Abstract
Background and Purpose
Silent brain infarctions are associated with an increased risk of stroke in healthy individuals. Risk of recurrent stroke in patients with both symptomatic and silent brain infarction (SBI) has only been investigated in patients with cardioembolic stroke in the European Atrial Fibrillation Trial. We assessed whether patients with recent non-cardioembolic stroke and SBI detected on MRI are at increased risk for recurrent stroke, other cardiovascular events, and mortality.
Methods
The prevalence of SBI detected on MRI was assessed in 1014 patients enrolled in the imaging substudy of the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial. The primary outcome was first recurrence of stroke in patients with both symptomatic stroke and SBI in comparison with age and sex matched stroke patients without SBI. Secondary outcomes were a combined vascular endpoint, other vascular events and mortality. The two groups were compared using conditional logistic regression.
Results
Silent brain infarction was detected in 207 (20.4%) patients of the 1014 patients. Twenty-seven (13.0%) patients with SBI and 19 (9.2%) without SBI had a recurrent stroke (odds ratio 1.42, 95% confidence interval 0.79 to 2.56; p=0.24) during a mean follow-op of 2.5 years. Similarly, there was no statistically significant difference for all secondary outcome parameters between patients with SBI and matched patients without SBI.
Conclusion
The presence of SBI in patients with recent mild non-cardioembolic ischemic stroke could not be shown to be an independent risk factor for recurrent stroke, other vascular events, or a higher mortality.
Keywords: Cerebral infarction, silent brain infarction, ischemic stroke, magnetic resonance imaging, mortality
Introduction
Brain infarction is classified as silent if it lacks clinically overt stroke-like symptoms. The prevalence of silent brain infarction (SBI) defined on magnetic resonance imaging (MRI) in the general population ranges from 8% to 28%, with a higher prevalence with increasing age.1–3 Patients with SBI are at increased risk of stroke in population based studies of healthy elderly people.4–5 There is little information, however, on the prevalence of SBI among patients with ischemic stroke. In studies of consecutive ischemic stroke patients, the prevalence of SBI ranged from 13% in young adults aged 15 to 49 years with first-ever stroke up to 57% in a cohort of Japanese patients with a mean age of 72 years.6–9 The risk of recurrent stroke in patients with both symptomatic and silent ischemic stroke (identified on computed tomography) has only been investigated in the European Atrial Fibrillation Trial in the early 1990s which found a non-significant increased risk of recurrent stroke and vascular events in patients with SBI.10 However, medical secondary stroke prevention treatment has substantially improved since this time and computed tomography was used as imaging modality which has a lower sensitivity for the detection of SBI compared with MRI.1 No study to date has evaluated the risk of recurrent stroke and other cardiovascular events in patients with non-cardioembolic ischemic stroke and SBI. The Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial is the largest trial to date that has investigated the prevention of recurrent stroke in 20 332 patients with a recent ischemic stroke and compared, in a factorial design, the combination of aspirin and extended-released dipyridamole with clopidogrel.11–12 The PRoFESS Imaging Substudy was designed to assess the prevalence of SBI detected on MRI in a subset of patients with a recent non-cardioembolic ischemic stroke enrolled in the main PRoFESS trial and subsequently whether patients with SBI are at increased risk for recurrent stroke and other cardiovascular events.
