ABSTRACT
The acquisition, delivery, and incorporation of metals into their respective metalloproteins are important cellular processes. These processes are tightly controlled in order to prevent exposure of cells to free-metal concentrations that could yield oxidative damage. Copper (Cu) is one such metal that is required as a cofactor in a variety of proteins. However, when present in excessive amounts, Cu is toxic due to its oxidative capability. Cytochrome c oxidases (Coxs) are among the metalloproteins whose assembly and activity require the presence of Cu in their catalytic subunits. In this study, we focused on the acquisition of Cu for incorporation into the heme-Cu binuclear center of the cbb3-type Cox (cbb3-Cox) in the facultative phototroph Rhodobacter capsulatus. Genetic screens identified a cbb3-Cox defective mutant that requires Cu2+ supplementation to produce an active cbb3-Cox. Complementation of this mutant using wild-type genomic libraries unveiled a novel gene (ccoA) required for cbb3-Cox biogenesis. In the absence of CcoA, the cellular Cu content decreases and cbb3-Cox assembly and activity become defective. CcoA shows homology to major facilitator superfamily (MFS)-type transporter proteins. Members of this family are known to transport small solutes or drugs, but so far, no MFS protein has been implicated in cbb3-Cox biogenesis. These findings provide novel insights into the maturation and assembly of membrane-integral metalloproteins and on a hitherto-unknown function(s) of MFS-type transporters in bacterial Cu acquisition.
IMPORTANCE
Biogenesis of energy-transducing membrane-integral enzymes, like the heme copper-containing cytochrome c oxidases, and the acquisition of transition metals, like copper, as their catalytic cofactors are vital processes for all cells. These widespread and well-controlled processes are poorly understood in all organisms, including bacteria. Defects in these processes lead to severe mitochondrial diseases in humans and poor crop yields in plants. In this study, using the facultative phototroph Rhodobacter capsulatus as a model organism, we report on the discovery of a novel major facilitator superfamily (MFS)-type transporter (CcoA) that affects cellular copper content and cbb3-type cytochrome c oxidase production in bacteria.
Introduction
Cells require copper (Cu) as a cofactor for many metalloproteins, including cytochrome c oxidases (Coxs) (1), superoxide dismutases (2), and multicopper oxidases (3). Among them, Cox enzymes terminate the electron transfer chains of aerobic organisms by catalyzing the four-electron reduction of dioxygen (O2) to water during respiration. Cu is an essential catalytic and structural cofactor of this important energy transduction enzyme (1), but an excess amount of it is toxic, as it can activate O2 via the Fenton reaction to generate dangerous reactive oxygen radicals (4, 5). Indeed, its shortage or surplus leads to severe human illnesses, like Menkes, Wilson’s, and Alzheimer’s diseases (6–9). Thus, cells need to precisely control Cu acquisition, trafficking, and incorporation into the target proteins.
Cox enzymes have a conserved catalytic subunit (i.e., subunit I) that contains a low-spin heme and a binuclear metal center, composed of a high-spin heme Fe and a Cu atom (i.e., CuB) (10). They also have additional subunits: the mammalian mitochondrial enzyme contains 13, whereas the bacterial Cox has 3 or 4 subunits in total (11). Subunit II is the primary electron acceptor and harbors extra cofactors, like another binuclear Cu center (CuA) in the mitochondrial aa3-type Cox (10, 11). In the bacterial cbb3-type Cox (cbb3-Cox), which is important for aerobic respiration, the onset of photosynthesis, rhizobial symbiosis, and pathogenesis in various species (12–14), subunit II contains covalently bound hemes instead of a CuA center. The presence of various cofactors renders Cox biogenesis a complex process. Synthesis and maturation of individual subunits, their insertions into the membrane, and assembly of mature subunits into active enzymes are coordinated temporally and spatially (15). Regulated expression of the subunits and accurate insertion of the cofactors during biogenesis are required to control the concentration of potentially toxic, free redox-active cofactors or reactive assembly intermediates (5).
The purple nonsulfur facultative phototrophic bacterium Rhodobacter capsulatus contains only two terminal O2 reductases, a cbb3-Cox and a hydroquinone oxidase (Qox), that allow cells to grow under respiratory conditions even in the absence of an active Cox (16). Unlike in many other bacterial species, cbb3-Cox is the only Cox in this species, making it an organism of choice for investigating cbb3-Cox biogenesis. The R. capsulatus cbb3-Cox has four subunits encoded by the ccoNOQP operon and, like all Cox enzymes, contains a heme CuB catalytic center located in subunit I (CcoN). It naturally lacks a CuA center; hence, the only Cu atom is that of the CuB center. Instead of a CuA center, cbb3-Cox contains two membrane-bound c-type cytochrome subunits: the monoheme cytochrome c (CcoO or cytochrome co) and the diheme cytochrome c (CcoP or cytochrome cp) as subunits II and III, respectively (17). Both CcoO and CcoP are required for electron transfer from the electron donor cytochromes c (i.e., cytochromes c2 and cy) to the heme CuB center at CcoN. The enzyme also has a fourth subunit (CcoQ) without any cofactor, but its absence hardly affects the enzymatic activity (18, 19).
Currently, a number of components involved in mitochondrial Cox biogenesis are known, and several of them are linked to human diseases (20–22). A smaller number of biogenesis components have been identified so far in the case of bacterial cbb3-Cox. Earlier genetic screens for loss of Cox activity identified several genes affecting cbb3-Cox biogenesis, including ccoGHIS (23), olsAB (24), and senC (25, 26). Recent proteomic approaches also associated dsbA and degP (27) with this process. However, many of the components and steps governing biogenesis of an active cbb3-Cox still remain undefined. In particular, those involved in forming the universally conserved heme CuB binuclear center of Cox enzymes are unknown. In this work, we report on the molecular characterization of a cbb3-Cox-defective mutant that requires Cu2+ supplementation to produce an active cbb3-Cox. This mutant unveiled a novel transporter, CcoA, of the major facilitator superfamily (MFS), whose absence greatly diminishes the levels of intracellular Cu content and active cbb3-Cox in R. capsulatus. To our knowledge, CcoA is the first example of an MFS-type transporter required for efficient Cu acquisition and cbb3-Cox production in bacteria.
RESULTS
Cu2+ supplement-dependent cbb3-Cox mutants.
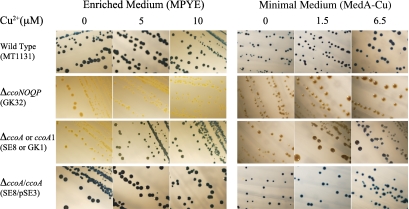
Previously, R. capsulatus mutants (e.g., GK1 [see Table S1 in the supplemental material]) that exhibited very low or no cbb3-Cox activity in a growth medium-dependent manner were isolated following mutagenesis with ethyl methanesulfonate (28). These mutants had a NADI (α- naphthol plus dimethylphenylene diamine → indophenol blue plus H2O)-negative (i.e., no cbb3-Cox activity) phenotype when grown on enriched peptone yeast extract (MPYE) medium (23) but exhibited a NADIslow (i.e., very low cbb3-Cox activity) phenotype on minimal (MedA) medium (Fig. 1). Subsequently, additional similar mutants (e.g., HY70 [see Table S1 in the supplemental material]) were also obtained. Two of these mutants (GK1 and HY70) were retained for further studies.
FIG 1 .
Growth medium-dependent NADI phenotypes of R. capsulatus mutants. The wild-type (MT1131), the ΔccoNOQP (GK32), the ΔccoA or ccoA1 (SE8 or GK1, respectively), and the complemented ΔccoA/ccoA+ (SE8/pSE3) strains were grown at 35°C under respiratory conditions on MPYE or MedA media supplemented with 0, 5, or 10 µM Cu2+, as indicated. Colonies that contain an active cbb3-Cox (NADI+) turn blue immediately (<0.5 min) when exposed to a NADI stain, and those that have no (NADI−) or low (NADIslow) cbb3-Cox activity remain green or become bluer upon longer (>10-min) exposure times. MedA with no Cu addition (MedA-Cu) was prepared by omitting 1.5 µM CuSO4 from the regular MedA (indicated as 1.5 µM) and MedA with 6.5 µM by adding 5 µM CuSO4 to the regular MedA (indicated as 0, 1.5, and 6.5, respectively).
