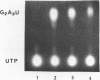
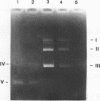
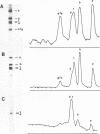
Abstract
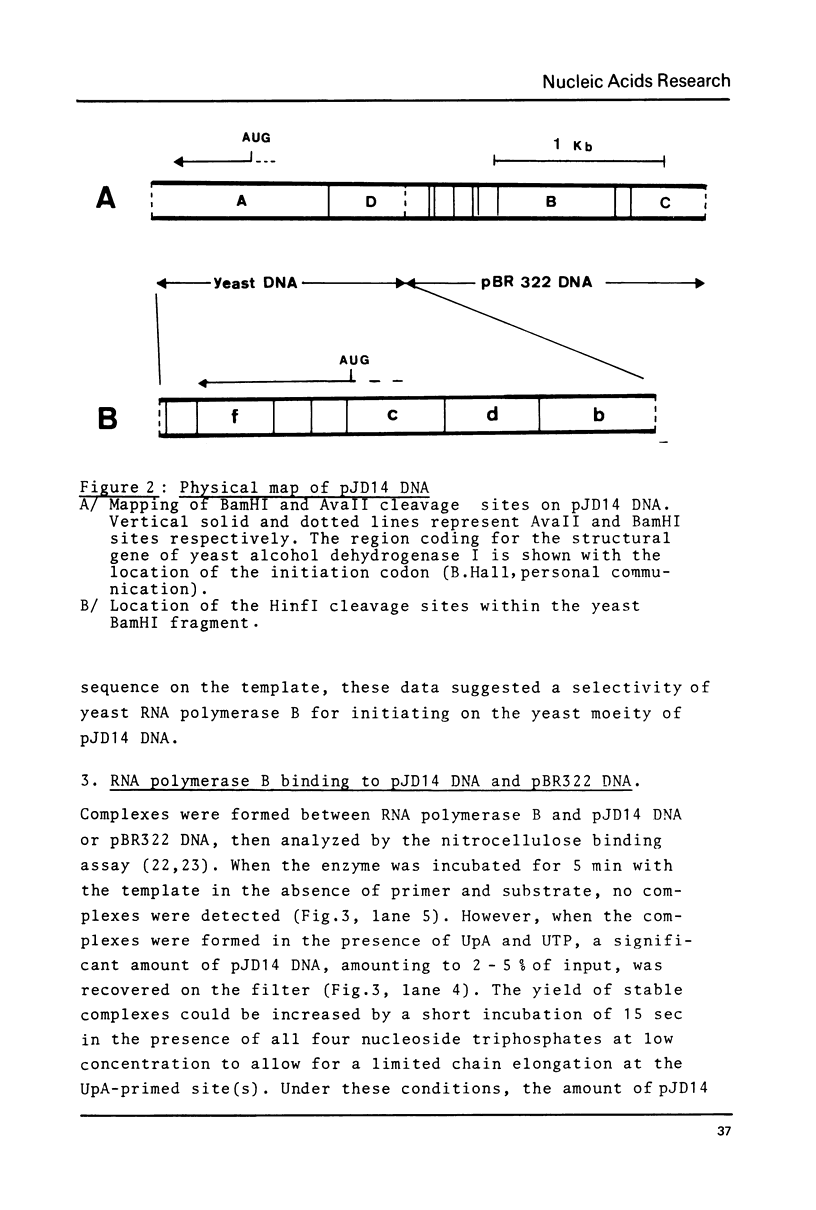
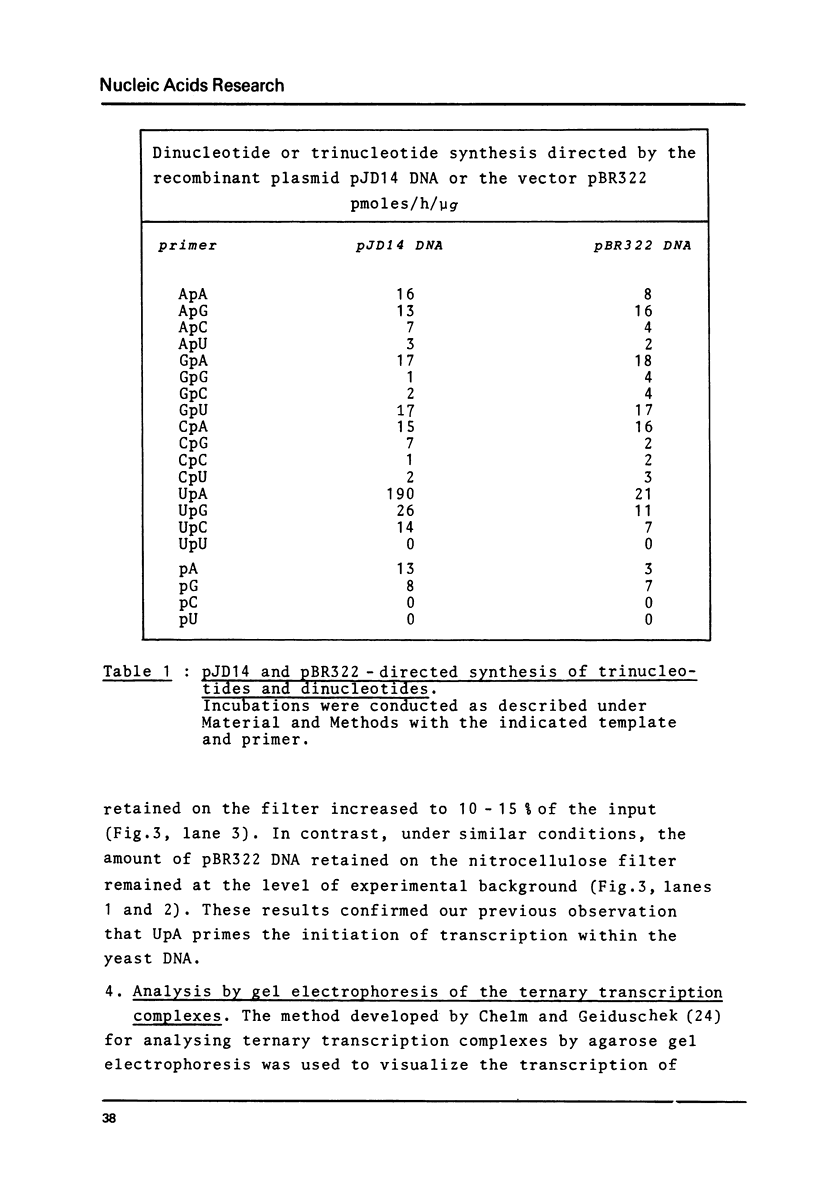
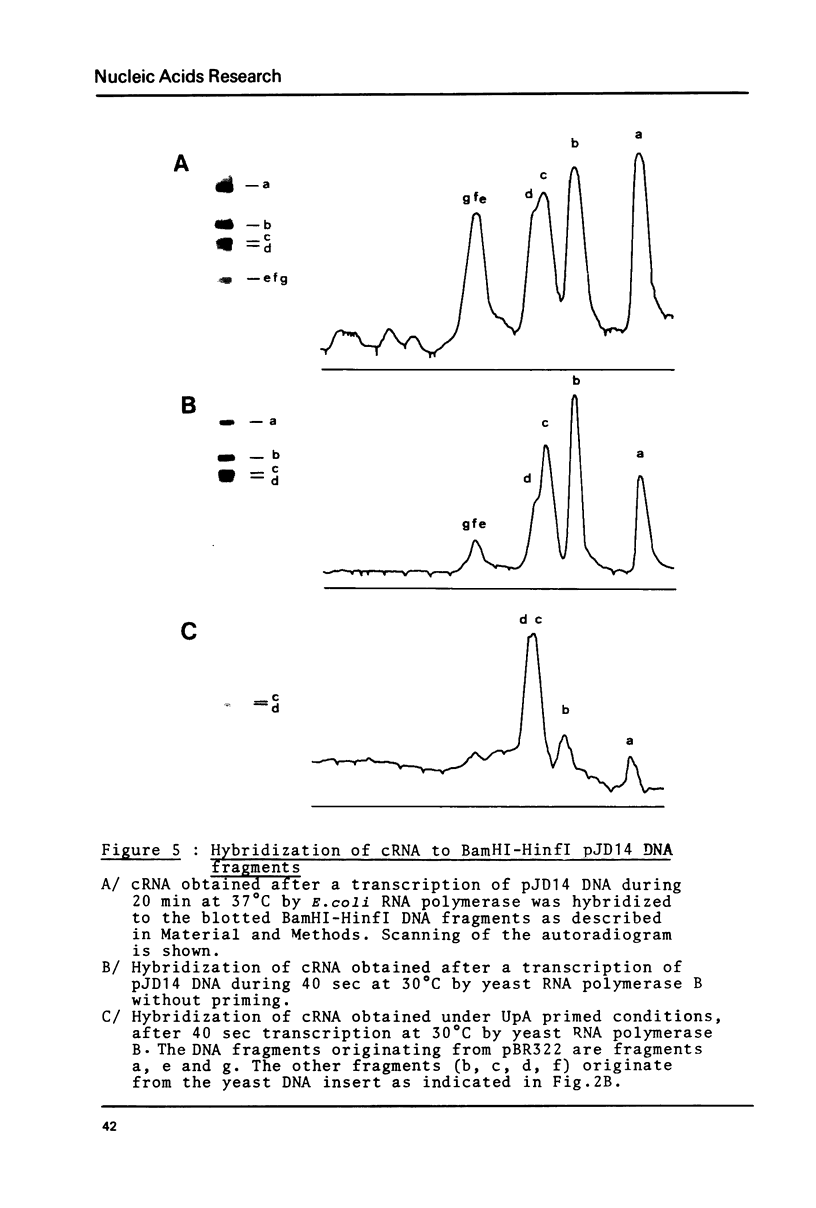
Yeast RNA polymerase B catalyzes an efficient abortive initiation on double-stranded DNA templates using the appropriate combination of primer and substrate. The specificity of initiation was investigated using a recombinant plasmid (pJD14 DNA) containing the structural gene for yeast alcohol dehydrogenase I (ADHI). The combination of the dinucleotide UpA and UTP was 10 fold more efficient with pJD14 DNA than with the vector pBR322 DNA to direct the synthesis of the trinucleotide UpApU. Under these conditions, stable enzyme-DNA complexes were formed and could be retained on nitrocellulose filters. Using the UpA-primed system and a short pulse of RNA synthesis, transcription complexes were located on the yeast part of pJD14 DNA as evidenced by agarose gel electrophoresis. Southern hybridization of the pulsed RNA was restricted to a region, within the yeast DNA fragment, upstream to the initial region of the ADHI gene.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkenmeier E. H., Brown D. D., Jordan E. A nuclear extract of Xenopus laevis oocytes that accurately transcribes 5S RNA genes. Cell. 1978 Nov;15(3):1077–1086. doi: 10.1016/0092-8674(78)90291-x. [DOI] [PubMed] [Google Scholar]
- Chelm B. K., Geiduschek E. P. Gel electrophoretic separation of transcription complexes: an assay for RNA polymerase selectivity and a method for promoter mapping. Nucleic Acids Res. 1979 Dec 11;7(7):1851–1867. doi: 10.1093/nar/7.