Methods
Patients and sample size calculation
The PRoFESS trial protocol and primary results have been published elsewhere.11–13 In brief, 20 332 patients who were at least 50 years of age with recent ischemic stroke within 120 days of study entry were randomized to either aspirin (25 mg BID) plus extended-release dipyridamole (ER-DP, 200 mg BID) or clopidogrel (75 mg daily), and telmisartan (80 mg daily) or placebo in a 2x2 factorial design and treated for 2 years. Mean duration of follow-up was 2.5 years. All patients received best medical care independent of treatment assignment. Apart from previous clinical stroke symptoms before the qualifying ischemic stroke as an exclusion criterion, and an MRI after the qualifying stroke as an additional inclusion criterion, patient inclusion and exclusion criteria were the same as for the main PRoFESS trial. We hypothesized that patients with both symptomatic and silent brain infarction had a higher risk of recurrent stroke compared with patients without evidence of SBI on MRI. All analyses in this imaging substudy were predefined. We estimated a prevalence of 30% of SBI in our study population and calculated with a sample size of 300 patients with SBI and 300 age-and sex-matched controls without SBI. Male and female patients with SBI were matched with patients of the same gender without evidence of SBI with dates of birth that were closest together chronologically. If a direct age match was not found, patients were matched if their ages were within 5 years of each other. If this resulted in more than one match, then the two patients with dates of birth that were closest together chronologically were matched. The Imaging substudy of the PRoFESS trial was approved in global amendment no. 3 by the ethics committees of the participating centers. Participating patients signed the informed consent form for the main PRoFESS trial, as well as a separate consent form for the imaging substudy.
MRI analysis and definition of silent brain infarction
MRI data were sent to the Clinical Adjudication Center of the University Duisburg-Essen and each case was independently rated by two experienced neuroradiologists blinded for treatment allocation. SBI were defined as a focal hyperintense lesion on T2-weighted images and/or fluid-attenuated inversion recovery (FLAIR) with no corresponding symptoms in the clinical history of the patient that could be attributed to the lesion. SBI were distinguished from non-specific subcortical and periventricular white matter lesions by the presence of a corresponding hypointense lesion on T1-weighted images. All ischemic strokes (the qualifying symptomatic stroke and SBI) were classified according to location. Lacunar infarcts were defined as a hypointense lesion that were 3 mm or larger on T1-weighted images. Cortical border zone infarctions were defined as infarctions of the cortex located at the border zones between the anterior, middle, and posterior cerebral arteries and separated from hemodynamic lesions in the roof of the lateral ventricles or the centrum semiovale.14 Subcortical infarctions were defined as infarctions in the basal ganglia and subcortical white matter supplied by the anterior cerebral, middle cerebral, posterior cerebral, lenticulostriate/choroidal, and thalamic arteries. Information regarding stroke symptoms of the qualifying ischemic stroke was elicited using baseline case report forms from the main PRoFESS trial.
Statistical Analysis
The primary outcome variable was first recurrence of stroke of any type. Secondary outcomes were a composite endpoint of vascular events (stroke, myocardial infarction, vascular death), other vascular events (pulmonary embolism, retinal vascular accidents that were not a retinal arterial occlusion, deep vein thrombosis, central venous thrombosis, peripheral arterial occlusion or transient ischemic attack), and death. The primary and secondary outcomes were adjudicated by a central committee.
The two groups (presence/absence of SBI at study entry) were compared using conditional logistic regression. This analysis was confirmed by logistic regression with age (in years) as a continuous covariate and sex (male/female). Statistical significance was set at p<0.05. Analyses were performed with SAS® Version 8.2.
Results
A total of 1057 patients who were randomized in the PRoFESS study had given consent to enter the imaging substudy of which 1014 (95.9%) patients had an evaluable MRI scan performed within 120 days of their qualifying stroke. Mean age was 66.1 years, and 63.9% of the patients were male. T1-weighted images were available for evaluation in 928 (91.5%), T2-weighted images in 954 (94.1%), FLAIR images in 926 (91.3%), diffusion-weighted images in 887 (87.5%), T1-weighted contrast-enhanced images in 186 (18.3%), and gradient echo MRI in 204 (20.1%) of these patients. Mean time from qualifying ischemic stroke to baseline MRI scan was 8.0 (± 15.7) days. Mean time from baseline MRI to study drug randomization was 18.4 (± 24.1) days in 956 patients. 58 patients got their baseline MRI after study drug randomization with a mean delay of 15.7 (± 24.6) days, but none of these patients experienced a recurrent stroke between study drug randomization and MRI.
