Abstract
Objective
To identify the frequency of pregnancy and neonatal complications in pregnancies carrying fetuses affected with trichothiodystrophy (TTD).
Methods
We identified pregnancy and neonatal complications and serum screening results from mothers of TTD patients in a DNA repair diseases study from 2001 to 2011.
Results
Pregnancy reports of 27 TTD patients and their 23 mothers were evaluated and81% of the pregnancies had complications: 56% had preterm delivery, 30% had preeclampsia, 19% had placental abnormalities, 11% had HELLP syndrome, 4% had an emergency c-section for fetal distress; while44% had two or more complications. Only19% of the pregnancies delivered at term without complications. Eight of the 10 pregnancies tested had abnormal multiple marker results including elevated levels of human chorionic gonadotropin. Eighty-five percent of the neonates had complications: 70 % were low birth weight (<2500g), 35% had birth weight <10 centile for gestational age, 70% had NICU admission, 67% had a collodion membrane, and 31% of the 16 males had cryptorchidism. Cataracts were present in 54% of the TTD patients examined.
Conclusion
TTD is a multisystem disease that predisposes mothers of affected patients to substantial risks for pregnancy complications and TTD neonates have a high incidence of multiple abnormalities.
Keywords: Trichothiodystrophy, Pregnancy, Maternal Serum Screening, hCG, Preeclampsia, HELLP syndrome
INTRODUCTION
Patients with the rare recessive disorder, trichothiodystrophy (TTD) (frequency about 1 per million(Kleijer, Laugel, Berneburg, et al. 2008)) have sulfur-deficient brittle hair and developmental abnormalities in association with defects in genes involved in DNA repair and transcription (Itin, Sarasin, & Pittelkow 2001; Lehmann 2003; Kraemer, Patronas, Schiffmann, et al. 2007; Stefanini, Botta, Lanzafame, et al. 2010). At birth, neonates with TTD commonly present with abnormalities such as erythroderma and a collodion membrane of the skin that peels away within the first 1–2 weeks of life(Figure 1A). The hair of TTD patients has shaft abnormalities and diagnostic “tiger tail” banding with polarized microscopy (Figure 1B–D) (Liang, Kraemer, Morris, et al. 2005; Liang, Morris, Schlucker, et al. 2006; Faghri, Tamura, Kraemer, et al. 2008; Zhou, Khan, Tamura, et al. 2010). TTD affected pregnancies frequently exhibit complications (Faghri, Tamura, Kraemer, et al. 2008; Moslehi, Signore, Tamura, et al. 2010; Zhou, Khan, Tamura, et al. 2010).
Figure 1.
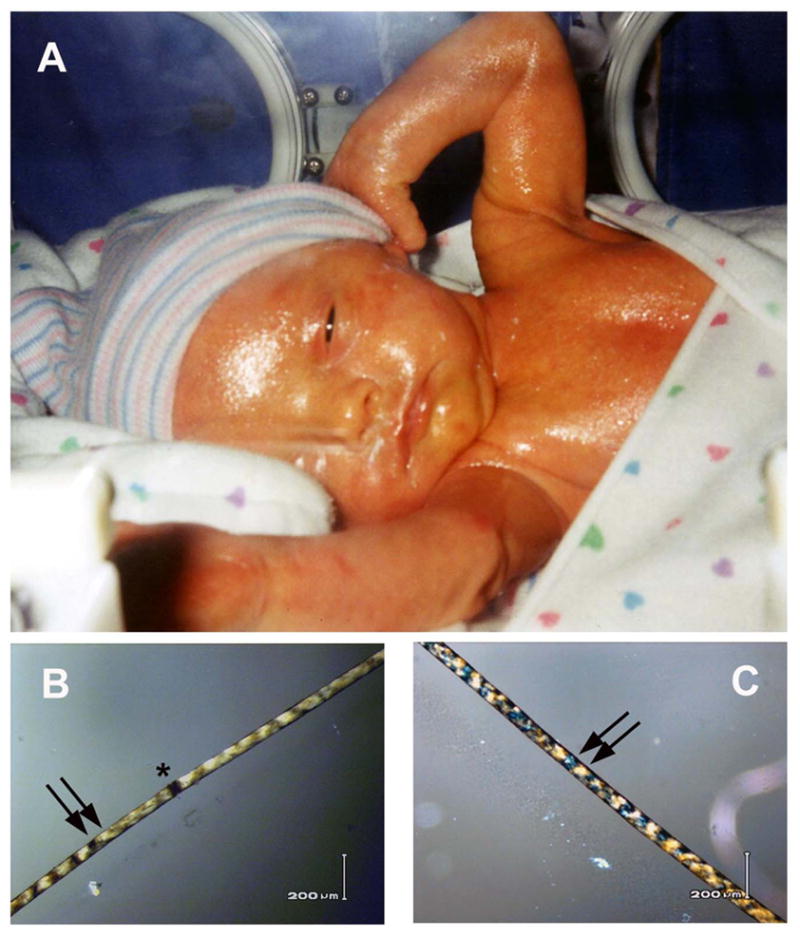
Neonate with TTD. A. Patient TTD353BE in family M at birth had a glistening collodion membrane covering her skin that persisted for several days. B–C. Her scalp hair viewed with polarized microscopy shows alternating dark and light “tiger tail” banding (arrows) and broken hair shaft (trichoschisis) (*).