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée S., Sentenac A., Fromageot P. Role of deoxyribonucleic acid-ribonucleic acid hybrids in eukaryotes. Study of the template requirements of yeast ribonucleic acid polymerases and nature of the ribonucleic acid product. J Biol Chem. 1974 Sep 25;249(18):5971–5977. [PubMed] [Google Scholar]
- Dezélée S., Sentenac A. Role of DNA-RNA hybrids in eukaryotes. Purification and properties of yeast RNA polymerase B. Eur J Biochem. 1973 Apr 2;34(1):41–52. doi: 10.1111/j.1432-1033.1973.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Dezélée S., Wyers F., Sentenac A., Fromageot P. Two forms of RNA polymerase B in yeast. Proteolytic conversion in vitro of enzyme BI into BII. Eur J Biochem. 1976 Jun 1;65(2):543–552. doi: 10.1111/j.1432-1033.1976.tb10372.x. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. P., Holland M. J. The primary structure of a glyceraldehyde-3-phosphate dehydrogenase gene from Saccharomyces cerevisiae. J Biol Chem. 1979 Oct 10;254(19):9839–9845. [PubMed] [Google Scholar]
- Huet J., Dezélée S., Iborra F., Buhler J. M., Sentenac A., Fromageot P. Further characterization of yeast RNA polymerases. Effect of subunits removal. Biochimie. 1976;58(1-2):71–80. doi: 10.1016/s0300-9084(76)80357-4. [DOI] [PubMed] [Google Scholar]
- Humphries P., McConnell D. J., Gordon R. L. A procedure for the rapid purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. Biochem J. 1973 May;133(1):201–203. doi: 10.1042/bj1330201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel D. A., Tilghman S. M., Leder P. The sequence of the chromosomal mouse beta-globin major gene: homologies in capping, splicing and poly(A) sites. Cell. 1978 Dec;15(4):1125–1132. doi: 10.1016/0092-8674(78)90040-5. [DOI] [PubMed] [Google Scholar]