An ischemic stroke on MRI attributable to the clinical presentation of the qualifying ischemic stroke was detected in 821 (81.0%) patients. A SBI was detected in 207 (20.4%) patients. The mean age of patients with a SBI was 66.2 years and 150 (72.5%) were males. These patients were age- and sex-matched with 207 patients without evidence of SBI. Baseline demographic characteristic, vascular risk factors and treatment are shown in table 1. There were no significant differences between patients with SBI and matched patients without SBI except for a significantly higher rate of current smokers and a higher proportion of qualifying strokes classified as small-artery occlusion. The frequency of cerebral microbleeds did not differ between patients with and without SBI in the 82 patients in whom gradient echo MRI was available (41 patients with and 41 patients without SBI). Eight patients in the SBI group and 7 patients in the non-SBI group had evidence of cerebral microbleeds.
Table 1.
Patient characteristics at enrollment in 414 stroke patients with and without silent brain infarction on MRI and comparison with patient population in the PRoFESS imaging substudy and the whole trial
| Silent brain infarction |
No silent brain infarction |
MRI substudy |
Whole trial | |
|---|---|---|---|---|
| Characteristic | n = 207 | n = 207 | n = 1014 | n = 20 332 |
| Demographics | ||||
| Age, y, mean (SD) | 66.2 (8.5) | 66.2 (8.5) | 66.1 (8.4) | 66.1 (8.6) |
| Age group, n (%) | ||||
| ≤ 75 years | 173 (83.6) | 173 (83.6) | 862 (85.0) | 17 114 (84.2) |
| > 75 years | 34 (16.4) | 34 (16.4) | 152 (15.0) | 3218 (15.8) |
| Sex, male, n (%) | 150 (72.5) | 150 (72.5) | 648 (63.9) | 13 022 (64.0) |
| Ethnicity, n (%) | ||||
| Asian | 131 (63.3) | 134 (64.7) | 644 (63.5) | 6660 (32.8) |
| White | 55 (26.6) | 53 (25.6) | 264 (26.0) | 11 697 (57.5) |
| Black | 2 (1.0) | 8 (3.9) | 30 (3.0) | 816 (4.0) |
| Other | 19 (9.1) | 12 (5.8) | 76 (7.5) | 1159 (5.7) |
| Clinical history, n (%) | ||||
| TIA | 19 (9.2) | 25 (12.1) | 97 (9.6) | 1762(8.7) |
| Hypertension | 162 (78.3) | 155 (74.9) | 774 (76.3) | 15 048 (74.0) |
| Hypertension, treated | 90 (43.5) | 88 (42.5) | 428 (42.2) | 9553 (47.0) |
| Atrial fibrillation | 8 (3.9) | 6(2.9) | 28 (2.8) | 540 (2.7) |
| Diabetes mellitus | 67 (32.4) | 63 (30.4) | 320 (31.6) | 5743 (28.2) |
| Hyperlipidemia | 99 (47.8) | 111 (53.6) | 502 (49.5) | 9493 (46.7) |
| Smoker† | ||||
| Current | 67 (32.4) | 41 (19.8) | 245 (24.2) | 4308 (21.2) |
| Former | 68 (32.9) | 89 (43.0) | 331 (32.6) | 7352 (36.2) |
| Never | 72 (34.8) | 77 (37.2) | 438 (43.2) | 8663 (42.6) |
| Clinical details | ||||
| Baseline NIHSS score, median | 2 | 2 | 2 | 2 |
| Body mass index, kg/m2, mean (SD) | 25.6 (4.2) | 26.1 (3.8) | 26.0 (4.3) | 26.8 (5.0) |
| TOAST classification of qualifying stroke, n (%) | ||||
| Large-artery atherosclerosis | 69 (33.3) | 82 (39.6) | 327 (32.2) | 5805 (28.6) |
| Cardioembolism | 5 (2.4) | 3 (1.4) | 17 (1.7) | 369 (1.8) |
| Small-artery occlusion | 114 (55.1) | 94 (45.4) | 539 (53.2) | 10578 (52.0) |
| Other determined etiology | 1 (0.5) | 8 (3.9) | 18 (1.8) | 416 (2.0) |
| Undetermined etiology | 18 (8.7) | 20 (9.7) | 112 (11.0) | 3148 (15.5) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (0.1) | 16 (0.1) |
| Medication | ||||
| Blinded antiplatelet therapy during treatment, n (%) | ||||
| Aspirin/ER-DP | 108 (52.2) | 91 (44.0) | 510 (50.3) | 10181 (50.1) |
| Clopidogrel | 99 (47.8) | 116 (56.0) | 504 (49.7) | 10151 (49.9) |
| Antihypertensive therapy, n (%) | ||||
| Open-label | 97 (46.9) | 95 (45.9) | 466 (46.0) | 10 526 (51.8) |
| Blinded antihypertensive treatment with telmisartan | 105 (50.7) | 113 (54.6) | 516 (50.9) | 10146 (49.9) |
TOAST indicates Trial of ORG 10172 in Acute Stroke Treatment. Data for the imaging substudy and whole PRoFESS trial are given for comparison.
