IODINE PHYSIOLOGY DURING PREGNANCY
Beginning in early gestation, maternal thyroid hormone production normally increases by approximately 50% in response to increased levels of serum thyroxine-binding globulin (resulting from the increase in estrogen levels) and because of stimulation of thyrotropin (TSH) receptors by human chorionic gonadotropin.1 The placenta is a rich source of the type 3 inner ring deiodinase, which enhances the degradation of thyroxine (T4) to bioinactive reverse triiodothyronine (T3).2 Thus, thyroid hormone demand increases, which requires an adequate iodine supply that is obtained primarily from the diet and/or as supplemental iodine (Fig. 1). In addition, fetal thyroid hormone production increases during the second half of pregnancy, further contributing to increased maternal iodine requirements because iodide readily crosses the placenta.
Fig. 1.
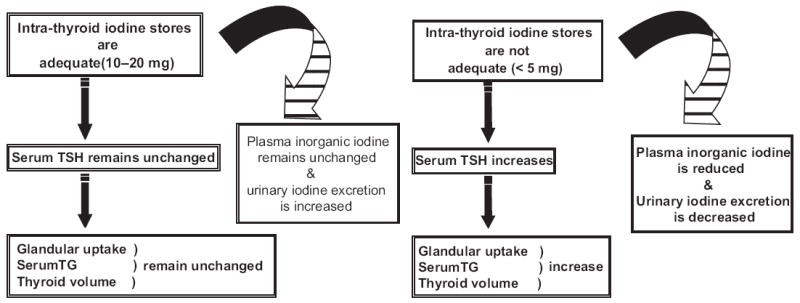
Conceptual models of adequate (left panel) and inadequate (right panel) iodine nutrition and thyroid function. (Adapted from Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutr 2007;10(12A):1543; with permission.)
After oral ingestion, iodide is rapidly absorbed through the stomach and duodenum.3 Iodide, in its pure form, is 100% bioavailable and fully absorbed. Plasma inorganic iodide is then transported through the circulation to be either taken up by the thyroid in varying amounts (5%–100% of absorbed iodine), depending on the iodine supply and the functional state of the thyroid,3 or it is renally excreted. The normal thyroid gland contains approximately 15 g of iodine.4 The inability to compensate for the increased iodine demand of pregnancy is associated with the development of maternal goiter due to TSH stimulation.5
The primary route of iodine excretion is through the kidney,6 which accounts for more than 90% of ingested iodine.3 Beginning in early pregnancy, the glomerular filtration rate of iodide increases by 30% to 50%,1 thereby further decreasing the circulating pool of plasma iodine.7 Stilwell and colleagues8 reported that median urinary iodine levels in Tasmania, a region of mild iodine deficiency, decline after the elevated excretion seen in early pregnancy. A comparison of pregnant women from various countries demonstrated that peak gestational urinary iodine levels vary, thus suggesting differences in renal excretion thresholds by regional dietary iodine intake.9
Because of increased thyroid hormone production, increased renal iodine losses, and fetal iodine requirements in pregnancy, dietary iodine requirements are higher in pregnant adults than in nonpregnant adults.10 Guidelines for daily dietary iodine intake of pregnant women, based on several studies that assessed the effect of iodine supplementation on maternal thyroid volume,11 indicate a higher iodine requirement in these women than that for nonpregnant nonlactating adolescents and adults (Table 1).
Table 1.
Guidelines for daily dietary iodine intake
METHODS TO ASSESS IODINE SUFFICIENCY
There are several accepted methods used in the monitoring of population iodine sufficiency.11 Median spot urinary iodine concentrations (as a biomarker for dietary iodine intake)12 reflect iodine intake over the recent few days. Thresholds for median urinary iodine sufficiency have been identified for populations but not for individuals, given significant day-to-day variation of iodine intake.13 As shown in Table 2, population iodine sufficiency is defined by median urinary iodine concentrations of 100 μg/L or more in nonpregnant women and children younger than 2 years and 150 μg/L or more in pregnant women.14
Table 2.
Thresholds for population iodine sufficiency based on median urinary iodine concentrations
| Pregnancy (μg/L) | Lactation (μg/L) | Children Younger Than 2 Y (μg/L) | |
|---|---|---|---|
| Insufficient | <150 | <100 | <100 |
| Adequate | ≥150 | ≥100 | ≥100 |
Data from Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab 2010;24(1):63–75.