Testing of the chemical constituents of different growth media indicated that the growth medium-dependent NADI phenotype of GK1 correlated with the Cu2+ content of the medium used (Fig. 1). Earlier inductively coupled plasma mass spectrometry (ICP-MS) analyses indicated that, in the absence of CuSO4 supplementation or chemical chelation of Cu, both MPYE and Med A lacking Cu (MedA-Cu) media contained approximately 150 nM Cu2+ as a “contaminant,” but the level of bioavailable Cu was much lower in MPYE medium (23). When Cu2+ was omitted from MedA, which contains 1.5 µM CuSO4, GK1 became NADI minus. Conversely, when MPYE that has no Cu2+ supplement contained 5 µM or more CuSO4, GK1 regained a NADIslow phenotype (Fig. 1). Membranes of GK1 exhibited ~2% or 15% of the wild type’s cbb3-Cox activity (monitored as O2 consumption activity in the presence of ascorbate and tetramethylphenylene diamine [TMPD]) when cells were grown on MPYE (i.e., lacking Cu) and MedA (i.e., containing Cu) media, respectively (28). Addition of metal ions other than Cu2+, including Fe3+, Zn2+, and Mn2+, or redox-active chemicals, such as cysteine/cystine or oxidized/reduced glutathione, did not affect the NADI phenotypes of GK1, indicating that cbb3-Cox activity in GK1 responded specifically to increased exogenous Cu2+ availability. Moreover, R. capsulatus structural (ccoNOQP) or assembly (ccoGHIS) genes of cbb3-Cox (23, 28) or other genes (dsbAB, senC, and olsAB) known to affect its biogenesis (23, 24, 27) were unable to complement GK1 or HY70 for a NADI+ phenotype in the absence of a Cu2+ supplement. We therefore surmised that these mutants might reveal a novel component(s) involved in cbb3-Cox biogenesis.
New gene responsible for active cbb3-Cox production.
The gene that was defective in GK1 was identified by complementation with chromosomal libraries that were constructed using either the EcoRI or HindIII restriction enzyme. These crosses yielded the plasmids pSE1 and pSE2, which complemented GK1 to a NADI+ phenotype without Cu2+ supplementation and carried 8.0-kb EcoRI and 4.8-kb HindIII fragments, respectively (see Fig. S1A in the supplemental material). DNA sequence determinations of the end portions of these fragments and their alignments with the R. capsulatus reference genome (http://www.ncbi.nlm.nih.gov) identified the chromosomal region that complemented GK1. The EcoRI fragment contained six intact open reading frames (ORFs), annotated as follows: a protein of unknown function (DUF88, GenBank accession no. RCAP_RCC02189), a heavy-metal-translocating P-type ATPase (RCAP_RCC02190), a transcriptional regulator of the MerR family (RCAP_RCC02191), a major facilitator superfamily member (RCAP_RCC02192), DNA-3-methlyadenine glycosylase II (RCAP_RCC02193), and a phospholipase/carboxylesterase family protein (RCAP_RCC02194). The HindIII fragment, which was contained within the EcoRI fragment, carried only the ORFs RCC02190, RCC02191, and RCC02192 (Fig. S1A). Three derivatives of pSE2 were constructed, namely, pSE201, pSE202, and pSE203, which contain deletions in RCC02190, in RCC02191, and in both, respectively. All three plasmids complemented GK1 to NADI+ in the absence of a Cu2+ supplement. Concurrently, the knockout mutants SE4, SE5, and SE6, with inactive copies of RCC02190, RCC02191, and both RCC02190 and RCC02191, respectively, were constructed by interposon mutagenesis (see Text S1 in the supplemental material). All three mutants showed a NADI+ phenotype (Fig. S1A). The plasmids pSE204 and pSE3 contained only RCC02192 (see Fig. S1A and -B in the supplemental material) and complemented both GK1 and HY70, whereas its derivatives with internal deletions in RCC02192, pSE5 and pSE6, were unable to do so (see Fig. S1B and Table S1 in the supplemental material). These data showed that RCC02192 was defective in GK1 and HY70.
Chromosomal knockout allele of RCC02192.
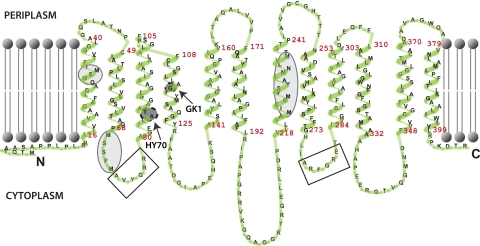
A chromosomal deletion-insertion allele of RCC02192 was obtained using the gene transfer agent with pSE5 (ΔRCC02192::spe) as a donor (Text S1), to yield the mutant SE8 (Fig. S1B). Like GK1 and HY70, SE8 was NADI− on media lacking a Cu2+ supplement and NADIslow on Cu-containing media. It was fully complemented to a NADI+ phenotype in the absence of Cu2+ supplementation by pSE3 but not by pRK-GK1 (Table S1), which carries an identical chromosomal DNA fragment derived from GK1. Thus, the defect in cbb3-Cox activity seen in GK1 and HY70 was confined to RCC02192, which we subsequently named ccoA to recognize its role in cbb3-Cox biogenesis. DNA sequencing of appropriate chromosomal regions encompassing ccoA defined the molecular bases of the mutation(s) in GK1 and HY70. A single base pair change (C to T) at nucleotide position 345 of ccoA, converting glycine 116 of CcoA to aspartate, and a 6-base-pair-long insertion at nucleotide position 257, resulting in an in-frame insertion of a threonine-alanine dipeptide between positions 86 and 87 of CcoA, were found in GK1 and HY70, respectively (Fig. 2).
FIG 2 .
Topological model of the CcoA (RCAP_RCC02192) protein. The CcoA protein is predicted to have 12 transmembrane helices, like the MFS-type transporters. The topology model was drawn by using the TmRPres2D program (64). The mutations present in GK1 and HY70, the motifs similar to the conserved motifs found in MFS transporters (rectangles), and the metal-binding Mets motifs (ellipses) are also indicated.
CcoA belongs to the major facilitator superfamily of transporters.
In the R. capsulatus reference genome, ccoA (RCC02192) is annotated as a protein of the major facilitator superfamily (MFS). Like the MFS-type transporters, CcoA is an integral membrane protein (predicted to be 405 amino acids), with 12 putative transmembrane helices split into two subdomains of six helices each, separated by a large cytoplasmic loop (Transporter Classification Database [TCDB], http://www.tcdb.org) (29, 30) (Fig. 2). These transporters contain two highly conserved DRXGRR motifs between transmembrane helices two-three and eight-nine (AVYGRR and ARFGRE in CcoA) (31). Remarkably, mutations that inactivated ccoA in GK1 and HY70 are located near the first motif (Fig. 2), suggesting that this portion of CcoA is important for its function. A striking feature of CcoA is the presence of several Mets motifs (M30SM, M70SSFM, M223ICGM, and M233NLVM) associated with Cu binding and transport in Ctr-type Cu importers (32, 33). Earlier, mutating a conserved and functionally important tyrosine residue located in the same transmembrane helix as a Mets motif (YFLMLIFMT) was shown to decrease Cu transport by a Ctr-type Cu importer (33). Remarkably, a similar sequence (Y230ALMNLVMT) is also present on the seventh helix of CcoA (Fig. 2).
Properties of a mutant lacking CcoA.
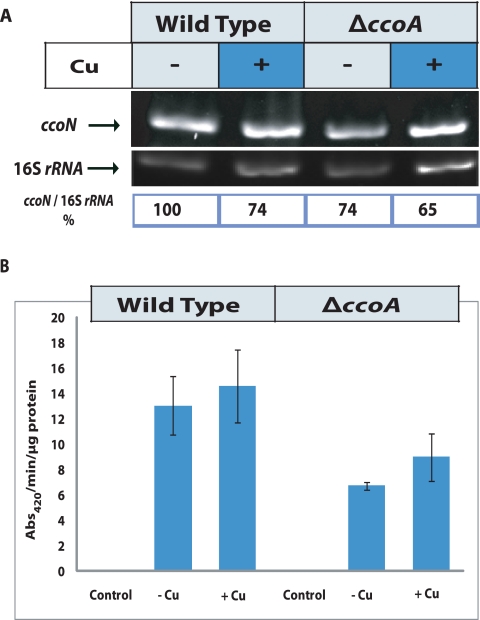
In order to gain mechanistic insights into how the absence of CcoA decreases the activity of cbb3-Cox in R. capsulatus, we compared the transcription levels of the ccoNOQP gene cluster between a wild type (MT1131) and its ΔccoA derivative (SE8) in the presence and absence of a Cu2+ supplement. Total cellular RNA isolated from appropriate strains grown in MPYE medium with or without a Cu2+ supplement were subjected to reverse transcription-PCR (RT-PCR) using ccoN-specific primers (see Materials and Methods). Analyses of the amplification products (Fig. 3A) indicated that the amount of ccoN mRNA was slightly lower (~74% of the wild-type amount) in a mutant lacking ccoA. In the presence of a Cu2+ supplement, these amounts further decreased slightly and comparably in the ccoA mutant and the wild-type strain. The data indicated that the lack of CcoA had no significant effect on ccoN transcription and thus most likely on the entire ccoNOQP cluster, which is initiated from a promoter located immediately upstream of ccoN (28).