p-values for all comparisons between patients with silent brain infarction and matched patients without silent brain infarction were greater than 0.05 except for smoking status (p = 0.01).
Comparison of matched patients with and without silent brain infarction were based on the χ2 test for qualitative variables and Student’s t test for quantitative variables.
A total of 501 ischemic brain infarctions (symptomatic and silent) were diagnosed in the 207 patients with SBI on the qualifying MRI. The localization of these ischemic brain infarctions are shown in figure 1.
Figure 1.
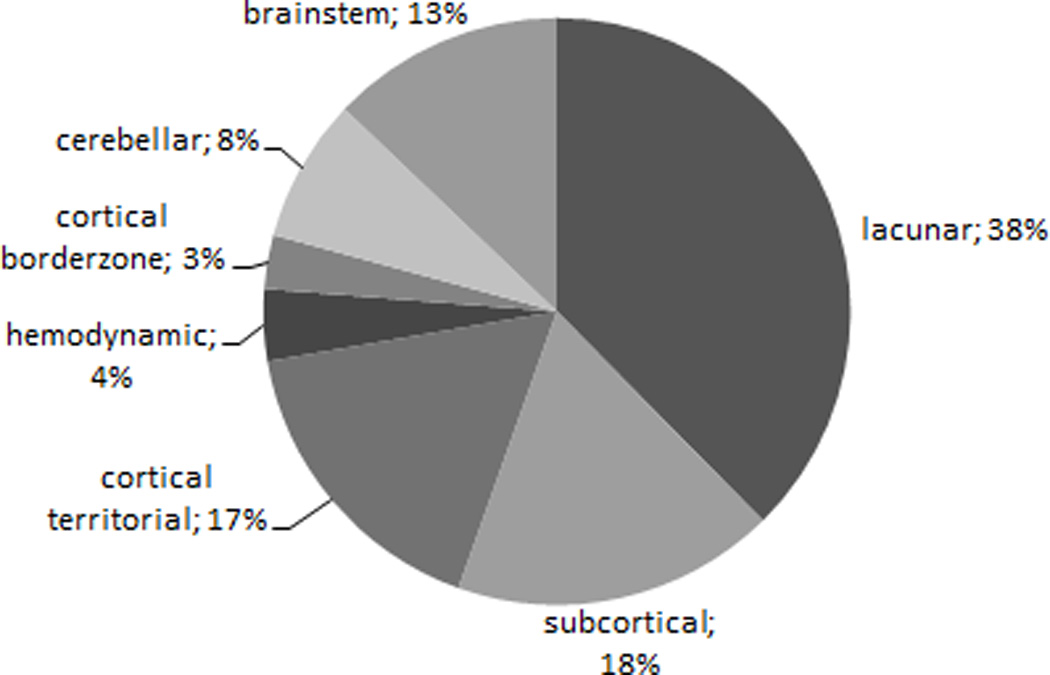
Localization of 501 symptomatic and silent ischemic brain infarcts in 207 patients with presence of silent brain infarction on MRI.