Serum levels of TSH and thyroglobulin increase over weeks to months of iodine deficiency, although these concentrations often remain in the normal range and are thus not a good measure of mild iodine deficiency. The World Health Organization (WHO) guideline of using the upper limit (3%) of neonatal TSH values of more than 5 mIU/L has been regarded as one method to define population iodine sufficiency, although it has been suggested to be unreliable.15 Goiter size, assessed by palpation or ultrasonography, is used to assess long-term iodine sufficiency. The WHO has established international reference ranges for serum thyroglobulin and thyroid gland volumes to be used in the monitoring of iodine deficiency in school-aged children.16,17
IMPORTANCE OF ADEQUATE IODINE NUTRITION
Consequences of iodine deficiency include endemic goiter, cretinism, intellectual impairments, growth retardation, neonatal hypothyroidism, and increased pregnancy loss and infant mortality,18 many of which were recognized beginning in the 1970s by Pharoah and colleagues19 in Papua New Guinea. Research since then has shown that thyroid hormone plays a particularly vital role in fetal and infant neurodevelopment in in utero and in early life because it is required for oligodendrocyte differentiation and myelin distribution.20 Animal studies have demonstrated that low levels of thyroid hormone in early pregnancy up to midgestation, when the developing fetus is completely reliant on maternal thyroid hormone stores, impair radial migration of neurons to the cortex and hippocampus and result in behavior changes.21
Insufficient iodine levels during pregnancy and the immediate postpartum period result in neurologic and psychological deficits in children.22,23 The prevalence of attention deficit and hyperactivity disorders is higher in the offspring of women living in iodine-deficient areas than those in iodine-replete regions.24 Intelligence quotient (IQ) levels of children living in severely iodine-deficient areas are an average of 12.45 points lower than those living in iodine-sufficient areas and are improved with iodine supplementation.25 In Spain, a region of mild iodine deficiency, children with urinary iodine levels more than 100 μg/L have significantly higher IQ levels than those with urinary iodine levels less than this threshold.26 Although public health efforts have improved iodine nutrition over the past few decades, iodine deficiency affects more than 2.2 billion individuals (38% of the world’s population)27 and remains the leading cause of preventable mental retardation worldwide.14
IODINE SUPPLEMENTATION DURING PREGNANCY
Many studies have established the benefits of iodine supplementation during pregnancy in areas of severe iodine deficiency.28 One of the earliest studies was a randomized controlled trial during the early 1970s in Papua New Guinea, in which pregnant women living in the remote highlands were administered injections of Lipiodol, a solution of iodinated poppy seed oil, and found to have decreased rates of fetal death and endemic cretinism for up to 5 years compared with untreated women.19
There have been 6 controlled trials to assess the effects of iodine supplementation during pregnancy in several moderately iodine-deficient European regions. In Italy, 18 iodine-untreated women had larger thyroid volumes than 17 women who received 120 to 180 μg/d of iodine beginning during the first trimester.29 Investigators in Denmark reported that iodine-treated women have decreased maternal TSH levels compared with women who received no supplementation.30,31 Among 180 pregnant women in Belgium, the infants of those who received 100 μg/d potassium iodide had decreased thyroid volumes.32 Liesenkotter and colleagues33 reported similar findings among the infants of 38 pregnant women in Germany who received 300 μg/d potassium iodide starting at 10 to 12 weeks’ gestation. In contrast, Antonangeli and colleagues34 reported that there were no differences in maternal thyroid function or thyroid gland size between 86 iodine-supplemented and iodine-unsupplemented women.
In a mildly iodine-deficient area of Italy, consumption of iodized salt in the 24 months preceding pregnancy, compared with initiation of iodized salt ingestion on becoming pregnant, decreased the risk of maternal thyroid dysfunction in women with negative thyroid antibody titers.35 In the only 2 studies of iodine supplementation in mildly and moderately iodine-deficient women evaluating neurobehavioral outcomes, infants born to mothers who received iodine during pregnancy had improved psychological and neurocognitive measures compared with those born to nonsupplemented mothers. Berbel and colleagues36 reported that children of women who were both mildly hypothyroxinemic from a mildly iodine-deficient region and supplemented with 200 μg potassium iodide per day beginning at 12 to 14 gestational weeks compared with women who received no supplementation had delayed neurocognitive performance at 18 months of age compared with children of women who received supplementation at 4 to 6 gestational weeks. Similarly, Velasco and colleagues37 found that infants aged 3 to 18 months of mildly iodine-deficient mothers who received 300 μg potassium iodide per day during the first trimester had higher neuropsychological assessment scores than those of mothers who received no iodine supplementation.