FIG 3 .
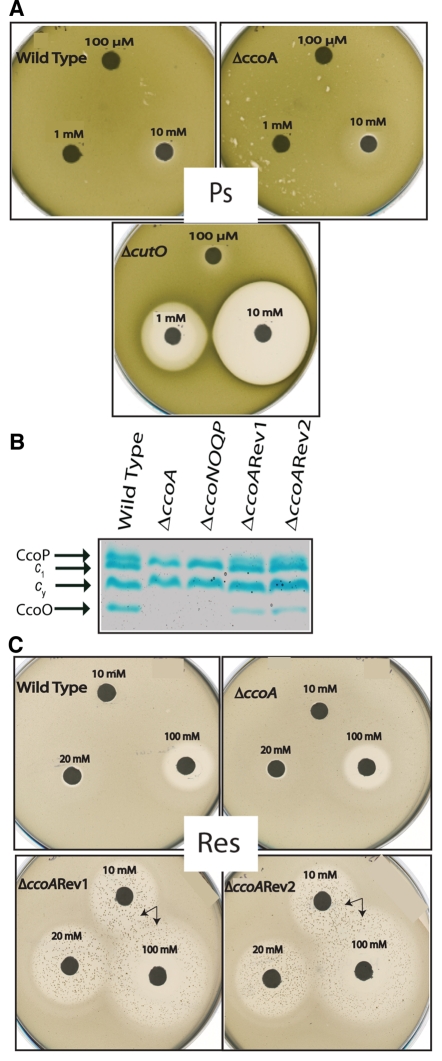
Effects of ccoA mutation on the expression of ccoNOQP structural genes of cbb3-Cox. (A) The top row shows the results of RT-PCR of total RNA from wild-type (MT1131) and ΔccoA mutant (SE8) strains grown in MPYE media supplemented with 0 or 5 µM Cu2+. The middle row shows 16S rRNA gene expression, which is not affected by the ccoA mutation, and the bottom row shows the ratios of ccoN to 16S rRNA gene expression for comparison. The intensities of the bands were determined by ImageJ software (NIH). A control PCR omitting reverse transcriptase enzyme was performed in each case to check for DNA contamination (data not shown). (B) β-Galactosidase activities measured in cell extracts derived from the wild type (MT1131) and ΔccoA mutant (SE8) strain carrying the ccoN::lacZ gene fusion, grown by respiration on MPYE media without Cu2+ (−Cu) or with (+Cu) 5 µM Cu2+ supplementation. Control reactions refer to the assays performed with cell extracts from the wild type and ΔccoA mutant carrying the pXCA601 plasmid, which contains a promoterless lacZ gene used to construct the ccoN::lacZ fusion. The assays were performed at least in duplicate, and the activities are presented in nanomoles of ONPG hydrolyzed per minute per microgram of total proteins.
Next, a transcriptional-translational ccoN::lacZ fusion construct (pXG1) that carried the 220 bp 5′ of the ATG start codon and the first 13 amino-terminal codons of ccoN (28) was conjugated to the wild-type strain MT1131 and its ΔccoA derivative SE8. The absence of CcoA decreased roughly 2-fold the amounts of β-galactosidase activity produced in these strains grown in MPYE medium without any Cu2+ supplement (Fig. 3B). Upon 5 µM Cu2+ supplementation, this activity increased slightly and comparably in both strains. Thus, neither the transcription nor the translation initiation of ccoN in R. capsulatus was abolished by the absence of CcoA, and Cu2+ supplementation enhanced it only marginally.
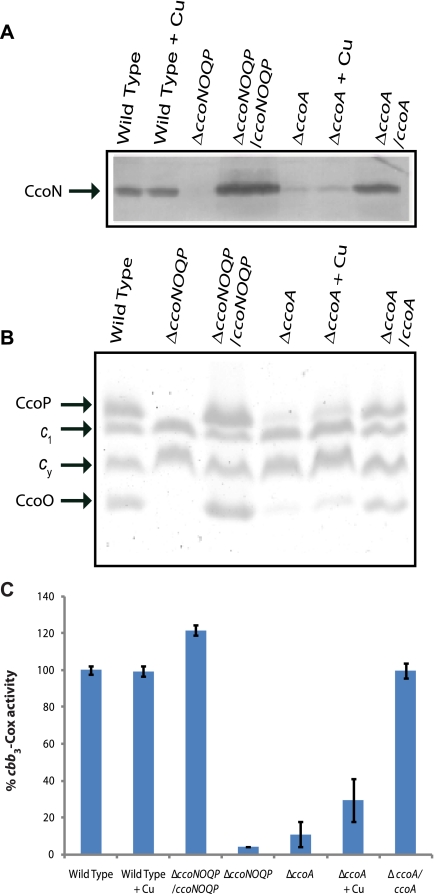
The steady-state amounts of cbb3-Cox subunits were examined in membranes of appropriate strains grown with or without Cu2+ supplementation. Subunit I of cbb3-Cox, CcoN, was monitored by immunoblot analyses using polyclonal anti-R. capsulatus CcoN antibodies (28). Subunits II and III, CcoO and CcoP, were visualized using SDS-PAGE with tetramethylbenzidine (TMBZ) staining, which reveals specifically membrane-bound c-type cytochromes (see Materials and Methods). The amount of CcoN in SE8 lacking CcoA was much lower than that seen in the wild-type strain, MT1131, and addition of 5 µM Cu2+ supplement increased this amount in both strains (Fig. 4A). Similarly, the amounts of CcoO and CcoP (cytochromes co and cp, respectively) were lower in the absence of CcoA than in the wild-type strain. Cu2+ supplementation increased these amounts (Fig. 4B), even though no effect was seen with other cbb3-Cox-unrelated membrane-bound c-type cytochromes (e.g., cytochromes c1 and cy). Thus, in the absence of CcoA, the steady-state amounts of the structural subunits of cbb3-Cox in membranes were highly decreased, and Cu2+ supplementation palliated this defect(s) partially.
FIG 4 .
cbb3-Cox subunit profiles and enzyme activity in membranes of various R. capsulatus strains. Chromatophore membranes derived from the wild-type (MT1131), ΔccoNO (GK32), ΔccoNO/ccoNOQP (GK32/pOX15), ΔccoA (SE8), and ΔccoA/ccoA (SE8/pSE3) strains grown at 35°C under respiratory conditions on MPYE medium supplemented (+Cu) or not supplemented with 5 µM Cu were prepared as described in Materials and Methods. (A) Immunoblot analysis to define the amounts of cbb3-Cox subunit I CcoN. Approximately 50 µg of chromatophore membranes were separated by 12% SDS-PAGE and treated as described in Materials and Methods, using anti-CcoN polyclonal antibodies. (B) Comparison of membrane-associated cytochrome c profiles of various strains. Approximately 50 µg chromatophore membranes prepared from appropriate strains, grown as described above, were separated using 16.5% SDS-PAGE, and the c-type cytochromes were visualized using TMBZ staining as described in Materials and Methods. CcoO and CcoP refer to subunits II and III of cbb3-Cox, and c1 and cy correspond to the cytochrome c1 subunit of the cytochrome bc1 complex and membrane-attached electron carrier cytochrome cy, respectively. (C) cbb3-Cox activities of various strains determined by monitoring the rate of oxidation of reduced horse heart cytochrome c, as described in Materials and Methods. The Cox activity exhibited by the wild-type strain, MT1131 (1.5 µmol of cytochrome c oxidized per min and per mg of total proteins), was taken as 100 to determine the relative amounts of Cox activities of appropriate strains. At least two independent duplicates were performed for each assay.
