Abstract
The easy accessibility of skin makes it an excellent target for gene transfer protocols. To take advantage of skin as a target for gene transfer, it is important to establish an efficient and reproducible delivery system. Electroporation is an established technique for enhancing plasmid delivery to many tissues in vivo. A critical component of this technique is the electrode configuration. Electroporation parameters were optimized for transgene expression with minimal tissue damage with a novel electrode. The highest transgene expression and efficiency of individual cell transformation with minimal damage was produced with eight 150 ms pulses at field strength of 100 V/cm. This electrode design offers the potential for easier and more reproducible electrically mediated cutaneous plasmid delivery than the simple electrodes currently commercially available. This electrode can be a valuable tool in determining the applicability of electrically mediated cutaneous gene transfer.
Keywords: electroporation, skin, electrode design
Introduction
Skin is an advantageous tissue for gene therapy, primarily due to its accessibility. Expressed proteins can be detected locally or systemically. Cutaneous plasmid DNA delivery aided by in vivo electroporation was first attempted in a newborn mouse model.1 Exponential pulses were delivered using a clip electrode and analysis of transgene expression was performed ex vivo. Since that time, several studies exploring plasmid delivery using skin electroporation, primarily with square wave pulses, have been published.
For electroporation gene delivery to skin, plasmid DNA may be injected intradermally1–10 or biolistically.11 Pulses are applied to the skin surface or through penetrating (needle) electrodes. Some electrode configurations are commercially available, although investigators often specifically design electrodes for in vivo delivery. Caliper, plate, tweezer or clip type electrodes grip a fold of skin.1,4–6,9,10 The meander7,11 and the N1/N2 electrode8 are simply pressed against the injected region. The applied pressure may vary. Invasive electrodes used in cutaneous gene delivery include 2-needle,5 6-needle,12 and 7-pin arrays.2,3
Comparisons show that penetrating electrodes induce higher levels of transgene expression,2,3,5 but these electrodes raise the specter of needle insertion. Previous studies have also shown that delivery is more efficient when the field is applied in more than one direction.12–14 This approach permeabilizes more areas of the cell membrane as well as more cells, which results in increased expression and better distribution.14 When nonpenetrating electrodes are used, better delivery is obtained by rotating the plates 90° between sets of pulses.13 To accomplish this, the electrodes are lifted off the skin, repositioned, and a second set of pulses is delivered. This delays the time between injection and pulsing and also demands precise repositioning of the electrodes with respect to the injection site. To alleviate these problems, a new electrode designated four-plate electrode (4PE, Figure 1) was developed.15 This electrode contains four plates that grip a fold of skin and are arranged to allow pulses in two electric field orientations at a 90° angle to be administered without removing the electrode from the skin. A nonconductive ‘stopper’ is placed in the center to establish this constant distance. The space between the electrodes, and therefore the voltage setting, is consistent.
Figure 1.
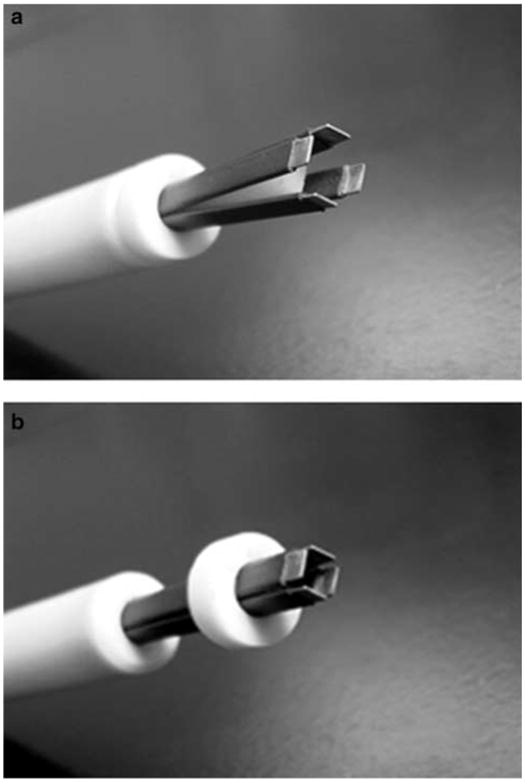
4PE: (a) open position; (b) closed position. A 6 mm gap is maintained between plates.