CONSEQUENCES OF HYPOTHYROIDISM IN PREGNANCY
Maternal hypothyroidism, as characterized by elevated TSH levels, occurs in an estimated 2.5% of all pregnancies in the United States.38 The fetal thyroid does not begin to concentrate iodine until 10 to 12 weeks of gestation and is not controlled by fetal pituitary TSH until approximately 20 weeks of gestation.39 Before this, the fetus is reliant on maternal T4 that crosses the placenta in very small quantities.40
Because thyroid hormone is required for normal neurodevelopment,41 even mildly low maternal T4 and/or elevated maternal serum TSH levels during pregnancy may result in cognitive delays in the offspring. Haddow and colleagues42 reported that 7- to 9-year-old children whose pregnant mothers had untreated mild hypothyroidism have an average of 7 IQ points lower than those of matched euthyroid control mothers. Subclinical hypothyroidism and low free T4 concentrations in women during pregnancy are independent predictors of impaired neurodevelopment in their children.43-47 The most recent study supporting these data was the Dutch Generation R study of 3659 women and their infants, in which severe maternal hypothyroxinemia was associated with both expressive language delay and nonverbal cognitive delay in their infants at 30 months.47
IODINE PHYSIOLOGY DURING LACTATION
Thyroidal iodine turnover rate is more rapid in infants.11 Thus, adequate breast milk iodine levels are particularly important for proper neurodevelopment in nursing infants. Iodine is secreted into breast milk at a concentration gradient 20 to 50 times that of plasma48 through increased expression of the sodium/iodide symporter (NIS) present on lactating breast cells.49 In iodine-sufficient areas, breast milk iodine concentrations are generally adequate to meet infants’ iodine nutritional needs based on iodine balance studies.50 Even compensatory mechanisms may not be adequate to meet the increased demands for thyroid hormone production and iodine intake for mothers living in iodine-deficient areas. In a recent study, the iodine needs for breastfed infants in iodine-deficient New Zealand remained inadequate even when their mothers were supplemented with 150 μg/d of iodine during the first 6 postpartum months.51
IODINE NUTRITION IN LACTATION
Because breastfed infants are reliant on maternal dietary iodine intake, recommendations for dietary iodine intake during lactation range from 250 to 290 μg/d, higher than the 150 μg/d recommended for nonpregnant and nonlactating adolescents and adults (see Table 1). These thresholds were determined based on a mean breast milk iodine concentration of 146 μg/L, as was measured in 37 women in the United States,52 and the assumption that infants ingest an average of 0.78 L/d of breast milk during 0 to 6 months and 0.60 L/d during 6 to 12 months.53
Data regarding breast milk iodine levels in lactating women in the United States are extremely limited. Small studies (57 women in the largest sample)54 have demonstrated that median breast milk iodine levels in women in the United States range from 35 to 155 μg/L.52,54-57 Furthermore, breast milk iodine levels vary temporally, as was reported by Kirk and colleagues56 in a study of 10 lactating women in the United States. Among 108 total samples, there was considerable variation of breast milk iodide levels (which comprises 89%–90% of breast milk iodine [S. Pino, BS, unpublished data, 2004]) within and between individuals over a 3-day period. Larger studies are needed to determine the iodine sufficiency of lactating women in the United States and their breastfed infants.
IODINE NUTRITION IN THE UNITED STATES
Since the 1920s, dietary iodine in the United States has been considered adequate. However, sources of iodine in the US diet have been difficult to identify because there are a wide variety of potential sources and a wide variation in the iodine content of some common foods and food iodine content is not listed on packaging. In the United States, population iodine deficiency has been eliminated by means of silent prophylaxis. Sources of dietary iodine include iodized salt (due to the addition of iodine to table salt as a public health measure), dairy foods (due to the iodophor cleansers of milk cans and teats), and bread dough (due to the use of iodate as bread conditioners in some bakeries).58
Results of the Total Diet Studies by the US Food and Drug Administration (FDA) have demonstrated that iodine is found mostly in grain products, milk, and cheese in the United States.59 Most foods contain 3 to 75 μg of iodine per serving.53 Based on the most recent US FDA’s Total Diet Study, the estimated average daily iodine intake ranges from 138 to 353 μg per person.60 A survey of more than 700 schoolchildren in the United States during the early 1970s using diet frequency diaries and urinary iodine levels found that milk, iodized salt, and iodated bread were the primary sources of iodine intake.61 In addition, sources of iodine exposure may include use of iodine-rich medications (such as amiodarone), topical antiseptics, multivitamins, radiographic contrast agents, and water purification tablets.
According to data from the National Health and Nutrition Examination Survey (NHANES), the median urinary iodine concentration in adults in the United States decreased by more than 50% from the early 1970s to the late 1990s (Fig. 2).62 Of particular concern in the NHANES data is that the prevalence of urinary iodine values less than 50 μg/L among women of childbearing age increased by almost 4-fold, from 4% to 15%, during this period. The most recent NHANES survey (2005–2008) demonstrated that 35.3% of pregnant women had urinary iodine levels less than 100 μg/L,63 which suggests mild iodine sufficiency.14 Reductions in the US dietary iodine level have been variously ascribed to a possible reduction in the iodine content of dairy products, the removal of iodate dough conditioners in commercially produced bread, new recommendations for reduced salt intake for blood pressure control, and the increasing use of noniodized salt in manufactured or premade convenience foods.64
Fig. 2.
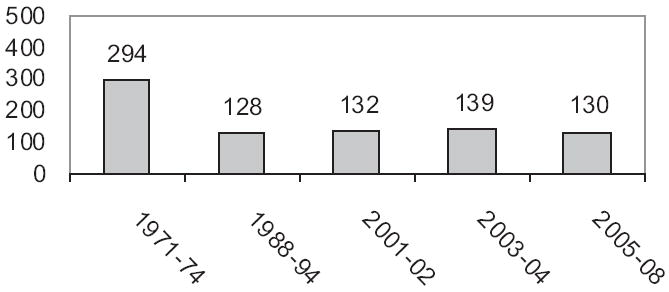
US dietary iodine in women of childbearing age: NHANES data from 1971 to 2008. (Data from Refs.62,63,88,89)
A study of aggregate NHANES data from 2001 to 2006 showed that the included pregnant women in the United States were marginally iodine sufficient (N = 326; median urinary iodine = 153 μg/L) and lactating (N = 53; median urinary iodine = 115 μg/L) and nonpregnant nonlactating women (N = 1,437; median urinary iodine = 130 μg/L) were both iodine deficient.60 We reported that the median urinary iodine concentration of a sample of 100 women in the Boston area in their first or second trimesters of pregnancy was 149 μg/L, including 9% with values less than 50 μg/L.65
PUBLIC HEALTH EFFORTS OF IODINE SUPPLEMENTATION
A public health approach to iodine supplementation in the United States has been advocated. The American Thyroid Association has recommended that women in North America receive dietary supplements containing 150 μg of iodine daily during pregnancy and lactation and that all prenatal vitamins contain 150 μg of iodine.66 These recommendations have not yet been widely adopted. Only 20.3% of pregnant and 14.5% of lactating women in the United States take a supplement containing iodine, according to the NHANES data.67 At present, 114 of 223 (51%) brands of prescription and nonprescription prenatal multivitamins marketed in the United States list iodine as a constituent, and many of those which contain iodine do not contain the labeled amount, especially when kelp is the iodine source.68 The US Women, Infants, and Children Nutrition Program has recommended that all prenatal multivitamins administrated to women in its program contain 150 μg of iodine per daily serving beginning in 2010.
