIP-chip and FAIRE-chip data analysis.
Raw ChIP chip and FAIRE chip hybridization intensities were analyzed using R/Bioconductor packages. In brief, genome-wide ChIP-chip enrichment was estimated as follows. (i) Log2-scaled hybridization intensities from the input genomic DNA (5 replicas) were quantile normalized and averaged. (ii) Log2-scaled ratios of ChIP hybridization intensities to input were taken and quantile normalized, giving log2(ChIP/input)norm values. (iii) The same was done for the mock ChIP hybridization intensities, giving log2(mock/input)norm. (iv) Averaged log2(ChIP/input) and log2(mock/input) ratios were scaled by the median, and the log2(mock/input)norm was subtracted from the log2(ChIP/input)norm, giving the ChIP-chip enrichment score. It should be noted that input is not cancelled and provides a minor correction to the final ChIP score, which accounts for the differences in hybridization for distinct oligonucleotides on the array. For FAIRE-chip analysis, enrichment score was calculated as quantile-normalized averaged log2(FAIRE/input)norm ratio, as this assay does not rely on antibodies. For histone H3 and H2B ChIP-chip and FAIRE-chip analyses, the resulting enrichment scores were smoothened by running the mean on the window of 250 bp. For FAIRE-chip analysis, the results were centered by the mean.
Binding sites of ATP-dependent chromatin remodelers were selected based on false discovery rate (FDR) estimation as described by Simonis et al. (52). For (P)BAP, NURD, and ISWI remodelers, two different antibodies were used, and only sites which met the threshold of an FDR of <0.05 for both antibodies were considered positive remodeler-bound regions. For each remodeler, the resulting number of overlapping loci for two different antibodies is ∼2 times higher than overlaps obtained after random permutations of the binding sites. The INO80 binding sites were selected at an FDR of <0.01.
For DNA analysis, genomic sequences were retrieved using the Bioconductor BSgenome package and piped to external programs for the analysis. Base pair stacking energies were calculated using the data for dinucleotides from reference 43 and previously described software and algorithms (2). DNA bending anisotropy was calculated with the EMBOSS banana program using a previously described method (18). The nucleosome scoring algorithm (nucleosome score 1) for predicting nucleosome occupancies from the sequence has been described (28, 49), and genome-wide predictions were taken from the work of Kaplan et al. (28). Percus nucleosome binding energies were calculated for the Drosophila genome as described previously (34) using an n = 2 position-independent model with dinucleotide energies fitted on in vivo nucleosome data for Caenorhabditis elegans (55) or on Saccharomyces cerevisiae in vitro reconstituted nucleosome arrays (34). Since both yielded similar Percus energies landscapes (r = 0.74), we used the n = 2 position-independent model fitted on the C. elegans nucleosome profile for further calculations. Predicted nucleosome occupancies (nucleosome score 2) were calculated from the Percus energies landscape by taking into account steric exclusions between neighboring nucleosome particles (34). Scatterplots, heatmaps, correlation and autocorrelation analysis, regression analysis, and clustering were done with R/Bioconductor tools, and the full scripts will be made available upon request.
Database accession number.
Data were deposited in Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=lxonpiueyssacri&acc=GSE32404) under accession number GSE32404.
RESULTS
Different remodelers have different genome-wide distributions.
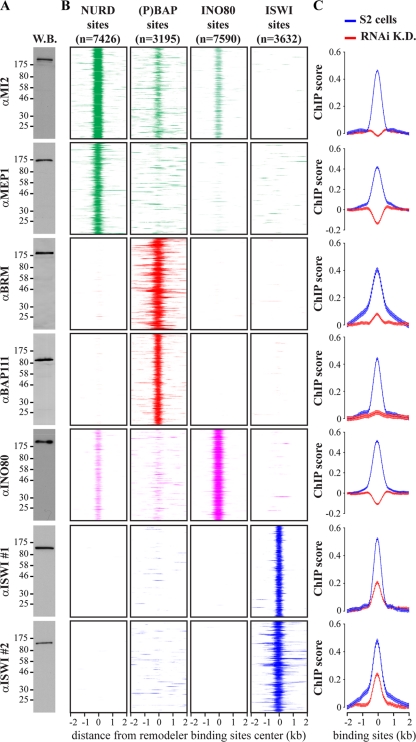
We determined the genomic binding sites in S2 cells of Drosophila remodelers representing the four major classes. These include the related BAP and PBAP [(P)BAP] remodelers representing the SWI/SNF class, the ACF, CHRAC, and NURF complexes of the ISWI class, the CHD/MI2 class, and INO80. We used specific antibodies directed against INO80, two distinct ISWI domains, and the (P)BAP common core subunits BRM and BAP111 (Fig. 1A). We also used antibodies directed against MI2 and MEP1, subunits of NURD and dMEC (32, 46). Following chromatin immunoprecipitations (ChIPs), isolated DNA fragments were mapped back to the genome by hybridization to Drosophila tiling arrays (see Fig. S1A in the supplemental material). To control for background, we subtracted hybridization intensities of mock ChIPs.
Fig 1.
Different remodelers have different genomic distributions. (A) Western blot (W.B.) analysis of embryo nuclear extracts with antibodies raised against indicated remodeler subunits. Positions of molecular weight markers are shown, in kDa. (B) Heatmaps displaying the ChIP chip enrichment on aligned NURD, (P)BAP, INO80, and ISWI loci using the indicated antibodies directed against selective remodeler subunits. Four-kilobase regions were aligned at the center of remodeler binding. The signals for the various remodelers are color coded [green, NURD; red, (P)BAP; magenta, INO80; blue, ISWI], and the numbers of binding sites are shown. (C) Subunits used for the remodelers mapping were depleted by RNAi-mediated knockdown (K.D.). Plots of the averaged ChIP chip enrichment at aligned NURD, (P)BAP, INO80, and ISWI loci are shown before (blue) and after (red) the knockdowns. Distance from the binding site center (±2 kb) is indicated. Whiskers indicate 95% confidence intervals for a mean (CI) calculated with a 50-bp steps from −2 to +2 kb from the center of remodeler loci.
