Abstract
The brain reserve hypothesis has been posited as being one important mediating factor for developing dementia, especially Alzheimer’s disease (AD). Evidence for this hypothesis is mixed though different methodologies have made these findings difficult to interpret. We examined imaging data from a large cohort (N=194) of mixed dementia patients and controls 65 yrs old and older from the Cache County, Utah Study of Memory and Aging for evidence of the brain reserve hypothesis using total intracranial volume (TICV) as a quantitative measure of premorbid brain size and a vicarious indicator of reserve. A broader spectrum of non-demented elderly control subjects from previous studies was also included for comparison (N=423). In addition, non-parametric classification and regression tree (CART) analyses were performed to model group heterogeneity and identify any subgroups of patients where TICV might be an important predictor of dementia. Parametrically, no main effect was found for TICV when predicting a dementia diagnosis, however, the CART analysis did reveal important TICV subgroups, including a sex differential wherein ε4 APOE allele presence in males and low TICV predicted AD classification. TICV, APOE, and other potential mediator/moderator variables are discussed in the context of the brain reserve hypothesis.
Keywords: Alzheimer’s disease, intracranial volume, cognitive or brain reserve, APOE, dementia
INTRODUCTION
A brain reserve hypothesis has been proposed by a number of investigators exploring etiological factors in the development of Alzheimer’s disease (AD; Alexander et al., 1997; Coffey et al., 1999; Jorm et al., 1997; Reynolds et al., 1999; Schmand et al., 1997; Schofield et al., 1995; 1997b; Roe et al., 2008; Fotenos et al., 2008). Within the limits of normality, the basic premise of the brain reserve hypothesis is that larger pre-morbid brain volume is protective against the development of dementia. The protective influence may be related to size-complexity and size-redundancy issues (Glassman, 1987), where a larger brain may have “reserve” capacity associated with increased complexity or redundancy (Satz, 1993; Stern, 2002; 2007). The oft cited study in support of this position is one by Katzman et al. (1988) where, at post mortem, senile plaques and neurofibrillary tangles were found in sufficient numbers to make the pathological diagnosis of AD in subjects who, prior to death, did not meet clinical criteria for dementia. Importantly, the brain size was larger in these subjects who met pathological criteria for AD yet did not meet the clinical criteria for dementia.
We know from developmental studies that the ultimate size of the cranial vault is primarily dependent on internal pressure from expanding brain parenchyma, which creates outward tensional forces on the pliable cranial bones and sutures. These tensional forces induce cranial expansion (Sperber, 2001) creating a dynamic relationship between head size and brain volume in development. This parallel dynamic relationship is probably the most significant reason for the robust circumference-brain growth relationships, particularly in early infancy and childhood (Bartholomeusz et al., 2002). In addition, we know that there are linear relationships between the size of the cranial vault, brain size, head, and body size (Courchesne et al., 2000; Dekaban et al., 1978; Vernon et al., 2000). This relationship has been the primary factor driving the development of various techniques including circumferential measures of the skull or head (HC) as well as magnetic resonance imaging (MRI) methods for total intracranial volume (TICV) as indices of premorbid brain size (Graves et al., 2001; 1996; Jenkins et al., 2000; Mori et al., 1997; Schofield et al., 1995). While the majority of studies that have examined pre-morbid head size have reported a relationship between smaller HC/TICV and AD, a recent well-designed study by Jenkins et al. (2000) found no relationship between sporadic AD subjects and TICV. However, these authors did observe smaller TICV in a group of familial AD subjects, although the differences were not significant. Edland and colleagues (2002) also reported similar findings, suggesting head size alone is not a critical determinant of AD (see also Drachman, 2002; Graves et al., 1996; Jenkins et al., 2000; MacLullich et al., 2002).
Brain size also relates to the complexity of cognitive tasks that can be accomplished by a given species (Armstrong et al., 1982; Jerison, 1987) and in humans, brain size is associated with psychometric intelligence (Andreasen et al., 1993; Vernon et al., 2000; Willerman et al., 1991) and general cognitive ability (MacLullich et al., 2002; Erten-Lyons et al., 2009; Drachman, 2002). There is also evidence that throughout this formative period, cranial capacity and brain size are relevant to cognitive outcome (Johnson et al., 1991; Peterson et al., 2000a; Vernon et al., 2000; Frisk et al., 2002). For example, Stathis et al. (1999) demonstrated that smaller HC at age eight months in low birth weight babies predicted lower IQ at age six. Another study by Martyn et al. (1996) demonstrated that a larger biparietal head diameter at birth positively correlated with measures of intelligence in adulthood. In addition, Peterson et al. (2000a) have shown that prematurity influences brain volume and cognitive outcome in preterm infants and Reddick et al. (1998) demonstrated volume differences related to outcome in children receiving irradiation, where lower volume related to greater cognitive impairment.
It should be noted that in addition to the amount of reserve capacity of the brain, the presence of the ε4 allele of apolipoprotein E (APOE) is another established risk factor for AD (Cummings et al., 2002; Berlau et al., 2009; Agosta et al. 2009; Cosentino et al., 2008) although recent studies of APOE genotype appear to demonstrate a less direct affect of APOE on the development of dementia. For example, studies from the Cache County Memory and Aging study demonstrated a relationship between the age of onset of dementia symptoms and not diagnosis of dementia. This might be interpreted as meaning any effects of APOE would necessarily occur very early, perhaps even in early development. Because head size is in part driven by brain growth during development, researchers have examined the relationship between head size and APOE. For example, Schofield et al. (1997a) found an association between smaller HC and AD, but did not find a relationship between APOE genotype and HC. Similarly, Espinosa et al. (2006) demonstrated a main effect for HC, which discriminated between AD patients and healthy controls. There was no relationship between HC and APOE status and in the prospective arm of this study; APOE allele status conferred additional risk for AD while HC did not. By contrast, Graves et al. (2001; 1996) found a relationship between HC and presence of the ε4 allele in their patient population suggesting a link between brain reserve, HC, and APOE. In addition, Kim et al. (2008) findings suggest the possibility that the presence of APOE affects cognitive function when brain reserve is low, supporting the brain reserve hypothesis.
These sometimes equivocal results make it difficult to understand the true relationship between these variables. However, it is noteworthy that HC is an indirect measure of cranial capacity correlating only moderately (r ~ 0.6, R2 ~ 0.36) with actual brain size in typically developing adolescents and adults (Lainhart et al., 1997). In contrast, TICV among typically aging adults as measured by our lab is significantly associated with brain volume (r = 0.89) accounting for approximately 79% of the variability associated with total brain volume with the difference between TICV and brain volume being less than 191 ml3 in healthy controls regardless of age (Bigler et al., 2001; Blatter et al., 1995). This represents a methodological improvement over external measures of HC that is sometimes complicated by variable amounts of fascia, skin, and bone captured in this measures (Tate et al., 2007).
