Abstract
Treatment strategies for heart failure remain a high priority for ongoing research due to the profound unmet need in clinical disease coupled with lack of significant translational progress. The underlying issue is the same whether the cause is acute damage, chronic stress from disease, or aging: progressive loss of functional cardiomyocytes and diminished hemodynamic output. To stave off cardiomyocyte losses, a number of strategic approaches have been embraced in recent years involving both molecular and cellular approaches to augment myocardial structure and performance. Resultant excitement surrounding regenerative medicine in the heart has been tempered by realizations that reparative processes in the heart are insufficient to restore damaged myocardium to normal functional capacity and that cellular cardiomyoplasty is hampered by poor survival, proliferation, engraftment and differentiation of the donated population. To overcome these limitations, a combination of molecular and cellular approaches needs to be adopted involving use of genetic engineering to enhance resistance to cell death and increase regenerative capacity. This review will highlight biological properties of approached to potentiate stem cell-mediated regeneration to promote enhanced myocardial regeneration, persistence of donated cells, and long lasting tissue repair. Optimizing cell delivery and harnessing the power of survival signaling cascades for ex vivo genetic modification of stem cells prior to reintroduction into the patient will be critical to enhance the efficacy of cellular cardiomyoplasty. Once this goal is achieved, then cell-based therapy has great promise for treatment of heart failure to combat the loss of cardiac structure and function associated with acute damage, chronic disease or aging.
Keywords: regeneration, stem cell, infarction, myocardium
Prologue
Perplexity is the beginning of knowledge.
-Khalil Gibran
Substantial resources have been expended over the last decade in pursuit of interventional strategies to treat the unmet need of heart failure patients to restore myocardial structure and function. In the wake of thousands of research reports and hundreds of clinical studies we remain perplexed, which is reassuring in the context of the Gibran quote that begins this review. Although there remains a lot to learn, knowledge is coalescing into understanding that, in turn, refines the search for answers into ever more fruitful investigations. However, it has become abundantly clear from both clinical and basic research studies is that full restoration of myocardial structure and function in the wake of pathological injury remains outside our reach at present, but may be achievable with a combination of ongoing research, creativity, perseverance, and maybe a little luck. This review will endeavor to summarize the run up to current understanding, where road is blocked or splits apart, and how utilization of enhanced stem cells may provide the means to surpass current results and further the efficacious implementation of regenerative cell therapy for heart failure.
Part 1: In the beginning there were a couple ideas
Ideas are like rabbits. You get a couple and learn how to handle them, and pretty soon you have a dozen.
-John Steinbeck
Today in a new age of enlightenment, students and trainees regard their mentors with bemused incredulousness when told that, until recently, the prevailing dogma held the myocardium as a fully post-mitotic tissue incapable of regeneration. At the turn of this century, cell therapy approaches were essentially limited to adoptive transfer of various non-cardiac cell types into the pathologically injured heart in the hopes of stimulating chimeric engraftment and modicum of repair1-4. The transplantation of skeletal myoblasts into the myocardium of a patient with severe ischemic heart failure in 2001 and subsequent arrythmogenic complications raised concern over the safety of adoptive transfer cell therapy5. Despite this setback the concept of adoptive cell transfer remained an attractive one, especially in a tissue considered post-mitotic. Finding a cell type that was safe, efficacious, and durable for mediating repair remained the holy grail of cardiac regenerative medicine. Coincidentally, while skeletal myoblast transfer studies stalled in 2001, a new era was concurrently dawning with the advent of bone marrow adoptive cell transfer for repair of the infarcted heart6, 7 Regardless of the maelstrom of debate which ensued about the findings of these seminal studies,8, 9 these publications represented a turning point in the perspective of how myocardial repair could be effected. The following decade witnessed numerous clinical trials with bone marrow and bone marrow derived cells to assess the clinical application of stem cells as summarized in excellent reviews and meta-analyses10-13. In brief, cardiac clinical trials from the past decade have mainly been based on different cell subsets of autologous bone marrow. The general conclusion is that bone-marrow stem cell therapy is safe and associated with a moderate (1.93%- 5.40%) increase in ejection fraction. This improvement appears to be temporary11 presumably due to limitation of remodeling or relief of angina through paracrine effects, rending this approach possibly efficacious in biologically old patients but a suboptimal choice for the majority of the mid-life patient population. Long-term functional improvement requires application of stem cells possessing true cardiomyogenic and vascular differentiation potential and contributing to new cell and vessel formation in the myocardium. This rationale underpinned the announcement that resident cardiac progenitor cells (CPCs) derived from human samples capable of generating myocardium and vasculature14 had been isolated and, as a consequence of experimental studies and published reports now numbering in the thousands, the reputation of the heart as an organ incapable of cell regeneration has been transformed15, 16. No longer slumbering in post-mitotic quiescence, the heart is a dynamic organ capable of repair, cellular replacement over aging, and a fertile milieu for the panoply of stem cells sourced from adults, embryos, and induced fibroblasts. With subtypes of each cell category seemingly multiplying like proverbial rabbits, the field has morphed from a lack of suitable regenerative cell populations to an overabundance of possibilities. A brief examination of the embryonic / inducible pluripotent camp versus adult cells is in order to understand the empowerment issues involved.