The major concerns regarding the global burden of iodine deficiency are related to goiter; neurocognitive impairments; and, in severe deficiency, hypothyroidism resulting in cretinism. In 1990, the United Nations World Summit for Children set forth the goal of eliminating iodine deficiency worldwide,69 and considerable progress has since been achieved. This goal has largely been led by programs of universal salt iodization in various countries, in line with the recommendations by the WHO and the International Council for the Control of Iodine Deficiency Disorders27,70 and use of oral potassium iodide drops or intramuscular iodized oil injections. Median urinary iodine concentrations of infants residing in iodine-sufficient countries range from 90 to 170 μg/L, which suggests optimal iodine nutrition by criteria as defined by the WHO.14 Although these efforts have been successful in many countries, the WHO estimates that 2 billion people, including 285 million school-aged children, remain iodine deficient.71
The WHO has proposed that an iodine intake of 500 μg/d poses no excessive risk,14 and the European Food Safety Agency and the US Institute of Medicine have recommended 600 μg/d and 1100 μg/d, respectively, as the tolerable upper limit for iodine per day.53,72 Thus, although the overall US adult population remains iodine sufficient by WHO standards, a subset of pregnant and lactating women may have inadequate dietary iodine intake.
SUBSTANCES INTERFERING WITH IODINE USE
Competitive inhibitors of NIS, such as perchlorate, thiocyanate, and nitrate, can decrease the entry of iodine into the thyroid and lactating breast, thereby potentially exacerbating the effects of dietary iodine insufficiency. Low-level perchlorate exposure seems to be ubiquitous in the US, European, and South American population. Environmental perchlorate comes from a variety of sources, is extremely stable as an inorganic salt, and persists in low levels in soil and groundwater over long periods.73 In the United States, perchlorate has been found in many substances, including tobacco, alfalfa, tomato, cow’s milk,55 cucumber, lettuce, soybeans, eggs, and multivitamins (including prenatal multivitamins).74 Following the development of sensitive detection methods, perchlorate has been measured in the drinking water of communities around the United States.
When given in pharmacologic doses, perchlorate decreases the active transport of iodine into the thyroid and possibly breast milk by competitively inhibiting the NIS with 30 times its affinity for iodide.75 Furthermore, recent studies in lactating mice have suggested that perchlorate is actively transported into breast milk,76 thus potentially decreasing infants’ thyroidal iodine uptake. In the NHANES 2001 to 2002 survey, perchlorate was detected in all 2820 spot urine samples (median urine perchlorate concentration, 3.6 μg/L) and was a significant negative predictor of total T4 values and a positive predictor of TSH values in women, primarily those with urine iodine concentrations less than 100 μg/L.77,78 However, these relationships were not seen in men,78 a follow-up subset analysis of this dataset (which analyzed only women of childbearing age) using creatinine-adjusted urinary iodine values,79 or a large European study assessing serum thyroid function tests of iodine-deficient pregnant women.80 Recently, the US FDA reported in a Total Diet Study that infants and children have the highest estimated intakes of perchlorate by body weight.81
There are limited data regarding breast milk iodine82 and perchlorate concentrations in women in the United States. We reported that the median breast milk iodine concentration in 57 women in Boston area was 155 μg/L,54 similar to that of a 1984 study of 37 women (178 μg/L).52 However, the median breast milk iodine levels in our study were far higher than those (33.5, 43.0, and 55.2 μg/L) observed recently by Kirk and colleagues55,56 in 3 studies.57 Kirk and colleagues55 also measured perchlorate levels in the breast milk of 36 women from 18 states and found detectable levels in all the samples (range, 0.6–2.2 μg/L). Breast milk iodide and perchlorate levels were inversely correlated in the 6 samples with perchlorate concentrations of at least 10 μg/L, although there were no correlations between breast milk iodide and perchlorate in the full data set.55 We reported no correlation between breast milk and colostrum iodine and perchlorate concentrations, even in those breast milk samples with perchlorate concentrations of 10 μg/L or more.54,83
Similarly, exposures to thiocyanate, a metabolite of cyanide that is produced as a byproduct of cigarette smoke, and nitrate, which is produced naturally and is present in many prepared foods, are able to decrease NIS activity, thereby decreasing iodine availability. Naturally goitrogenic foods include those that contain cyanogenic glucosides (which become metabolized to thiocyanate), such as cassava, millet, maize, sweet potatoes, lima beans, and cruciferous vegetables (which contain thiocyanates and isothiocyanates), such as cabbage, Brussels sprouts, cauliflower, broccoli, and horseradish.18 However, the thiocyanate content of these foods is low, and their ingestion does not usually produce clinically significant sequelae unless severe iodine deficiency is present.