The purpose of this study is to examine associations between TICV, APOE genotype, and dementia diagnosis in a sample of participants from the Cache County Memory and Aging Study for evidence supporting the cerebral reserve hypothesis. Unique to this study is the inclusion of participants from a population sample that not only include AD patients but also participants with other types of dementia. To our knowledge, this is the first study to specifically examine the relationships between TICV, APOE genotype in a mixed dementia diagnostic group. The focus of past research has been primarily on AD though in the context of the cerebral reserve hypothesis, TICV may plausibly play a role in other types of dementia. To address these issues of TICV, dementia and APOE genotype within a population based sample, we used the magnetic resonance (MR) imaging studies from the Cache County, Utah study (Breitner et al., 1999; Steffens et al., 2000; Tschanz et al., 2000) to calculate TICV using well established published methods (Bigler et al., 2000; Bigler & Tate, 2001).
This paper then reports the descriptive findings of TICV in this population as it relates to dementia type, age of onset, intellectual status, education, and APOE genotype. We expected that smaller TICV values would be associated with a diagnosis of dementia, especially in the presence of the ε4 allele. For non-demented control comparisons, the sample was enriched by including normative TICV data from other studies (Bigler et al., 1997; Blatter et al., 1995; 1997), including a post-mortem study where intracranial capacity was reported for 87 normal individuals (Davis et al., 1977). Using these additional control comparisons provided a very large control sample (N = 423) of TICV values. In addition, we also utilized a non-parametric classification and regression tree (CART; Breiman et al., 1983; Zhang et al., 1998; 1999) analysis to examine the sensitivity and specificity of TICV and APOE genotype to determine dementia classification with the expectation that regardless of dementia type, patients with smaller TICV and the presence of at least one ε4 allele would be more readily classified as having dementia.
RESULTS
TICV and Diagnostic Classification
TICV values for the different groups are presented in FIGURE 1, where the broader control sample size of TICV is compared to all Cache County groups. Considerable variability in TICV size is noted in this figure with distinct overlap of median quartiles. Consequently, no significant differences were observed between the groups even when age, height and sex are controlled. Within the Cache County sample alone, a two-way ANOVA using sex and diagnosis as predictors of TICV was performed (see Table 1). Results of the ANOVA demonstrated significant male/female differences in TICV (F1, 173 = 101.05, p < 0.001), but no significant difference in TICV based on diagnosis (F 4,173 = 1.91, p = 0.11). Examination of the post hoc results did reveal a significant difference between the female control groups and the mixed neuropsychiatric group of patients (p<0.001) with the mixed neuropsychiatric dementia group having the smaller TICV. No other post hoc analyses were significant.
Figure 1.
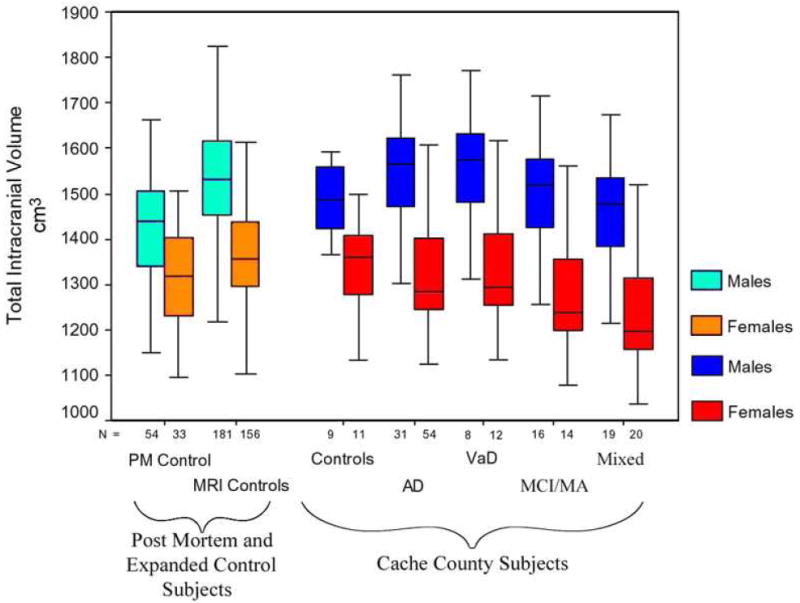
Box plots displaying the distribution of total intracranial volume (TICV) for each group (medium, quartiles, and range). The subjects in the magnetic resonance imaging (MRI) controls and Post-Mortem (PM) Controls are from other studies (see Bigler, Tate, 2001) and were used to establish the appropriateness of the 20 control subjects in the Cache County, Utah project. As can be seen there is considerable overlap between the control groups but the differences were not significant. Note the ordinate is truncated, starting at 1,000 cm3. No significant main effect of TICV X diagnostic group was observed. By examination of the range of variability, it is obvious that there is considerable overlap resulting in no significant mean difference in TICV between subjects with dementia and/or neuropsychiatric disorder and controls.
Table 1.
Descriptive statistics for the experimental groups for TICV and APOE genotype
| AD | VaD | MA/MCI | Mixed | Control | F-Statistic | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Education-years (s.d.) | 13.12 (2.97) | 12.19 (2.43) | 13.3 (3.06) | 12.72 (3.33) | 12.95 (2.37) | 0.44 (p=.78) | |||||
| Age (s.d.) | 83.17 (6.59) | 83.88 (6.97) | 83.78 (6.87) | 81.14 (6.98) | 76.7 (6.38) | 4.83 (p=.001) | |||||
| M/F | 32/52 | 8/12 | 16/14 | 19/20 | 8/12 | ||||||
| APOE | ε4+ (n=60) | ε4- (n=24) | ε4+ (n=6) | ε4- (n=14) | ε4+ (n=22) | ε4- (n=8) | ε4+ (n=18) | ε4- (n=21) | ε4+ (n=14) | ε4- (n=6) | |
|
|
|||||||||||
| TICV-cm3 | 1413.58 | 1469.36 | 1485.89 | 1483.66 | 1416.89 | 1350.76 | 1506.99 | 1462.81 | 1533.12 | 1445.19 | |
| Males (s.d.) | (103.36) | (128.16) | (223.79) | (87.24) | (88.92) | (99.47) | (187.94) | (138.7) | (46.85) | (94.11) | .98 (p=.43) |
| n=21 | n=10 | n=2 | n=6 | n=12 | n=4 | n=6 | n=13 | n=4 | n=5 | ||
| Total (Males) | 1431.58 (112.92) | 1484.22 (112.21) | 1400.36 (93.01) | 1476.76 (151.93) | 1484.27 (86.02) | 1.58 (p=.19) | |||||
| n=31 | n=8 | n=16 | n=19 | n=9 | |||||||
| TICV-cm3 | 1270.7 | 1333.11 | 1280.27 | 1306.9 | 1361.23 | 1258.32 | 1260.52 | 1188.33 | 1393.02 | 1324.41 | |
| Females (s.d.) | (101.67) | (135.28) | (68.45) | (132.49) | (116.51) | (80.04) | (117.41) | (97.17) | (47.5) | (120.6) | 1.47 (p=.22) |
| n=40 | n=13 | n=3 | n=9 | n=10 | n=4 | n=12 | n=8 | n=2 | n=9 | ||
| Total (Females) | 1286.01 (112.74) | 1300.25 (117.32) | 1260.4 (104.3) | 1231.64 (113.03) | 1336.88 (112.39) | 1.81 (p=.13) | |||||
| n=53 | n=12 | n=14 | n=20 | n=11 | |||||||
| Total TICV-cm3 M/F (s.d.) | 1339.73 (132.54) | 1373.83 (145.46) | 1335.04 (119.98) | 1351.06 (180.91) | 1403.21 (124.19) | .77 (p=..55) | |||||
Legend: TICV-cm3 = total intracranial volume in cubic centimeters; APOE = Apolipoprotein-E; M/F = male/female; AD = Alzheimer disease; VaD = vascular dementia; Mixed = mixed neuropsychiatric group; Control = control group; (s.d.) = standard deviation
TICV and other covariates
Using traditional ANOVA methods, there was no significant relationship between TICV and MMSE, Shipley IQ, age of disease onset, dementia severity, and/or APOE genotype for males or females. TICV was minimally related to education attainment for males only (r=0.15, p=0.05).