The cbb3-Cox activity present in detergent-dispersed membranes from cells lacking CcoA was determined using reduced horse heart cytochrome c (see Materials and Methods). The total amount of cbb3-Cox activity detected in SE8 lacking CcoA was ~5 to 10% of that seen in the wild-type strain, MT1131 (Fig. 4C). Upon addition of 5 µM Cu2+ supplement, the cbb3-Cox activity of SE8 increased to ~20 to 30% of that of MT1131, which was unchanged under these conditions. As expected, upon complementation with a plasmid carrying ccoA (e.g., SE8/pSE3), the NADI phenotypes, steady-state amounts, and activities of cbb3-Cox reached wild-type levels (Fig. 1 and 4). Therefore, the absence of CcoA affected a step(s) that was subsequent to transcription and translation initiation of ccoN during biogenesis of R. capsulatus cbb3-Cox under respiratory growth conditions. This defect(s) decreased drastically the steady-state amount and activity of cbb3-Cox, and Cu2+ supplementation alleviated it partially.
The absence of CcoA decreases the total Cu content of R. capsulatus cells.
In order to determine whether the absence of CcoA affected the intracellular Cu content, cells grown in MPYE medium with or without 5 µM Cu2+ supplement were analyzed by inductively coupled plasma dynamic-reaction cell mass spectrometry (ICP-DRC-MS) (see Materials and Methods). In the absence of a Cu2+ supplement, washed and lyophilized cells of SE8 lacking CcoA contained ~20% less Cu than its wild-type parent, MT1131, whereas the amounts of Fe, Mn, and Zn found in these strains were unchanged (Table 1). Both wild-type and CcoA-lacking cells grown in the presence of 5 µM Cu2+ contained larger amounts of Cu, but the amount found in the absence of CcoA was again ~60% smaller than that seen in the wild-type parent, MT1131. Thus, in the absence of CcoA, the intracellular Cu accumulation in R. capsulatus cells grown by respiration was significantly decreased but not completely abolished.
TABLE 1 .
Total Cu contents of the R. capsulatus wild type and of the ΔccoA strain and its revertants as determined by ICP-DRC-MSb
| Strain | 5 µM Cu2+ | Relative level of: |
|||
|---|---|---|---|---|---|
| Cu | Mn | Zn | Fe | ||
| Wild type (MT1131) | − | 100a | 100a | 100a | 100a |
| + | 363 | 111 | ND | ND | |
| ΔccoA mutant (SE8) | − | 79 | 107 | 101 | 97 |
| + | 146 | 118 | ND | ND | |
| ΔccoARev1 (SE8R1) | − | 126 | 102 | 101 | 96 |
| + | 490 | 137 | ND | ND | |
| ΔccoARev2 (SE8R2) | − | 150 | 100 | 93 | 92 |
| + | 433 | 129 | ND | ND | |
A mean value of 12 µg Cu, 6.3 µg Mn, 34.5 µg Zn and 268 µg Fe per gr of lyophilized cells was determined and is referred to as 100% for the wild-type strain grown in the absence of Cu2+ in MPYE medium under respiratory growth conditions.
For each strain, two sets of independently grown cells were analyzed; for each measurement, at least two repeats were done, and differences of ~10 to 20% were observed between the measurements. For a given strain, the absolute amounts of metals determined varied from culture to culture in MPYE medium, but the trend of metal contents of different strains remained unchanged between the cultures. In each case, the mean value of all measurements was presented as a percentage of the value obtained with the wild-type cells treated under the same conditions, as described in Materials and Methods. + or − refers to cells grown in the presence or absence of 5 µM Cu2+ added to MPYE medium.
The absence of CcoA does not affect the production of multicopper oxidase.
Rhodobacter species contain, in addition to cbb3-Cox, other Cu cofactor-containing enzymes, like Cu/Zn superoxide reductase (34) or multicopper oxidase (laccase or CutO) (35). Unlike Rhodobacter sphaeroides, R. capsulatus does not contain a Cu/Zn superoxide dismutase but has a twin-arginine translocation (TAT) signal sequence containing periplasmic CutO, which confers resistance to Cu2+. Mutants lacking CutO exhibit increased sensitivity to Cu2+ toxicity, and its protective effect is readily observed under anoxygenic photosynthetic growth conditions (35). In order to test whether CutO enzyme was defective in the absence of CcoA, the R. capsulatus cutO gene (RCC02110) was cloned and an insertion-deletion allele (cutO::kan) was constructed and introduced into both wild-type R. capsulatus strain MT1131 and its ΔccoA derivative SE8 (see Text S1 in the supplemental material). The sensitivity to Cu2+ of a ΔcutO single (SE15) and a ΔccoA ΔcutO double (SE16) mutant (Table S1) was determined by a plate growth inhibition assay (see Materials and Methods) and compared to the Cu2+ sensitivity of SE8. The sizes of growth inhibition zones surrounding filter disks soaked with various concentrations of Cu2+ were determined. As expected, a ΔcutO mutant (SE15) was sensitive to Cu2+ under photosynthetic growth conditions, unlike the wild-type strain (MT1131). Unlike the ΔcutO mutant, a ΔccoA mutant (SE8) was not sensitive to Cu2+, like the wild-type strain (MT1131) (Fig. 5A). Similarly, a ΔcutO ΔccoA double mutant was not more sensitive to Cu2+ than a ΔcutO mutant (not shown). Thus, the data inferred that CutO must still be functional to confer Cu2+ tolerance in the absence of CcoA in R. capsulatus.
FIG 5 .
Cu2+ response of R. capsulatus strains lacking CcoA and its derivatives. (A) Cu2+ sensitivity or resistance phenotypes of the R. capsulatus wild-type (MT1131), ΔccoA (SE8), and ΔcutO (SE15) strains under anaerobic photosynthetic growth conditions on MPYE medium were determined as described in Materials and Methods. Filter paper discs were soaked in 100 µM, 1 mM, and 10 mM Cu2+, as indicated, and plates were incubated for 2 days under photosynthetic growth conditions (Ps) to visualize the growth inhibition zones. (B) Cytochrome c profiles of appropriate R. capsulatus strains grown in enriched MPYE medium. See Fig. 4B for details. (C) The Cu2+ sensitivity or resistance phenotypes of the R. capsulatus wild-type (MT1131) and ΔccoA (SE8) strains and ΔccoA revertant 1 (ΔccoARev1; SE8R1) and 2 (ΔccoARev2; SE8R2) under respiratory growth conditions (Res) on MPYE medium were determined as described above for panel A, except that filter paper discs were soaked in 10 mM, 20 mM, and 100 mM Cu2+, as R. capsulatus is more tolerant to Cu2+ under aerobic growth conditions. Arrows indicate Cu-resistant derivatives of SE8R1 and SE8R2.
Bypass suppressors of ccoA are hypersensitive to Cu2+.
During the complementation experiments using genomic libraries, we noticed that GK1 reverted to the NADI+ phenotype at unusually high frequencies (~10−3 to 10−4) when grown by respiration without the Cu2+ supplement. DNA sequence analyses of the ccoA locus in several such revertants indicated that these revertants still retained the initial mutation (a C-to-T change at position 345 of ccoA) carried by GK1 and produced cbb3-Cox enzyme (Fig. 5B). Similar high frequencies of reversion to the NADI+ phenotype were observed with SE8, which carried a deletion-insertion allele of ccoA, suggesting that the revertants restored the ability to produce cbb3-Cox activity without any need for Cu2+ supplementation, thus bypassing the role of CcoA. Unexpectedly, when tested for their response to the Cu2+ supplement, the ccoA suppressors SE8R1 [Δ(ccoA::spe)rev1] and SE8R2 [Δ(ccoA::spe)rev2] (see Table S1 in the supplemental material) showed hypersensitivity to Cu2+ under both respiratory (Res) (Fig. 5B and C) and photosynthetic (Ps) (not shown) growth conditions. The R. capsulatus wild-type strain MT1131 and its derivative SE8, lacking CcoA, are tolerant up to a millimolar concentration of Cu2+, but the ccoA suppressors SE8R1 and SE8R2 were sensitive to micromolar amounts of a Cu2+ supplement for respiratory growth inhibition. Indeed, these mutants were partially growth inhibited in MedA medium containing 1.5 µM Cu2+ and completely inhibited by addition of ~25 µM Cu2+ supplement to MPYE medium. This hypersensitivity was specific to Cu2+ only, as no similar effect was seen with other metals, including Fe3+, Mn2+, Zn2+, and Ag+, and oxidants, such as cystine or glutathione. We therefore concluded that SE8R1 and SE8R2 regained the ability to produce cbb3-Cox at the expense of decreased tolerance to Cu2+ toxicity.