Although the rate of recurrent stroke was slightly increased among patients with SBI on baseline MRI compared to patients without SBI, the difference was not significant. Twenty-seven (13.0%) patients with SBI and 19 (9.2%) without SBI had a recurrent stroke (adjusted odds ratio [OR] 1.42, 95% confidence interval [CI] 0.79 to 2.56; p=0.24) during mean follow-up of 2.5 years (figure 2). The results of this analysis were consistent with the confirmatory model. Recurrent ischemic stroke occurred in 24 (11.6%) patients with SBI and 17 (8.2%) matched patients without SBI. A hemorrhagic stroke occurred in 3 (1.4%) patients with SBI and 2 (1%) matched patients without SBI. There was no apparent difference in stroke recurrence between Asian (17 [13.0%] of 131 patients) and non-Asian (10 [13.2%] of 76 patients) patients with SBI. Similarly, there was no statistically significant difference for all secondary outcome parameters between patients with SBI and matched patients without SBI. The combined vascular endpoint occurred in 33 patients (15.9%) with SBI compared to 24 (11.6%) in the matched group (OR 1.38, 95% CI 0.81 to 2.33; p=0.24). Other vascular events occurred in 8 patients (3.9%) with SBI compared to 9 (4.3%) in the matched group (OR 0.88, 95% CI 0.32 to 2.41; p=0.80). Fourteen patients with SBI (6.8%) died compared to 6 (2.9%) in the group without SBI (OR 2.33, 95% CI 0.90 to 6.07; p=0.08).
Figure 2.
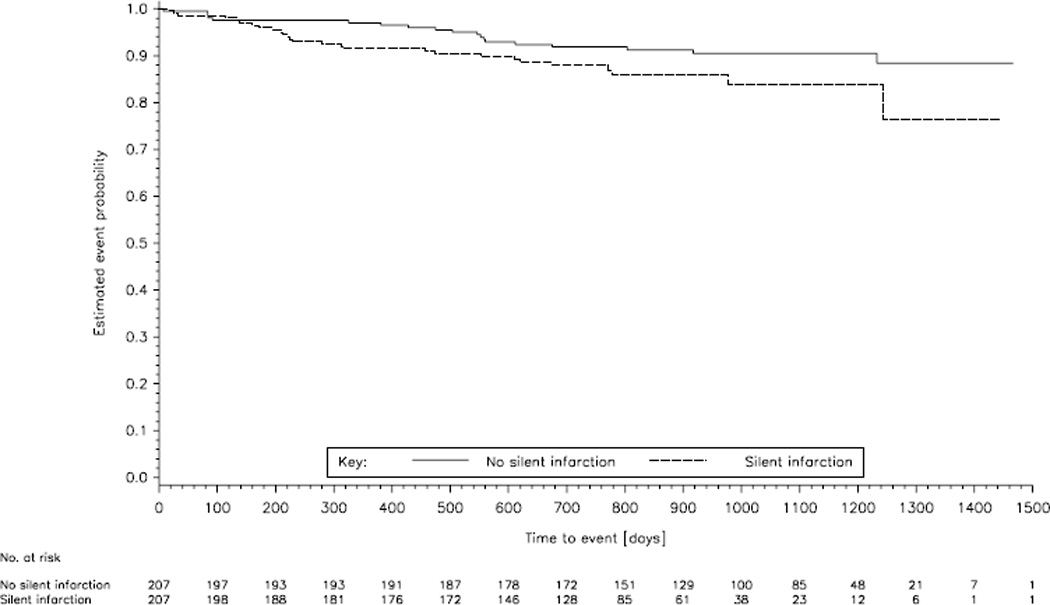
Kaplan-Meier estimates of the time to first recurrent stroke in the 207 patients with evidence of silent brain infarction on MRI and 207 age and sex matched patients without silent brain infarction. Time point zero indicates the date of baseline MRI. No recurrent stroke occurred before baseline MRI.