The purpose of these experiments was to explore localized cutaneous plasmid delivery with the 4PE electrode and to develop electroporation parameters that would result in high expression levels. Two basic classes of square wave electroporation parameters differing in pulse length and amplitude were tested. Both localized transgene expression and histological damage were compared after the electrically mediated delivery of plasmid DNA.
Results and discussion
Comparison of 2-plate versus 4-plate electrode designs
The 4PE was designed with a 6 mm distance between parallel plates to fit around a 50 μl intradermal injection, which results in a 6 mm bubble on the surface of the skin. To determine if the 4PE could be used for skin transgene delivery, expression of a plasmid encoding luciferase delivered with the 4PE was compared to the commonly used and commercially available caliper electrode type. After injection of 100 μg pCMVLuc+ in 50 μl saline, eight 2 ms pulses at field strength of 800 V/cm were applied. To duplicate delivery with the 4PE electrode, the caliper electrode was placed in two perpendicular positions with four pulses applied in each position. Luciferase activity was determined as described in Figure 2. Injection of plasmid without electroporation resulted in production of 59±27 total pg luciferase (n = 16). Delivery with the caliper electrode significantly (P < 0.05) increased transgene expression to 1544±555 total pg (n = 16), while delivery with the 4PE significantly increased expression to 2186±1079 total pg (n = 16). The difference in transgene expression between the two electrodes was not statistically significant.
Figure 2.
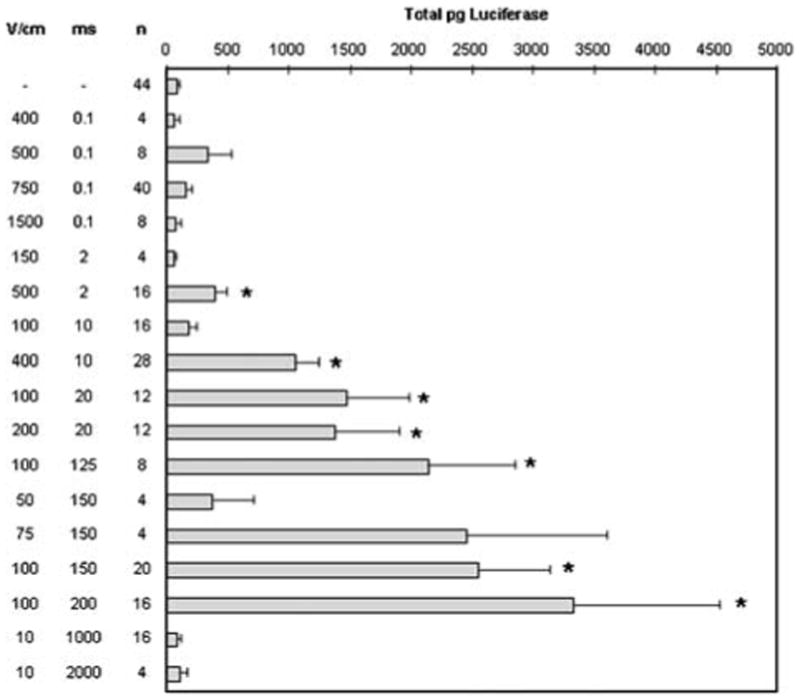
Comparison of luciferase expression after plasmid delivery with two types of pulsing conditions. The flank skin of 6–7 weeks old female C57/Bl6 mice (NCI) was shaved and then injected intradermally with 50 μl of 2 μg/μl pCMVLuc+ (a gift of Claude Nicolau, Harvard Medical School, Boston, MA, USA), which was prepared using Endo-free Megaprep kits (Qiagen, Valencia, CA, USA). Eight electric pulses were administered with a 90° rotation between sets of four pulses at a frequency of 1 Hz with a BTX T830 (BTX Molecular Delivery Systems, Holliston, MA, USA) using the 4PE electrode. Mice were anesthetized in an induction chamber charged with 3% isoflurane in O2 then fitted with a standard rodent mask and kept under general anesthesia during treatment. At 48 h after plasmid delivery, animals were humanely euthanized, and the tissue samples were excised and analyzed. For luciferase quantification, the tissue samples were homogenized in buffer (50 mM K3PO4, 1 mM EDTA, 1 mM DTT, 10% glycerol) using a Tissumizer (Tekmar, Cincinnati, OH, USA). Extracts were assayed for luciferase activity32 and quantified using an MLX microtiter plate luminometer (Dynex Technologies, Chantilly, VA, USA). Activity is expressed in total pg luciferase per tissue sample. Values represent mean and standard error. Due to the simplex scheme, some pulsing conditions were repeated multiple times as indicated. *P<0.05.