With a goal of recreating tissue in mind, employment of cells that give rise to all the tissue types in our bodies in early development seems a logical and promising choice. Indeed, embryonic stem cells (ESC) derived from human blastocysts have been around since the end of the last century17. These pluripotent cells exhibit normal karyotypes, high telomerase activity and express cell surface markers that characterize embryonic stem cells (ESCs) prior to lineage commitment. Human embryonic stem cells (hESCs) can, conceptually, give rise to cells in any somatic cell line. Differentiation of hESCs can be regulated by different culture conditions and growth factors18, 19. Animal studies using ESCs have demonstrated restoration of cardiac function but teratoma formation and immunological rejection restricts therapeutic utility of this cell type, in addition to ethical considerations20, 21. Tumorigenic potential of ESCs persists in various differentiated stages regardless of cell population leading to teratoma formation22 which clearly illustrates safety concerns associated with purportedly “differentiated” hESC-derived material intended for clinical application, with chromosomal instability reported in later passages of these cells in culture23. Human induced pluripotent cells (hiPSCs) are similar to embryonic cells in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell specific genes, telomerase activity24 and cardiac potential25. Along with these attributes, hiPSCs also show similarities with hESCs regarding teratoma formation, tumorogenicity and somatic coding mutations26. In addition, incomplete reprogramming or accumulation of genetic abnormalities during the iPSCs derivation process may render even autologous iPSCs lines immunogenic27. iPSCs co-culture studies with various cell types have revealed that until now, no cell type has been able to generate the cell type of interest with higher than 95% purity. Heterogeneity in the iPSC population raises risk of cellular transdifferentiation and susceptibility to teratogenesis. However, the dilemma of heterogeneity is not limited to pre-differentiation, as maturation stages are also not uniform in iPSC culture, including cardiomyocyte phenotypes27, 28. Variable differentiation is of major concern in the heart where synchrony and structure is of fundamental importance. In summary, at the time of this review, clinical cardiac application of ESC and hiPSCs populations must traverse a deep chasm that can only be bridged by harnessing overenthusiastic proliferative potential, gaining control over cell fate determination signals, and coping with issues of allogenic rejection for the ESC. As such, these cell types have yet to make an appearance in a clinical trial for treatment of heart failure.
Safety concerns over the utilization of ESC or hiPSCs contrasts with the lack of adverse events associated with administration of adult stem cells derived from bone marrow or cardiac tissue explants. Although ontogeny of adult cardiac stem cells remains unresolved, collective findings from multiple laboratories validate the cardiogenic potential of these cells29-32. The presumption for presence of tissue resident adult stem cells is their participation in normal cellular renewal due to consequences of aging over the lifetime of an organism. Therein lies the crux of the problem, since the resident adult cell population never evolved for rapid creation of new tissue in the wake of injury. The positive aspect of an adult stem cell’s limited proliferative potential is the fact that not a single incidence of oncogenic transformation has been documented, and this distinction from their embryonic brethren has enabled clinical trials with adult stem cells to move forward. In the SCIPIO trial (cardiac Stem Cell Infusion in Patients with Ischemic cardiOmyopathy), cardiac stem cells are isolated from patients undergoing a coronary artery bypass grafting (CABG) procedure for autologous reintroduction following expansion in culture when they are percutanously infused into the scar tissue four months after CABG. (http://clinicaltrials.gov/ct2/show/study/NCT00474461) Although the SCIPIO trial is mainly based on determining the feasibility and safety of harvesting adult cardiac progenitors for autologous reintroduction, there is also optimism toward obtaining functional hemodynamic improvement.
The good news is that clinical utilization of adult stem cells is a reality today and the results appear promising as well as safe as shown by a SWOT analysis of stem cell types (Fig. 1). The caveat is that whereas embryonic or induced pluripotent cells possess an inherently youthful phenotype, heart failure patients who provide tissue for autologous stem cells isolation are usually above the age of sixty years and suffer from coronary occlusions, possibly multiple events, and previous cardiac procedures. Indeed, aging may be, in part, a “stem cell disease” characterized by the ravages of time upon the resident adult cell population that renders them increasingly stressed in the progressively dysfunctional tissue environment of aging myocardium. Stem cells would be well suited for regeneration if they clung to the exuberance of youth while also maintaining self-control that comes with maturity.
Figure 1. SWOT analysis of Different Stem Cells and Their Possible Clinical Application.