In comparison with the total number of 788 patients included in the PRoFESS imaging substudy who had no evidence of SBI on MRI, the 207 patients with SBI had a significantly higher stroke recurrence rate (13.0% vs. 8.0%; OR 0.58, 95% CI 0.36 to 0.94; p=0.03) and mortality rate (6.8% vs. 3.2%; OR 0.45, 95% CI 0.23 to 0.89; p=0.02).
Discussion
Patients with both symptomatic and silent ischemic brain infarction detected on MRI had a numerically higher risk of recurrent stroke, other vascular events, and a higher mortality compared with stroke patients without evidence of SBI in this imaging substudy of the PRoFESS trial. The differences were not statistically significant in age and sex matched patients.
Two previous population-based studies and one study in 933 neurologically normal Japanese adults had consistently found a significantly increased risk of symptomatic stroke in patients with SBI on MRI who did not have a history of symptomatic stroke at study entry.4–5, 15 Symptomatic stroke occurred in 7.3% of 923 patients with SBI compared with 3.8% of 2401 patients without evidence of SBI (hazard ratio [HR] 1.5, 95% CI 1.1 to 2.1) during a 4-year follow-up period in the Cardiovascular Health Study.4 Similar to this study, we found a non-significant odds ratio for recurrent stroke of 1.42 in patients with SBI. In the Rotterdam Scan Study, SBI at baseline MRI was associated with an increased risk of both a new SBI and a symptomatic infarct during a mean interval of 3.4 years. Presence of SBI increased the risk of stroke more than 3-fold, independently of other stroke risk factors (adjusted HR 3.9, 95% CI 2.3 to 6.8).5 Both aforementioned population-based studies reported a 30–40% higher prevalence of SBI among women compared with men, whereas 72.5% of patients with SBI in our patient cohort were male. Furthermore, most of the participants in the population-based studies were white, whereas the majority of our patients were Asians. Thus, we cannot rule out a bias by selection of stroke patients in our substudy. There was however no difference in stroke recurrence between Asian and non-Asian stroke patients with SBI in our patient cohort. Data from a retrospective cohort study in 104 acute ischemic stroke patients who underwent initial MRI within 24 hours of symptom onset and subsequent MRI on day 5 and between 30 and 90 days suggested that patients with late SBI recurrence between 30 and 90 days (22.1%) had a significantly increased risk of recurrent symptomatic ischemic stroke during a mean follow-up of 19.3 ± 9.0 months (OR 6.55; 95% CI 1.09 to 39.55).16 The combined vascular endpoint of recurrent symptomatic ischemic stroke, TIA, and vascular death was independently predicted by both early (OR 3.10, 95% CI 1.02 to 10.00) and late (OR 8.09; 95 CI 1.29 to 50.91) SBI recurrences.
The only prospective study that investigated the risk of stroke recurrence in patients with both symptomatic and silent stroke was the European Atrial Fibrillation Trial.10 985 patients with non-rheumatic atrial fibrillation with a mean age of 73 years who had suffered a TIA or non-disabling ischemic stroke were included for this analysis. Fourteen percent had evidence of SBI on computed tomography and these patients had a non-significantly increased risk for recurrent stroke (HR 1.18; 95% CI 0.79 to 1.77) and recurrent vascular events (HR 1.2, 95% CI 0.9 to 1.6) compared with patients without SBI.