Classification and Regression Tree Analysis by TICV and APOE
The CART analysis supported the lack of a TICV main effect by diagnosis (using TICV as a predictor for AD; see Figures 2a, 2b). Male and female subjects were analyzed separately with similar results. However, within the CART analysis, there appear to be pockets where smaller TICV had relevance in classification. In females, for example, only one control had a TICV less than 1200.6 cm3 (one standard deviation below the mean), but 30 of the dementia, MA/MCI and mixed neuropsychiatric subjects had values below this cut-score. Likewise, in males the 15 subjects with the smallest TICV (<1318.3 cm3) were all AD, MA/MCI, or mixed neuropsychiatric disorder cases.
Figure 2.

(a) Classification and Regression Tree (CART) analysis by total intracranial volume (TICV) and diagnostic classification for females. The title of each line represents the cut-point where the model initially classifies by the Alzheimer disease (AD) diagnosis. The classification criterion is read to the left. Thus, for the first classification, TICV < 1203.8 is satisfied to the left. The numbers beneath each cut-score represent the number classified as AD for each category, read from left to right, where the first category is controls, followed by AD, vascular dementia (VaD), and mild ambiguous/mild cognitive impairment (MA/MCI), and mixed neuropsychiatric subjects. The ‘tree’ evolves as it attempts to classify by the diagnosis of AD at each ‘branch’ by TICV in the beginning of the CART analysis. In so doing the model looks at the best fit and classification rate at each branch and continues to branch until all subjects have been classified. Note that initially each heading is AD, because the model parameters for this CART analysis were based solely on TICV and AD classification until the lower branches were reached and then it classified by other diagnostic categories. (b) CART analysis by TICV and diagnostic classification for males. The decision tree is read in an identical manner as described above.
Additional support is had by calculating the odds ratio for the final branches of the CART analysis. For example, at “TICV < 1157.4 cm3” only one of 20 control subjects (5%) was classified as AD but 10 of the 54 female subjects (18.5%) were classified as AD. In the expanded female control sample there were 13 subjects out of a total 189 who had TICV ≤ 1157.4 cm3, or 7%. Applying the ratio of smaller TICV in the expanded control sample resulted in an odds ratio of 3.08 (95% confidence interval (CI) 1.27-7.47, p = .013) indicating a higher risk for being classified as dementia when the TICV value was below 1157.4 cm3. As with the female subjects, males with the smallest TICV had either AD, VaD, MA/MCI, or neuropsychiatric disorder with no controls found in that branch (i.e., TICV < 1318 cm3). For the broader sample of controls, there were 2 out of 35 males who had TICV < 1318.3 cm3, or 5%, but 6 of 31 AD subjects (19.4%) had TICV < 1318.3 cm3, or an odds ratio of 3.96 (95% CI 0.74-21.30, p = 0.11). Though non-significant, the odds ratio is similar in magnitude to that of females and is likely non-significant due to sample size.
The discriminate trees based on CART analysis for males and females analyzed separately for TICV and occurrence of ε4 allele is presented in Figure 3. For females, the majority of AD subjects were classified by presence of the ε4 allele with considerable misclassification of all other diagnostic categories (Figure 3a). In the context of APOE genotype, TICV appeared to have no relevance to AD classification in females. For males, there was one classification branch that appeared to have relevance for AD and that was for subjects with at least one ε4 allele who had TICV less than 1482 cm3. This decision tree correctly classified 18 of the 21 (86%) male AD subjects who had the ε4 allele. No controls were misclassified but several other diagnostic categories were.
Figure 3.
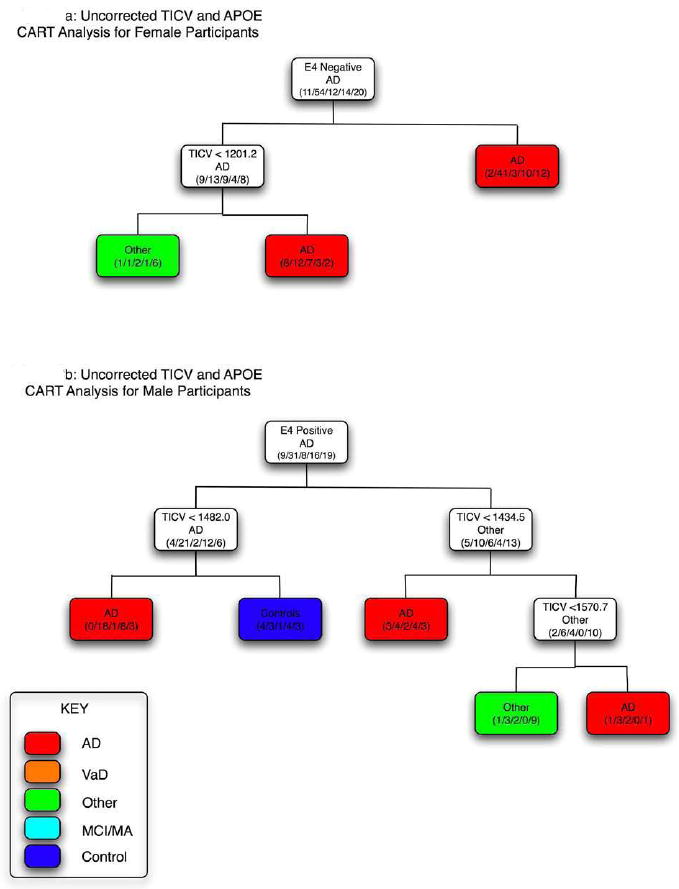
Classification and Regression Tree (CART) analysis by total intracranial volume (TICV) and presence of the ε4 allele for females (a) and males (b). As in Figure 2, when the criterion heading for each line is met, the direction is to the left. Accordingly, there is a cluster (asterisk) where Alzheimer disease (AD) subjects had brain volume ≤ 1482.04 and no controls were in this branch. No branching occurred by TICV that appeared meaningful in females, as the CART analysis classified AD strictly by apolipoprotein E (APOE) genotype on the first branch and those who were ε4+ (right branch) were not further subdivided by TICV. Likewise almost all other subjects who were classified by the ε4- condition had TICV > 1201.2 cm3.