As part of a natural history/genetic epidemiology study of DNA repair diseases(Moslehi, Signore, Tamura, et al. 2010; Bradford, Goldstein, Tamura, et al. 2011)mothers of patients with TTD(Kraemer, Patronas, Schiffmann, et al. 2007) were surveyed about their pregnancies. The mothers of TTD patients had more complications with their affected pregnancies compared to their pregnancies resulting in unaffected children. The affected pregnancies frequently had preeclampsia, decreased fetal movement, and HELLP (hemolysis elevated liver enzymes and low platelets) syndrome. Some of the mothers also reported having abnormal prenatal serum screening results only with the affected pregnancies. The TTD neonates were significantly more often low birth weight, small for gestational age and required NICU admission compared with pregnancies of the same mothers resulting in unaffected live births. We have now increased the number of TTD pregnancies reported (from 17to 27), the number of families evaluated (from 13 to 23), and present new data on the high frequency of multiple complications in each pregnancy and among the TTD neonates.
MATERIALS AND METHODS
TTD patients and their families were recruited to the NIH as part of a natural history/genetic epidemiology study of DNA repair diseases(Liang, Kraemer, Morris, et al. 2005; Liang, Morris, Schlucker, et al. 2006; Moslehi, Signore, Tamura, et al. 2010; Zhou, Khan, Tamura, et al. 2010; Bradford, Goldstein, Tamura, et al. 2011). We evaluated hair samples from all patients for the presence of the TTD diagnostic “tiger tail” banding and hair shaft abnormalities as described previously (Liang, Kraemer, Morris, et al. 2005; Liang, Morris, Schlucker, et al. 2006; Zhou, Khan, Tamura, et al. 2010). We interviewed all of the mothers during their visit to NIH and by telephone as described previously (Moslehi, Signore, Tamura, et al. 2010). We examined the medical records on all 27 patients. We were able to obtain the prenatal, delivery and neonatal records on 15 mothers and patients, and reviewed pregnancy and infancy related information from other medical sources including pediatric and medical genetics charts on an additional 12 of the patients. We examined these medical records for the presence of pregnancy abnormalities, neonatal complications, laboratory abnormalities, and dermatologic findings. Small for gestational age (SGA) was defined as <10 centile according to Olsen et al (Olsen, Groveman, Lawson, et al. 2010). Normal references values for preeclampsia, HELLP syndrome, preterm delivery, and low birth weight were from (Moslehi, Signore, Tamura, et al. 2010)and citations therein. Only patients with tiger tail banding in the hair samples and meeting the clinical criteria for TTD (Faghri, Tamura, Kraemer, et al. 2008)were included in this study(except for patient TTD426BE who had atypical TTD with rudimentary hair (Table 1)). In this paper we include 17 of the previously-reported TTD patients(Moslehi, Signore, Tamura, et al. 2010)plus 10 additional TTD patients and present new data on the high frequency of multiple complications in each pregnancy and among the TTD neonates. In contrast, the earlier paper(Moslehi, Signore, Tamura, et al. 2010) included patients with TTD and associated disorders(the rare overlap syndromes of XP/TTD (4 patients in one family) and COFS/TTD (1 patient))but the numbers were too small to distinguish between the pregnancy risks associated with each of the different disorders. We also excluded a set of twins in one family who died prior to hair documentation of TTD diagnosis.
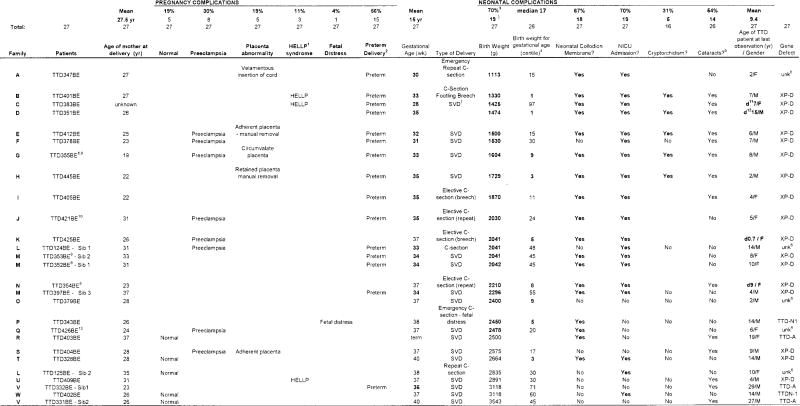
Table 1.