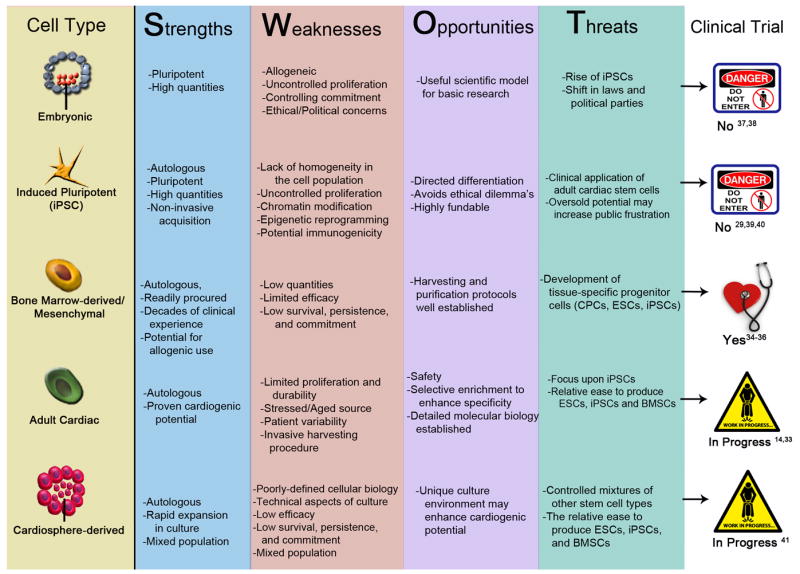
Matrix assessment delineating a SWOT analysis (Strengths, Weaknesses, Opportunities and Threats) of various stem cell types and their clinical implementation.
Part 2: Getting older, not necessarily better
By the time we’ve made it, we’ve had it.
-Malcom Forbes
In adult mammalian tissue, stem cells participate in normal tissue homoeostasis through repair and regeneration upon damage33. Stem cell niches are profoundly affected by signals and growth factors from the local and systemic environment34. Thus, a younger niche is exposed to a different local milieu than an older or injured niche. Since normal regeneration is a function of local stem cell niches, the accretion of age–related changes such as DNA damage, impaired catabolism, altered epigenetics, and environmental stress prompt decline in stem cell function. In the process of DNA replication, alterations such as single- and double-strand DNA breaks, chromosomal translocations, telomere shortening, and single base mutations35-37 can occur and lead to replicative cellular senescence38-41. In addition to replicative senescence, adult stem cells in the heart are susceptible to chronological aging, reflected by aggregation of damaged proteins, lipids and other macromolecules due to a decrease in cellular autophagy42. Inefficient catabolism leads to accumulation of dysfunctional organelles and cellular substructures over time, which in turn reduces quality and efficiency of cellular and molecular biological processes required to maintain homeostasis and survival42-44. As an organ matures, the well-orchestrated regulation of sequential expression timing and intensity for genes such as Wnt, Notch and Hedgehog in the stem cell pool can be epigenetically disrupted leading to changes cell progeny45. Disturbance of gene expression cascades into production of misprogramed daughter cell progeny that fail to maintain tissue structure and function. The accumulation of aberrant cells can be significant with advancing age, as predictive calculations reveal the entire myocyte compartment is replaced 15 times in women and 11 times in men from 20 to 100 years of age, meaning an average of 13 replications in 80 years46, 47. As an indication of repetitive rounds of replication, shortening of telomeres in the adult cardiac stem cell pool was paralleled by appearance of myocytes with severe telomere attrition46 suggesting that older CPCs are the likely source for phenotypically old myocyte progeny. Last, but not least, in this cavalcade of detrimental insults are the exogenous stresses that stem cells endure in a pathologically compromised heart. For example, cardiac stem cells from a CABG patient have not only likely suffered from replicative and chronological aging, but have also been forced to persevere in a genotoxic environment of reactive oxygen species and chemical substances, promoting a process called stress induced premature senescence44. Stress induced premature senescence, in turn, leads to DNA damage and mitochondrial DNA destruction, which ultimately influences stem cell replicative capacity and progeny44. Premature senescence also occurs through the renin-angiotensin-aldosterone system (RAAS) and chronic elevation of angiotensin II levels.48-51 Since the majority of the target patient population for stem cell therapy suffers from sympathetic hyperactivity, such patients also carry a stem cell pool compromised by adverse repercussions of RAAS. The emerging paradigm of cellular senescence also portrays senescent cells as active participants in communicating their decrepitude by profoundly affecting their microenvironment in a paracrine fashion through an altered secretome that inhibits proliferation and modulates immune responses52, 53. These processes initiate a vicious circle of negative events on stem cell function and progeny, ultimately compromising the regenerative potential of the tissue as a whole.
Taken collectively, evidence indicates that adult stem cells are unlikely to be equivalent in their regenerative potential. Moreover, the very target population of aged and infirmed patients destined to be at the forefront of interventional therapy also possess the most compromised stem cell population in terms of functional capacity and regenerative potential. Like so many biological problems, the solution is conceptually simple but fraught with technical challenges. Simply put, we would want to metaphorically “turn back the clock” on aged adult stem cells and empower them with the phenotypic characteristics of youthful vigor while not obviating their programming for context-dependent recognition of the environment and appropriate integration into the local environment in a salubrious fashion.
Part 3: May-December wedding between science and stem cells
You’ve got to go out on a limb sometimes because that’s where the fruit is.