In addition, there was a slightly higher mortality in patients with symptomatic and silent brain infarction in our study. Likewise, 3-year mortality was increased in a population-based study in 239 85 year-old stroke-free individuals,17 while there was no effect on 1-year mortality in patients with SBI in a study of 755 consecutive patients with first-ever stroke.6
Due to the lower than expected prevalence of SBI in patients with recent ischemic stroke, our study was underpowered to detect a significantly increased cardiovascular risk or mortality in stroke patients with SBI when compared to age and sex matched stroke patients without SBI. The low prevalence of SBI can be explained by the mean age of 66.2 years in our patient group while the prevalence of SBI has been shown to be strongly age dependent in population based studies.1 The mean age of 66.1 years in the main study PRoFESS was almost identical to the mean age in this substudy. An almost identical prevalence of 20% SBI in 226 Asian patients with first-ever stroke and a similar age distribution (mean age 68.8 years) was reported by Ong et al.18 In contrast, the prevalence of SBI in 171 Japanese patients with acute first-ever ischemic stroke with a mean age of approximately 72 years was as high as 56.7%.7 Furthermore, mean duration of follow-up was 2.5 years in our study which is substantially lower compared with the follow-up period in the Cardiovascular Health Study (4 years) and the Rotterdam Scan Study (3.4 years). These studies also had larger sample sizes compared to our study. Increasing the study sample size by comparing the 207 patients with SBI with the total number of 788 non-matched patients without evidence of SBI resulted in a statistically significantly higher rate of symptomatic stroke recurrence and mortality rate in patients with SBI.
There is a wide variation in classification, detection and discrimination of SBI, especially lacunar ischemic lesions, and white matter lesions on brain imaging.19 Strengths of our study include the blinded assessment of MRI scans by two experienced neuroradiologists and the use of multimodal MRI. The combination of T1-, T2-weighted, and FLAIR images, which was available in more than 90% of our included patients, has been shown to accurately discriminate between small white matter infarction and non-specific white matter lesions in a multicenter observer performance study.20 Furthermore, diffusion-weighted images which allow differentiation of the acute qualifying stroke from chronic stroke and white matter lesions21 were also available for evaluation in 87.5% of patients.
In summary, the presence of SBI in patients with recent mild non-cardioembolic ischemic stroke could not be shown to be an independent risk factor for recurrent stroke, other vascular events, or a higher mortality.
Supplementary Material
Acknowledgments
Source of funding
Boehringer Ingelheim sponsored and funded the PRoFESS trial and this imaging substudy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Participating investigators of the PROFESS Imaging substudy are listed in the supplementary webappendix
- Ralph Weber, Isabel Wanke, Claudia Möller-Hartmann, Elke R. Gizewski, Michael Forsting, and Steven Warach report no conflict of interest.
- Christian Weimar received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Boehringer Ingelheim, Bristol-Myers Squib, Daiichi Asubio, Novartis, Novo-Nordisk, Sanofi-Aventis.
- Jonathan Blatchford and Karin Hermansson are employees of Boehringer Ingelheim.
- Andrew M. Demchuk received honoraria for contribution to advisory boards or oral presentations from Boehringer Ingelheim and Merck.
- Ralph L. Sacco received grants from NINDS for the Northern Manhattan Study. He served as a consultant to Boehringer Ingelheim during the conduct of the PRoFESS trial.
- Jeffrey L. Saver is an employee of the University of California, which received payments based on clinical trial contracts for the number of subjects enrolled from Boehringer Ingelheim and AGA Medical, and received payments for faculty participation on scientific advisory boards from AGA Medical.
- Hans-Christoph Diener received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, CoAxia, D-Pharm, Fresenius, GlaxoSmithKline, Janssen-Cilag, MSD, MindFrame, Novartis, Novo-Nordisk, Paion, Parke-Davis, Pfizer, Sanofi-Aventis, Sankyo, Servier, Solvay, Thrombogenics, Wyeth, Yamaguchi. Financial support for research projects was provided by Astra/Zeneca, GSK, Boehringer Ingelheim, Novartis, Janssen-Cilag, Sanofi-Aventis.
- Anke Diehl received honoraria for contribution to advisory boards or oral presentations from Boehringer Ingelheim and Bristol-MyersSquib.