Pregnancy and neonatal complications of 27 trichothiodystrophy patients
|
Hemolysis elevated liver enzymes and low platelets syndrome
Preterm delivery <37 weeks
Low birth weight <2500g
Olsen et al Pediatrics 125: e214–e224 (2010)
modified from Brooks et al (2011)
Unknown - not XPB, XPD, TTDA or TTDN1
Spontaneous vaginal delivery
Liang et al J Invest Dermatol 126: 2210–2216 (2006)
Boyle et al Human Mutation 29: 1194–1208 (2008)
Zhou et al J American Acad Dermatol 63: 323–8 (2010)
Age at death
Death due to infection with respiratory failure following hip surgery
Atypical TTD with absence of hair
This study was approved by the National Cancer Institute IRB(99C-0099) and informed consent was obtained from all participants. The living TTD patients and their mothers were evaluated at the National Institutes of Health Clinical Center over a period of 3–5 days(Zhou, Khan, Tamura, et al. 2010). Medical evaluations of the TTD patients included dermatology, immunology, neurology, ophthalmology, gastroenterology and rehabilitation medicine and gynecologic consultations as appropriate. Imaging studies included brain MRI, skeletal series and bone age x-rays, or dexa scans as clinically indicated. The patients also received extensive laboratory examinations including complete blood count, chemistry, liver and lipid panels, vitamin D and iron levels, parathyroid and thyroid function tests and assessments of immunologic status. One patient (TTD425BE) (Table 1) died prior to coming to the NIH for formal evaluation; however patient hair, extensive medical records (including an autopsy report and tissue specimens obtained at autopsy) and patient photographs were available for review.
Fibroblast and/or lymphoblastoid cell cultures were established from TTD patients and, when possible, from their parents, and assayed for DNA repair complementation groups by use of the plasmid host cell reactivation as say or by DNA sequencing as previously described(Boyle, Ueda, Oh, et al. 2008; Emmert, Ueda, Zumsteg, et al. 2009).
RESULTS
Twenty-seven TTD patients and their 23 mothers were evaluated at the NIH from 2001 to March 2011 (Table 1). Twenty mothers each had one TTD affected pregnancy. Two mothers (families L and V) had 2 TTD affected children (one pregnancy of mother L had discordant twins with one affected with TTD (TTD124BE) and the other unaffected) and all 3 pregnancies of one mother (family M) resulted in TTD affected children. The age of the mothers at time of delivery ranged from 19 to 37 years with a mean of 27.5 years. All mothers in the study were healthy at the inception of the pregnancies and none of the mothers had a history of hypertension, diabetes or autoimmune disease.
PREGNANCY COMPLICATIONS
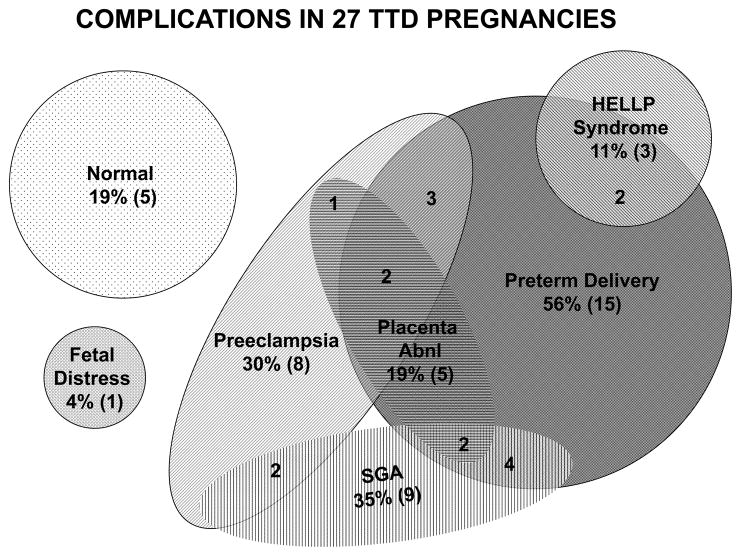
Twenty-two (81%)of the 27 pregnancies were reported to have complications: 15(56%) had preterm delivery (<37 weeks gestation) and 8 (30%) had preeclampsia. These are substantially greater than the normal reference values of 10.9% for preterm delivery and 6.5% for preeclampsia. Placental abnormalities were reported at birth in5 (19%): 3 had an adherent placenta requiring manual removal, 1 had a circumvalate placenta and 1 had velamentous insertion of the umbilical cord. There were 2(8%) pregnancies with documented intrauterine growth restriction (IUGR):TTD347BEhad IUGR with growth at the 20th percentile but the head circumference was significantly smaller than the other parameters and TTD379BE had a single live intrauterine pregnancy average fetal age - 33 weeks +1 day -mild IUGR. Three mothers (11%) developed HELLP syndrome[normal reference 0.35%]: the mother of patient TTD401BE had an uneventful pregnancy until 33 weeks when she developed vaginal bleeding and HELLP syndrome, the mother of TTD383BE had no prenatal care and presented at 28 weeks with preterm labor and HELLP syndrome; the mother of patient TTD409BE presented at term in labor with HELLP syndrome, however she had been experiencing intermittent epigastric pain since 37 weeks gestation. The mother of patient TTD342BE had an emergency c-section for non-reassuring fetal heart rate tracing at 38 weeks gestation. Only5 (19%) of the pregnancies were delivered at term without complications. Twelve (44%) of the pregnancies had 2 or more complications (Table 1 and Figure 2).