It is important to note that although the 4PE electrode covers a smaller surface area (0.8 cm2) than the caliper electrode (2 cm2), the same level of transgene expression was observed after delivery. Moreover, pulse application was smoother and easier with the 4PE. The electrode remains in contact with the tissue area injected throughout pulsing. The timing between each set of pulses is consistent and because the electrode spacing is preset, the applied voltage is also consistent from one application to the next.
Optimization of electroporation parameters with 4PE
Using the 4PE electrode, pulsing parameters were optimized for the largest level of transgene expression while minimizing tissue damage. Two different simplex optimizations16 were tested to accommodate the two classes of square waves used for in vivo electroporation. Simplex A focused on low electric fields (25–800 V/cm) and long pulses (1–200 ms), while simplex B focused on higher electric fields (700–2000 V/cm) and short pulses (10–1000 μs). Each simplex optimized on electric field strength and pulse duration. Multiple iterations of the simplex were run and more than 70 different pulsing conditions were evaluated to determine the optimal conditions. Some electroporation conditions were used repeatedly for this optimization. The electroporation pulsing conditions from both optimization schemes resulting in the highest transgene expression were then compared (Figure 2). Most of the conditions tested yielded expression levels that were higher than injection alone. Using the 4PE, short, high-voltage pulses increased gene expression to as high as four-fold over injection alone, while long, low-voltage pulses increased in expression as high as 36-fold over injection alone.
Reporter expression peaked in a small range of pulse field strengths (100–200 V/cm), while increasing pulse length up to 200 ms increased transgene expression (Figure 2). These data agree with expression optimization studies performed in other tissues.17–20 A model based on previously collected data21 analyzes viability (V) as well as number of molecules taken up (N) after electroporation delivery. For multiple pulses, the model predicts that at field strengths of <1000 V/cm, the NV increases as pulse length increases. The data shown here tends to indicate a general increase in expression as pulse length increases, which is in agreement with this model. It is also interesting to note that expression levels could be manipulated by careful selection of pulsing parameters.
Electroporation parameters can also be applied in two distinct steps to include an electroporation pulse to permeabilize the membrane followed by an electrophoretic pulse to move the plasmid through the membrane.22–25 Previous studies have evaluated this approach for in vivo plasmid DNA delivery to muscle.26–27 In skin delivery, the electroporative–electrophoretic pulse combination significantly increased gene expression levels (P < 0.05) when compared to each component pulse (Figure 3). However, these expression levels can be obtained using a single pulse type (Figure 2). Therefore, plasmid delivery can be performed either by combining these two steps into a single pulse or administering two distinct pulses. While early studies have presented a strong case for the necessity of the electrophoretic component,26–28 a recent study has presented evidence to the contrary.29 The results presented in this study question the necessity of the electrophoretic pulse for electroporation gene transfer as significant increase in expression was obtained with short (μs) pulses. Although the mechanism of pulse combination may not be fully understood, the advantage of applying the pulses in two steps is that the amplitude of the electric pulses can be reduced.