DISCUSSION
In this study, there does not appear to be a main statistical effect for TICV on the development of dementia, including AD. This is consistent with the Jenkins et al. (2000) observations, despite the fact that in both studies AD subjects as a group had smaller average TICV. In the CART analysis, AD classification based on TICV alone results in correct classification of more AD subjects for males and females alike though this finding is more difficult to evaluate given the small number of control participants in the Cache County sample. Odds ratios utilizing data from the expanded control group seem to improve this observation by demonstrating that individuals with the smallest TICV volumes were three (females) to four (males) times as likely to be classified with dementia. Taken together, the smaller average TICV and elevated odds ratios within the spectrum of those who develop dementia seem to emphasize the relevance of premorbid brain size, but clearly additional studies of this relationship are needed. In addition, there may be a sex difference associated with presence of APOE ε4 allele, where smaller TICV and presence of the ε4 allele may be significant for males.
When using the CART statistical method to classify AD by TICV, it is insightful to note that the greatest misclassification occurred with the MA/MCI subjects. This may be significant given the fact that MA/MCI patients are often considered prodromal AD (Daly et al., 2000; Elias et al., 2000; Peterson et al., 2000b; Small et al., 2000). Consequently, for AD it appears (and potentially MA/MCI subjects at risk for AD) that the TICV effect may be nested within a larger group of demented subjects where premorbid size is a factor. In addition, the TICV effect may merely be an epiphenomenon where total brain size actually relates to component structures of the brain and it is the size of the component structure—i.e., mesial temporal cortex, hippocampus or some other target structure—where the true size effect occurs (Bigler, 2001). Thus, a reduction in the size of a given target structure (i.e., hippocampus), not necessarily the whole brain, may be critical in who develops dementia.
The observations of the potential nested effects of TICV in this sample and that in male AD subjects there was a subgroup where presence of the ε4 allele and smaller TICV were associated with AD, raises the question of early effect of ε4 on brain development. Total intracranial capacity is set by adolescence (Reiss et al., 1996). However, because most intracranial growth occurs by age 6 in response to the functional growth matrix of the expanding brain (Moore et al., 1974; Ranly, 1980), any influence of the ε4 allele would have to occur early in development though this clearly would need to be examined more directly. The determinants of brain size, and therefore intracranial capacity, are undoubtedly complex (Baare et al., 2001; DeMeyer, 1994; Moore Lavelle, 1974; Ranly, 1980) and any number of genetic, perinatal, and postnatal influences could contribute to biological parameters affecting brain and skull development (Bigler et al., 2000; Diamond, 1986; Scheibel et al., 1990).
Nonetheless, evolutionary influences on brain size appear to relate size to function (Glassman, 1987; Haug, 1987; Jerison, 1987; Pagel et al., 1988; Stern, 2002) and therefore, from a ‘brain reserve’ perspective (Satz, 1993; Stern, 2002), smaller size suggests vulnerability (Peterson et al., 2000a). Smaller brain size may also be associated with early injury, perinatal stressors and nutritional deficits and is associated with development of neuropsychiatric disorder, particularly schizophrenia (Gosch et al., 1997; Gur et al., 2000a; Gur et al., 2000b; Peterson et al., 2000a). However, in the present study there was no apparent premorbid expression of cognitive difference among those with smaller TICV as smaller TICV was not associated with various measures of cognitive ability. Though the relationships of education to incidence of dementia has been the focus of several studies (Katzman, 1993) where lower education is either a risk factor for dementia (Ganguli et al., 2000) or a risk factor for deleterious socioeconomic or environmental influences that may in turn relate to risk factors associated with dementia (Hall et al., 2000), it should be noted that the three AD subjects with the highest education (doctoral degree) were all ε4+ and all had TICV values below the mean for their sex. Thus, in this sample, even those with smaller TICV had similar levels of education and time since onset of dementia. This also argues that whatever effect TICV may have on the prediction of premorbid brain development, it is unlikely a simple size relationship.
There are several limitations to this current study that should be acknowledged. The first limitation lies in the fact that TICV is only a proxy measure of premorbid brain volume or size. Though studies in the literature have consistently demonstrated significant relationships between TICV and brain volume in healthy typically developing adolescents and adults (Bigler & Tate, 2001; Courchesne, et al., 2000), it is not a perfect one-to-one relationship. Practically speaking, this means that TICV does not always capture possible demographic, nutritional, social, and educational factors that may also operate to impact premorbid brain volume and that this fact may actually make these results more difficult to interpret. However, because other studies have used TICV in a similar way, direct comparisons can be made between these studies. Somewhat related is the second limitation which is the use of controls subjects from cohorts recruited outside the Cache County Memory and Aging Study. Though there are likely cohort effects that might result in differences in premorbid brain size, we attempted to control for these effects by first comparing the various groups statistically. There were no significant differences in either the variance or the means for TICV between the various studies noted even after controlling for additional variables such as age, gender, and height. Though this does not completely eliminate the possibility of cohort effects, it suggests that TICV is comparable across the various groups. We acknowledge that this is not the best possible scenario though the inclusion of these additional groups did allow us to determine the sensitivity of the predictions derived from the CART analysis. Second, scanning was accomplished for each cohort using different equipment from scanners with different field strengths. It should be noted that in spite of the difference in field strength, the protocols for the various studies had similar resolution parameters, thus minimizing the potential adverse effects field strength might have on a gross morphologic measures such as TICV. Ideally, data should have been collected concurrently though this was not possible at the time of the collection of the original Cache County Memory and Aging Study data.
In spite of these limitations, it should be noted that to date, the three largest studies utilizing MR-based direct measurement of TICV in AD are the Jenkins et al. (2000) study, the Edland et al. (2002) study, and the current one. None find a main effect of TICV, yet in the current study we do find some subjects where head size may be a nested factor within a larger group and there also appears to be a potential interaction between the presence of the ε4 allele and TICV for males. The conflicting results of previous research may be that within patients who develop AD, only for a subset does premorbid brain size and APOE genotype play a role. Obviously, considerable research is needed to more fully explore these issues. It may also be that using TICV alone is an incorrect approach to address the issue of pre-morbid brain size and subsequent development of dementia. Efforts to determine the premorbid size of more clinically relevant anatomical structures may present a better chance of understanding the impact cognitive reserve might have on the onset of dementing illnesses.