Figure 2.
Complications in 27 TTD pregnancies. The shaded shapes represent different complications. The numbers in parentheses (n) indicate the total number of pregnancies with the complication. The isolated numbers within two or more shapes indicate the number of pregnancies with the indicated multiple complications.
Eight of 10TTD affected pregnancies tested had elevated levels of human chorionic gonadotropin (hCG)(>2.50 Multiple of Median(MOM)or greater than the 95th percentile); 3of these pregnancies also had elevated levels of alpha fetoprotein (AFP)(>2.50 MOM or greater than the 95th percentile) and 2 pregnancies also had low levels of unconjugated estriol (<0.30 MOM or less than the 5th percentile). (Table 2). Nine of the 10 screened pregnancies had complications. All 3 TTD affected pregnancies in family M had elevated hCG and preterm delivery at 34 weeks gestation; the newborns had erythroderma/collodion presentation and birth weight about 50 %-ile for gestational age (Table 1). One pregnancy with normal maternal serum values (TTD354BE) had a low birth weight (2210g) infant delivered at 37 weeks gestation with birth weight 8 centile for gestational age(Table 1). The other pregnancy with normal maternal serum values (TTD402BE) had preterm labor at 32 weeks but the baby was delivered at 37 weeks, had a normal birth weight and was not reported to have a collodion membrane or neonatal cataracts. However, he was hypotonic at birth, developed hypoglycemia, apnea, bradycardia and feeding difficulties and was transferred to the NICU.
Table 2.
Maternal screening values of 10 trichothiodystrophy pregnancies
| Family | Patients | Human Chorionic gonadotropin <2.50 MoM1 | alpha- Fetoprotein <2.50 MoM1 | Unconjugated Estriol >0.30 MoM1 |
|---|---|---|---|---|
| K | TTD425BE | 6.27 | 2 | 0.08 |
| U | TTD409BE | 5.82 | 0.95 | 0.99 |
| M | TTD352BE - Sib 1 | 5.5 | 2.1 | 0.9 |
| O | TTD379BE | 4.78 | 1.78 | 0.47 |
| G | TTD355BE | 4.15 | 4.85 | 0.28 |
| J | TTD421BE | 3.32 | 2.54 | 0.99 |
| M | TTD397BE - Sib 3 | 3.28 | 2.8 | 0.95 |
| M | TTD353BE - Sib 2 | 2.7 | 1.3 | 0.8 |
| N | TTD354BE | 1.56 | 0.59 | 0.7 |
| W | TTD402BE | 0.88 | 0.84 | 1.16 |
Normal values multiple of the median
NEONATAL COMPLICATIONS
There were a total of 27 TTD patients in the study: 16 (59%) males and 11 (41%) females (Table 1). Their birth weights ranged from 1113 to 3543 g (median weight 2180g) with19 (70%) of the patients having low birth weight (<2500 grams)[normal reference 6.9%]. The birth weight of 9(35%) of the 26 patients with known gestational age had weight <10centile for gestational age. Three (12%) were <3centile. The median weight for gestational age was 17centileand 15 (58%) were < 25centile. Nineteen (70%) of the infants were admitted to the NICU and 7 (26%) of these infants had stays of greater than 1 month. Erythroderma/collodion presentation of the skin was noted in 18 (67%) infants (Figure 1A). It typically persisted for 1 to 2 weeks; the patients were treated with humidified air and emollients. Cryptorchidism was reported in 5 (31%) of the male infants. Other birth anomalies noted were: cardiac defects [ventricular septal defect (TTD425BE) and patent ductus arteriosus (TTD332BE)], abnormal placement of the ureters (TTD355BE)(Liang, Morris, Schlucker, et al. 2006; Boyle, Ueda, Oh, et al. 2008), and cavernous hemangioma of the leg (TTD421BE)(Zhou, Khan, Tamura, et al. 2010). Multiple complications were frequent in these infants: for example 14 had low birth weight, erythroderma/collodion membrane and NICU admission. Only 3 TTD neonates had none of these complications. Cataracts were reported in 14 (54%) of the TTD patients when examined at NIH at age 2 to 29 (mean 9.4years) and many were not visually significant (Brooks et al manuscriptin press).