Figure 3.
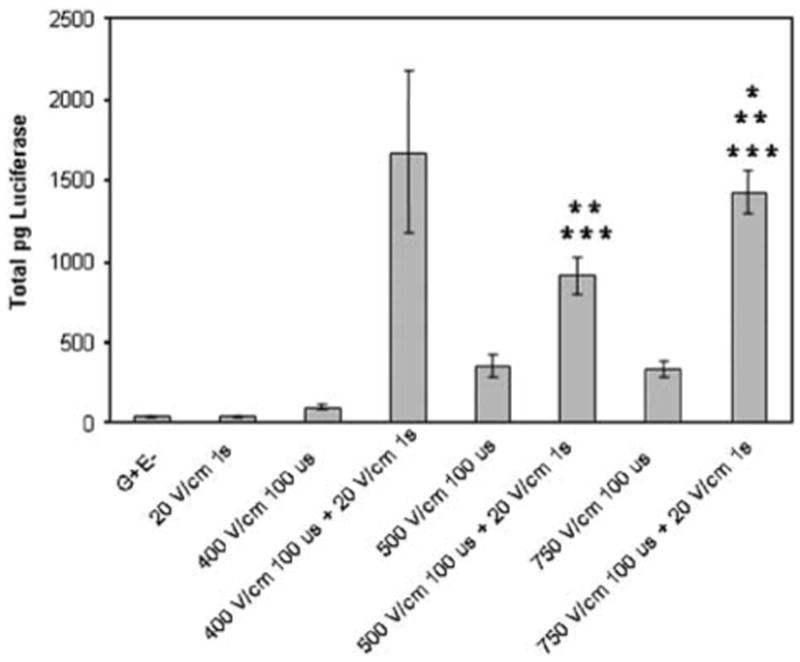
Combination of electroporation and electrophoresis. One hundred micrograms of pCMVLuc+ were delivered and luciferase assays were performed as described in Figure 2. Bars represent the mean of the means and standard error of the means for two replicate experiments. Each experiment contained four samples for each group. *P < 0.05 with respect to G+E-; **P < 0.05 with respect to electroporation pulse; ***P < 0.05 with respect to electrophoretic pulse.
Luciferase expression levels do not predict whether a small number of cells have been transfected with a large plasmid copy number or the reverse. In order to determine delivery efficiency, a plasmid encoding GFP was delivered to the skin using four pulsing conditions that generated high luciferase expression (Table 1). Cells (20–30%) were positive after delivery with pulses ranging from 100 to 200 V/cm at 20–150 ms. Therefore, although high luciferase expression was seen using eight 150 ms pulses at 100 V/cm, the total number of cells expressing the transgene was not significantly increased. These conditions may result in a larger plasmid copy number entering each electroporated cell or in better nuclear delivery. Finally, with field strength of 400 V/cm, the positive cell number decreased significantly.
Table 1. Transfected cell distribution in skin samples after plasmid delivery with electroporation conditions resulting in high and consistent luciferase expression.
| Pulsing conditions | Total pg per tissue | % GFP positive cells |
|---|---|---|
| Injection only | 93±17 | 0 |
| 100 V/cm 20 ms | 1485±496 | 19.75±12.74 |
| 100 V/cm 150 ms | 2558±581 | 32.06±4.55 |
| 200 V/cm 20 ms | 1379±526 | 32.25±11.16 |
| 400 V/cm 10 ms | 1057±194 | 8.5±4.57 |
pEGFP-N1 (Clontech, Palo Alto, CA, USA), encoding green fluorescent protein (GFP), was prepared using Endo-free Qiagen Megaprep kits (Qiagen, Valencia, CA, USA) and was injected intradermally followed by electroporation at the specified parameters (mice were anesthetized and treatment performed as described in Figure 2). At 48 hours after procedure, the entire treated area was removed and evaluated for GFP expression. Each tissue sample was evaluated (entire 6 mm diameter area, unsectioned) using a fluorescent microscope and the percentage of each sample that was fluorescent was determined visually. Results represent the mean and s.d. for two replicate experiments with n of 4 for each experiment.