All ChIPs were performed with at least two independent biological replicates. ChIP-chip peaks were selected at a false discovery rate (FDR) of <0.05 per individual antibody, based on random permutation (52). To derive NURD, (P)BAP, and ISWI binding sites, we selected only those loci that were significant for both antibodies directed against the complex. INO80 binding sites were derived from ChIPs with three biological replicates using one antibody, and peaks were selected more stringently at an FDR of <0.01. In this way, we identified 7,426 NURD loci, 3,195 (P)BAP loci, 7,590 INO80 loci, and 3,632 ISWI loci. The ChIP enrichment detected with each antibody at the aligned binding sites is shown as a heatmap (Fig. 1B). Each row of panels represents the binding of a remodeler to distinct sets of loci, corresponding to respective NURD, (P)BAP, INO80, or ISWI binding sites. The heatmaps show the well-defined binding profiles of the remodelers, with a median width ranging between ∼300 and 470 bp (see Fig. S1B in the supplemental material). They also illustrate the limited overlap in binding between distinct remodelers. Due to our stringent criteria, it is possible that the actual number of remodeler loci is higher than that reported here. To ascertain the reliability of our binding site assignments, we repeated the ChIP-chip experiments after RNAi-mediated depletion of the targeted remodeler subunits (see Fig. S2A in the supplemental material). In all cases, loss of the target protein led to a dramatic drop in ChIP signals, as shown by plotting of the averaged ChIP-chip enrichment at aligned loci (Fig. 1C). In addition, we performed ChIP-qPCR for several loci, comparing S2 cells, mock depleted cells and cells depleted of a specific remodeler (see Fig. S2B to E in the supplemental material). Together, these experiments underscore the quality of our remodeler binding site identification.
Remodeler binding sites are dispersed throughout the genome and do not display a clustered distribution. This is illustrated by a genomic view of remodeler loci across a 60-kb region of chromosome arm 3R (see Fig. S1A in the supplemental material). We did not detect substantial enrichment of remodelers at promoters or gene bodies (see Fig. S1D and E in the supplemental material). Only NURD and INO80 are enriched at promoters ∼2-fold relative to the level that would be expected by random chance (see Fig. S1D in the supplemental material). Despite stringent peak selection criteria, we observed a high genomic density of remodelers. On average, every ∼2.8 kb there is a major peak of one of the remodelers analyzed (see Fig. S1C in the supplemental material). Thus, remodelers are likely to play a major role in genomic chromatin organization.
Signature subunits are crucial for (P)BAP function.
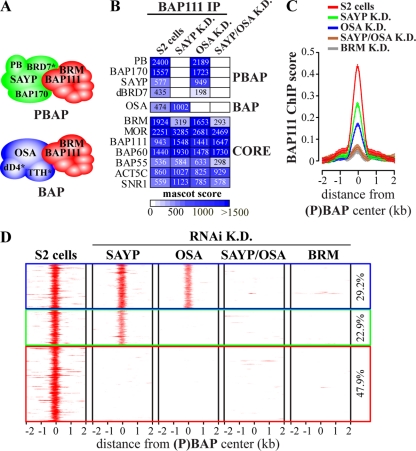
BAP and PBAP share 7 core subunits, including the BRM ATPase, but differ in a number of signature subunits (4, 38–39). Purification of (P)BAP complexes from embryo nuclear extracts revealed that BAP is composed of the core complex associated with OSA, dD4, and TTH, whereas the PBAP-specific module comprises SAYP, BAP170, POLYBROMO, and BRD7 (Fig. 2A; also, see Fig. S3A to E and G in the supplemental material). Thus, Drosophila (P)BAP complexes are closely related to their mammalian (P)BAF counterparts (20, 23, 26, 33). We also immunopurified (P)BAP from S2 cells using antibodies against BAP111. In (P)BAP from S2 cells, TTH was not detected and only few dD4 peptides were identified, suggesting that these subunits are absent or significantly substoichiometric in these cells (see Fig. S3F and G in the supplemental material).
Fig 2.
Signature subunits SAYP and OSA are essential for (P)BAP targeting. (A) The Drosophila SWI/SNF class comprises BAP and PBAP, which share the BRM ATPase and six additional core subunits but differ in selective signature subunits. BAP is composed of the core associated with OSA and the newly identified dD4 and TTH (asterisks). PBAP is formed by the core associated with SAYP, BAP170, POLYBROMO, and the newly identified BRD7 (asterisk). We note that in S2 cells, TTH is absent from (P)BAP and dD4 is incorporated into BAP at a significantly smaller amounts than in embryo purifications (see Fig. S3F and G in the supplemental material). (B) Mass spectrometry analysis of (P)BAP complexes immunopurified from S2 cells depleted of SAYP or OSA (K.D.) with antibodies against BAP111. A heatmap depicting mascot scores for (P)BAP subunits is shown. Due to their very low abundance in S2 cells, we left dD4 and TTH out of this analysis. (C) Average ChIP enrichment of BAP111 binding at (P)BAP loci before or after RNAi-mediated depletion of SAYP, OSA, SAYP plus OSA, or BRM. Distance from the (P)BAP binding center is indicated (kb). Whiskers indicate the 95% CI. (D) Heatmaps of BAP111 ChIP chip enrichment at (P)BAP binding sites before or after RNAi-mediated knockdown of signature subunits or BRM.
BAP and PBAP perform common as well as unique functions (4, 38–39). Earlier, we reported that in the absence of its signature subunits, (P)BAP is largely dysfunctional for transcription control (39). To test if this is due to defective targeting, we combined RNAi-mediated depletion of SAYP and OSA with genome-wide ChIP-chip analysis of the BAP111 core subunit. However, we first examined the effect of SAYP or OSA depletion on (P)BAP composition. Immunopurification of BAP111 followed by mass spectrometric analysis revealed that SAYP depletion or simultaneous SAYP and OSA depletion led to a reduced amount of BRM association with the remaining core assemblage (Fig. 2B). We noted that after SAYP loss, BRM was still present in S2 cells (4) (data not shown), suggesting a role for SAYP in BRM incorporation. OSA depletion did not affect PBAP composition or stability of the core assemblage. Next, we examined the effect of the loss of OSA or SAYP on (P)BAP targeting. Averaged plots of BAP111 ChIP enrichment revealed reduced binding to (P)BAP loci upon either SAYP or OSA knockdown (Fig. 2C). Simultaneous knockdown of SAYP and OSA or depletion of the BRM ATPase resulted in complete loss of BAP111 binding to (P)BAP loci. Examination of BAP111 ChIP enrichment at individual (P)BAP loci identified three types of binding sites (Fig. 2D). Type 1 sites (29.2% of all loci) require the presence of either OSA or SAYP, suggesting that BAP and PBAP bind them independently. Type 2 loci (22.9%) depend on OSA but not SAYP, indicating that they are BAP targets. Binding to type 3 loci (47.9%) depends on both OSA and SAYP, indicating cooperative binding of BAP and PBAP.