METHODS
Subjects and the Cache County, Utah Study
Subjects were drawn from the Cache County, Utah, elderly population. Detailed description of the Cache County Memory and Aging Study methods have been published elsewhere (Anthony et al., 2000; Bigler et al., 2002a; 2002b; Breitner et al., 1999; Lyketsos et al., 2000; Steffens et al., 2000; Tschanz et al., 2000). Briefly, fieldwork identified 5,677 permanent residents over the age of 65—5,092 (89.7%) of whom were enrolled in the larger parent study. Participants were screened for dementia using a brief cognitive examination and/or a structured telephone dementia questionnaire (Breitner et al., 1999). Ultimately, a sample of 1196 individuals was selected for full clinical assessment that included a detailed standard examination for detection and differential diagnosis of dementia or neuropsychiatric disorder. From this population, 335 individuals were diagnosed as demented (DSM-III-R criteria) by a consensus diagnostic approach in the initial phases of the study (others were diagnosed later as the study progressed). AD was diagnosed using NINCDS-ADRDA criteria and VaD by NINDS-AIREN criteria (Breitner et al., 1999). Although imaging was sought on all subjects diagnosed with dementia or its prodrome, only 174 subjects had scans sufficient for performing the image analyses undertaken in this study. Of these subjects, 85 were diagnosed with Probable or Possible AD (40% of all AD subjects) and 20 with VaD (25% of all VAD subjects). Thirty more subjects who were classified originally as “mild/ambiguous” (MA) were also scanned. This designation was assigned when the pattern of clinical symptoms or the results of neuropsychological testing of prodromal AD and there were no medical or neuropsychiatric disorders to preclude an eventual AD diagnosis (Tschanz et al., 2006). Many of these MA subjects likely meet the criteria for what is now operationalized and termed as Mild Cognitive Impairment (MCI; Peterson et al., 2000b).
The last dementia group of 39 subjects included a wide spectrum of disorders not meeting AD or VaD criteria and was termed the “Mixed Neuropsychiatric Group” and included patients with a variety of diagnoses (e.g., Parkinson’s disease, frontotemporal dementia, major depression, etc). Lastly, a control sample of 20 individuals from non-demented enrollees was selected for the full clinical assessment. Unfortunately, funding limitations to perform additional MR imaging precluded a more comprehensive sample of the non-demented enrollees in the clinical assessment, but healthy controls from other studies were included in the analyses to expand the control sample (see below). All the subjects from the Cache County Memory and Aging Study with MRI imaging data also had APOE genotyping (Breitner et al., 1999) and the allele information was utilized in the final analyses as an independent variable. Though the rate of APOE e4 positive individuals in this sample exceeds what is typically expected in the general population (63%), it should be noted that the sample for which imaging was available represents a select group of individuals who were screened and found to have many symptoms consistent with dementia. Thus, APOE could be expected to occur more frequently in this sub-sample given the high concentration of dementia patients represented (van der Flier et al., 2008).
To enrich the control sample, we added healthy controls from a separate normative study (N=184; M/F=98/86; mean age=32.76 (SD=9.64); age range=16 to 68) previously reported (Blatter et al., 1995) and a large sample of patients (N=152; M/F = 83/69; mean age = 27.13 (SD = 9,62); age range=16 to 50) who had sustained traumatic brain injury (TBI; Bigler et al., 1997; Blatter et al., 1997). Both the healthy controls and TBI patients were sampled from the same geographical region of the country. We felt justified in using TBI patients as one would not expect head injury to influence TICV, since all the TBI subjects from this sample were 16 and older—a point where TICV has already reached maximum (Bigler & Tate, 2001; Courchesne et al., 2000). Initial comparisons between these control participants and the small Cache County control sample revealed no significant differences in the mean TICV and/or the variability in the distribution. Thus, the two groups were combined into a single group labeled “MRI controls” for some of the analyses (n = 336). Also, the post-mortem study by Davis and Wright (1977) were added as a control comparison because they reported directly measured (cranial cavity was filled with fluid and then measured) TICV values in 87 adult (M/F = 54/33; mean age = 63.32 (SD ± 18.05); age range = 22 to 94) individuals who died of natural causes. Again, examination of the TICV values from this post-mortem study and the other control groups revealed no significant differences between any of the groups. This group was labeled as a separate control group called “post-mortem.” We did not have APOE genotype on these additional control subjects so analyses examining APOE genotype did not include these expanded control groups.
Since the control sample from the Cache County study consisted of only 20 subjects, we used the larger “MRI controls” group and the post-mortem control group to ensure proper representation of the range of possible TICVs. Furthermore, this “MRI control” group was used to establish a normative sample so z-scores, by sex, could be calculated for TICV for each diagnostic group, including controls, in the Cache County sample. Since these z-score corrected TICV values (TICV-corrected) remove the effect of sex differences, direct comparison across all subjects and diagnostic groups by APOE genotype was possible. Lastly, the large sample of MRI controls was used to calculate odds ratios to determine the rate of occurrence of small head size in the dementia groups for the CART analyses.
For AD and VaD subjects, year of disease onset was assigned retrospectively as the point where each subject unambiguously met DSM-III-R criteria for dementia or in the case of MA/MCI and mixed neuropsychiatric patients the age at when they began to experience their first cognitive symptoms. Dementia severity was determined by the Clinical Dementia Rating (CDR, Morris, 1993) reference scale, which was also examined for associations with TICV. Since there are inherent sex differences in TICV (Blatter et al., 1995), male and female subjects were analyzed separately for some of the analyses (especially those analyses examining the raw uncorrected data).
MR imaging and Quantitative Analysis
For the Cache County subjects, MR imaging was performed on a 0.5 Tesla Philips scanner following a standard protocol as detailed elsewhere (Bigler et al., 2000; Bigler & Tate, 2001). Quantitative analyses were performed using image analysis protocols previously published (see Bigler et al., 1997; Blatter et al., 1995). Briefly, using the commercial imaging package ANALYZE© (see Robb et al., 1989; Mayo Clinic Software), the images for each subject were segmented using a two-channel segmentation routine based on the dual acquisition T2 and proton-density (PD) weighted images. Using the segmentation results, the inner table of the skull was defined as the outer limit of brain parenchyma and cortical CSF dorsal to the foramen magnum. As indicated, the “MRI controls” group (Bigler & Tate, 2001) was created for comparison based on previously published data and all quantitative analyses were identical except image acquisition was based on a 1.5 Tesla scanner (see Bigler et al., 1997; Blatter et al., 1995; 1997) using a protocol with similar resolution.
Statistical Analysis
Descriptive statistics for the experimental groups is provided in Table 1. Statistical main effects for TICV and APOE genotype were examined using analysis of variance (ANOVA). Comparison between diagnostic groups and controls was also accomplished using ANOVA methods. The 20 control subjects from the Cache County study were significantly younger (see Table 1) than the other diagnostic groups. However, age was not used as a variable in the analyses as it is known to have little effect on measures of TICV at maturity (Blatter et al., 1995; Courchesne et al., 2000).
We used classification and regression tree (CART) analysis to assess the bivariate structure of multiple variables simultaneously (Breiman et al., 1983; Zhang et al., 1998). CART is a flexible nonparametric method that used with either quantitative or categorical response variables. The underlying theory behind CART for categorical response variables is that it will sequentially partition the data into subgroups based on binary partitions of predictor variables, where the subgroups are chosen in such a way that they are “purer” with respect to the response variable. In other words, the goal is to have subgroups as homogenous as possible with observations from the same category (for example, having all observations from one category is ideal). CART is also called “recursive” partitioning (see Zhang and Singer, 1999) since subgroups can always be further partitioned and predictor variables may be used more than once as splits in the tree.