Four (15%)of the 27 TTD children in this study died(TTD425BE at ages 0.7 yr, TTD383BE at 7 yr, TTD354BE(Liang, Morris, Schlucker, et al. 2006)at 9 yr and TTD351BE at 15 yr)(Table 1), all as a result of infections, underscoring the seriousness of this condition.
GENETIC ABNORMALITIES
DNA repair complementation groups were determined to beXP-D (15 patients)and TTD-A(3 patients), and 2patientshad abnormalities in TTDN1 a gene with unknown function. In5 patients abnormalities were not found in any of these genes (Table 1). Seven of the 8 screened pregnancies in XP-D complementation group demonstrated abnormal maternal serum screening values(Table 2). One pregnancy with a TTDN1 mutation (TTD402BE)had normal maternal serum results.
In two sibs (TTD124BE -Sib 1TTD125BE -Sib 2) mutations were not found in any of the known TTD genes. TTD124BEhad an unaffected fraternal twin. His pregnancy was complicated by preeclampsia and his delivery was by C-section at 33 weeks gestation. He and his twin were admitted to the NICU; he was not reported to have a collodion membrane but was noted to have unusual hair. His affected younger sister (TTD125BE) was delivered at 38 weeks by repeat C-section; she was also noted to have unusual hair; she developed feeding difficulties and was admitted to the NICU. Three patients from two families(TTD331BE–Sib1, TTD331BESib -2, and TTD403BE) had mutations in the TTDA gene, a subunit of TFIIH. There were no reported complications in either of the siblings’ pregnancies and neither sibling was low birth weight, had a collodion membrane or required special care following delivery. However, the older sibling was found to have a patent ductus arteriosus at one month of age and had surgical repair at 1 year of age. The third TTDA patient delivered at term following an uncomplicated pregnancy; however she did have a collodion membrane and had neonatal cataracts.
DISCUSSION
Pregnancy complications were described in 28% of TTD patients in a literature review (Faghri, Tamura, Kraemer, et al. 2008). These TTD-associated pregnancy complications may be under-reported (Itin, Sarasin, & Pittelkow 2001; Stefanini, Botta, Lanzafame, et al. 2010)as evidenced by a recent genetic epidemiologic study of gestational complications in TTD families enrolled in the NIH natural history study(Moslehi, Signore, Tamura, et al. 2010). In the current report 81% of the TTD affected pregnancies had abnormalities and 44%had two or more abnormalities(Table 1 and Figure 2).
A higher incidence of pregnancy complications has been observed in mothers carrying fetuses with several other rare inherited diseases (Witters, Legius, Devriendt, et al. 2001; Witters, Moerman, Van, et al. 2002). Preeclampsia, HELLP syndrome and acute fatty liver of pregnancy have been reported in pregnancies where the infants are subsequently diagnosed with disorders of fatty acid oxidation (Ibdah, Bennett, Rinaldo, et al. 1999; Preece and Green 2002; Shekhawat, Matern, & Strauss 2005). Preeclampsia has been reported in pregnancies with fetuses having argininosuccinic aciduria (Donn and Thoene 1985). Some of the TTD patients were diagnosed before prenatal screening or more sensitive maternal follow-up were commonly used. One TTD case report in the literature noted an elevated maternal serum AFP during the pregnancy with a positive maternal antiphospholipid antibody but no preeclampsia (Petrin, Meckler, & Sybert 1998). The infant had severe fetal growth restriction, failure to thrive, angioendothelioma of the liver and died of sepsis at 6 months of age. Since the advent of generalized prenatal screening, several studies have suggested an increased incidence of pregnancy complications where levels of hCG and/or AFP are elevated in the absence of an identifiable fetal anomaly. These complications include pregnancy-induced hypertension, preterm labor, IUGR and fetal death(Milunsky and Nebiolo 1996; Yaron, Cherry, Kramer, et al. 1999; Krause, Christens, Wohlfahrt, et al. 2001; Lepage, Chitayat, Kingdom, et al. 2003; Driscoll 2004; Audibert, Benchimol, Benattar, et al. 2005; Alkazaleh, Chaddha, Viero, et al. 2006; Kang, Farina, Park, et al. 2008). Preeclampsia and abnormal maternal screening has also been reported in pregnancies with fetuses that have triploidy and Beckwith-Wiedemann syndrome (Huang, Alberman, Wald, et al. 2005; Aagaard-Tillery, Buchbinder, Boente, et al. 2007). In the current study 8 of 10 TTD affected pregnancies tested had elevated hCG (Table 2). This suggests that in a small percentage of pregnancies, non chromosomal fetal disease/defects may be a strong contributing factor to the screening abnormality and development of pregnancy complications.