These results revealed the importance of the signature subunits for (P)BAP targeting. Loss of OSA had no effect on the composition of PBAP or the core complex but eliminated binding to ∼70% of target loci. Interpretation of the effects of SAYP depletion is confounded by its role in BRM incorporation into the complex. Nevertheless, it is clear that the signature subunits are crucial for the association of (P)BAP with the majority of its genomic targets and for (P)BAP's transcriptional output (39). Together, these observations support the notion that (P)BAP functions as a holoenzyme that depends on its signature subunits for functional specificity.
Remodeler loci have characteristic DNA sequence features.
Having identified the genomic loci of distinct remodelers, we wondered if they displayed any unique DNA sequence characteristics, which could influence nucleosome formation. First, we plotted the averaged AT content across the aligned remodeler loci (Fig. 3A). The ChIP enrichment of the indicated remodelers is shown in Fig. 3 as a filled yellow plot. We noted a marked increase in AT richness at the NURD and INO80 sites and, albeit less pronounced, at (P)BAP loci. In contrast, ISWI loci are characterized by a marked increase in GC content. Next, we plotted two sequence-dependent biophysical DNA parameters that are important for nucleosome placement: DNA bending anisotropy and base pair stacking energy (2). On average, NURD, (P)BAP, and IN080 loci are characterized by low base pair stacking energy and low bending anisotropy (Fig. 3B and C). Note that a decreased base pair stacking energy is plotted as a peak. In contrast to the other remodelers, ISWI binding sites display strong base pair stacking energy and high bending anisotropy. Next, we used an algorithm developed by Locke and colleagues (34) to infer the free energies of nucleosome formation at remodeler loci, referred to as Percus energies (Fig. 3D). The Percus energy plots for aligned remodeler loci suggest that NURD, (P)BAP, and INO80 loci are less favorable for nucleosome placement than average. In contrast, the average ISWI locus is predicted to favor nucleosome formation.
Fig 3.
Remodeler loci display characteristic DNA sequence and predicted histone-binding profiles. (A) Average AT content of aligned NURD (green), (P)BAP (red), INO80 (magenta), and ISWI (blue) binding sites. Average ChIP-chip enrichment scores for MI2, BAP111, INO80, and ISWI (antibody 1) on corresponding remodeler loci are shown as filled yellow plots. (B) Plot of the averaged DNA base pair stacking energy (ΔG/base step [kcal/mol]) of aligned remodeler loci. (C) Averaged intrinsic DNA bending anisotropy of aligned remodeler loci. (D) Plot of the averaged nucleosome binding energy (Percus energy) of aligned remodeler loci. Nucleosome binding energy was estimated from the sequence for each remodeler locus as described by Locke et al. (34). (E) Plot of the averaged nucleosome score of aligned remodeler loci based on a genome-wide prediction made as described by Kaplan et al. (28) (here, referred to as nucleosome score 1). Nucleosome score 1 provides a prediction of nucleosome occupancy. (F) Plot of the averaged nucleosome score 2 of aligned remodeler loci based on the prediction of nucleosome occupancy derived from the Percus nucleosome binding energy shown above, as described by Locke et al. (34). For panels A to F, distance from the binding sites center is indicated in kb and whiskers indicate 95% CI. For panels A to F, values were scaled to 0 for the genomic average and shown as arbitrary units (a.u.).
We applied two models that were developed to predict nucleosome occupancy and positioning. First, we used a probabilistic model that calculates a “nucleosome score” based on results of in vitro nucleosome deposition by salt dialysis to a wide variety of different DNA sequences (49). One key feature determining high nucleosome occupancy in this model is the periodic distribution of AA/TT/TA dinucleotides facing the histone core, alternating with GC dinucleotides facing out. A high nucleosome score (referred to here as 1) represents a good fit to the sequence periodicity of octamer binding sites and predicts a high probability for the formation of well-positioned nucleosomes (28, 49). Nucleosome score 2 was derived from an alternative model in which nucleosome placement is inferred from the Percus energies by taking into account steric exclusion between neighboring particles (34). Applying both algorithms, we plotted the averaged nucleosome scores of the aligned remodeler loci (Fig. 3E and F). Both models yielded similar results: the nucleosome scores decrease sharply at NURD and INO80 loci and also drop at (P)BAP loci. In contrast, the nucleosome scores peak at the aligned ISWI sites. Thus, according to these models, the average NURD, (P)BAP, and INO80 loci intrinsically disfavor assembly of well-positioned nucleosomes, whereas ISWI sites are predicted to be favorable for such nucleosome formation. On the other hand, lower base pair stacking energies diminish the deformation energy required for DNA wrapping. Thus, it is important to realize that these sequences would also be expected to form nucleosomes but with decreased precision of positioning (2, 60). We note that the various parameters discussed here are not fully independent of each other, as is clear from the genome-wide correlations between them (see Fig. S4 in the supplemental material). We conclude that remodeler loci display class-specific DNA sequence characteristics that are known to influence nucleosome formation.
Remodelers differentially antagonize histone-DNA sequence preferences.
To test the role of remodelers in determining chromatin architecture at their binding sites, we determined nucleosome occupancy and DNA accessibility before and after RNAi-mediated depletion of their ATPases. We chose sonication over micrococcal nuclease (MNase) digestion to fragment chromatin for the following reasons. (i) Sonication allowed us to isolate both nucleosome-bound and free DNA. (ii) Compared to sonication, MNase cleavage is biased toward AT-rich sequences (24, 34). (iii) Extensive MNase digestion affects identification of remodeled nucleosomes (61). We performed ChIP-chip assays using antibodies directed against histones H3 and H2B to determine nucleosome occupancy. Below, we show only the H3 results because those obtained for H2B were similar (see Fig. S5A in the supplemental material). In parallel, we used FAIRE-chips, which rely on a phenol-chloroform extraction of uncross-linked DNA to independently map accessible genomic regions depleted of nucleosomes (15). FAIRE showed a robust negative correlation with histone occupancy, and both techniques identified open chromatin at active promoters (see Fig. S5A and C in the supplemental material). In addition, autocorrelation analysis revealed that histone ChIP-chip and FAIRE-chip profiles are highly comparable across all samples and differ significantly from the “white” noise (see Fig. S5B in the supplemental material). Note that we measured nucleosome occupancy, not precise positioning. Nucleosome occupancy refers to the average density of histones at a given genomic location but does not reflect the strict rotational or translational position of nucleosomes. Nonetheless, with regard to DNA accessibility for transcription factors, local occupancy provides the relevant measure of chromatin state (17).