One recent study in Denmark concluded that cigarette smoking decreases breast milk iodine concentrations,84 and we demonstrated the same effect in a small cohort of lactating women in Boston area.54 Comparatively, perchlorate is a potent inhibitor of NIS; its effects are 15-fold greater than thiocyanate, 30-fold compared with iodide, and 240-fold compared with nitrate.75 Nonetheless, because exposure to thiocyanate and nitrate is ubiquitous, the additive effects on iodide uptake may be important when assessing iodine availability.
The urinary levels of selenium and iodine in pregnant women are closely correlated.85 Selenium is an important component of glutathione peroxidase and selonoproteins, which include the 3 thyroid hormone deiodinases. Thus, insufficient selenium may result in the accumulation of damaging peroxides in the thyroid and impair the peripheral deiodination process required to generate the active thyroid hormone, T3, from T4. Although selenium nutrition is generally adequate in humans, rare conditions resulting in selenium deficiency may be important in the pathogenesis of hypothyroidism.86 A recent study by Negro and colleagues87 reported that selenium supplementation of 200 μg/d during pregnancy and in the postpartum period reduced the prevalence of permanent maternal hypothyroidism (11.7%) compared with women who did not receive supplementation (20.3%) (P<.01). The findings of this study are very preliminary, and currently there are no recommendations for selenium supplementation during pregnancy and lactation.
SUMMARY
Adequate iodine nutrition during pregnancy and lactation is needed for thyroid hormone synthesis and normal neurodevelopment of the developing fetus in utero and in the breastfed infant. Iodine deficiency during pregnancy has been associated with impairments of infant neurologic and psychological outcomes. Studies of maternal iodine supplementation in severe iodine deficiency have demonstrated reductions in the rates of fetal death and endemic cretinism. Iodine supplementation in areas of moderate iodine deficiency has shown decreased maternal thyroid volumes and TSH levels and, in areas of mild iodine deficiency, improvements in infants’ neurocognitive measures.
Although the overall adult population in the United States remains iodine sufficient in recent national surveys, a subset of pregnant and lactating women may have inadequate dietary iodine intake. A public health approach has been undertaken to achieve recommended median urinary iodine concentrations during pregnancy and lactation. Recent guidelines have recommended that women in the United States take a multivitamin containing 150 μg of iodine during pregnancy and lactation. Further studies are needed to assess the impact of environmental exposures to substances that may interfere with iodine use.
Footnotes
All authors have nothing to disclose.
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Glinoer D. The importance of iodine nutrition during pregnancy. Public Health Nutr. 2007;10(12A):1542–6. doi: 10.1017/S1368980007360886. [DOI] [PubMed] [Google Scholar]
- 2.Roti E, Fang SL, Emerson CH, et al. Placental inner ring iodothyronine deiodination: a mechanism for decreased passage of T4 and T3 from mother to fetus. Trans Assoc Am Physicians. 1981;94:183–9. [PubMed] [Google Scholar]
- 3.Zimmermann MB, Jooste PL, Pandav CS. Iodine-deficiency disorders. Lancet. 2008;372(9645):1251–62. doi: 10.1016/S0140-6736(08)61005-3. [DOI] [PubMed] [Google Scholar]
- 4.Fisher DA, Oddie TH. Thyroid iodine content and turnover in euthyroid subjects: validity of estimation of thyroid iodine accumulation from short-term clearance studies. J Clin Endocrinol Metab. 1969;29(5):721–7. doi: 10.1210/jcem-29-5-721. [DOI] [PubMed] [Google Scholar]
- 5.Smyth PP, Hetherton AM, Smith DF, et al. Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal iodine intake. J Clin Endocrinol Metab. 1997;82(9):2840–3. doi: 10.1210/jcem.82.9.4203. [DOI] [PubMed] [Google Scholar]
- 6.Wayne EJ, Koutras DA, Alexander WD. Clinical aspects of iodine metabolism. Philadelphia: Oxford, Blackwell; 1964. Excretion of iodine; pp. 73–83. [Google Scholar]
- 7.Dafnis E, Sabatini S. The effect of pregnancy on renal function: physiology and pathophysiology. Am J Med Sci. 1992;303(3):184–205. doi: 10.1097/00000441-199203000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Stilwell G, Reynolds PJ, Parameswaran V, et al. The influence of gestational stage on urinary iodine excretion in pregnancy. J Clin Endocrinol Metab. 2008;93(5):1737–42. doi: 10.1210/jc.2007-1715. [DOI] [PubMed] [Google Scholar]
- 9.Smyth PP. Variation in iodine handling during normal pregnancy. Thyroid. 1999;9(7):637–42. doi: 10.1089/thy.1999.9.637. [DOI] [PubMed] [Google Scholar]
- 10.Glinoer D. Pregnancy and iodine. Thyroid. 2001;11(5):471–81. doi: 10.1089/105072501300176426. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann MB. Iodine deficiency. Endocr Rev. 2009;30(4):376–408. doi: 10.1210/er.2009-0011. [DOI] [PubMed] [Google Scholar]
- 12.Vought RL, London WT. Iodine intake, excretion and thyroidal accumulation in healthy subjects. J Clin Endocrinol Metab. 1967;27(7):913–9. doi: 10.1210/jcem-27-7-913. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen LB, Ovesen L, Christiansen E. Day-to-day and within-day variation in urinary iodine excretion. Eur J Clin Nutr. 1999;53(5):401–7. doi: 10.1038/sj.ejcn.1600762. [DOI] [PubMed] [Google Scholar]