TTD patients have a broad spectrum of abnormalities in infancy and early childhood including short brittle, sulfur-deficient hair, ichthyosis, short stature, immune deficiency, dysmyelination of the brain, developmental delayand, in some patients, marked skin sun sensitivity without an increase in skin cancer (Itin, Sarasin, & Pittelkow 2001; Kraemer, Patronas, Schiffmann, et al. 2007; Faghri, Tamura, Kraemer, et al. 2008; Zhou, Khan, Tamura, et al. 2010). Strikingly, in this study, 70% of cases required neonatal intensive care(Table 1), with several infants requiring prolonged stays. They may have early childhood feeding problems, slow growth and recurrent infections (otitis media, pneumonia and gastroenteritis) secondary to an impaired immune response requiring frequent hospitalizations. TTD children may require surgery for infantile cataracts and males often have surgery to repair cryptorchidism. These multisystem problems result in morbidity and mortality, as identified in a survey of all 112 identified TTD cases from the literature, where a high frequency of mortality in the first 10 years of life (20-fold higher than expected) was reported (Faghri, Tamura, Kraemer, et al. 2008). The high frequency of mortality is confirmed in this study.
Trichothiodystrophy is caused by mutations in the XPD, XPB, TTDA and TTDN1 genes(Table 1 and(Itin, Sarasin, & Pittelkow 2001; Kraemer, Patronas, Schiffmann, et al. 2007; Faghri, Tamura, Kraemer, et al. 2008)). Three of these genes (XPD, XPB and TTDA) are components of nucleotide excision repair (NER) and the basal transcription pathways(Lehmann 2003; Stefanini, Botta, Lanzafame, et al. 2010). NER repairs damage to DNA such as ultraviolet photoproducts (thymine dimers) and some forms of oxidative damage. They are also subunits of the transcription factor TFIIH, which is required for transcription by RNA polymerase II. Mice with TTD type mutations in the XPD gene have defects in activation of multiple nuclear receptors including thyroid, estrogen, retinoid and peroxisome proliferator-activated receptors (Compe, Malerba, Soler, et al. 2007). Pregnancy complications observed in mothers of TTD patients may be related to insufficiency of transcription of essential genes in the placenta (see discussion in (Moslehi, Signore, Tamura, et al. 2010)). Placental abnormalities were reported in 19% (5/27) of the TTD pregnancies (Table 1). A detailed histopathological examination and/or molecular analysis of placental gene expression may reveal an anatomic or metabolic basis of these abnormalities.
For at-risk families, where a prior case of TTD has been confirmed, prenatal diagnosis may be considered. In utero biopsy of fetal eyebrow hairs was reported showing typical “tiger-tail” banding pattern on polarized microscopy and low content of the sulfur containing amino acid cystine in a family with TTD and normal DNA repair (Quintero, Morales, Gilbert-Barness, et al. 2000). If the molecular defect is known, DNA based methods can be used. Prenatal diagnosis of TTD has been reported based on analysis of the DNA repair gene defect in chorionic villi or in amniocenteses samples in families with an affected child (Sarasin, Blanchet-Bardon, Renault, et al. 1992; Kleijer, van der Sterre, Garritsen, et al. 2007).
This current study has several limitations. The small number of patients may not reflect the pregnancy or prenatal screening findings of the majority of TTD pregnancies. In rare disorders, the most phenotypically severe patients are often the first to be identified and studied. Pregnancies and neonates with milder manifestations of the disease may not be recognized. Since only 10 of 27 TTD pregnancies had maternal serum screening, more screened pregnancies with TTD will need to be assessed. However the 17 TTD pregnancies where screening results were not available had similar frequencies of complications.
TTD is quickly and easily diagnosed by identification of tiger tail banding with simple examination of hair shafts under polarizing microscopy. We hope that increased awareness of these TTD related pregnancy and neonatal complications will raise the index of suspicion for TTD diagnosis. Early diagnosis of TTD in a neonate may be helpful for the detection of co-morbidities and for counseling in subsequent pregnancies.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research and the Division of Cancer Epidemiology and Genetics, National Cancer Institute, the National Human Genome Research Institute, and the National Eye Institute, National Institutes of Health (NIH), Bethesda, MD. Support for author Zhou was made possible through the Clinical Research Training Program, a public-private partnership supported jointly by the NIH and Pfizer Inc (via a grant to the Foundation for NIH from Pfizer Inc). We thank Dr. Amy Thompson for assistance in compiling data on eye abnormalities in the patients.