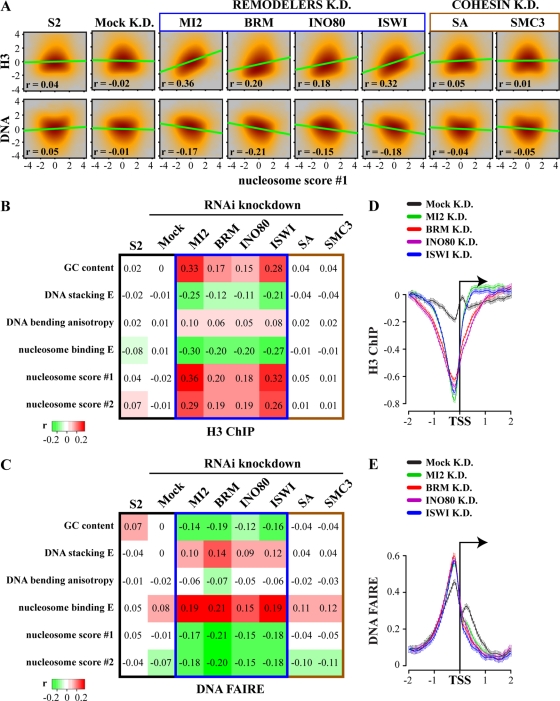
We plotted the averaged H3 ChIP signals against the aligned remodeler binding loci before and after remodeler depletion and after treatment with an irrelevant dsRNA (Fig. 4A). MI2 depletion caused a massive loss of H3 at NURD sites. We also observed a strongly reduced average nucleosome occupancy at (P)BAP and INO80 loci, following depletion of their respective ATPases. In contrast, ISWI depletion caused a strong increase in histone density. Complementing these results, DNA becomes more accessible at NURD, (P)BAP, and INO80 loci, while at ISWI loci the strong peak in DNA accessibility was completely lost upon the knockdown of their respective ATPases (Fig. 4B). As expected, mock depletion did not cause appreciable changes in chromatin structure at remodeler loci. We conclude that different remodelers differentially regulate nucleosome occupancy and DNA accessibility. On average, the net effect of NURD, (P)BAP, or INO80 activity is to increase the nucleosome density at their binding sites. In contrast, ISWI removes nucleosomes from its loci. We note that although in general, nucleosome occupancy correlates negatively with DNA accessibility, these two measures of chromatin state do not mirror each other at all loci. These observations might reflect the presence of noncanonical, remodeled nucleosomes or increased nucleosome turnover (7, 10–11, 51).
Fig 4.
Remodelers alter chromatin structure at their binding sites in a class-specific manner. (A) Average histone H3 occupancy at NURD, (P)BAP, INO80 and ISWI binding sites in S2 cells (blue) or after RNAi knockdown (K.D.) of their respective ATPase subunits (red) or mock RNAi knockdown (gray). On average, depletion of NURD, (P)BAP, and INO80 ATPases causes nucleosome loss at their binding sites, whereas ISWI knockdown results in gain of nucleosome occupancy. Distance from the binding site center is indicated, in kb. Whiskers indicate 95% CI. Values were scaled to 0 for the genomic average. (B) Average DNA accessibility, as determined by FAIRE-chips at remodeler loci in S2 cells (blue) or after knockdown of their respective ATPases (red) or mock knockdown (gray). (C) Correlations between H3 and DNA accessibility profiles at remodeler loci (±250 bp from the binding site center) and predicted nucleosome occupancy scores and biophysical DNA parameters. S2 cells were either mock treated with dsRNA against GFP (mock) or depleted of the indicated remodeler ATPases by RNAi. H3 and DNA accessibility profiles of S2 cells display correlations with DNA parameters at the remodeler loci similar to those of mock-treated cell chromatin profiles (data not shown).
There is a striking relationship between nucleosome repositioning due to remodeler loss and DNA sequence properties. After depletion, nucleosomes at remodeler loci move in accordance with intrinsic histone-DNA sequence preferences, which is reflected by an increased correlation between histone occupancy, DNA accessibility and DNA parameters (Fig. 4C; also, see Fig. S5D in the supplemental material). In other words, depletion of NURD, (P)BAP, or INO80 activity generally caused a decrease in nucleosome density at their binding sites, which disfavor positioned nucleosome formation. Conversely, ISWI depletion resulted in higher nucleosome occupancy at its loci, which favor precise nucleosome placement. These results suggest that remodelers antagonize the intrinsic nucleosome formation properties of the DNA sequence at their binding loci.
Remodelers impact global nucleosome organization.
Given their high genomic density, we wondered if remodelers influence global chromatin organization. We found that depletion of remodeler ATPases also affected nucleosome organization at nontarget loci. Remodeler loss resulted in increased correlations between DNA sequence-based predictions and the experimentally determined histone occupancy and DNA accessibility (see Fig. S6 in the supplemental material). In fact, remodeler-dependent nucleosome repositioning changed the genome-wide correlation between DNA sequence-derived predictions and actual nucleosome occupancy. A scatterplot comparison of the genome-wide histone density or DNA accessibility prior to remodeler depletion versus the nucleosome score 1 (Fig. 5A), or other DNA sequence parameters (Fig. 5B and C), revealed the absence of any appreciable correlation. In other words, none of these sequence parameters appears to be a major determinant of global nucleosome occupancy in S2 cells or mock-treated cells. Following depletion of individual remodelers, however, we observed a clear increase in correspondence between DNA sequence parameters and the experimentally determined genome-wide nucleosome density (Fig. 5A to C; also, see Fig. S7A in the supplemental material). We excluded the possibility that these changes were due to cell cycle effects. Whereas both loss of BRM and depletion of cohesin subunit SA or SMC3 caused a G2/M arrest, only BRM depletion affected nucleosome distribution (Fig. 5A to C; also, see Fig. S7 in the supplemental material). Depletion of other remodelers did not change the cell cycle profiles significantly (see Fig. S7B in the supplemental material). Finally, simultaneous depletion of SAYP and OSA, causing loss of (P)BAP (Fig. 2), caused a chromatin reorganization similar to that observed with loss of BRM (see Fig. S7A in the supplemental material).
Fig 5.