- Lescure B., Dauguet C., Yaniv M. Transcription of polyoma virus DNA in vitro. III. Localization of calf thymus RNA polymerase II binding sites. J Mol Biol. 1978 Sep 5;124(1):87–96. doi: 10.1016/0022-2836(78)90149-3. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. Analysis of the RNAs synthesized on Simian virus 40 superhelical DNA by mammalian RNA polymerases AI and B. Eur J Biochem. 1974 Jan 16;41(2):379–395. doi: 10.1111/j.1432-1033.1974.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. Animal DNA-dependent RNA polymerases. Analysis of the RNAs synthesized on Simian virus 40 superhelical DNA by mammalian RNA polymerases AI and B. Eur J Biochem. 1974 Jan 16;41(2):379–395. doi: 10.1111/j.1432-1033.1974.tb03280.x. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Cech C. L. On the mechanism of rifampicin inhibition of RNA synthesis. J Biol Chem. 1978 Dec 25;253(24):8949–8956. [PubMed] [Google Scholar]
- Meilhac M., Chambon P. Animal DNA-dependent RNA polymerases. Initiation sites on calf-thymus DNA. Eur J Biochem. 1973 Jun 15;35(3):454–463. doi: 10.1111/j.1432-1033.1973.tb02859.x. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Sentenac A., Fromageot P. Selective blotting of restriction DNA fragments on nitrocellulose membranes at low salt concentrations. Nucleic Acids Res. 1980 Jun 11;8(11):2453–2460. doi: 10.1093/nar/8.11.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. Y., Parker C. S., Roeder R. G. Transcription of cloned Xenopus 5S RNA genes by X. laevis RNA polymerase III in reconstituted systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):136–140. doi: 10.1073/pnas.76.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oen H., Wu C. W., Haas R., Cole P. E. T7 deoxyribonucleic acid directed, rapid-turnover, single-step addition reactions catalyzed by Escherichia coli ribonucleic acid polymerase. Biochemistry. 