For future clinical considerations, it is important to document any tissue damage that may be caused by the application of electric pulses. Tissue health following electric pulse application is an important criterion to evaluate delivery. The four electroporation conditions used in Table 1 were used to assess histological damage following removal of the 6 mm diameter treated area of skin. Multiple sections of each sample were examined following hematoxylin and eosin staining. Samples were graded for percent damage using a schema including surface damage (burning and or necrosis), inflammation and subepidermal necrosis.30 The total amount of damage (surface and subepidermal area affected) was determined for each sample and expressed as a percentage of the total treatment area. Although minimal levels of damage were observed under any pulsing conditions, the primary effect observed was surface burns or scars and the appearance of localized sub-epidermal necrosis (Figure 4). Low damage levels were observed with pulses ranging from 100 to 200 V/cm at 20–150 ms (Table 2). In this limited study, field strength appeared to be the most important variable in determining levels of cutaneous damage, since even relatively short pulses (10 ms) at the highest field strength (400 V/cm) tested resulted in detectable tissue damage. This does not agree with a previous study exploring the effect of pulse delivery on skin integrity in hairless rats.31 In that study, no significant inflammation or necrosis was observed histologically after electroporation with 10 pulses at a similar field strength (558 V/cm) and pulse length (5 ms). This may be due to the specific definition of damage, the difference in skin thickness in the animals compared or to the electrode used in the delivery.
Figure 4.
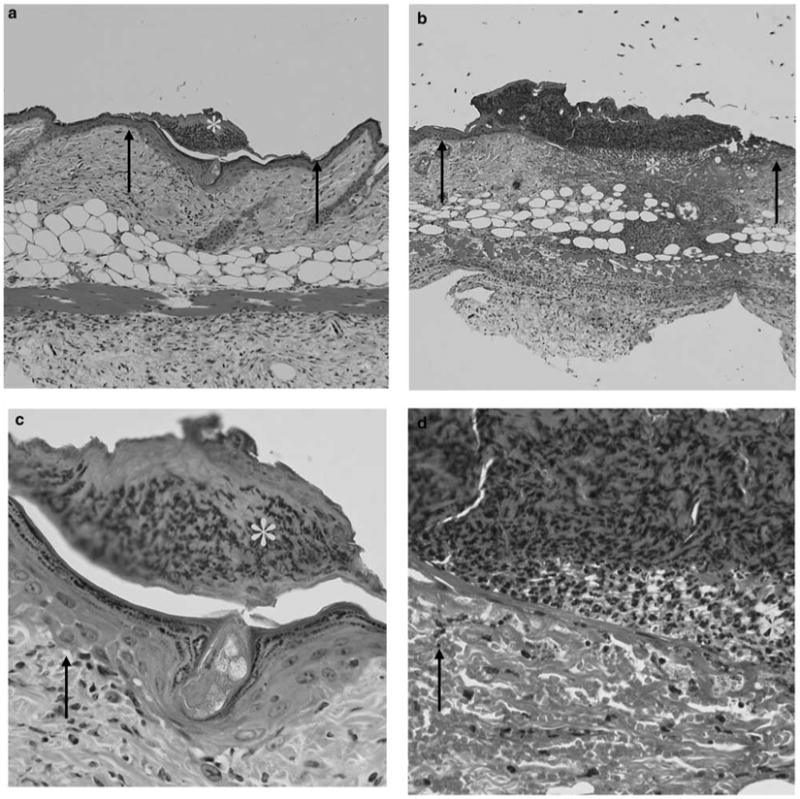
Tissue damage following electroporation. Photographs of representative sections from study described in Table 2 illustrate examples of surface damage and subepidermal necrosis. (a and c) An area with surface damage is shown. (a) × 100 magnification and (c) × 400 magnification. (b and d) An area containing subepidermal necrosis is shown. (b) × 100 magnification and (d) × 400 magnification. Arrows point to the areas of normal tissue and stars are placed in the areas of damage.