Remodelers counteract genome-wide DNA sequence-driven nucleosome organization. (A) Remodeler depletion strongly increases the correlation between DNA sequence-derived nucleosome occupancy predictions and experimentally determined endogenous nucleosome organization. Smooth scatterplots of genome-wide histone H3 density or DNA accessibility versus nucleosome score 1 for cells depleted of the indicated remodeler ATPase are shown. The cohesin subunits stromalin (SA) and SMC3, as well as GFP (mock), were depleted as controls. Correlations (r) and linear regression lines are shown. (B and C) Heatmaps of the correlations between experimentally determined chromatin structure and DNA sequence-derived predictions genome-wide. Correlation between a variety of DNA sequence parameters and genome-wide H3 ChIP-chip occupancy (B) and FAIRE-chip-derived DNA accessibility profiles (C), before (S2 cells) or after depletion of the indicated remodeler ATPases, is shown. SA, SMC3, and GFP (mock) were depleted as controls. (D and E) Remodeler depletion affect chromatin conformation at promoters. Plots of averaged H3 occupancy (D) and DNA accessibility (E) at promoters of S2 cells, which were either mock treated (mock K.D.) or depleted of the indicated remodeler ATPases, are shown. Distance to the TSS is indicated, and whiskers for 95% CI are shown.
We conclude that remodelers impact the genome-wide nucleosome distribution. This also has consequences for chromatin organization at promoters, in spite of the lack of significant remodeler enrichment there (see Fig. S1D and E in the supplemental material). On average, DNA sequences just upstream of the transcription start site (TSS) disfavor nucleosome placement (see Fig. S8 in the supplemental material). In agreement with the antagonism between remodelers and intrinsic histone-DNA sequence preferences, remodeler depletion caused the overall displacement of nucleosomes from promoters and increased DNA accessibility (Fig. 5D and E). As expected, this reflects an increased correlation between DNA sequence-based predictions at promoters and the determined chromatin structure (data not shown).
In summary, remodeler depletion reveals the influence of predicted histone-DNA sequence preferences on genomic nucleosome organization. Therefore, remodelers do not simply facilitate the formation of nucleosomes at intrinsically favored positions. Rather, they counteract and overrule DNA sequence effects on chromatin structure. This translates into a global effect on chromatin organization because of the high genomic density of remodeler sites and statistical nucleosome positioning.
Intrinsic histone-DNA sequence preferences are overruled during development.
Are there in vivo conditions where the influence of DNA sequence on global nucleosome placement would be more dominant? In many metazoans, the early stages of development are characterized by rapid, synchronous cycles of replication and mitosis and the absence of transcription. Midblastula transition (MBT) is a developmental switch that marks the onset of zygotic transcription and progressive restriction of cellular divisions. In Drosophila, this occurs ∼2 h after oviposition, when the first 13 synchronous nuclear divisions have been completed. To follow the relationship between DNA sequence and nucleosome organization during development, we compared chromatin of 0- to 1-h embryos with that of later embryonic and larval stages. Autocorrelation analysis of histone ChIP-chip profiles for different developmental stages revealed similar types of oscillations, suggesting that the quality of these profiles is comparable (see Fig. S9A in the supplemental material). Early embryonic chromatin showed a remarkably robust correlation with DNA sequence properties, as revealed by a scatterplot of genome-wide H3 ChIP signals versus nucleosome score 1 (Fig. 6A). Likewise, there was a strong correlation between H3 density and other DNA parameters (Fig. 6B). The genome-wide DNA accessibility profile also showed the strong influence of DNA sequence on chromatin organization in pre-MBT embryos. Following MBT, the impact of intrinsic DNA sequence properties on nucleosome placement gradually disappeared. This appears to happen even faster when DNA accessibility is being monitored, which might be due to the binding of nonhistone proteins, such as the transcription machinery. We note that tissue heterogeneity might be a confounding factor in our developmental chromatin analysis. However, in contrast to S2 cells, which are late embryonic cells, the earlier embryonic Kc cells do show a modest but significant correlation with DNA parameters. For example, our analysis of genome-wide nucleosome organization by histone ChIPs and FAIRE revealed that the correlation between H3 density and nucleosome score 1 is 0.26 for Kc cells but only 0.03 for S2 cells (see Fig. S9B in the supplemental material). Thus, the nucleosomal organization of Kc and S2 cells seems to reflect the developmental stage from which they were derived.
Fig 6.
Global changes in chromatin architecture during Drosophila development. (A) The correlation between the DNA sequence-predicted nucleosome occupancy and endogenous chromatin organization disappears during development. Smooth scatterplots of genome-wide H3 density and DNA accessibility versus nucleosome score 1 at different developmental stages are shown. Chromatin was analyzed in Drosophila embryos 0 to 1 h, 2 to 12 h or 21 to 24 h after oviposition and in 3rd-instar larvae. Midblastula transition (MBT) is about 2 h after oviposition. MBT marks the transition from rapid, synchronous cycles of replication and mitosis to progressive restriction of mitotic domains and the onset of zygotic transcription. Correlations (r) and linear regression lines are shown. (B) Correlations of DNA sequence parameters with the experimentally determined genome-wide histone H3 occupancy and DNA accessibility during Drosophila development. Whereas in 0- to 1-h embryos, chromatin organization clearly correlates well with DNA sequence-derived predictions, this correspondence disappears later in development. (C) Prior to zygotic transcription, promoters have an open chromatin conformation. Average H3 enrichment (blue) and DNA accessibility (green) at promoters at different developmental stages are shown. Distance to the TSS is indicated, and whiskers for 95% CI are shown.
We also monitored general promoter accessibility through development. Reflecting that on average promoter sequences disfavor nucleosome placement, we found that promoters in 0- to 1-h embryos were nucleosome depleted and highly accessible (Fig. 6C). After MBT, DNA accessibility at the TSS is lost rapidly, accompanied by a more gradual increase in nucleosome occupancy. Our results suggest that intrinsic histone-DNA sequence preferences significantly influence nucleosome organization of fast-replicating, nontranscribed chromatin. Paradoxically, while the genome is silent prior to MBT, promoters have an open, highly accessible chromatin conformation. Following MBT, when replication becomes restricted and the genome differentially expressed, the influence of DNA sequence properties on nucleosome density becomes blurred and eventually is no longer detectable.
DISCUSSION
We explored the effect of ATP-dependent remodelers on the nucleosomal organization of the Drosophila genome. We analyzed the molecular mechanisms underlying the functional specialization of remodelers. We found that remodelers of different classes have unique sets of targets and generate distinct chromatin signatures. Next, we explored the relationship between DNA sequence, histone occupancy, and remodeler activity. A priori, remodelers might act as regular enzymes and accelerate nucleosome (dis)assembly, without changing the thermodynamically preferred locations dictated by the underlying DNA sequence. Alternatively, in addition to stimulating the rate of the (dis)assembly reaction, remodelers might determine nucleosome positions. Our results favor the latter model. Remodelers determine cellular chromatin structure by counteracting intrinsic DNA-driven nucleosome placement preferences. We propose that the antagonism between remodeler action and DNA sequence effects on nucleosome formation is a crucial component of chromatin dynamics.