References
- Aagaard-Tillery KM, Buchbinder A, Boente MP, Ramin KD. Beckwith-Wiedemann syndrome presenting with an elevated triple screen in the second trimester of pregnancy. Fetal Diagn Ther. 2007;22:18–22. doi: 10.1159/000095837. [DOI] [PubMed] [Google Scholar]
- Alkazaleh F, Chaddha V, Viero S, Malik A, Anastasiades C, Sroka H, Chitayat D, Toi A, Windrim RC, Kingdom JC. Second-trimester prediction of severe placental complications in women with combined elevations in alpha-fetoprotein and human chorionic gonadotrophin. Am J Obstet Gynecol. 2006;194:821–827. doi: 10.1016/j.ajog.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Audibert F, Benchimol Y, Benattar C, Champagne C, Frydman R. Prediction of preeclampsia or intrauterine growth restriction by second trimester serum screening and uterine Doppler velocimetry. Fetal Diagn Ther. 2005;20:48–53. doi: 10.1159/000081369. [DOI] [PubMed] [Google Scholar]
- Boyle J, Ueda T, Oh KS, Imoto K, Tamura D, Jagdeo J, Khan SG, Nadem C, DiGiovanna JJ, Kraemer KH. Persistence of repair proteins at unrepaired DNA damage distinguishes diseases with ERCC2 (XPD) mutations: cancer-prone xeroderma pigmentosum vs. non-cancer-prone trichothiodystrophy. Hum Mutat. 2008;29:1194–1208. doi: 10.1002/humu.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, Emmert S, Pike KM, Raziuddin A, Plona TM, DiGiovanna JJ, Tucker MA, Kraemer KH. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. Journal of Medical Genetics. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compe E, Malerba M, Soler L, Marescaux J, Borrelli E, Egly JM. Neurological defects in trichothiodystrophy reveal a coactivator function of TFIIH. Nat Neurosci. 2007;10:1414–1422. doi: 10.1038/nn1990. [DOI] [PubMed] [Google Scholar]
- Donn SM, Thoene JG. Prospective prevention of neonatal hyperammonaemia in argininosuccinic acidura by arginine therapy. J Inherit Metab Dis. 1985;8:18–20. doi: 10.1007/BF01805478. [DOI] [PubMed] [Google Scholar]
- Driscoll DA. Second trimester maternal serum screening for fetal open neural tube defects and aneuploidy. Genet Med. 2004;6:540–541. doi: 10.1097/00125817-200411000-00013. [DOI] [PubMed] [Google Scholar]
- Emmert S, Ueda T, Zumsteg U, Weber P, Khan SG, Oh KS, Boyle J, Laspe P, Zachmann K, Boeckmann L, Kuschal C, Bircher A, Kraemer KH. Strict sun protection results in minimal skin changes in a patient with xeroderma pigmentosum and a novel c. 2009delG mutation in XPD (ERCC2) Exp Dermatol. 2009;18:64–68. doi: 10.1111/j.1600-0625.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghri S, Tamura D, Kraemer KH, DiGiovanna JJ. Trichothiodystrophy: a systematic review of 112 published cases characterises a wide spectrum of clinical manifestations. J Med Genet. 2008;45:609–621. doi: 10.1136/jmg.2008.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Alberman E, Wald N, Summers AM. Triploidy identified through second-trimester serum screening. Prenatal Diagnosis. 2005;25:229–233. doi: 10.1002/pd.1115. [DOI] [PubMed] [Google Scholar]
- Ibdah JA, Bennett MJ, Rinaldo P, Zhao Y, Gibson B, Sims HF, Strauss AW. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med. 1999;340:1723–1731. doi: 10.1056/NEJM199906033402204. [DOI] [PubMed] [Google Scholar]
- Itin PH, Sarasin A, Pittelkow MR. Trichothiodystrophy: update on the sulfur-deficient brittle hair syndromes. Journal of the American Academy of Dermatology. 2001;44:891–920. doi: 10.1067/mjd.2001.114294. [DOI] [PubMed] [Google Scholar]
- Kang JH, Farina A, Park JH, Kim SH, Kim JY, Rizzo N, Elmakky A, Jun HS, Hahn WB, Cha DH. Down syndrome biochemical markers and screening for preeclampsia at first and second trimester: correlation with the week of onset and the severity. Prenatal Diagnosis. 2008;28:704–709. doi: 10.1002/pd.1997. [DOI] [PubMed] [Google Scholar]
- Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, Jaspers NG, Sarasin A, Stefanini M, Lehmann AR. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Kleijer WJ, van der Sterre ML, Garritsen VH, Raams A, Jaspers NG. Prenatal diagnosis of xeroderma pigmentosum and trichothiodystrophy in 76 pregnancies at risk. Prenatal Diagnosis. 2007;27:1133–1137. doi: 10.1002/pd.1849. [DOI] [PubMed] [Google Scholar]
- Kraemer KH, Patronas NJ, Schiffmann R, Brooks BP, Tamura D, DiGiovanna JJ. Xeroderma pigmentosum, trichothiodystrophy and Cockayne syndrome: A complex genotype-phenotype relationship. Neuroscience. 2007;145:1388–1396. doi: 10.1016/j.neuroscience.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause TG, Christens P, Wohlfahrt J, Lei U, Westergaard T, Norgaard-Pedersen B, Melbye M. Second-trimester maternal serum alpha-fetoprotein and risk of adverse pregnancy outcome(1) Obstet Gynecol. 2001;97:277–282. doi: 10.1016/s0029-7844(00)01109-1. [DOI] [PubMed] [Google Scholar]
- Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. Biochimie. 2003;85:1101–1111. doi: 10.1016/j.biochi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Lepage N, Chitayat D, Kingdom J, Huang T. Association between second-trimester isolated high maternal serum maternal serum human chorionic gonadotropin levels and obstetric complications in singleton and twin pregnancies. Am J Obstet Gynecol. 2003;188:1354–1359. doi: 10.1067/mob.2003.278. [DOI] [PubMed] [Google Scholar]
- Liang C, Kraemer KH, Morris A, Schiffmann R, Price VH, Menefee E, DiGiovanna JJ. Characterization of tiger-tail banding and hair shaft abnormalities in trichothiodystrophy. J Am Acad Dermatol. 2005;52:224–232. doi: 10.1016/j.jaad.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Liang C, Morris A, Schlucker S, Imoto K, Price VH, Menefee E, Wincovitch SM, Levin IW, Tamura D, Strehle KR, Kraemer KH, DiGiovanna JJ. Structural and molecular hair abnormalities in trichothiodystrophy. J Invest Dermatol. 2006;126:2210–2216. doi: 10.1038/sj.jid.5700384. [DOI] [PubMed] [Google Scholar]
- Milunsky A, Nebiolo L. Maternal serum triple analyte screening and adverse pregnancy outcome. Fetal Diagn Ther. 1996;11:249–253. doi: 10.1159/000264310. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Signore C, Tamura D, Mills JL, DiGiovanna JJ, Tucker MA, Troendle J, Ueda T, Boyle J, Khan SG, Oh KS, Goldstein AM, Kraemer KH. Adverse effects of trichothiodystrophy DNA repair and transcription gene disorder on human fetal development. Clinical Genetics. 2010;77:365–373. doi: 10.1111/j.1399-0004.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–e224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- Petrin JH, Meckler KA, Sybert VP. A new variant of trichothiodystrophy with recurrent infections, failure to thrive, and death. Pediatr Dermatol. 1998;15:31–34. doi: 10.1046/j.1525-1470.1998.1998015031.x. [DOI] [PubMed] [Google Scholar]
- Preece MA, Green A. Pregnancy and inherited metabolic disorders: maternal and fetal complications. Ann Clin Biochem. 2002;39:444–455. doi: 10.1258/000456302320314458. [DOI] [PubMed] [Google Scholar]
- Quintero RA, Morales WJ, Gilbert-Barness E, Claus J, Bornick PW, Allen MH, Ackerman J, Koussef B. In utero diagnosis of trichothiodystrophy by endoscopically-guided fetal eyebrow biopsy. Fetal Diagn Ther. 2000;15:152–155. doi: 10.1159/000020995. [DOI] [PubMed] [Google Scholar]
- Sarasin A, Blanchet-Bardon C, Renault G, Lehmann A, Arlett C, Dumez Y. Prenatal diagnosis in a subset of trichothiodystrophy patients defective in DNA repair. British Journal of Dermatology. 1992;127:485–491. doi: 10.1111/j.1365-2133.1992.tb14845.x. [DOI] [PubMed] [Google Scholar]
- Shekhawat PS, Matern D, Strauss AW. Fetal fatty acid oxidation disorders, their effect on maternal health and neonatal outcome: impact of expanded newborn screening on their diagnosis and management. Pediatr Res. 2005;57:78R–86R. doi: 10.1203/01.PDR.0000159631.63843.3E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini M, Botta E, Lanzafame M, Orioli D. Trichothiodystrophy: from basic mechanisms to clinical implications. DNA Repair (Amst) 2010;9:2–10. doi: 10.1016/j.dnarep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Witters I, Legius E, Devriendt K, Moerman P, Van SD, Van AA, Fryns JP. Pregnancy outcome and long term prognosis in 868 children born after second trimester amniocentesis for maternal serum positive triple test screening and normal prenatal karyotype. J Med Genet. 2001;38:336–338. doi: 10.1136/jmg.38.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters I, Moerman P, Van AA, Fryns JP. Physical and psychomotor development of 1799 children born after second trimester amniocentesis for maternal serum positive triple test screening and normal prenatal karyotype. J Med Genet. 2002;39:e75. doi: 10.1136/jmg.39.12.e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron Y, Cherry M, Kramer RL, O’Brien JE, Hallak M, Johnson MP, Evans MI. Second-trimester maternal serum marker screening: maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999;181:968–974. doi: 10.1016/s0002-9378(99)70334-0. [DOI] [PubMed] [Google Scholar]
- Zhou X, Khan SG, Tamura D, Patronas NJ, Zein WM, Brooks BP, Kraemer KH, DiGiovanna JJ. Brittle hair, developmental delay, neurologic abnormalities, and photosensitivity in a 4-year-old girl. Journal of the American Academy of Dermatology. 2010;63:323–328. doi: 10.1016/j.jaad.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]