Total intracellular Cu contents of SE8R1 and SE8R2 cells grown in MPYE medium with and without 5 µM Cu2+ supplement were also determined using ICP-DRC-MS analyses (see Materials and Methods). In the absence of a Cu2+ supplement, the total intracellular Cu contents of these mutants were greater than in their parent, SE8, which lacks CcoA, and similar or greater than in the wild-type strain, MT1131 (Table 1). In the presence of a Cu2+ supplement, all strains accumulated larger amounts of intracellular Cu. The levels found in SE8R1 and SE8R2 were much higher than those seen in the wild-type strain MT1131, although the intracellular amounts of Mn used as an internal control were unchanged in all cases (Table 1). Thus, the suppressor mutation(s) bypassed the absence of CcoA by increasing specifically intracellular Cu accumulation at the expense of compromised cellular tolerance to this toxic metal. The molecular basis of this suppression, which is beyond the scope of this work, remains to be identified.
DISCUSSION
The impetus behind this work was to understand how cells assemble catalytic metal cofactors into the heme CuB binuclear center of Cox, a process that is largely uncharacterized. Using R. capsulatus, we initiated a genetic approach to investigate bacterial Cox biogenesis and isolated various cbb3-Cox-defective mutants. In this work, we focused on mutants that produced an active cbb3-Cox only upon exogenous Cu2+ supplementation. Studies of these mutants uncovered a novel gene, ccoA, which was distinct from ccoGHIS (23), senC (26), olsAB (24), and dsbA and degP (27), known to affect this process. Mutants lacking CcoA were unable to produce normal amounts of cbb3-Cox activity because the steady-state amounts of the subunits of this enzyme were drastically decreased in membranes. However, neither the transcription of the structural genes ccoNOQP nor the translation initiation of CcoN was abolished in the absence of CcoA, indicating that cbb3-Cox assembly was defective. Whether ccoA affects only cbb3-Cox is not known. Its absence does not inactivate the periplasmic Cu-containing multicopper oxidase CutO in R. capsulatus. The putative TAT signal sequence of CutO suggests that this enzyme might acquire its Cu cofactor in the cytoplasm prior to translocation, in a manner similar to that of its Escherichia coli homologue, CueO (3). Investigation of bacterial species that have CcoA homologues, like R. sphaeroides or Bradyrhizobium japonicum, and that also produce aa3-Cox might further elucidate its role in the biogenesis of other Cox enzymes.
A major finding of this work was that the ccoA gene encodes a member of the MFS-type transporters, which have not been implicated hitherto into cbb3-Cox biogenesis in bacteria. Remarkably, CcoA homologues with Mets motifs are present in most bacteria that contain cbb3-Cox (except the epsilonproteobacteria and the Cytophaga-Flexibacter-Bacteroides [CFB] group) (see Table S2 in the supplemental material), suggesting that they are important for the production of this enzyme. How CcoA affects cbb3-Cox assembly is intriguing. The MFS-type secondary transporters use the electrochemical potential difference generated by ion or solute gradients and transport a diverse range of substrates in and out of cytoplasm (36). Some MFS proteins have been implicated as importers or exporters of siderophores, including E. coli EntS (37), Vibrio parahaemolyticus PvsC (38), and Sinorhizobium meliloti RhtX (39), which secrete enterobactin, vibrioferrin, and rhizobactin, respectively. R. capsulatus mutants lacking CcoA produce various c-type cytochromes that rely on efficient siderophore trafficking and Fe supply for heme production, indicating that CcoA is not involved in Fe uptake. This is also supported by the unchanged intracellular Fe concentration in the CcoA knock out strain (Table 1). Instead, several findings suggest that CcoA is involved in cellular Cu acquisition for cbb3-Cox assembly. First, mutants lacking CcoA exhibit enhanced cbb3-Cox activity upon increased exogenous Cu2+ supplementation. This enhancement is specific to Cu2+ only, as Zn2+, Mn2+, or Fe3+ addition has no similar effects. Mass spectrometry measurements indicated that in cells lacking CcoA, only the total Cu (and not Zn, Mn, or Fe) content is lower than that of a wild-type cell under normal growth conditions. Upon Cu2+ supplementation, cellular Cu content increased in mutants lacking CcoA, although it never reached wild-type levels, whereas the cellular contents of metals other than Cu, like Zn, Mn, or Fe, remained unaffected. These findings are consistent with CcoA being involved in a Cu influx rather than a Cu efflux pathway. It is noteworthy that R. capsulatus mutants lacking CcoA still contain cellular Cu, indicating that unrelated and currently unknown Cu acquisition pathways also exist in this species.
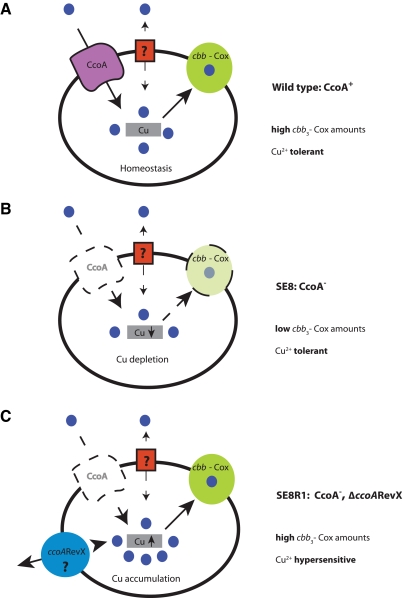
Second, mutants lacking CcoA are not more sensitive to Cu2+ supplementation than a wild-type R. capsulatus strain under various growth conditions, suggesting that CcoA is not involved in Cu detoxification, unlike, for example, the P1B-type Cu exporters (40–42). Moreover, suppressor mutants that bypass the need for CcoA to recover cbb3-Cox activity are extremely sensitive to very small amounts (~25 µM) of Cu2+ supplement in the medium. Again, this sensitivity is specific to Cu2+, as these revertants exhibit normal tolerance towards Zn2+, Mn2+, Fe3+, and even Ag+, known to mimic Cu+ (42, 43). Mass spectrometry measurements indicated that in these suppressor mutants, Cu content was similar to that seen in the wild-type cells, suggesting that the suppressors overcame the function of CcoA by enhancing Cu acquisition or retention. Indeed, upon Cu2+ supplementation, the suppressor mutants accumulated much larger amounts of intracellular Cu (and not Mn) than wild-type cells. Based on the overall findings, it is therefore compelling to rationalize that the absence of CcoA induces an intracellular Cu2+ shortage to decrease cbb3-Cox production (Fig. 6). Consequently, the availability of an increased exogenous Cu2+ supply or the occurrence of an additional mutation(s) overcomes this shortage to yield normal amounts of cbb3-Cox at the expense of a cell’s tolerance of Cu, which is compromised.
FIG 6 .
Hypothetical model of how CcoA affects cellular Cu content and the production of cbb3-Cox in R. capsulatus. (A) A wild-type strain (MT1131) requires CcoA to produce normal amounts of active cbb3-Cox and exhibits tolerance to Cu2+. (B) In the absence of CcoA (SE8), cbb3-Cox formation is abolished and intracellular Cu amounts are decreased, but cells remain tolerant to Cu2+. (C) The absence of CcoA can be suppressed by a second mutation(s), referred to as ΔccoARevX (e.g., SE8R1 or SE8R2), that restores the production of active cbb3-Cox at the expense of increased intracellular Cu amounts and renders cells sensitive to Cu2+.