Remodeler targeting.
Remodelers are highly enriched at defined loci (40, 41, 48; also this study). There appears to be no single mechanism that directs remodelers to their target sites. For example, recruitment through binding to sequence-specific transcription factors or by recognition of specific histone modifications are both well established (6, 23). Although the observation was outside the scope of this study, we found that loci of distinct remodelers display characteristic histone modification patterns (data not shown). This supports the idea of cross talk between remodelers and histone-modifying enzymes. We found that the signature subunits SAYP and OSA are crucial for correct targeting of (P)BAP to the majority of binding loci. These results support our earlier hypothesis, based on the effects of subunit depletion on genome-wide gene expression, that (P)BAP complexes act as holoenzymes (4, 39).
Remodeler loci display striking DNA sequence features. Thus far, the role of direct DNA binding by remodelers in recruitment has received little attention. However, remodelers harbor multiple DNA binding domains, including AT hooks, SANT domains, HMG boxes, and zinc fingers. SWI/SNF can bind both naked and nucleosomal DNA, and extensive DNA interactions have been identified (1, 6, 23, 44). Likewise, ISWI recognizes intrinsically curved DNA sequences via its SANT and SLIDE domains (47, 62). Although sequence specificity might be relaxed and the binding of remodelers to chromatin dynamic, we suspect that direct recognition of DNA sequence or structure (e.g., flexibility, intrinsic bending, etc.) contributes significantly to remodeler targeting.
Remodelers alter relative affinities of DNA sequences for histone octamer binding.
The contribution of intrinsic histone-DNA sequence preferences to in vivo nucleosome organization is debated, because experimental tests of this hypothesis have yielded variable results (25, 28, 53, 55, 64–65). Here, we identified remodelers as factors that obscure intrinsic DNA sequence effects on nucleosome positioning in vivo. Remodeler binding sites display defined DNA sequence properties. On average, NURD and INO80 sites, and to a lesser degree (P)BAP loci, are AT rich and have a low base pair stacking energy, low DNA bending anisotropy, and a low predicted nucleosome binding energy. ISWI sites are characterized by a high GC content, strong base pair stacking energy, high DNA bending anisotropy, and high nucleosome binding energy.
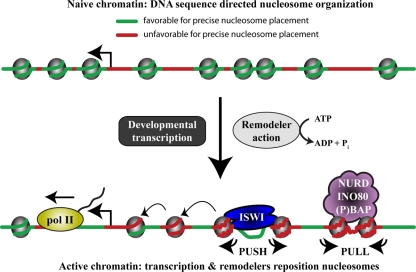
Two distinct probabilistic models similarly predict that (P)BAP, INO80, and NURD loci disfavor positioned nucleosome formation. In contrast, ISWI binding sites are expected to favor precise nucleosome placement. Although each remodeler class analyzed has characteristic properties, we can broadly recognize two modes of chromatin remodeling (Fig. 7). The common feature of NURD, (P)BAP, and INO80 is that all three “pull” nucleosomes onto DNA sequences that are unfavorable for intrinsic positioning. In contrast, ISWI “pushes” nucleosomes away from DNA sequences that favor precise nucleosome placement. In vitro, ACF has been shown to diminish the effects of intrinsic histone-DNA interactions (47, 62, 64). Our in vivo results with ISWI are expected to reflect the activity of both ACF/CHRAC and NURF. However, we suspect that the influence of ACF/CHRAC-mediated de novo chromatin assembly after replication (13) will be limited in our analysis because only a minority of the S2 cells are in S phase.
Fig 7.
Model for remodeler action. A simplified model illustrating the major conclusions from this study is shown. Replication-coupled histone deposition during early development favors DNA sequences that are characterized by strong base pair stacking energy and high bending anisotropy (green). These sequences can adopt the nucleosomal DNA trajectory, thus minimizing the entropic penalty for binding. Following the start of developmental gene expression, the combined activities of the transcription machinery and remodelers drive nucleosome repositioning. Consequently, the influence of DNA sequence properties on nucleosome organization density diminishes dramatically. The collective result of remodeler action is to increase nucleosome formation on flexible sequences with low base pair stacking energy (red). Wrapping these DNA sequences around the histone octamer requires lower deformation energy, but this would be expected to decrease the precision of nucleosome positioning and might facilitate the generation of remodeled nucleosomes. Although each remodeler class has its own characteristics, there are two main modes of chromatin remodeling. NURD, (P)BAP, and INO80 pull nucleosomes onto DNA sequences that are intrinsically unfavorable for positioning. In contrast, ISWI pushes nucleosomes away from its binding loci that innately favor nucleosome placement. Finally, remodeling can propagate from target loci and reposition neighboring nucleosomes, referred to as statistical positioning.
Remodeled nucleosomes are prone to MNase digestion, suggesting that in spite of having a high spatial resolution, most of the current nucleosomal maps might be biased toward identification of tightly wrapped, well-positioned nucleosomes (34, 54, 61). Consequently, this bias will influence nucleosome-predicting algorithms trained on such maps. Thus, there seems to be a trade-off between high-resolution mapping and the identification of a larger portion of nucleosomes. Limited MNase digestion or sonication has a lower resolution but is expected to better capture loosely positioned and remodeled nucleosomes. Future studies should provide a more definitive distinction between “naive” and remodeled chromatin (61).
Our conclusions, based on genome-wide remodeler mapping and analysis of endogenous chromatin, dovetail well with selective studies by others (21, 42, 47, 58, 65). These analyses provided examples of remodeler-mediated nucleosome repositioning away from strong nucleosome positioning sequences, although the position of the remodelers on the chromatin template was not considered in these studies. Based on our genome-wide analysis of chromatin at remodeler loci, we conclude that remodelers use the energy derived from ATP hydrolysis to actively reposition nucleosomes, counteracting innate nucleosome sequence preferences.
Remodelers and genomic nucleosome organization.
Remodelers are abundant and bind chromatin at a high density. The high-confidence binding peaks of the remodelers analyzed here have a median spacing of only ∼2.8 kb. In other words, in Drosophila, for every ∼14 to 18 nucleosomes there is at least one remodeler complex present, which conforms to estimations of remodeler abundance in yeast (14, 47). Within a nucleosomal array, a remodeling event is expected to have a knock-on effect on more distant nucleosomes. Indeed, remodeler depletion in S2 cells caused global nucleosome repositioning and a remarkable shift from a negligible correlation between nucleosome organization and the nucleosome score, to a robust positive one. Combined, the high genomic density of remodelers and statistical nucleosome positioning provides a plausible explanation for the global impact of remodelers on chromatin. In our view, the physiological nucleosome landscape is the resultant of the balance between intrinsic DNA affinities for histones and active nucleosome repositioning by remodelers and the transcription machinery.