Importantly, CART is much more flexible than traditional parametric regression tools. In a situation with multiple response categories, the advantage that CART offers over multinomial logistic regression is first, that no distributional assumptions are made, and second, it is not assumed that the relationship between class membership and response variables is reflected through a single coefficient. CART readily exposes nonlinear relationships between predictor variables, which is very helpful in understanding the structure in complex heterogeneous data sets with groups of participants who might not always meet the assumptions of parametric statistics. Lastly, using the end-points of the CART permits comparison to the expanded “MRI controls” sample, where odds ratios were calculated to determine the likelihood of TICV values of certain levels occurring in the control sample. In our CART analyses, we examined a combination of variables including APOE genotype and TICV, by gender.
Research Highlights.
There are a subset of patients for which total intracranial volume (TICV) appear to have an impact of predicting dementia diagnosis when examining data in a non-parametric fashion.
There were no significant main effects for TICV when comparing dementia patients and healthy controls (parametric testing).
APOE genetype appears to be more relevant for males with a smaller TICV when predicting dementia diagnosis.
Acknowledgments
Supported in part by National Institutes of Health Grant AG-1380 and the Ira Fulton Foundation. The support from the Radiology Department at Logan Regional Medical Center Logan, Utah, where the imaging studies were performed, is gratefully acknowledged along with the support of John C.S. Breitner, M.D. and other investigators of the Cache County Memory in Aging Project. We would like to acknowledge the following funding sources as being instrumental in the completion of this project: RO1-AG11380 (KWB), RO1-AG21136 (JTT), K23MH073416 (DFT) and P30-AG013486 (Boston University Alzheimer’s Disease Core Center).
We would like to make the following disclaimer: Lara Jill Wolfson is a staff member of the World Health Organization. She alone is responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of the World Health Organization. The technical assistance of Tracy J. Abildskov and the manuscript assistance of Jo Ann Petrie, M.S. are gratefully acknowledged.
Footnotes
DISCLOSURE STATEMENT There are no actual or potential conflicts on interest to be disclosed by any of the authors. Recruitment and consent procedures for human subjects and subsequent data usage were approved by the respective institutional review boards.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
E.S. Neeley, Email: sneeley@stat.byu.edu.
M.C. Norton, Email: maria.norton@usu.edu.
J.T. Tschanz, Email: joann.tschanz@usu.edu.
L. Wolfson, Email: wolfsonl@who.int.
K.A. Welsh-Bohmer, Email: kwe@duke.edu.
B. Plassman, Email: brenda.plassman@duke.edu.
Erin D. Bigler, Email: erin_bigler@byu.edu.
References
- Agosta F, Vossel KA, Miller BL, Migliaccio R, Bonasera SJ, Filippi M, Boxer AL, Karydas A, Possin KL, Gorno-Tempini ML. Apolipoprotein E ε4 is associated with disease-specific effects on brain atrophy in Alzheimer’s disease and frontotemporal dementia. PNAS. 2009;106(6):2018–2022. doi: 10.1073/pnas.0812697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease. Am J Psychiatry. 1997;154(2):165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V, II, O’Leary D, Alliger R, Cohen G, Ehrhardt J, Yuh WT. Intelligence and brain structure in normal individuals. Am J Psychiatry. 1993;150:130–134. doi: 10.1176/ajp.150.1.130. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Breitner JCS, Zandi PP, Meyer MR, Jurasova I, Norton MC, Stone SV. Reduced prevalence of AD in users of NSAIDs and H2 blockers. The Cache County Study. Arch Gen Psychiatry. 2000;54(11):2066–2071. doi: 10.1212/wnl.54.11.2066. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Falk D. Primate Brain Evolution: Methods and Concepts. New York: Plenum Press; 1982. [Google Scholar]
- Baare WFC, Hulshoff Pol HE, Boomsma DI, Postuma D, de Geus EJC, Schnack HG, van Haren NEM, van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Barboriak DP, Doraiswamy PM, Krishnan KRR, Vidyarthi S, Sylvester J, Charles HC. Hippocampal sulcal cavities on MRI: Relationship to age and apolipoprotein E genotype. Neurology. 2000;54:2150–2153. doi: 10.1212/wnl.54.11.2150. [DOI] [PubMed] [Google Scholar]
- Barnes J, Scahill RI, Schott JM, Frost C, Rossor MN, Fox NC. Does Alzheimer’s disease affect hippocampal asymmetry? Evidence from a cross-sectional and longitudinal volumetric MRI study. Dement Geriatr Cogn Disord. 2005;19(5-6):338–344. doi: 10.1159/000084560. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz HH, Courchesne E, Karns CM. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics. 2002;33(5):239–241. doi: 10.1055/s-2002-36735. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72:829–34. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Premorbid brain volume and dementia. Arch Neurol. 2001 May;58:831–833. doi: 10.1001/archneur.58.5.831. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, Burnett B. Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Kerr B, Victoroff J, Tate D, Breitner JCS. White matter lesions, quantitative magnetic resonance imaging, and dementia. Alzheimer Dis Assoc Disord. 2002a;16(3):161–170. doi: 10.1097/00002093-200207000-00006. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Lowry CM, Anderson CV, Johnson SC, Terry J, Steed M. Dementia, quantitative neuroimaging, and apolipoprotein E genotype. AJNR Am J Neuroradiol. 2000;21:1857–1868. [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Tate DF. Brain volume, intracranial volume and dementia. Invest Radiol. 2001;36(9):539–546. doi: 10.1097/00004424-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Tate DF, Miller MJ, Rice SA, Hessel CD, Heath DE, Tschanz JT, Plassman BL, Welsh-Bohmer KA. Dementia, asymmetry of temporal lobe structures, and Apolipoprotein E genotype: Relationships to cerebral atrophy and neuropsychological impairment. J Int Neuropsychol Soc JINS. 2002b;8:925–933. doi: 10.1017/s1355617702870072. [DOI] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn S. Quantitative volumetric analysis of brain MR: Normative database spanning five decades of life. AJNR Am J Neuroradiol. 1995;16(1):241–251. [PMC free article] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Ryser D, Macnamara SE, Bailey BJ. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: Correlation with neuropsychological outcome. AJNR Am J Neuroradiol. 1997;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobio Aging. 2000;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont: Wadsworth; 1983. [Google Scholar]
- Breitner JCS, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Tschanz JT, Plassman BL, Meyer MR, Skoog I, Khachaturian A. APOE-4 count predicts age when prevalence of AD increases, then declines: The Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Bullock TH. How are more complex brains different? Brain Behav Evol. 1993;41:88–96. doi: 10.1159/000113826. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage. 2008;41(4):1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurol. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M, Blacker D, Stern Y. APOE ε4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurol. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiol. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Cole G. Alzheimer disease. J A M A. 2002;287(18):2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert MS. Predicting conversion to Alzheimer Disease using standardized clinical information. Arch Neurol. 2000;57(5):675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- Davis PJM, Wright EA. A new method for measuring cranial cavity volume and its application to the assessment of cerebral atrophy at autopsy. Neuropathol Appl Neurobiol. 1977;3:341–358. [Google Scholar]