-Will Rogers
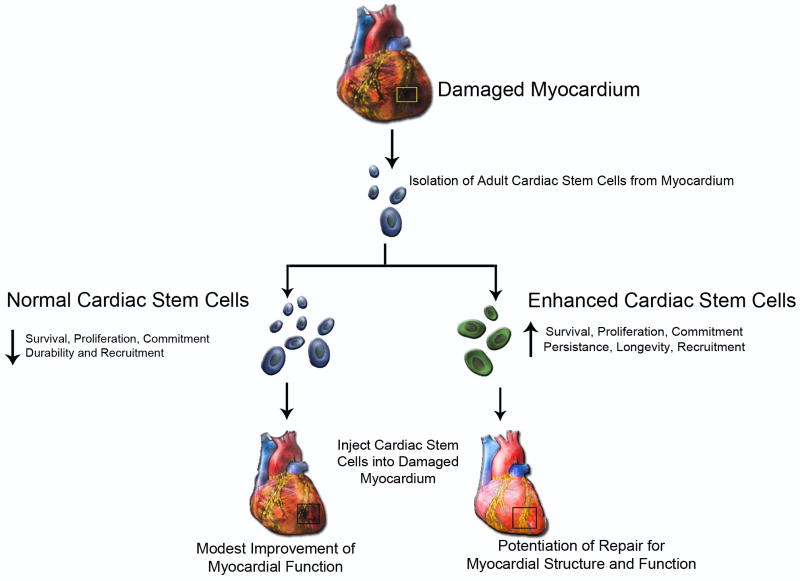
As researchers pursue the ultimate goal of therapeutic implementation for regenerative medicine, the journey slowly yields hard won fruits of knowledge gathered through innovation and creativity. Transformational ideas alter longstanding paradigms and redefine approaches to creating and delivering stem cells, but major issues concerning the therapeutic application of stem cells still remain unresolved. Success of adoptively transferred adult stem cells remains modest primarily as a consequence of three factors: poor survival, marginal proliferation, and limited functional engraftment / commitment within the host tissue. Adoptively transferred stem cells need to be primed against apoptotic, necrotic and hypoxic conditions prevalent within the damaged tissue. Furthermore, the aforementioned deterioration of proliferative capacity in old age adversely affects the stem cell regenerative capacity. Finally, if cells persist and even proliferate but are functionally incapable of appropriate lineage commitment and functional integration, then the end result is a cell predisposed to oncogenic transformation. Therefore, combating a constellation of negative factors affecting stem cell mediated regeneration must be balanced against the need for restraint and appropriate participation in direct or indirect tissue repair. Threading this figurative “eye of the needle” is the purview of stem cell empowerment as detailed in the remainder of the review wherein current concepts, research efforts and problems associated with stem cell modification to enhance function are enumerated (Fig. 2).
Figure 2. Adult Cardiac Stem Cell Requires Empowerment.
Schematic representation of enhanced cardiac stem cells (CPCs) and their potentiation for repair to damaged myocardium relative to normal CPCs.
Survival
Poor survival and marginal retention of adoptively transferred cells into the pathologically challenged heart is widely accepted as a significant barrier to enhancing efficacy of regenerative therapy, and there is no controversy over the assertion that live cells do a better job of mediating biologically relevant effects than dead ones. And yet, researchers readily acknowledge massive losses of donated stem cells and failure to engraft in the damaged organ takes place within the first few days after delivery 54,55. If most of the effects we observe are mediated by cells that disappear within a week, then imagine the possibilities for enhanced repair if the donated population persisted for weeks, months, or even became incorporated permanently into the heart tissue? Clearly this is one of the front lines in the battle to enhance efficacy of adoptive transfer cell therapy. Stem cell survival is influenced by a number of factors such as ischemic conditions, inflammatory response56 and quality of donor cells 57, and research has focused on enhancement of stem cell survival within host environment to augment repair.
“Preconditioning” in the context of stem cells refers to treatment with growth factors, hypoxic shock, or anti-aging compounds for augmentation of stem cell potency. Preconditioning promotes cyto-protection that enhances resistance stem cell survival against oxidative stress in vitro and in vivo58,59,60,61 as well as promotes migration and recruitment to ischemic myocardium60. Cytokines and chemokine preconditioning strategies augment stem cell recruitment to injured tissue after intra-cardiac delivery of erythropoietin62, hepatocyte growth factor (HGF), or vascular endothelial growth factor (VEGF)63. Other factors used to enhance stem cell function included BMP-2, IGF-1 FGF-264, HGF, Hsp70 and atorvastatin65,66,67,68,69. Similarly, mesenchymal stem cells under hypoxic conditions exhibit increased proliferation and differentiation70 associated with pro-survival and pro-angiogenic signaling. The mechanistic signal transduction basis for these preconditioning effects promoting cell survival involves activation of PI3K/AKT and ERK1/2 signal transduction and activation of STAT356 as well as scenarios involving ERK1/2 expression71. Preconditioning can be initiated by multiple different cytokines that differentially influence downstream targets; therefore, multiple signaling pathways participate in mediating stem cell survival. The advantage of preconditioning is that the treatments are often simple, take advantage of cellular endogenous responses, and do not depend upon genetic manipulation that is time consuming and introduces foreign DNA into the treatment regimen. The duration of the protective response is a significant limitation of the preconditioning approach, as cell surface receptors are down-regulated, desensitized, or internalized in response to stimulation. Therefore, protection afforded by ex vivo preconditioning treatment prior to delivery will likely improve donated cell survival, but only by hours to days.