Bacteria utilize multiple transporters to achieve the movement of metals across the membrane and maintain homeostasis with metals in nontoxic concentrations (44). In the case of Cu, homeostatic pathways are multiple and complex (45, 46). For example, energy-dependent primary transporters of the P1B-type ATPases are involved in Cu+ efflux from the cytoplasm to the periplasm. Of these, CopA1-type transporters have high efflux rates and are involved in Cu detoxification. Their expressions are induced by an excess of Cu, and their absence induces Cu sensitivity (40, 42, 47, 48). In contrast, CopA2-type transporters, like R. capsulatus CcoI of the ccoGHIS gene cluster, have low efflux rates and no role in Cu toxicity but are involved in cbb3-Cox biogenesis (23, 40). RND (resistance-nodulation-cell division protein family)-type transporters are also involved in the efflux of Cu ions from both the cytoplasm and the periplasm to the extracellular milieu to detoxify cells (49). Clearly, while Cu efflux and detoxification pathways are elaborate, how Cu is imported into the bacterial cytoplasm is less well known (41, 46). Only a few proteins, including Enterococcus hirae CopA (48), Pseudomonas aeruginosa HmtA (50), Bacillus subtilis YcnJ (51), Pseudomonas syringae CopCD (52), the cyanobacterial P1B-type ATPases (CtaA and PacS located in the cytoplasmic and thylakoid membranes, respectively) (53), and two plant chloroplast P1B-type ATPases (PAA1/HMA6 and PAA2/HMA8 of the inner and thylakoid membranes, respectively) (54), have been implicated in Cu import. In eukaryotic microbes like Saccharomyces cerevisiae, mainly the plasma membrane-located Ctr-type transporters are known to import Cu with very high affinity into the cytoplasm (45, 46). So far, no bacterial Ctr homologue has been reported, and until very recently, no MFS-type Cu transporter was known. Very recently, Beaudoin et al. (56) reported a novel forespore membrane Cu transporter, Mfc1, which is distinct from the Ctr-type transporters (55) and involved in meiotic and sporulating cells of Schizosaccharomyces pombe. Mfc1 is a member of the MFS-type transporters, and it functions as a specific Cu importer during meiotic differentiation under Cu-limiting conditions in S. pombe (56). Excitingly, S. pombe Mfc1 is homologous to R. capsulatus CcoA, has a topology similar to CcoA’s (with 12 transmembrane helices with cytoplasmic amino- and carboxyl-terminal ends), and contains other landmarks of MFS-type transporters. Moreover, both Mfc1 and CcoA contain the Mets motifs (MXM, MXXM, and MXCXM) involved in Cu transport. The Cu2+ importer function of Mfc1 was established by direct-transport assay using radioactive 64Cu and by observation of its ability to complement appropriate S. cerevisiae mutants lacking the known Cu-importing Ctr-type transporters. The similarities found between CcoA and Mfc1 suggest that CcoA also acts as a Cu importer, although direct proof of this is lacking. Finally, as in some methane-oxidizing bacteria (methanotrophs), Cu is imported into the cytoplasm, which is associated with siderophore-like molecules (called chalkophores or methanobactins) (57, 58), and whether CcoA-mediated Cu acquisition involves additional compounds (59) needs investigation.
In summary, our findings establish for the first time that the MFS-type transporter CcoA is required for maintaining normal amounts of intracellular Cu and cbb3-Cox in R. capsulatus and possibly in other bacterial species. Future work will hopefully elucidate the links between CcoA, cellular Cu acquisition, and cbb3-Cox biogenesis.
MATERIALS AND METHODS
Strains, culture conditions, phenotypes, and molecular genetic techniques.
Detailed descriptions of strains, culture conditions, phenotypes, and molecular genetic techniques used in this study are presented in the supplemental material (Text S1).
Cu2+ sensitivity assays.
Strains to be tested for Cu2+ sensitivity were grown to the exponential phase (optical density at 630 nm [OD630] of ~0.5) in MPYE medium under appropriate growth conditions. Cells for respiratory (1.7 × 107 cells) and photosynthetic (2.6 × 107 cells) growth conditions (estimated with 1.0 OD630 unit equal to 7.5 × 108 R. capsulatus cells per ml) were added to 4 ml of the same medium containing 0.7% top agar and poured on top of regular plates containing 10 ml medium. Whatman 3 MM paper discs (3 mm in diameter), soaked with 8 µl per disc of desired concentrations of CuSO4 solution, were placed on the surfaces of the plates after solidification of the top agar. Plates were incubated under the desired growth conditions and scanned at the end of incubation period, and the sizes of the growth incubation zones exhibited by different mutants were measured to estimate their responses to CuSO4 toxicity. Each assay was repeated at least three times.
RNA isolation and RT-PCR.
R. capsulatus cultures were grown semiaerobically in MPYE medium until mid-log phase (OD630 of approximately 0.5), and total RNA was isolated from about 2 × 108 cells using the Qiagen RNeasy minikit, digested with DNase I for 25 min at room temperature, and ethanol precipitated. Fifty nanograms of total RNA was used per RT-PCR with the Qiagen OneStep RT-PCR kit, and the ccoN BHK20B (5′ CCAGTCGGGCAGCGCGGTAT 3′) and N3-RT (5′ CGGCAACGGGATGCTGAACTTC 3′) primers amplified a 652-bp-long region internal to ccoN that corresponds to positions 299 and 972 of ccoN. The primers 16SrRNA-F (5′ ATATTCGGAGGAACACCAGTGGC 3′) and 16SrRNA-R (5′ CAGAGTGCCCAACTGAATGATGG 3′) were used as a control for amplification of a 450-bp region of the 16S rRNA gene. In each case, RT-PCR controls were prepared by omitting the reverse transcriptase enzyme from the reaction mixture, the amplification products were separated using 1% agarose gels, and their intensities were compared by ImageJ (NIH).
Cell extract preparation.
Cells were grown in 10 ml of MPYE medium by respiration and harvested at 4,000 pm for 10 min. Pellets were resuspended in 200 µl of CellLytic B 2× cell lysis solution (Sigma Inc.) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM EDTA, 50 µg lysozyme, 20 µg DNase, and 10 mM MgCl2, incubated at room temperature for 15 min, and centrifuged at 14,000 rpm for 10 min. Supernatants thus obtained were taken as whole-cell extracts. Intracytoplasmic membrane vesicles (chromatophore membranes) were prepared in 50 mM MOPS (pH 7.0) containing 100 mM KCl and 1 mM PMSF as described earlier (17). Protein concentrations were determined using the bicinchoninic acid assay according to the supplier’s recommendations (Sigma Inc.; procedure TPRO-562).
Enzyme activity measurements.
Cytochrome c oxidase activity was measured spectrophotometrically using reduced horse heart cytochrome c (Sigma, St. Louis, MO) in a stirred cuvette at 25°C. Horse heart cytochrome c was reduced by incubation for 15 min at room temperature with a 1 mM final concentration of fresh sodium dithionite (100 mM stock solution), which was then removed using a PD10 desalting column (GE Healthcare Life Sciences). R. capsulatus chromatophore membranes were detergent solubilized with 1 mg (wt/wt) dodecyl β-d-maltoside per mg of membrane proteins added to the assay buffer (10 mM Tris-HCl, pH 7.0, 120 mM KCl, and 25 µM reduced cytochrome c). The enzymatic reaction was started and stopped by the addition of solubilized membranes and 100 µM KCN, respectively. The linear range of the assay was controlled by using different amounts of solubilized membranes, and KCN-sensitive Cox activity was calculated as micromoles of cytochrome c oxidized per milligram of membrane protein per minute using an absorption coefficient (ε550) of 20, as described earlier (17, 60). The β-galactosidase activities of whole-cell extracts prepared using 10-ml cultures of appropriate strains were measured spectrophotometrically at 420 nm using o-nitrophenyl galactoside (ONPG), as described earlier (23), and specific activity in nanomoles of ONPG hydrolyzed per minute per milligram of protein was determined using an absorption coefficient (ε420) of 21,300 M−1 cm−1.
SDS-PAGE, immunoblotting, and heme staining.
For CcoN immunodetection, chromatophore membrane proteins (50 µg) in 62.5 mM Tris-HCl (pH 6.8), 2% (wt/vol) SDS, 25% (vol/vol) glycerol, 0.01% (wt/vol) bromophenol blue, and 5% β-mercaptoethanol were incubated at room temperature for 15 min prior to being loaded and were separated by 12% SDS-PAGE (61). The gels were electroblotted onto Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) and probed with R. capsulatus CcoN rabbit polyclonal antibodies (28). Alkaline phosphatase-conjugated monoclonal anti-rabbit IgG (clone RG-16) was used as the secondary antibody (Sigma-Aldrich, Saint Louis, MO), with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (NBT) as a substrate (Sigma-Aldrich, Saint Louis, MO) for the detection. For detection of the c-type cytochromes, ~50 µg of total membrane proteins was separated by 16.5% SDS-PAGE (62), and the gels were stained for endogenous peroxidase activity of the c-type cytochromes by using 3,3′,5,5′-tetramethylbenzidine (TMBZ) and H2O2 (63).