This balance changes dramatically during Drosophila development. In rapidly proliferating early Drosophila embryos, prior to expression of the zygotic genome, the relationship between DNA sequence and nucleosome occupancy is readily detected. Following the start of zygotic transcription and cell differentiation, however, this correlation becomes obscured. During early development promoters have an open chromatin conformation, reflecting their DNA sequence properties. This allows easy access for early transcription factors to initiate zygotic transcription. Once differential expression of the genome commences, nucleosomes will reposition due to the combined activities of the transcription machinery, remodelers and histone modifying enzymes. Diversified chromatin states will then help to reinforce gene expression programs. A recent study found that introduction of a strong synthetic nucleosome positioning sequence in mice initially drove nucleosome formation on this sequence but that this effect disappeared during development (19). These, and related observations (53, 57–58, 64), indicate that differential gene expression has a major impact on cellular chromatin and overrules DNA sequence-instructed nucleosome organization.
Concluding remarks.
Sequence selection by the histone octamer depends strongly on DNA bending anisotropy and on DNA deformability (54). It is important to realize that the relative contribution of DNA parameters to nucleosome formation in vitro is contingent on conditions such as temperature or histone concentration (60). Consequently, sequence-dependent affinity is an ambiguous term because it depends on the mechanism of assembly. Keeping this caveat in mind, in our view, both de novo cellular and in vitro nucleosome assembly favor DNA sequences that are characterized by strong base pair stacking energy and high bending anisotropy. These sequences can adopt the nucleosomal DNA trajectory while minimizing the entropic penalty for binding. We found that the net result of remodeler action is to increase nucleosome formation on flexible sequences with low base pair stacking energy (Fig. 7). Wrapping these DNA sequences around the histone octamer requires lower deformation energy, making the enthalpic contribution to binding energy more significant. This would be expected to decrease the precision of nucleosome positioning and could allow the generation of remodeled nucleosomes involving loosening of histone-DNA contacts (3, 8, 11, 35, 51), as well as changes in nucleosome turnover (7). In summary, our results provide a framework for further studies to understand the molecular mechanisms of remodeling and how these control chromatin organization and diverse biological pathways.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jurg Muller for the generous gift of anti-INO80 antibodies, Jesper Svejstrup for insightful discussions and suggestions, Robert-Jan Palstra for suggesting FAIRE, and Tokameh Mahmoudi and Rob Maeda for comments on the manuscript.
This work was supported by an EU grant (EUtracc; C.P.V.), an EMBO fellowship (Y.M.M.). A.A.T. thanks l'Agence Nationale de la Recherche for an award of a Chaire d'Excellence.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Badis G, et al. 2008. A library of yeast transcription factor motifs reveals a widespread function for Rsc3 in targeting nucleosome exclusion at promoters. Mol. Cell 32:878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caserta M, et al. 2009. A translational signature for nucleosome positioning in vivo. Nucleic Acids Res. 37:5309–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaban Y, et al. 2008. Structure of a RSC-nucleosome complex and insights into chromatin remodeling. Nat. Struct. Mol. Biol. 15:1272–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chalkley GE, et al. 2008. The transcriptional coactivator SAYP is a trithorax group signature subunit of the PBAP chromatin remodeling complex. Molecular Cell. Biol. 28:2920–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalkley GE, Verrijzer CP. 2004. Immuno-depletion and purification strategies to study chromatin-remodeling factors in vitro. Methods Enzymol. 377:421–442 [DOI] [PubMed] [Google Scholar]
- 6. Clapier CR, Cairns BR. 2009. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 78:273–304 [DOI] [PubMed] [Google Scholar]
- 7. Deal RB, Henikoff JG, Henikoff S. 2010. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science 328:1161–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dechassa ML, et al. 2010. SWI/SNF has intrinsic nucleosome disassembly activity that is dependent on adjacent nucleosomes. Mol. Cell 38:590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drew HR, Travers AA. 1985. DNA bending and its relation to nucleosome positioning. J. Mol. Biol. 186:773–790 [DOI] [PubMed] [Google Scholar]
- 10. Engeholm M, et al. 2009. Nucleosomes can invade DNA territories occupied by their neighbors. Nat. Struct. Mol. Biol. 16:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Floer M, et al. 2010. A RSC/nucleosome complex determines chromatin architecture and facilitates activator binding. Cell 141:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuda NJ, Ardehali MB, Lis JT. 2009. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fyodorov DV, Blower MD, Karpen GH, Kadonaga JT. 2004. Acf1 confers unique activities to ACF/CHRAC and promotes the formation rather than disruption of chromatin in vivo. Genes Dev. 18:170–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghaemmaghami S, et al. 2003. Global analysis of protein expression in yeast. Nature 425:737–741 [DOI] [PubMed] [Google Scholar]
- 15. Giresi PG, Kim J, McDaniell RM, Iyer VR, Lieb JD. 2007. FAIRE (formaldehyde-assisted isolation of regulatory elements) isolates active regulatory elements from human chromatin. Genome Res. 17:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gkikopoulos T, et al. 2011. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science 333:1758–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goh WS, Orlov Y, Li J, Clarke ND. 2010. Blurring of high-resolution data shows that the effect of intrinsic nucleosome occupancy on transcription factor binding is mostly regional, not local. PLoS Comp. Biol. 6:e1000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodsell DS, Dickerson RE. 1994. Bending and curvature calculations in B-DNA. Nucleic Acids Res. 22:5497–5503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gracey LE, et al. 2010. An in vitro-identified high-affinity nucleosome-positioning signal is capable of transiently positioning a nucleosome in vivo. Epigenet. Chromatin 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hargreaves DC, Crabtree GR. 2011. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 21:396–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartley PD, Madhani HD. 2009. Mechanisms that specify promoter nucleosome location and identity. Cell 137:445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henikoff S. 2008. Nucleosome destabilization in the epigenetic regulation of gene expression. Nat. Rev. 9:15–26 [DOI] [PubMed] [Google Scholar]