Alternative to preconditioning, genetic modification of stem cells to express pro-survival factors also enhances endurance of stem cells in the hostile environment of a pathologically damaged heart. Moreover, genetic manipulation allows for cells to serve as a source of growth factors that initiate intracrine, autocrine and paracrine effects, which augment activity of the donated population, endogenous cells, and their local environment. Candidate molecules employed for genetic modification of cells include canonical mediators of cell survival in the context of cardiomyocytes or oncogenically transformed cells (see Table 1-3) and will be briefly delineated in the next few paragraphs.
Table 1.
Preconditioning by factors to empower stem cells to augment myocardial repair
| Factor used | Delivery Method | Model | Outcome | References |
|---|---|---|---|---|
| Hepatocyte Growth Factor (HGF) | Intravenous injection | Mice | Improved cardiac function, promotes migration, proliferation and angiogenesis | 155, 156 |
| Vascular Endothelial Growth Factor (VEGF) | Preconditioning of MSCs | Mice | Improved cardiac function, reduced fibrosis | 157, 158 |
| IGF-1 | Intramyocardial injection, MSCs preconditioning | Mice | Improved cardiac function, promotes engraftment and differentiation | 66, 114, 159 |
| TGF-1α | MSCs preconditioning | Rat | Improved cardiac function. | 160 |
| Atorvastatin | Oral | Pigs | Improved cardiac function, promotes cell survival. | 68 |
| Erythropoietin | Intra cardiac injection | Rat | Improved cardiac function, promotes migration | 62 |
Table 3.
Pharmacological treatment with chemicals to empower stem cells in-vitro
| Factor used | Delivery Method | Model | Outcome | References |
|---|---|---|---|---|
| 5-azacytidine | In culture media | In vitro cells (MSCs, bone marrow derived cells) | Formation of myotubes and cardiomyocyte like structure | 89,90, 91 |
| TGF-β1 | In culture media | In vitro cells (CPCs) | Beating myocytes | 93 |
| eNOS | Genetic deletion of Nos-3 | Nos 3-/- mice | Inhibit mobilization of stem cells | 118 |
| MCP-3-CCR1/2 | In culture media and through cardiac fibroblast expressing MCP-3-CCR1/2 | MSCs, Rats | Mobilization of MSCs | 113 |
Apoptosis is a serious threat faced by transplanted cells into a hostile environment, so modifying stem cells to circumvent apoptotic signaling increases cell survival. The Bcl-2 protein family regulates caspase activation and mitochondrial integrity through dual actions of anti- and pro-apoptotic members. Bcl-2 engineering of mesenchymal stem cells (MSCs) increases survival after acute myocardial infarction72. Bcl-2 modified mesenchymal stem cells ameliorated LV remodeling and improved LV function. Exogenous delivery of Bcl-2 in MSCs activates a survival pathway that is sufficient to suppress hypoxia-induced apoptosis72 and adenoviral Bcl-2 transgene expression attenuated early donor cell death in cardiomyoblast transplantation73. Heme oxygnase-1 (HO-1) is an anti-apoptotic stress-inducible enzyme with anti-oxidant cytoprotective activity under ischemic conditions74. Overexpression of HO-1 in mesenchymal stem cells promotes angiogenesis and reduces fibrotic area 74 after transplantation in ischemic myocardium. Transplantation of survivin-engineered mesenchymal stem cells also enhanced cellular survival after transplantation 75. Similarly, other survival molecules including SDF-176, Ang-177 and CXCR478 significantly improve survival of transplanted cells.
This approach has proven successful with MSCs expressing myristolated AKT that augments heart function resulting in significant infarct size reduction79 and inhibition of ventricular remodeling 72 hrs after transplantation80 despite the fact that donated cells did not significantly contribute to formation of new myocardium 81. Paracrine effects of these AKT-expressing modified cells were postulated to play an important role in protection, with identification of genes including VEGF, FGF-2, HGF, IGF, and notably thymosin β4 that complexes with PINCH and integrin-linked kinase (ILK) resulting in the activation of AKT within cardiomyocytes of the border zone. Secreted frizzled related protein 2 (Sfrp 2) was also identified as a key paracrine factor mediating myocardial survival and repair after ischemic injury since protection of injured myocardium by AKT-modified mesenchymal stem cells was lost following suppression of Sfrp276.
Proliferation
Another important factor for consideration to improve the efficacy of cellular therapy is to augment the rate of proliferation of transplanted adult stem cells, which leads to persistence and expansion of the donated cell population and increases the number of cells available for engraftment. Combined with enhanced survival, increasing proliferation can serve as a powerful combinatorial approach to expand the impact of donated stem cells, as shown in studies using cardiac progenitor cells modified to express Pim-1 kinase32,82,83. Similarly, over expression of nucleostemin in cultured cells cardiac stem cells increased proliferation accompanied by preservation of telomere length84. However, an important caveat is that enhancing proliferation at the expense of lineage commitment and functional engraftment may not provide significant long term benefits, as was the case when cardiac progenitor cells were modified to express nuclear-targeted Akt resulting in expansion and persistence of the donated cells85. This study points out the importance of balancing the trifecta of desirable stem cell properties judiciously, as the optimal outcome can only be effected when appropriate cell phenotypic properties accompany enhanced survival, proliferation, and commitment to cardiogenic fate.