- 14.WHO, UNICEF, ICCIDD. Assessment of the iodine deficiency disorders and monitoring their elimination. Geneva (Switzerland): World Health Organization; 2007. WHO/NHD/01.1. [Google Scholar]
- 15.Li M, Eastman CJ. Neonatal TSH screening: is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab. 2010;24(1):63–75. doi: 10.1016/j.beem.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann MB, Hess SY, Molinari L, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group report. Am J Clin Nutr. 2004;79(2):231–7. doi: 10.1093/ajcn/79.2.231. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann MB, de Benoist B, Corigliano S, et al. Assessment of iodine status using dried blood spot thyroglobulin: development of reference material and establishment of an international reference range in iodine-sufficient children. J Clin Endocrinol Metab. 2006;91(12):4881–7. doi: 10.1210/jc.2006-1370. [DOI] [PubMed] [Google Scholar]
- 18.Boyages SC. Clinical review 49: iodine deficiency disorders. J Clin Endocrinol Metab. 1993;77(3):587–91. doi: 10.1210/jcem.77.3.8370679. [DOI] [PubMed] [Google Scholar]
- 19.Pharoah PO, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet. 1971;1(7694):308–10. doi: 10.1016/s0140-6736(71)91040-3. [DOI] [PubMed] [Google Scholar]
- 20.Younes-Rapozo V, Berendonk J, Savignon T, et al. Thyroid hormone deficiency changes the distribution of oligodendrocyte/myelin markers during oligodendroglial differentiation in vitro. Int J Dev Neurosci. 2006;24(7):445–53. doi: 10.1016/j.ijdevneu.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 21.de Escobar GM, Obregon MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10(12A):1554–70. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 22.Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331(26):1739–44. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- 23.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16(10):809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 24.Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89(12):6054–60. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- 25.Qian M, Wang D, Watkins WE, et al. The effects of iodine on intelligence in children: a meta-analysis of studies conducted in China. Asia Pac J Clin Nutr. 2005;14(1):32–42. [PubMed] [Google Scholar]
- 26.Santiago-Fernandez P, Torres-Barahona R, Muela-Martinez JA, et al. Intelligence quotient and iodine intake: a cross-sectional study in children. J Clin Endocrinol Metab. 2004;89(8):3851–7. doi: 10.1210/jc.2003-031652. [DOI] [PubMed] [Google Scholar]
- 27.International Council for the Control of Iodine Deficiency Disorders (ICCIDD) [August 31, 2011]; Available at: http://www.iccidd.org.
- 28.Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab. 2010;24(1):39–50. doi: 10.1016/j.beem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Romano R, Jannini EA, Pepe M, et al. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol. 1991;164(2):482–5. doi: 10.1016/s0002-9378(11)80004-9. [DOI] [PubMed] [Google Scholar]
- 30.Nohr SB, Laurberg P. Opposite variations in maternal and neonatal thyroid function induced by iodine supplementation during pregnancy. J Clin Endocrinol Metab. 2000;85(2):623–7. doi: 10.1210/jcem.85.2.6391. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen KM, Laurberg P, Iversen E, et al. Amelioration of some pregnancyassociated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab. 1993;77(4):1078–83. doi: 10.1210/jcem.77.4.8408456. [DOI] [PubMed] [Google Scholar]
- 32.Glinoer D, De Nayer P, Delange F, et al. A randomized trial for the treatment of mild iodine deficiency during pregnancy: maternal and neonatal effects. J Clin Endocrinol Metab. 1995;80(1):258–69. doi: 10.1210/jcem.80.1.7829623. [DOI] [PubMed] [Google Scholar]
- 33.Liesenkotter KP, Gopel W, Bogner U, et al. Earliest prevention of endemic goiter by iodine supplementation during pregnancy. Eur J Endocrinol. 1996;134(4):443–8. doi: 10.1530/eje.0.1340443. [DOI] [PubMed] [Google Scholar]
- 34.Antonangeli L, Maccherini D, Cavaliere R, et al. Comparison of two different doses of iodide in the prevention of gestational goiter in marginal iodine deficiency: a longitudinal study. Eur J Endocrinol. 2002;147(1):29–34. doi: 10.1530/eje.0.1470029. [DOI] [PubMed] [Google Scholar]
- 35.Moleti M, Lo Presti VP, Campolo MC, et al. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93(7):2616–21. doi: 10.1210/jc.2008-0352. [DOI] [PubMed] [Google Scholar]
- 36.Berbel P, Mestre JL, Santamaria A, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19(5):511–9. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 37.Velasco I, Gonzalez-Romero S, Soriguer F. Iodine and thyroid function during pregnancy. Epidemiology. 2010;21(3):428–9. doi: 10.1097/EDE.0b013e3181d742a5. [DOI] [PubMed] [Google Scholar]
- 38.Klein RZ, Haddow JE, Faix JD, et al. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol (Oxf) 1991;35(1):41–6. doi: 10.1111/j.1365-2265.1991.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 39.Brown RS. Minireview: developmental regulation of thyrotropin receptor gene expression in the fetal and newborn thyroid. Endocrinology. 2004;145(9):4058–61. doi: 10.1210/en.2004-0458. [DOI] [PubMed] [Google Scholar]