- 23. Ho L, Crabtree GR. 2010. Chromatin remodelling during development. Nature 463:474–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Horz W, Altenburger W. 1981. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 9:2643–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang C, Pugh BF. 2009. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. 10:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaeser MD, Aslanian A, Dong MQ, Yates JR, 3rd, Emerson BM. 2008. BRD7, a novel PBAF-specific SWI/SNF subunit, is required for target gene activation and repression in embryonic stem cells. J. Biol. Chem. 283:32254–32263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kal AJ, Mahmoudi T, Zak NB, Verrijzer CP. 2000. The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev. 14:1058–1071 [PMC free article] [PubMed] [Google Scholar]
- 28. Kaplan N, et al. 2009. The DNA-encoded nucleosome organization of a eukaryotic genome. Nature 458:362–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kornberg RD. 1977. Structure of chromatin. Annu. Rev. Biochem. 46:931–954 [DOI] [PubMed] [Google Scholar]
- 30. Kornberg RD, Stryer L. 1988. Statistical distributions of nucleosomes: nonrandom locations by a stochastic mechanism. Nucleic Acids Res. 16:6677–6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kristjuhan A, Svejstrup JQ. 2004. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 23:4243–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kunert N, Brehm A. 2009. Novel Mi-2 related ATP-dependent chromatin remodelers. Epigenetics 4:209–211 [DOI] [PubMed] [Google Scholar]
- 33. Lange M, et al. 2008. Regulation of muscle development by DPF3, a novel histone acetylation and methylation reader of the BAF chromatin remodeling complex. Genes Dev. 22:2370–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Locke G, Tolkunov D, Moqtaderi Z, Struhl K, Morozov AV. 2010. High-throughput sequencing reveals a simple model of nucleosome energetics. Proc. Natl. Acad. Sci. U. S. A. 107:20998–21003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lorch Y, Maier-Davis B, Kornberg RD. 2010. Mechanism of chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A. 107:3458–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. 1997. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389:251–260 [DOI] [PubMed] [Google Scholar]
- 37. Mohd-Sarip A, et al. 2006. Architecture of a polycomb nucleoprotein complex. Mol. Cell 24:91–100 [DOI] [PubMed] [Google Scholar]
- 38. Mohrmann L, et al. 2004. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol. Cell. Biol. 24:3077–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moshkin YM, Mohrmann L, van Ijcken WF, Verrijzer CP. 2007. Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Mol. Cell. Biol. 27:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ng HH, Robert F, Young RA, Struhl K. 2002. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes Dev. 16:806–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parnell TJ, Huff JT, Cairns BR. 2008. RSC regulates nucleosome positioning at Pol II genes and density at Pol III genes. EMBO J. 27:100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pham CD, He X, Schnitzler GR. 2010. Divergent human remodeling complexes remove nucleosomes from strong positioning sequences. Nucleic Acids Res. 38:400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Protozanova E, Yakovchuk P, Frank-Kamenetskii MD. 2004. Stacked-unstacked equilibrium at the nick site of DNA. J. Mol. Biol. 342:775–785 [DOI] [PubMed] [Google Scholar]
- 44. Quinn J, Fyrberg AM, Ganster RW, Schmidt MC, Peterson CL. 1996. DNA-binding properties of the yeast SWI/SNF complex. Nature 379:844–847 [DOI] [PubMed] [Google Scholar]
- 45. Rando OJ, Chang HY. 2009. Genome-wide views of chromatin structure. Annu. Rev. Biochem. 78:245–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reddy BA, et al. 2010. Drosophila transcription factor Tramtrack69 binds MEP1 to recruit the chromatin remodeler NuRD. Mol. Cell. Biol. 30:5234–5244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rippe K, et al. 2007. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl. Acad. Sci. U. S. A. 104:15635–15640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sala A, et al. 2011. Genome-wide characterization of chromatin binding and nucleosome spacing activity of the nucleosome remodelling ATPase ISWI. EMBO J. 30:1766–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Segal E, et al. 2006. A genomic code for nucleosome positioning. Nature 442:772–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Segal E, Widom J. 2009. What controls nucleosome positions? Trends Genet. 25:335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shukla MS, et al. 2010. Remosomes: RSC generated non-mobilized particles with approximately 180 bp DNA loosely associated with the histone octamer. Proc. Natl. Acad. Sci. U. S. A. 107:1936–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simonis M, et al. 2006. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 38:1348–1354 [DOI] [PubMed] [Google Scholar]
- 53. Stein A, Takasuka TE, Collings CK. 2010. Are nucleosome positions in vivo primarily determined by histone-DNA sequence preferences? Nucleic Acids Res. 38:709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Travers A, Hiriart E, Churcher M, Caserta M, Di Mauro E. 2010. The DNA sequence-dependence of nucleosome positioning in vivo and in vitro. J. Biomol. Struct. Dynamics 27:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Valouev A, et al. 2008. A high-resolution, nucleosome position map of C. elegans reveals a lack of universal sequence-dictated positioning. Genome Res. 18:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weake VM, Workman JL. 2010. Inducible gene expression: diverse regulatory mechanisms. Nat. Rev. 11:426–437 [DOI] [PubMed] [Google Scholar]
- 57. Weiner A, Hughes A, Yassour M, Rando OJ, Friedman N. 2010. High-resolution nucleosome mapping reveals transcription-dependent promoter packaging. Genome Res. 20:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Whitehouse I, Tsukiyama T. 2006. Antagonistic forces that position nucleosomes in vivo. Nat. Struct. Mol. Biol. 13:633–640 [DOI] [PubMed] [Google Scholar]
- 59. Worby CA, Simonson-Leff N, Dixon JE. 2001. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci. STKE 2001:PL1. [DOI] [PubMed] [Google Scholar]
- 60. Wu C, Travers A. 2005. Relative affinities of DNA sequences for the histone octamer depend strongly upon both the temperature and octamer concentration. Biochemistry 44:14329–14334 [DOI] [PubMed] [Google Scholar]
- 61. Xi Y, Yao J, Chen R, Li W, He X. 2011. Nucleosome fragility reveals novel functional states of chromatin and poises genes for activation. Genome Res. 21:718–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamada K, et al. 2011. Structure and mechanism of the chromatin remodelling factor ISW1a. Nature 472:448–453 [DOI] [PubMed] [Google Scholar]
- 63. Zaret KS, et al. 2008. Pioneer factors, genetic competence, and inductive signaling: programming liver and pancreas progenitors from the endoderm. Cold Spring Harbor Symp. Quant. Biol. 73:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Y, et al. 2009. Intrinsic histone-DNA interactions are not the major determinant of nucleosome positions in vivo. Nat. Struct. Mol. Biol. 16:847–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Z, et al. 2011. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science 332:977–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.