Commitment
Ideally, donated stem cells will ultimately participate directly in repair of damaged tissue by becoming new myocardium through synthesis of de novo myocytes, vessels, and endothelium. Regulatory pathways involved in embryonic stem cell differentiation to cardiomyocytes provide insight into how such cell fate decisions might be controlled and influenced86-88. A commonly employed pharmacologic strategy to promote differentiation is exposure to the DNA demethylation reagent 5-azacytidine as performed upon mesenchymal stem cells, bone marrow derived stem cells 89-91, or cardiac progenitor cells92. Long term stimulation of cardiac stem cells with TGF-β1 also favors acquisition of a cardiomyocyte phenotype93. Such approaches are unlikely to have significant clinical implications due to regulatory concerns about the effects of such treatments upon stem cells, but examining molecular processes induced by such treatments facilitates unraveling the pathways involved in optimizing cardiac differentiation of transplanted cells. An interesting alternative approach is the delivery of cardiac transcription factors as chimeric proteins fused to cell penetrating peptides to promote differentiation into cardiac phenotypes94, 95. Paracrine factors secreted by adoptively transferred mesenchymal stem cells may play an important role in orchestrating recruitment and lineage commitment of endogenous responses by promoting vasculogenesis and inhibiting apoptosis via vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), Hepatocyte growth factor (HGF), Angiotensin- (Ang-1), GATA4, or angiopoetin 77, 96-99. However, at present the most intriguing multifaceted player in the signaling cascade of cardiac stem cell myogenic determination is Notch, which regulates commitment100 as well as survival101. Notch is also a key regulator in smooth muscle differentiation as noted in epicardium derived cells 102. Therefore, manipulating stem cells with Notch seems a likely avenue for enhancing stem cell commitment and persistence. Similarly, GSK3-β induces cardiomyocyte differentiation, with myocardial injection of bone marrow mesenchymal stem cells over expressing GSK3-β associated with cardiomyocyte differentiation and angiogenesis 103.
Rejuvenation
One additional consideration alluded to earlier in this review is the problem of stem cell exhaustion due to aging. Autologous cell therapy on an aging target population will likely be hampered by the biological limitations of endogenous stem cells and the advent of senescence in the myocardial cell pool. Ideally, empowering the explanted stem cell population requires attention to antagonizing senescence and “turning back the clock”. While relatively little has been accomplished in the myocardial context, there are signaling pathways that seem connected to reversing the passage of time. For example, experimental activation of Notch restored “youthful” myogenic responses to satellite muscle cells isolated from 70-year-old humans rendering them similar to cells from 20-year-old humans104. Declining proliferation in hepatic progenitor cells has been ascribed to formation of a complex involving cEBP-a and the chromatin remodelling factor brahma (Brm) that inhibits the regenerative capacity of aged liver105. The mTOR pathway has been studied in the context of hematopoietic stem cells where rapamycin increased life span and restored self-renewal and hematopoiesis in aged mice, implicating mTOR signaling in aging and showing the potential of mTOR inhibitors to restoring hematopoiesis in the elderly106. Manipulation of telomere-telomerase axis was suggested in 1998 when two different human cell lines; retinal pigment epithelial cells and foreskin fibroblasts were transfected with vectors encoding for human telomerase catalytic subunit. Overexpression of telomerase resulted in elongated telomeres, invigorated cell division, and reduced expression of senescence markers107. Increased telomerase activity correlates with telomere elongation in stem cell-derived activated T cells.108
Genetic modulation for guidance and trafficking
Stem cell homing through injured myocardium represents another relevant key facet for furthering stem cell based regeneration for both donated as well as endogenous cell populations. Multiple molecular players are involved in the journey from a niche or injection site to the battleground of border zone or infarct region. Adhesion molecules such as integrins 109 as well as proteases work in concert to facilitate migration of stem cells through damaged tissue. Several integrins have been identified on stem cells and found to be involved in the recruitment, mobilization and homing of stem cells to the site of injury110, 111. Directional motility for mesenchymal stem cells was enhanced by engineered expression of the SDF-1/ CXCR4 axis.112, 113,114. MSCs have also been involved in recruiting endogenous stem cells improving myocardial repair.115, 116 Similarly, cellular recruitment is enhanced for endothelial progenitor cells infarcted myocardium by CD18/ ICAM 117 or the MCP-3-CCR1/2 axis.113 Selected proteases and their inhibitors have been touted as candidates to influence stem cell trafficking such as PAI-1, a protease inhibitor that blunts trafficking of mobilized CD34+ bone marrow cells and influences ventricular remodeling.112 Endothelial nitric oxide synthetase (eNOS) also enhanced stem cell homing after acute myocardial infarction118 via increases in MMP-9 and SDF-1.119 Thus, engineering of stem cells to induce expression of stem cell mobilization and homing factors can augment recruitment and retention of prodigal stem cells in their effort to find the right place to exert their reparative effects.