Table 2. Histological assessment of surface damage following electroporation using selected electroporation conditions.
| Treatment | n | Mean % ± s.d. |
|---|---|---|
| No treatment | 6 | 0.13±0.30 |
| pND2Lux injection only | 6 | 0.4±0.4 |
| pND2Lux plus eight 100 V/cm 20 ms pulses | 6 | 1.90±1.50 |
| pND2Lux plus eight 100 V/cm 150 ms pulses | 6 | 5.12±3.50 |
| pND2Lux plus eight 200 V/cm 20 ms pulses | 6 | 2.28±1.26 |
| pND2Lux plus eight 400 V/cm 10 ms pulses | 6 | 20.89±12.98 |
One hundred micrograms of pCMVLuc+ in 50 μl saline was injected intradermally, followed immediately by pulses as described in Figure 2. After 48 h, treated areas were removed and examined histologically. Skin samples were fixed over night in 10% formal saline, and then processed for routine histopathological examination. Briefly, specimens were dehydrated through a sequence of 50, 70, 95 and 100% ethanol, cleared in xylene and then embedded in paraffin wax. Sections were cut with a microtome (three sections per specimen) and stained with hematoxylin–eosin. From each sample, 3–5 sections were examined for damage.
Kinetics of expression and dose response
Since 150 ms pulses at field strength of 100 V/cm resulted in the highest and most consistent transgene expression with minimal tissue damage, these pulses were tested in additional experiments. Transgene expression levels were increased over injection alone up to 2 weeks after electrically mediated delivery. A significant increase in mean expression was observed at 48 h (1859±696 total pg luciferase), 7 days (1627±757) and 14 days (1515±700) when compared to injection alone (110±23, 123±42 and 409±179 total pg luciferase respectively, n=12). Expression levels decreased at day 17. Interestingly, after subcutaneous injection alone, luciferase expression levels tended to increase slowly over the time period tested. By day 17, expression in samples that received plasmid injection alone (796±401 total pg) was not significantly different from expression levels with electroporation (645±234 total pg). If a subtle onset of expression is desirable, for example, in a specific therapeutic situation, simple injection may be the optimal form of plasmid delivery.
In a plasmid dose response, electroporation increased expression significantly at doses greater than 50 μg (Figure 5). Luciferase expression increased linearly up to 200 μg plasmid injected, indicating that maximum expression levels were not reached.
Figure 5.
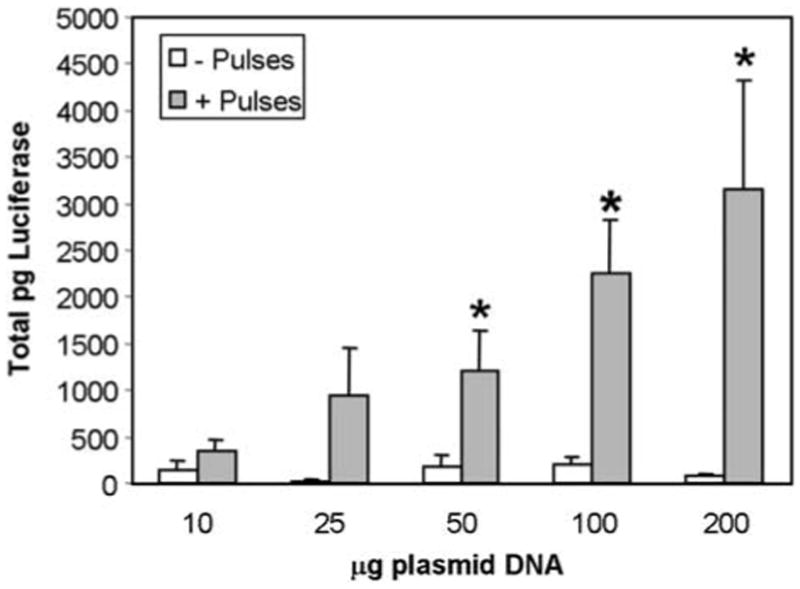
Plasmid dose response. pCMVLuc+ (P) was injected intradermally in 50 μl at increasing concentrations, pulses (E) applied, and luciferase assays performed as described in Figure 2. Data represent mean and standard error of three separate experiments each containing four samples for each time point (total of 12 samples/time point). *P < 0.05
Summary
The results presented here demonstrate that in vivo electroporation enhances intradermal delivery of plasmid DNA. An important consideration for optimal delivery is the configuration of the electrodes that are used to apply the electric fields. This electrode design offers the potential for easier and more rapid application of electrically mediated cutaneous plasmid delivery than the electrodes currently commercially available. Of the electroporation parameters tested with this electrode, 150 ms pulses with field strength of 100 V/cm produced the highest and most consistent transgene expression, a high efficiency of individual cell transformation, and minimal tissue damage. While these results are encouraging, the next step for this system will be an evaluation in an appropriate disease model and eventual translation into clinical trials.