Determination of cellular Cu content by ICP-DRC-MS.
The cellular Cu contents of various strains were determined using inductively coupled plasma-dynamic reaction cell-mass spectrometry (ICP-DRC-MS). In this technique, aliquots of sample digestions are introduced into a radio frequency plasma where energy transfer processes cause desolvation, atomization, and ionization. The ions thus formed are extracted from the plasma via a differentially pumped vacuum interface and travel through a pressurized chamber (DRC) containing a specific reactive gas that preferentially reacts with interfering ions of the same target mass-to-charge ratios (m/z). A solid-state detector detects ions transmitted through the mass analyzer on the basis of their mass-to-charge ratios, and the resulting current is processed by a data handling system. For sample preparation, at least 1 h prior to use, all containers, glassware, and tubes were washed with 2% nitric acid and rinsed with metal-free Milli-Q water to prevent metal contamination. Metal-free water and buffers were prepared by stirring them for 1 h at room temperature with 5 g Chelex 100 per liter. For each strain, a 1-liter culture was grown by respiration in MPYE medium to an OD630 of 0.8 to 0.9, and cells were harvested by centrifugation and washed three times with a metal-free buffer of 20 mM Tris-HCl, pH 8.0, and once with metal-free Milli-Q water. Cell pellets were lyophilized until complete dryness and shipped to Applied Speciation and Consulting, LLC (WA), for determination of total Cu, Zn, Mn, and Fe contents. Fifty milligrams of lyophilized cells was digested completely with aliquots of concentrated HNO3 and H2O2 at 95°C. The digests were diluted to a known final volume (50 ml) with metal-free reagent water and analyzed via ICP-DRC-MS according to a standard procedure of this company. The data were provided in µg of metal of interest per g of cells (ppm).
Chemicals.
All chemicals were of reagent grade and were obtained from commercial sources.
SUPPLEMENTAL MATERIAL
Supplemental methods. Download Text S1, DOCX file, 0 MB.
Restriction map of plasmids pSE1 and pSE2 and their derivatives used to complement GK1 and to construct chromosomal knockout mutants. Various plasmids, isolated or constructed as described in Materials and Methods, are shown on the left, and their ability to complement the NADI+ phenotype of GK1 is shown on the right. When appropriate, R. capsulatus strains carrying the related chromosomal knockout alleles are also indicated under the plasmids. (A) Plasmids derived from pSE1 and pSE2. See the text for the annotations of the ORFs RCAP_RCC02189, RCC02190, RCC02191, RCC02192, RCC02193, and RCC02194, located on pSE1 and pSE2. (B) The RCAP_RCC02192 gene corresponds to ccoA, which complements GK1, HY70, and SE8 and encodes a major facilitator superfamily (MFS)-type transporter responsible for a Cu2+ supplement-independent NADI phenotype and cbb3-Cox production in these mutants. E, H, Ns, C, B, Bl, As, Ba, Bg, XB, and K correspond to the restriction endonuclease sites for the EcoRI, HindIII, NsiI, ClaI, BstBI, BlpI, AsiSI, BamHI, BglII, XbaI, and KpnI enzymes, respectively. Superscript commas indicate partial genes. Download Figure S1, DOCX file, 0.1 MB.
Strains and plasmids used in this work.
Phylogenetic distribution of CcoA among various bacterial genomes.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM38239 and DOE grant 91ER20052 to F.D. and Deutsche Forschungsgemeinschaft grant GRK1478 and German-French Ph.D. College grant UFA0407 to H.-G.K.
Footnotes
Citation Ekici S, Yang H, Koch H, Daldal F. 2012. Novel transporter required for biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. mBio 3(1):e00293-11. doi:10.1128/mBio.00293-11.
REFERENCES
- 1. Tsukihara T, et al. 1995. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science 269:1069–1074 [DOI] [PubMed] [Google Scholar]
- 2. Tainer JA, Getzoff ED, Richardson JS, Richardson DC. 1983. Structure and mechanism of copper, zinc superoxide dismutase. Nature 306:284–287 [DOI] [PubMed] [Google Scholar]
- 3. Roberts SA, et al. 2002. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:2766–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halliwell B, Gutteridge JM. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. 1993. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 3:7–13 [DOI] [PubMed] [Google Scholar]
- 7. Tanzi RE, et al. 1993. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 5:344–350 [DOI] [PubMed] [Google Scholar]
- 8. Gaggelli E, Kozlowski H, Valensin D, Valensin G. 2006. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 106:1995–2044 [DOI] [PubMed] [Google Scholar]
- 9. Hung YH, Bush AI, Cherny RA. 2010. Copper in the brain and Alzheimer’s disease. J. Biol. Inorg. Chem. 15:61–76 [DOI] [PubMed] [Google Scholar]
- 10. García-Horsman JA, Barquera B, Rumbley J, Ma J, Gennis RB. 1994. The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 176:5587–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pereira MM, Sousa FL, Veríssimo AF, Teixeira M. 2008. Looking for the minimum common denominator in haem-copper oxygen reductases: towards a unified catalytic mechanism. Biochim. Biophys. Acta 1777:929–934 [DOI] [PubMed] [Google Scholar]
- 12. Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG. 2006. Multi-step assembly pathway of the cbb3-type cytochrome c oxidase complex. J. Mol. Biol. 355:989–1004 [DOI] [PubMed] [Google Scholar]
- 13. Preisig O, Zufferey R, Thöny-Meyer L, Appleby CA, Hennecke H. 1996. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 178:1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsukita S, et al. 1999. Characterization of a cb-type cytochrome c oxidase from Helicobacter pylori. J. Biochem. 125:194–201 [DOI] [PubMed] [Google Scholar]
- 15. Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. 2011. Biogenesis of cbb3-type cytochrome c oxidase in Rhodobacter capsulatus. Biochem. Biophys. Acta 1808:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marrs B, Gest H. 1973. Genetic mutations affecting the respiratory electron-transport system of the photosynthetic bacterium Rhodopseudomonas capsulata. J. Bacteriol. 114:1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gray KA, et al. 1994. Rhodobacter capsulatus contains a novel cb-type cytochrome c oxidase without a CuA center. Biochemistry 33:3120–3127 [DOI] [PubMed] [Google Scholar]
- 18. Peters A, Kulajta C, Pawlik G, Daldal F, Koch HG. 2008. Stability of the cbb3-type cytochrome oxidase requires specific CcoQ-CcoP interactions. J. Bacteriol. 190:5576–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pawlik G, et al. 2010. The putative assembly factor CcoH is stably associated with the cbb3-type cytochrome oxidase. J. Bacteriol. 192:6378–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tiranti V, et al. 1998. Mutations of SURF-1 in Leigh disease associated with cytochrome c oxidase deficiency. Am. J. Hum. Genet. 63:1609–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Valnot I, et al. 2000. A mutation in the human heme A: farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum. Mol. Genet. 9:1245–1249 [DOI] [PubMed] [Google Scholar]
- 22. Papadopoulou LC, et al. 1999. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat. Genet. 23:333–337 [DOI] [PubMed] [Google Scholar]
- 23. Koch HG, Winterstein C, Saribas AS, Alben JO, Daldal F. 2000. Roles of the ccoGHIS gene products in the biogenesis of the cbb(3)-type cytochrome c oxidase. J. Mol. Biol. 297:49–65 [DOI] [PubMed] [Google Scholar]
- 24. Aygun-Sunar S, et al. 2006. Ornithine lipid is required for optimal steady-state amounts of c-type cytochromes in Rhodobacter capsulatus. Mol. Microbiol. 61:418–435 [DOI] [PubMed] [Google Scholar]