Collectively, information in this section of the review shows that modification of adult stem cells can adopt many forms and vary in method of implementation, but always shares the singular goal of enhancing regeneration. Optimization of stem cell modification will depend upon an approach or combination of approaches that maximizes all aspects of the regenerative process encompassing survival, proliferation, trafficking, lineage commitment, and functional engraftment. Published results using various strategies such as Pim-1 kinase support the premise that engineering of stem cells is a viable option to enhance the reparative process. Pim-1 is unique among the molecules used thus far as a combinatorial mediator of enhanced survival, proliferation, lineage commitment, and functional engraftment.32, 120 Some might argue that using such powerful molecular interventions with adult stem cells is going out on a limb and taking a risk, but cellular reprogramming by genetic engineering yielded inducible pluripotency that is unquestionably one of the greatest advances of stem cell biology. And, just like inducible pluripotent cells, the challenge is not in seeing the destination for where adult stem cell engineering needs to go, but rather how to get there as quickly and safely as possible.
Part 4: Clinical implementation and the challenge of genetically engineered stem cell empowerment
If you find a path with no obstacles, it probably doesn’t lead anywhere.
-Anthony Michael Hall
The primary hurdle in empowering stem cells for clinical application does not rest primarily with lack of knowledge on molecular mechanisms and pathways, but rather how best to deliver the engineered solution to the stem cell population in an acceptable and feasible solution. Traditional gene delivery relies upon recombinant protein expression through viral vectors which possess the inherently desirable characteristics of easy cell delivery of the engineered construct, use of replication deficient vectors and cell-type specific vectors to limit spread and target delivery, and the persistent expression of introduced genetic material by incorporation into the genome or maintained episomal presence in non-dividing cells.
Adenoviral, adeno-associated, lentiviral, and retroviral vectors are widely employed for gene delivery in the experimental setting and to a limited extent in clinical trials121-125. Each type of delivery vector has a different set of strengths and weaknesses in the context of empowering stem cells. Lentiviruses have the ability to infect non-dividing cells, whereas retroviruses express in a proliferative cell population. The primary advantage of lentiviral and retroviral-based engineering is the persistent incorporation of the viral genome (and with it the gene of interest) into the host genome so that the genetic modification can be selected for and propagated in daughter cell progeny. Although incorporation of the transgene into the host cell genome makes these vectors an excellent choice for engineering cells, risk of insertional mutagenesis and difficulty in regulating expression of the introduced gene limits utilization of these vectors in the clinical setting. Ongoing research is focused upon addressing these issues126-129 in an effort to make the lentiviral and retroviral vectors more palatable to regulatory agencies. Adenoviruses deliver their genomes to the nucleus of both dividing and non-dividing cells, are relatively cheap to produce in high titers and have a broad tropism to target cells especially within the cardiovascular system, which makes them widely used in myocardial gene therapy130 However, virus-specific cellular immune responses eventually lead to destruction of the adenoviral genetically modified cells131 that can provoke adenoviral-induced myocarditis132. As such the temporal expression of adenoviral-encoded proteins is relatively short lived (10-14 days). As of May 2001, 532 adenoviral gene therapy protocols had been approved for evaluation in clinical trials conducted predominantly in oncologic patients; however, only five of these trials had been evaluated in phase III testing. Multiple side effects including fever, chills, shivering, myalgias and even death were reported in these clinical trials133. As long as the inherent problem of high immunogenicity of these vectors remains unsolved, their production and application will remain restricted essentially to experimental and academic purposes. The contemporary virus of choice is the adeno-associated virus (AAV) that is able to infect non-dividing human cells, stably integrate into a specific locus on chromosome 19134, shows serotype-specific tissue tropism to direct expression135, and thus far shows no reported pathologic consequences. AAVs have been employed in numerous clinical trials136-139 including treatment of heart failure by increasing cardiac myocyte contractility in 2007140. Balancing these positive attributes, AAV vectors are hampered by restricted carrying capacity for DNA, are more challenging to produce in high titer than other viral vector types, and their viral backbones render them susceptible to eventual epigenetic modification.
Development of minicircles for gene delivery shows promise as a viable option for DNA delivery in engineering of stem cells. Minicircles are episomal DNA vectors produced as circular expression cassettes devoid of any bacterial plasmid DNA backbone. Their smaller molecular size enables more efficient transfections and offers sustained expression over a period of weeks as compared to standard plasmid vectors that only work for a few days. By virtue of the production methodology in minicircle creation, the expression plasmid no longer contains the bacterial origin of replication or the antibiotic resistance markers. Thus, delivering only the minicircles to cells lengthens the expression of the transgene over traditional transient transfections of plasmids. For dividing cells, expression of the minicircles lasts up to 14 days. For non-dividing cells, expression drops slightly after the first week, but then can continue expressing the transgenes for months. The lack of a bacterial backbone, the small size of the vector, potential expression duration of months, lack of genomic integration, and low cost of production make this delivery technique superior to viral delivery methods for ex vivo gene delivery involved in autologous stem cell modification. Moreover, the ability to produce minicircle vectors in bacterial expression systems devoid of animal by-products such as serum together with the ability to perform high quality good manufacturing practice to control for batch-to-batch quality makes them attractive from a regulatory perspective. However, efficiency of transfection for stem cells may be variable and the persistence of transfected DNA in the context of an adoptively tranferred adult stem cell population remains unknown at present.