1979 Sep 18;18(19):4148–4155. doi: 10.1021/bi00586a015. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Jaehning J. A., Roeder R. G. Faithful gene transcription by eukaryotic RNA polymerases in reconstructed systems. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):577–587. doi: 10.1101/sqb.1978.042.01.060. [DOI] [PubMed] [Google Scholar]
- Schmidt O., Mao J. I., Silverman S., Hovemann B., Söll D. Specific transcription of eukaryotic tRNA genes in Xenopus germinal vesicle extracts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4819–4823. doi: 10.1073/pnas.75.10.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Smith M., Leung D. W., Gillam S., Astell C. R., Montgomery D. L., Hall B. D. Sequence of the gene for iso-1-cytochrome c in Saccharomyces cerevisiae. Cell. 1979 Apr;16(4):753–761. doi: 10.1016/0092-8674(79)90091-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Kédinger C., Corden J., Brison O., Chambon P. Specific in vitro initiation of transcription on conalbumin and ovalbumin genes and comparison with adenovirus-2 early and late genes. Nature. 1980 Jun 5;285(5764):367–373. doi: 10.1038/285367a0. [DOI] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Williamson V. M., Bennetzen J., Young E. T., Nasmyth K., Hall B. D. Isolation of the structural gene for alcohol dehydrogenase by genetic complementation in yeast. Nature. 1980 Jan 10;283(5743):214–216. doi: 10.1038/283214a0. [DOI] [PubMed] [Google Scholar]
- Wu G. J. Adenovirus DNA-directed transcription of 5.5S RNA in vitro. Proc Natl Acad Sci U S A. 1978 May;75(5):2175–2179. doi: 10.1073/pnas.75.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]