- 40.de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18(2):225–48. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Iskaros J, Pickard M, Evans I, et al. Thyroid hormone receptor gene expression in first trimester human fetal brain. J Clin Endocrinol Metab. 2000;85(7):2620–3. doi: 10.1210/jcem.85.7.6766. [DOI] [PubMed] [Google Scholar]
- 42.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 43.Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59(3):282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 44.Choudhury N, Gorman KS. Subclinical prenatal iodine deficiency negatively affects infant development in northern China. J Nutr. 2003;133(10):3162–5. doi: 10.1093/jn/133.10.3162. [DOI] [PubMed] [Google Scholar]
- 45.Kooistra L, Crawford S, van Baar AL, et al. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117(1):161–7. doi: 10.1542/peds.2005-0227. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010;72(6):825–9. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 47.Henrichs J, Schenk JJ, Roza SJ, et al. Maternal psychological distress and fetal growth trajectories: the Generation R Study. Psychol Med. 2010;40(4):633–43. doi: 10.1017/S0033291709990894. [DOI] [PubMed] [Google Scholar]
- 48.Eskandari S, Loo DD, Dai G, et al. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272(43):27230–8. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 49.Tazebay UH, Wapnir IL, Levy O, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6(8):871–8. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- 50.Bruhn JC, Franke AA. Iodine in human milk. J Dairy Sci. 1983;66(6):1396–8. doi: 10.3168/jds.S0022-0302(83)81950-X. [DOI] [PubMed] [Google Scholar]
- 51.Mulrine HM, Skeaff SA, Ferguson EL, et al. Breast-milk iodine concentration declines over the first 6 mo postpartum in iodine-deficient women. Am J Clin Nutr. 2010;92(4):849–56. doi: 10.3945/ajcn.2010.29630. [DOI] [PubMed] [Google Scholar]
- 52.Gushurst CA, Mueller JA, Green JA, et al. Breast milk iodide: reassessment in the 1980s. Pediatrics. 1984;73(3):354–7. [PubMed] [Google Scholar]
- 53.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes. Washington, DC: National Academy Press; 2006. [Google Scholar]
- 54.Pearce EN, Leung AM, Blount BC, et al. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J Clin Endocrinol Metab. 2007;92(5):1673–7. doi: 10.1210/jc.2006-2738. [DOI] [PubMed] [Google Scholar]
- 55.Kirk AB, Martinelango PK, Tian K, et al. Perchlorate and iodide in dairy and breast milk. Environ Sci Technol. 2005;39(7):2011–7. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- 56.Kirk AB, Dyke JV, Martin CF, et al. Temporal patterns in perchlorate, thiocyanate, and iodide excretion in human milk. Environ Health Perspect. 2007;115(2):182–6. doi: 10.1289/ehp.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dasgupta PK, Kirk AB, Dyke JV, et al. Intake of iodine and perchlorate and excretion in human milk. Environ Sci Technol. 2008;42(21):8115–21. doi: 10.1021/es801549w. [DOI] [PubMed] [Google Scholar]
- 58.Pearce EN, Pino S, He X, et al. Sources of dietary iodine: bread, cows’ milk, and infant formula in the Boston area. J Clin Endocrinol Metab. 2004;89(7):3421–4. doi: 10.1210/jc.2003-032002. [DOI] [PubMed] [Google Scholar]
- 59.Pennington JA, Schoen SA. Contributions of food groups to estimated intakes of nutritional elements: results from the FDA total diet studies, 1982-1991. Int J Vitam Nutr Res. 1996;66(4):342–9. [PubMed] [Google Scholar]
- 60.Perrine CG, Herrick K, Serdula MK, et al. Some subgroups of reproductive age women in the United States may be at risk for iodine deficiency. J Nutr. 2010;140(8):1489–94. doi: 10.3945/jn.109.120147. [DOI] [PubMed] [Google Scholar]
- 61.Kidd PS, Trowbridge FL, Goldsby JB, et al. Sources of dietary iodine. J Am Diet Assoc. 1974;65(4):420–2. [PubMed] [Google Scholar]
- 62.Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971-1974 and 1988-1994) J Clin Endocrinol Metab. 1998;83(10):3401–8. doi: 10.1210/jcem.83.10.5168. [DOI] [PubMed] [Google Scholar]
- 63.Caldwell KL, Makhmudov A, Ely E, et al. Iodine status of the U.S. population, national health and nutrition examination survey, 2005-2006 and 2007-2008. Thyroid. 2011;21(4):419–27. doi: 10.1089/thy.2010.0077. [DOI] [PubMed] [Google Scholar]
- 64.Lee SY, Leung AM, He X, et al. Iodine content in fast foods: comparison between two fast-food chains in the United States. Endocr Pract. 2010;16(6):1071–2. doi: 10.4158/EP10180.CO. [DOI] [PubMed] [Google Scholar]
- 65.Pearce EN, Bazrafshan HR, He X, et al. Dietary iodine in pregnant women from the Boston, Massachusetts area. Thyroid. 2004;14(4):327–8. doi: 10.1089/105072504323031013. [DOI] [PubMed] [Google Scholar]
- 66.Becker DV, Braverman LE, Delange F, et al. Iodine supplementation for pregnancy and lactation—United States and Canada: recommendations of the American Thyroid Association. Thyroid. 2006;16(10):949–51. doi: 10.1089/thy.2006.16.949. [DOI] [PubMed] [Google Scholar]
- 67.Gregory CO, Serdula MK, Sullivan KM. Use of supplements with and without iodine in women of childbearing age in the United States. Thyroid. 2009;19(9):1019–20. doi: 10.1089/thy.2009.0166. [DOI] [PubMed] [Google Scholar]
- 68.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl J Med. 2009;360(9):939–40. doi: 10.1056/NEJMc0807851. [DOI] [PubMed] [Google Scholar]
- 69.World Declaration on the Survival, Protection and Development of Children. [August 31, 2011];1990 Available at: http://www.unicef.org/wsc/declare.htm.