Acknowledgments
This work was supported by research grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R21 DK55588) and the University of South Florida Center for Molecular Delivery. The pulse generator and electrodes used in this work were supplied by Inovio Biomedical Corporation, San Diego, CA.
References
- 1.Titomirov AV, Sukharev S, Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim Biophys Acta. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- 2.Drabick JJ, Glasspool-Malone J, Somiari S, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electroporation. Mol Ther. 2000;3:249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 3.Glasspool-Malone J, Somiara S, Drabick JJ, Malone RW. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2:140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- 4.Chesnoy S, Huang L. Enhanced cutaneous gene delivery following intradermal injection of naked DNA in a high ionic strength solution. Mol Ther. 2001;5:57–62. doi: 10.1006/mthe.2001.0511. [DOI] [PubMed] [Google Scholar]
- 5.Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Therapy. 2001;8:1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- 6.Dujardin N, Van Deà Smissen P, Préat V. Topical gene transfer into rat skin using electroporation. Pharm Res. 2001;18:61–66. doi: 10.1023/a:1011026726938. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Nolan E, Kreitschitz S, Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta. 2002;1572:1–9. doi: 10.1016/s0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 8.Heller R, Schultz J, Lucas ML, Jaroszeski MJ, Heller LC, Gilbert RA, et al. Intradermal delivery of interleukin-12 plasmid DNA by in vivo electroporation. DNA Cell Biol. 2001;20:21–26. doi: 10.1089/10445490150504666. [DOI] [PubMed] [Google Scholar]
- 9.Medi BM, Hoselton S, Marepalli RB, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I: efficacy. Int J Pharm. 2005;294:53–63. doi: 10.1016/j.ijpharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Pavselj N, Preat V. DNA electrotransfer into the skin using a combination of one high- and one low-voltage pulse. J Control Release. 2005;106:407–415. doi: 10.1016/j.jconrel.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Babiuk S, Baca-Estrada ME, Foldvari M, Baizer L, Stout R, Storms M, et al. Needle-free topical electroporation improves gene expression from plasmids administered in porcine skin. Mol Ther. 2003;8:992–998. doi: 10.1016/j.ymthe.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RA, Jaroszeski MG, Heller R. Novel electrode designs for electrochemotherapy. Biochim Biophys Acta. 1997;1334:9–14. doi: 10.1016/s0304-4165(96)00119-5. [DOI] [PubMed] [Google Scholar]
- 13.Sersa G, Cemazar M, Semrov D, Miklavcic D. Changing electrode orientation improves the efficacy of electrochemotherapy of solid tumors in mice. Bioelectrochem Bioenergetics. 1996;39:61–66. [Google Scholar]
- 14.Faurie C, Phez E, Golzio M, Vossen C, Lesbordes JC, Delteil C, et al. Effect of electric field vectoriality on electrically mediated delivery in mammalian cells. Biochim Biophys Acta. 2004;1665:92–100. doi: 10.1016/j.bbamem.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert R, Heller R, Jaroszeski MJ. Nonpenetrating electroporation device and method. #6,314,316 B1. 2001