- 25. Buggy J, Bauer CE. 1995. Cloning and characterization of senC, a gene involved in both aerobic respiration and photosynthesis gene expression in Rhodobacter capsulatus. J. Bacteriol. 177:6958–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swem DL, Swem LR, Setterdahl A, Bauer CE. 2005. Involvement of SenC in assembly of cytochrome c oxidase in Rhodobacter capsulatus. J. Bacteriol. 187:8081–8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Onder O, Turkarslan S, Sun D, Daldal F. 2008. Overproduction or absence of the periplasmic protease DegP severely compromises bacterial growth in the absence of the dithiol: disulfide oxidoreductase DsbA. Mol. Cell. Proteomics 7:875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koch HG, Hwang O, Daldal F. 1998. Isolation and characterization of Rhodobacter capsulatus mutants affected in cytochrome cbb3 oxidase activity. J. Bacteriol. 180:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saier MH, Jr, Tran CV, Barabote RD. 2006. TCDB: the transporter classification database for membrane transport protein analyses and information. Nucleic Acids Res. 34:D181–D186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saier MH, Jr, Yen MR, Noto K, Tamang DG, Elkan C. 2009. The transporter classification database: recent advances. Nucleic Acids Res. 37:D274–D278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henderson PJ, Maiden MC. 1990. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 326:391–410 [DOI] [PubMed] [Google Scholar]
- 32. Puig S, Lee J, Lau M, Thiele DJ. 2002. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 277:26021–26030 [DOI] [PubMed] [Google Scholar]
- 33. Eisses JF, Kaplan JH. 2005. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J. Biol. Chem. 280:37159–37168 [DOI] [PubMed] [Google Scholar]
- 34. Kho DH, Yoo SB, Kim JS, Kim EJ, Lee JK. 2004. Characterization of Cu- and Zn-containing superoxide dismutase of Rhodobacter sphaeroides. FEMS Microbiol. Lett. 234:261–267 [DOI] [PubMed] [Google Scholar]
- 35. Wiethaus J, Wildner GF, Masepohl B. 2006. The multicopper oxidase CutO confers copper tolerance to Rhodobacter capsulatus. FEMS Microbiol. Lett. 256:67–74 [DOI] [PubMed] [Google Scholar]
- 36. Pao SS, Paulsen IT, Saier MH., Jr. 1998. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 62:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furrer JL, Sanders DN, Hook-Barnard IG, McIntosh MA. 2002. Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol. Microbiol. 44:1225–1234 [DOI] [PubMed] [Google Scholar]
- 38. Tanabe T, Nakao H, Kuroda T, Tsuchiya T, Yamamoto S. 2006. Involvement of the Vibrio parahaemolyticus pvsC gene in export of the siderophore vibrioferrin. Microbiol. Immunol. 50:871–876 [PubMed] [Google Scholar]
- 39. Cuív PO, Clarke P, Lynch D, O’Connell M. 2004. Identification of rhtX and fptX, novel genes encoding proteins that show homology and function in the utilization of the siderophores rhizobactin 1021 by Sinorhizobium meliloti and pyochelin by Pseudomonas aeruginosa, respectively. J. Bacteriol. 186:2996–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. González-Guerrero M, Raimunda D, Cheng X, Argüello JM. 2010. Distinct functional roles of homologous Cu+ efflux ATPases in Pseudomonas aeruginosa. Mol. Microbiol. 78:1246–1258 [DOI] [PubMed] [Google Scholar]
- 41. Solioz M, Abicht HK, Mermod M, Mancini S. 2010. Response of gram-positive bacteria to copper stress. J. Biol. Inorg. Chem. 15:3–14 [DOI] [PubMed] [Google Scholar]
- 42. Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl. Acad. Sci. U. S. A. 97:652–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winge DR, Nielson KB, Gray WR, Hamer DH. 1985. Yeast metallothionein. Sequence and metal-binding properties. J. Biol. Chem. 260:14464–14470 [PubMed] [Google Scholar]
- 44. Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313–339 [DOI] [PubMed] [Google Scholar]
- 45. Banci L, Bertini I, McGreevy KS, Rosato A. 2010. Molecular recognition in copper trafficking. Nat. Prod. Rep. 27:695–710 [DOI] [PubMed] [Google Scholar]
- 46. Banci L, Bertini I, Cantini F, Ciofi-Baffoni S. 2010. Cellular copper distribution: a mechanistic systems biology approach. Cell. Mol. Life Sci. 67:2563–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanamaru K, Kashiwagi S, Mizuno T. 1994. A copper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species PCC7942. Mol. Microbiol. 13:369–377 [DOI] [PubMed] [Google Scholar]
- 48. Odermatt A, Suter H, Krapf R, Solioz M. 1993. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 268:12775–12779 [PubMed] [Google Scholar]
- 49. Kim EH, Nies DH, McEvoy MM, Rensing C. 2010. Switch or funnel: how RND-type transport systems control periplasmic metal homeostasis. J. Bacteriol. 193:2381–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lewinson O, Lee AT, Rees DC. 2009. A P-type ATPase importer that discriminates between essential and toxic transition metals. Proc. Natl. Acad. Sci. U. S. A. 106:4677–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chillappagari S, Miethke M, Trip H, Kuipers OP, Marahiel MA. 2009. Copper acquisition is mediated by YcnJ and regulated by YcnK and CsoR in Bacillus subtilis. J. Bacteriol. 191:2362–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cha JS, Cooksey DA. 1993. Copper hypersensitivity and uptake in Pseudomonas syringae containing cloned components of the copper resistance operon. Appl. Environ. Microbiol. 59:1671–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tottey S, Rich PR, Rondet SA, Robinson NJ. 2001. Two Menkes-type ATPases supply copper for photosynthesis in Synechocystis PCC 6803. J. Biol. Chem. 276:19999–20004 [DOI] [PubMed] [Google Scholar]
- 54. Nouet C, Motte P, Hanikenne M. 2011. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 16:395–404 [DOI] [PubMed] [Google Scholar]
- 55. Beaudoin J, Thiele DJ, Labbé S, Puig S. 2011. Dissection of the relative contribution of the Schizosaccharomyces pombe Ctr4 and Ctr5 proteins to the copper transport and cell surface delivery functions. Microbiology 157:1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Beaudoin J, et al. 2011. Mfc1 is a novel forespore membrane copper transporter in meiotic and sporulating cells. J. Biol. Chem. 286:34356–34372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Balasubramanian R, Rosenzweig AC. 2008. Copper methanobactin: a molecule whose time has come. Curr. Opin. Chem. Biol. 12:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim HJ, et al. 2004. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science 305:1612–1615 [DOI] [PubMed] [Google Scholar]
- 59. Cobine PA, Ojeda LD, Rigby KM, Winge DR. 2004. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 279:14447–14455 [DOI] [PubMed] [Google Scholar]
- 60. Myllykallio H, Jenney FE, Jr, Moomaw CR, Slaughter CA, Daldal F. 1997. Cytochrome c(y) of Rhodobacter capsulatus is attached to the cytoplasmic membrane by an uncleaved signal sequence-like anchor. J. Bacteriol. 179:2623–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 62. Schägger H, von Jagow G. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368–379 [DOI] [PubMed] [Google Scholar]
- 63. Thomas PE, Ryan D, Levin W. 1976. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal. Biochem. 75:168–176 [DOI] [PubMed] [Google Scholar]
- 64. Spyropoulos IC, Liakopoulos TD, Bagos PG, Hamodrakas SJ. 2004. TMRPres2D: high quality visual representation of transmembrane protein models. Bioinformatics 20:3258–3260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods. Download Text S1, DOCX file, 0 MB.
Restriction map of plasmids pSE1 and pSE2 and their derivatives used to complement GK1 and to construct chromosomal knockout mutants. Various plasmids, isolated or constructed as described in Materials and Methods, are shown on the left, and their ability to complement the NADI+ phenotype of GK1 is shown on the right. When appropriate, R. capsulatus strains carrying the related chromosomal knockout alleles are also indicated under the plasmids. (A) Plasmids derived from pSE1 and pSE2. See the text for the annotations of the ORFs RCAP_RCC02189, RCC02190, RCC02191, RCC02192, RCC02193, and RCC02194, located on pSE1 and pSE2. (B) The RCAP_RCC02192 gene corresponds to ccoA, which complements GK1, HY70, and SE8 and encodes a major facilitator superfamily (MFS)-type transporter responsible for a Cu2+ supplement-independent NADI phenotype and cbb3-Cox production in these mutants. E, H, Ns, C, B, Bl, As, Ba, Bg, XB, and K correspond to the restriction endonuclease sites for the EcoRI, HindIII, NsiI, ClaI, BstBI, BlpI, AsiSI, BamHI, BglII, XbaI, and KpnI enzymes, respectively. Superscript commas indicate partial genes. Download Figure S1, DOCX file, 0.1 MB.
Strains and plasmids used in this work.
Phylogenetic distribution of CcoA among various bacterial genomes.