- 70.Iodine and health: eliminating iodine deficiency disorders safely through salt iodization. Geneva: World Health Organization (WHO); 1994. Report No.: WHO/NUT/94.4. [Google Scholar]
- 71.Andersson M, Takkouche B, Egli I, et al. Current global iodine status and progress over the last decade towards the elimination of iodine deficiency. Bull World Health Organ. 2005;83(7):518–25. [PMC free article] [PubMed] [Google Scholar]
- 72.Opinion of the Scientific Committee on Food on the tolerable upper intake level of iodine. [August 31, 2011];2002 Available at: http://ec.europa.eu/food/fs/sc/scf/ouT146_en.pdf.
- 73.Dasgupta PK, Dyke JV, Kirk AB, et al. Perchlorate in the United States. Analysis of relative source contributions to the food chain. Environ Sci Technol. 2006;40(21):6608–14. doi: 10.1021/es061321z. [DOI] [PubMed] [Google Scholar]
- 74.Renner R. Perchlorate found in vitamins and elsewhere. Environ Sci Technol. 2006;40(8):2498–9. [PubMed] [Google Scholar]
- 75.Tonacchera M, Pinchera A, Dimida A, et al. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14(12):1012–9. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 76.Dohan O, Portulano C, Basquin C, et al. The Na+/I− symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci U S A. 2007;104(51):20250–5. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blount BC, Valentin-Blasini L, Osterloh JD, et al. Perchlorate exposure of the US population, 2001-2002. J Expo Sci Environ Epidemiol. 2007;17(4):400–7. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- 78.Blount BC, Pirkle JL, Osterloh JD, et al. Urinary perchlorate and thyroid hormone levels in adolescent and adult men and women living in the United States. Environ Health Perspect. 2006;114(12):1865–71. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamm SH, Hollowell JG, Engel A, et al. Perchlorate, thiocyanate, and low iodine association not seen with low creatinine-adjusted urine iodine among women of child-bearing age. Thyroid. 2007;17:S51. [Google Scholar]
- 80.Pearce EN, Lazarus JH, Smyth PP, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95(7):3207–15. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- 81.Murray CW, Egan SK, Kim H, et al. US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008;18(6):571–80. doi: 10.1038/sj.jes.7500648. [DOI] [PubMed] [Google Scholar]
- 82.Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009;70(5):803–9. doi: 10.1111/j.1365-2265.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- 83.Leung AM, Pearce EN, Hamilton T, et al. Colostrum iodine and perchlorate concentrations in Boston-area women: a cross-sectional study. Clin Endocrinol (Oxf) 2009;70(2):326–30. doi: 10.1111/j.1365-2265.2008.03330.x. [DOI] [PubMed] [Google Scholar]
- 84.Laurberg P, Nohr SB, Pedersen KM, et al. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab. 2004;89(1):181–7. doi: 10.1210/jc.2003-030829. [DOI] [PubMed] [Google Scholar]
- 85.Szybinski Z, Walas S, Zagrodzki P, et al. Iodine, selenium, and other trace elements in urine of pregnant women. Biol Trace Elem Res. 2010;138(1–3):28–41. doi: 10.1007/s12011-009-8601-9. [DOI] [PubMed] [Google Scholar]
- 86.Gartner R. Selenium and thyroid hormone axis in critical ill states: an overview of conflicting view points. J Trace Elem Med Biol. 2009;23(2):71–4. doi: 10.1016/j.jtemb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 87.Negro R, Greco G, Mangieri T, et al. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab. 2007;92(4):1263–8. doi: 10.1210/jc.2006-1821. [DOI] [PubMed] [Google Scholar]
- 88.Caldwell KL, Jones R, Hollowell JG. Urinary iodine concentration: United States National Health and Nutrition Examination Survey 2001-2002. Thyroid. 2005;15(7):692–9. doi: 10.1089/thy.2005.15.692. [DOI] [PubMed] [Google Scholar]
- 89.Caldwell KL, Miller GA, Wang RY, et al. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003-2004. Thyroid. 2008;18(11):1207–14. doi: 10.1089/thy.2008.0161. [DOI] [PubMed] [Google Scholar]