- 16.Backman L, Shanbhag VP. Simplex optimization in biochemistry: application of the method in two-phase partition. Anal Biochem. 1984;138:372–379. doi: 10.1016/0003-2697(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 17.Mir LM, Bureau MF, Gehl J, Rangara R, Rouy D, Caillaud JM, et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc Natl Acad Sci USA. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas M, Heller R. Immunomodulation by electrically enhanced delivery of plasmid DNA encoding IL-12 to murine skeletal muscle. Mol Ther. 2001;3:47–53. doi: 10.1006/mthe.2000.0233. [DOI] [PubMed] [Google Scholar]
- 19.Heller R. Delivery of plasmid DNA using in vivo electroporation. Preclinica. 2003;1:198–208. [Google Scholar]
- 20.Andre F, Mir LM. DNA electrotransfer: its principles and an updated review of its therapeutic applications. Gene Therapy. 2004;11(Suppl 1):S33–S42. doi: 10.1038/sj.gt.3302367. [DOI] [PubMed] [Google Scholar]
- 21.Canatella PJ, Prausnitz MR. Prediction and optimization of gene transfection and drug delivery by electroporation. Gene Therapy. 2001;8:1464–1469. doi: 10.1038/sj.gt.3301547. [DOI] [PubMed] [Google Scholar]
- 22.Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenergetics. 1999;48:3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 23.Neumann E, Kakorin S, Tsoneva I, Nikolova B, Tomov T. Calcium-mediated DNA adsorption to yeast cells and kinetics of cell transformation by electroporation. Biophys J. 1996;71:868–877. doi: 10.1016/S0006-3495(96)79288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenchin VA, Sukharev SI, Serov SM, Chernomordik LV, Chizmadzhev YA. Electrically induced DNA uptake by cells is a fast process involving DNA electrophoresis. Biophys J. 1991;60:804–811. doi: 10.1016/S0006-3495(91)82115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev YA. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electro-pores. Biophys J. 1992;63:1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bureau MF, Gehl J, Deleuze V, Mir LM, Scherman D. Importance of association between permeabilization and electrophoretic forces for intramuscular DNA electrotransfer. Biochim Biophys Acta. 2000;1474:353–359. doi: 10.1016/s0304-4165(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 27.Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, et al. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- 28.Satkauskas S, Andre F, Bureau MF, Scherman D, Miklavcic D, Mir LM. Electrophoretic component of electric pulses determines the efficacy of in vivo DNA electrotransfer. Hum Gene Ther. 2005;16:1194–1201. doi: 10.1089/hum.2005.16.1194. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, Heston S, Shollenberger LM, Sun B, Mickle M, Lovell M, et al. Mechanism of in vivo DNA transport into cells by electroporation: electrophoresis across the plasma membrane may not be involved. J Gene Med. 2006;8:353–361. doi: 10.1002/jgm.851. [DOI] [PubMed] [Google Scholar]
- 30.Heller R, Coppola D, Pottinger C, Gilbert R, Jaroszeski MJ. Effect of electrochemotherapy on muscle and skin. Technol Cancer Res Treat. 2002;1:385–391. doi: 10.1177/153303460200100509. [DOI] [PubMed] [Google Scholar]
- 31.Dujardin N, Staes E, Kalia Y, Clarys P, Guy R, Preat V. In vivo assessment of skin electroporation using square wave pulses. J Control Release. 2002;79:219–227. doi: 10.1016/s0168-3659(01)00548-x. [DOI] [PubMed] [Google Scholar]
- 32.Brasier AR. Reporter system using firefly luciferase. In: Ausubel FM, et al., editors. Short Protocols in Molecular Biology. John Wiley and Sons; New York: 1992. pp. 9-21–9-23. [Google Scholar]