References
- 1.Vermeer SE, Longstreth WT, Jr., Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 2.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhakaran S, Wright CB, Yoshita M, Delapaz R, Brown T, DeCarli C, et al. Prevalence and determinants of subclinical brain infarction: the Northern Manhattan Study. Neurology. 2008;70:425–430. doi: 10.1212/01.wnl.0000277521.66947.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, et al. Silent MRI infarcts and the risk of future stroke: the cardiovascular health study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 5.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 6.Boon A, Lodder J, Heuts-van Raak L, Kessels F. Silent brain infarcts in 755 consecutive patients with a first-ever supratentorial ischemic stroke. Relationship with index-stroke subtype, vascular risk factors, and mortality. Stroke. 1994;25:2384–2390. doi: 10.1161/01.str.25.12.2384. [DOI] [PubMed] [Google Scholar]
- 7.Adachi T, Kobayashi S, Yamaguchi S. Frequency and pathogenesis of silent subcortical brain infarction in acute first-ever ischemic stroke. Intern Med. 2002;41:102–108. doi: 10.2169/internalmedicine.41.103. [DOI] [PubMed] [Google Scholar]
- 8.Oh SH, Kim NK, Kim SH, Kim JK, Kim HS, Kim WC, et al. The prevalence and risk factor analysis of silent brain infarction in patients with first-ever ischemic stroke. J Neurol Sci. 2010;293:97–101. doi: 10.1016/j.jns.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Putaala J, Kurkinen M, Tarvos V, Salonen O, Kaste M, Tatlisumak T. Silent brain infarcts and leukoaraiosis in young adults with first-ever ischemic stroke. Neurology. 2009;72:1823–1829. doi: 10.1212/WNL.0b013e3181a711df. [DOI] [PubMed] [Google Scholar]
- 10.Silent brain infarction in nonrheumatic atrial fibrillation. EAFT Study Group. European Atrial Fibrillation Trial. Neurology. 1996;46:159–165. doi: 10.1212/wnl.46.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359:1225–1237. doi: 10.1056/NEJMoa0804593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diener HC, Sacco R, Yusuf S. Rationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS) Cerebrovasc Dis. 2007;23:368–380. doi: 10.1159/000100105. [DOI] [PubMed] [Google Scholar]
- 14.Derdeyn CP, Khosla A, Videen TO, Fritsch SM, Carpenter DL, Grubb RL, Jr, et al. Severe hemodynamic impairment and border zone--region infarction. Radiology. 2001;220:195–201. doi: 10.1148/radiology.220.1.r01jl09195. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke. 1997;28:1932–1939. doi: 10.1161/01.str.28.10.1932. [DOI] [PubMed] [Google Scholar]
- 16.Kang DW, Lattimore SU, Latour LL, Warach S. Silent ischemic lesion recurrence on magnetic resonance imaging predicts subsequent clinical vascular events. Arch Neurol. 2006;63:1730–1733. doi: 10.1001/archneur.63.12.1730. [DOI] [PubMed] [Google Scholar]
- 17.Liebetrau M, Steen B, Hamann GF, Skoog I. Silent and symptomatic infarcts on cranial computerized tomography in relation to dementia and mortality: a population-based study in 85-year-old subjects. Stroke. 2004;35:1816–1820. doi: 10.1161/01.STR.0000131928.47478.44. [DOI] [PubMed] [Google Scholar]
- 18.Ong C-T, C W-P, Sung S-F, Wu C-S, Hsu Y-C. Silent infarction in patienst with first-ever stroke. Acta Neurol Taiwan. 2007;16:221–225. [PubMed] [Google Scholar]
- 19.Potter GM, Marlborough FJ, Wardlaw JM. Wide variation in definition, detection, and description of lacunar lesions on imaging. Stroke. 2011;42:359–366. doi: 10.1161/STROKEAHA.110.594754. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki M, Hirai T, Taoka T, Higano S, Wakabayashi C, Matsusue E, et al. Discriminating between silent cerebral infarction and deep white matter hyperintensity using combinations of three types of magnetic resonance images: a multicenter observer performance study. Neuroradiology. 2008;50:753–758. doi: 10.1007/s00234-008-0406-6. [DOI] [PubMed] [Google Scholar]
- 21.Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the "small stuff": the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39–45. doi: 10.1016/s1474-4422(03)00621-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