- Dekaban AS, Sadowsky D. Changes in brain weight during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- DeMeyer W. Microcephaly, micrencephaly, megalocephaly, and megalencephaly. In: Manning S, editor. Pediatric Neurology: Principles and Practice. II. St. Louis: Mosby; 1994. pp. 205–218. [Google Scholar]
- Diamond MC. Aging and the environmental influences on the rat forebrain. In: Scheibel AB, Wechsler AF, editors. The Biological Substrate of Alzheimer’s Disease. Academic Press; New York: 1986. pp. 55–63. [Google Scholar]
- Drachman DA. Hat size, brain size, intelligence, and dementia: What morphology can tell us about brain function and disease. Neurol. 2002;59:156–157. doi: 10.1212/wnl.59.2.156. [DOI] [PubMed] [Google Scholar]
- Eckerstrom C, Olsson E, Borga M, Ekholm S, Ribbelin S, Rolstad S, Starck G, Edman A, Wallin A, Malmgren H. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Goteborg MCI study. J Neurol Sci. 2008;272(1-2):48–59. doi: 10.1016/j.jns.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Edland SD, Xu Y, Plevak M, O’Brien P, Tangalos EG, Petersen RC, Jack CR., Jr Total intracranial volume: Normative values and lack of association with Alzheimer’s disease. Neurol. 2002;59:272–274. doi: 10.1212/wnl.59.2.272. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer Disease: A 22-Year Prospective Study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Erten-Lyons D, Woltjer RL, Dodge H, Nixon R, Vorobik R, Calvert JF, Leahy M, Montine T, Kaye J. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurol. 2009;72:354–360. doi: 10.1212/01.wnl.0000341273.18141.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa PS, Kryscio RJ, Mendiondo MS, Schmitt FA, Wekstein DR, Markesbery WR, Smith CD. Alzheimer’s disease and head circumference. J Alzheimers Dis. 2006;9(1):77–80. doi: 10.3233/jad-2006-9108. [DOI] [PubMed] [Google Scholar]
- Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Frisk V, Amsel R, Whyte HE. The importance of head growth patterns in predicting the cognitive abilities and literacy skills of small-for-gestational-age children. Dev Neuropsychol. 2002;22:565–593. doi: 10.1207/S15326942DN2203_2. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: The MoVIES Project. Neurol. 2000;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- Geroldi C, Laakso MP, C D, Beltramello A, Bianchetti A, Soininen H, Trabucchi M, Frisoni GB. Apolipoprotein E Genotype and hippocampal asymmetry in Alzheimer’s disease: a volumetric MRI study. J Neurol. 2000;68(1):93–96. doi: 10.1136/jnnp.68.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman RB. An hypothesis about redundancy and reliability in the brains of higher species: analogies with genes, internal organs, and engineering systems. Neurosci Biobehav Rev. 1987;11:275–285. doi: 10.1016/s0149-7634(87)80014-3. [DOI] [PubMed] [Google Scholar]
- Gosch A, Brambring M, Gennat H, Rohlmann A. Longitudinal study of neuropsychological outcome in blind extremely-low-birthweight children. Dev Med Child Neurol. 1997;39:295–304. doi: 10.1111/j.1469-8749.1997.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Graves AB, Mortimer JA, Bowen JD, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurol. 2001;57:1453–1460. doi: 10.1212/wnl.57.8.1453. [DOI] [PubMed] [Google Scholar]
- Graves AB, Mortimer JA, Larson EB, Wenzlow A, Bowen JD, McCormick WC. Head circumferences as a measure of cognitive reserve: association with severity of impairment in Alzheimer’s disease. B J P. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000a Aug;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Cowell PE, Finkelman C, Maany V, Grossman RI, Arnold SE, Bilker WB, Gur RC. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000b Aug;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. Structural brain variation and general intelligence. Neuroimage. 2004;23(1):425–33. doi: 10.1016/j.neuroimage.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Hall KS, Gao S, Unverzagt FW, Hendrie HC. Low education and childhood rural residence: Risk for Alzheimer’s disease in African Americans. Neurol. 2000;54:95–99. doi: 10.1212/wnl.54.1.95. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Halliday GM, Kril JJ. Variation in hippocampal neuron number with age and brain volume. Cereb Cortex. 1998;8(8):710–718. doi: 10.1093/cercor/8.8.710. [DOI] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Holzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul Sm, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurol. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rosser MN. Intracranial volume and Alzheimer disease: Evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57:220–224. doi: 10.1001/archneur.57.2.220. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Brain size. In: Adelman G, editor. Encyclopedia of Neuroscience. I. Birkhauser Boston; Boston: 1987. pp. 168–170. [Google Scholar]
- Johnson FP, Coppins DA, Brook IM. Fabrication of custom made tissue spacers for use in tissue augmentation. Br J Oral Maxillofac Surg. 1991;29(4):282–283. doi: 10.1016/0266-4356(91)90200-o. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Creasey H, Broe GA, Sulway MR, Kos SC, Dent OF. The advantage of being broad-minded: Brain diameter and neuropsychological test performance in elderly war veterans. Pers Indiv Differences. 1997;23:371–377. [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurol. 1993;43:12–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld PA, Renbing X, Peck A. Clinical, pathological and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- Kim KR, Lee KS, Kim EA, Cheong HK, Oh BH, Hong CH. The effect of the APoE genotype on the association between head circumference and cognition. Am J Geriatr Psychiatry. 2008;16(10):819–25. doi: 10.1097/JGP.0b013e3181800551. [DOI] [PubMed] [Google Scholar]
- Lainhart JE, Piven J, Wzorek M, Landa R, Santangelo SL, Coon H, Folstein SE. Macrocephaly in children and adults with Autism. J Am Acad Child Adolesc Psychiatry. 1997;36(2):282–290. doi: 10.1097/00004583-199702000-00019. [DOI] [PubMed] [Google Scholar]
- Levi O, Dolev I, Belinson H, Michaelson DM. Intraneuronal amyloid-beta plays a role in mediating the synergistic pathological effects of apoE4 and environmental stimulation. J Neurochem. 2007;103(3):1031–1040. doi: 10.1111/j.1471-4159.2007.04810.x. [DOI] [PubMed] [Google Scholar]
- Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J of Neurochem. 2007;100(1):202–210. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JCS. Mental and behavioral disturbances in dementia: Findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157(5):708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- MacLullich AMJ, Ferguson KJ, Deary IJ, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurol. 2002;59:169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Martyn CN, Gale CR, Sayer AA, Fall C. Growth in utero and cognitive function in adult life: Follow-up study of people born between 1920 and 1943. B M J. 1996;312:1393–1396. doi: 10.1136/bmj.312.7043.1393a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intell. 2004;33:337–346. [Google Scholar]
- McDowell I, Xi G, Lindsay J, Tierney M. Mapping the connections between education and dementia. J Clin Exp Neuropsychol. 2007;29(2):127–141. doi: 10.1080/13803390600582420. [DOI] [PubMed] [Google Scholar]
- Moore WJ, Lavelle CL. Growth of the facial skeleton in the hominoidea. Academic Press; London: 1974. [Google Scholar]
- Mori E, Hirono N, Yamashita H, Imamura T, Ikejiri Y, Ikeda M, Kitagaki H, Shimomura T, Yoneda Y. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s Disease. Am J Psychiatry. 1997;154(1):18–24. doi: 10.1176/ajp.154.1.18. [DOI] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR, Jr, Weiner MW, Thompson PM. Validation of a fully automated 3D hippocampal segmentation method using subjects with Alzheimer’s disease mild cognitive impairment, and elderly controls. Neuroimage. 2008a;43(1):59–68. doi: 10.1016/j.neuroimage.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra JH, Tu Z, Apostolova LG, Green AE, Toga AW, Thompson PM. Automatic subcortical segmentation using a contextual model. Int Conf Med Image Comput Comput Assist Interv. 2008b;11(Pt 1):194–201. doi: 10.1007/978-3-540-85988-8_24. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurol. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Pagel MD, Harvey PH. The taxon-level problem in the evolution of mammalian brain size: facts and artifacts. Am Nat. 1988;132(3):344–359. [Google Scholar]
- Pavlik VN, Doody RS, Massman PJ, Chan W. Influence of premorbid IQ and education on progression of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22(4):367–377. doi: 10.1159/000095640. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, ADolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. J A M A. 2000a;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Peterson RC, Jack CR, Xu Y-C, Waring SC, O’Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurol. 2000b;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JCS. Apolipoprotein E e4 and hippocampal volume in twins with normal cognition. Neurol. 1997;48:985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- Ranly DM. A synopsis of craniofacial growth. Appleton-Century-Crofts; New York: 1980. [Google Scholar]
- Reddick WE, Mulhern RK, Elkin RD, Glass JO, Merchant TE, Langston JW. A hybrid neural network analysis of subtle brain volume differences in children surviving brain tumors. Magn Reson Imaging. 1998;16(4):413–421. doi: 10.1016/s0730-725x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross jL, Denckla MB. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Reynolds MD, Johnston JM, Dodge HH, DeKosky ST, Ganguli M. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurol. 1999;53:228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP, Karwoski RA, Larson AG, Workman EL, Stacy MC. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Grant E, Miller JP, Morris JC. Education and reported onset of symptoms among individuals with Alzheimer disease. Arch Neurol. 2008;65(1):108–111. doi: 10.1001/archneurol.2007.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JP, Ankey CD. Brain size and cognitive ability: Correlations with age, sex, social class, arid race. Psychn Bull Rev. 1996;3:21–36. doi: 10.3758/BF03210739. [DOI] [PubMed] [Google Scholar]
- Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychol. 1993;7:273–295. [Google Scholar]
- Scheibel A, Conrad T, Perdue S, Tomiyasu U, Wechsler A. A quantitative study of dendrite complexity in selected areas of human cerebral cortex. Brain Cogn. 1990;12:85–101. doi: 10.1016/0278-2626(90)90006-a. [DOI] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerling MI, Lindeboom J. The effects of intelligence and education on the development of dementia A test of the brain reserve hypothesis. Psychol Med. 1997;27:1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurol. 1997a;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Mosesson RE, Stern Y, Mayeax R. The age at onset of Alzheimer’s disease and an intracranial area measurement: a relationship. Arch Neurol. 1995;52:95–98. doi: 10.1001/archneur.1995.00540250103019. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, Sano M, Stern Y, Mayeux R. Alzheimer’s disease after remote head injury: An incidence study. J Neurol Neurosurg Psychiatry. 1997b;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Fratiglioni L, Vittanen M, Winblad B, Backman L. The course of cognitive impairment in preclinical Alzheimer Disease: Three- and 6-Year follow-up of a population-based sample. Arch Neurol. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [DOI] [PubMed] [Google Scholar]
- Sperber GH. Craniofacial development. BC Decker, Inc.; London: 2001. [Google Scholar]
- Stathis SL, O’Callaghan M, Harvey J, Rogers Y. Head circumference in ELBW babies is associated with learning difficulties and cognition but not ADHD in the school-aged child. Dev Med Child Neurol. 1999;41:375–380. doi: 10.1017/s0012162299000833. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Skoog I, Norton MC, Hart AD, Tschanz JT, Plassman BL, Wyse BW, Welsh-Bohmer KA, Breitner JCS. Prevalence of depression and its treatment in an elderly population: The Cache County Study. Arch Gen Psychiatry. 2000;57:601. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Studies on Neuropsychology, Neurology, and Cognition. Taylor and Francis: Psychology Press; New York: 2007. Cognitive Reserve: Theory and Applications. [Google Scholar]
- Tate DF, Bigler ED, McMahon W, Lainhart J. The relative contributions of brain, cerebrospinal fluid-filled structures and non-neural tissue volumes to occipital-frontal head circumference in subjects with autism. Neuropediatrics. 2007:18–24. doi: 10.1055/s-2007-981450. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Lyketsos CG, Corcoran C, Green RC, Hayden K, Norton MC, Zandi PP, Toone L, West NA, Breitner JC. Conversion to dementia from mild cognitive disorder: the Cache County Study. Neurol. 2006;67(2):229–234. doi: 10.1212/01.wnl.0000224748.48011.84. [DOI] [PubMed] [Google Scholar]
- Tschanz JT, Welsh-Bohmer KA, Skoog I, West N, Norton MC, Wyse BW, Nickles R, Breitner JCS. Dementia diagnoses from clinical and neuropsychological date compared: The Cache County study (Supported by National Institutes of Health grant AG-11380) Am Acad Neurol. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- Vernon PA, Wickett JC, Bazana PG, Stelmack RM. The neuropsychology and psychophysiology of human intelligence. In: Sternberg RJ, editor. Handbook of Intelligence. Cambridge University Press; New York: 2000. pp. 245–264. [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal aging and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Starr JM, Athawes R, Hunter D, Pattie A, Dreary IJ. Childhood mental ability and dementia. Neurol. 2000;55:1455–1459. doi: 10.1212/wnl.55.10.1455. [DOI] [PubMed] [Google Scholar]
- Willerman L, Shultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence. Intell. 1991;15:223–228. [Google Scholar]
- Zhang H, Crowley J, Sox HC, Olshen RA. Tree structured statistical methods. In: Armitage P, Colton T, editors. The Encyclopedia of Biostatistics. John Wiley and Sons, Inc.; Chichester, UK: 1998. [Google Scholar]
- Zhang H, Singer B. Recursive Partitioning in the Health Sciences. Springer-Verlag; New York: 1999. [Google Scholar]