Intramyocardial injection of viral vectors has led to transgene expression in a variety of organs other than the hearts such as the thymus, lung and the liver141-144. Delivering specificity to myocardial gene therapy involves incorporation of targeting mechanisms to restrict transgene expression. Viral vector tropism has been altered through insertion of peptide ligands145, hyperfusogenic envelop glycoproteins146 or antibodies to specific cell targets147. Modification of viral envelope can disrupt natural fusion with the host cell leading to reduced viral titers and transduction efficiencies145-147. Cell-type-specific promoters, which avoid expression in non-target cells, improves efficacy and safety of gene transfer with myocardial specific promoters; myosin light chain (mlc-2), alpha-myosin heavy chain (alpha-MHC) and cardiac Troponin T (cTnT) 148,149, 150,151. Although specificity of tissue-specific promoters remain a major concern for in vivo use of vectors, genetically stable stem cell cell lines are engineered ex vivo and then subsequently introduced. As such, tissue-specificity of promoters might be an additional gain by restricting pro-survival or proliferative signals to myocardial or vascular progeny of the adoptively transferred cell population, thereby diminishing fears of oncogenic transformation for undifferentiated cells.
Epilogue
By prevailing over all obstacles and distractions, one may unfailingly arrive at his chosen goal or destination.
-Christopher Columbus
Success in the future of stem cell therapy for currently incurable conditions rests primarily in maintaining the unshakable faith espoused by Columbus. Advances in the field of regenerative medicine are coming fast and furious, both in the figurative and literal sense of those words. Controversies and disagreements are to be expected in any endeavor as complex and perplexing as stem cell research, especially in view of the high stakes placed on funding the “magic bullet” that will hit the target of clinically relevant intervention. This review has focused predominantly upon adult stem cells simply because from a clinical perspective that population is far more advanced than ESC or iPS cell types, not because adult-derived cells are the “best” type of cell. With decades of successful bone marrow reconstitution procedures now commonplace in hospitals it is clear that adult stem cell therapy works, but can it work in a structurally complex tissue such as the heart where proliferative activity is so limited? That question lies at the root of a massive international effort to understand the signals and cues necessary to coax stem cells into functionally relevant cardiac engraftment now entering the second decade of study.
The journey to a New World of regenerative medicine has been phenomenally productive as evidenced by a quick scan of the more than 7,500 references available today in a PubMed search for the keywords “cardiac, stem cell, heart”, with almost 1,800 of those references being review articles to summarize our current understanding. All this for a field of research that was essentially non-existent a little over a decade ago. In view of this overwhelming body of literature it seems pointless to debate whether cardiac regeneration occurs, as that question has now been asked and affirmatively answered in lower vertebrates152 the neonatal heart153 and even in adult hearts in response to injury154. While scientists in the laboratory benches unravel molecular pathways and mechanistic basis for curing the basis of heart disease, experts at bedside think in terms of individual patient indications, specific disease stages and co-morbidities. Zealous advocates and confirmed skeptics agree that the endogenous regenerative potential in adult human myocardium alone is not capable of mediating recovery from acute pathologic injury or long-term chronic stress. Toward that goal, empowering normal biological process of regeneration by potentiating stem cells to enhance repair works and provides improvement that is both structurally measurable and clinically relevant over non-primed cells. Thus, next question to be asked and answered is whether such enhancement can be done safely, reproducibly, and efficiently. Many alternatives have been presented in this review, yet we are still on the tip of the proverbial iceberg in terms of the possibilities and their implementation. We are in the Golden Age of translational stem cell research and achieving our shared goal of translational implementation looks more promising today than at any time in history.
Table 2.
Genetic modification by integrated or episomal DNA to empower stem cell
| Factor used | Delivery Method | Model | Outcome | References |
|---|---|---|---|---|
| AKT | Retrovirus | Rat | Improved cardiac function and prevent remodeling | 79,81 |
| Hepatocyte Growth Factor (HGF) | Liposome mediated cell transfection | Mice, Rat | Improved cardiac function, promotes migration, proliferation and angiogenesis | 161,155, 156 |
| Vascular Endothelial growth factor (VEGF) | Adenovirus | Rats | Improved cardiac function, reduce fibrosis | 157, 158 |
| IGF-1 | Adenovirus | Rats | Improved cardiac function, promotes engraftment, differentiation and cardiac function | 162 |
| Pim-1 | Lentivirus | Mice | Improved cardiac function, promotes engraftment, cell survival and differentiation | 32 |
| Ang-1 | Adenovirus | Rats | Improved cardiac function and angiogenesis | 162 |
| GSK3-ß | Adenovirus | Mice | Improved cardiac function, promotes survival and angiogenesis | 103 |
| GATA4 | Retrovirus | Rat | Improved cardiac function, increase angiogenesis and cell survival | 98 |
| CXCR4 | Adenovirus | Mice | Improved cardiac function reduced fibrosis, and angiogenesis | 163 |
| Survivin | Lentivirus | Rat | Improved cardiac function, increased capillary density, reduced infarct | 75 |
| Heme oxygenase-1 (HO-1) | Adenovirus | Rats | Improved cardiac function, promotes angiogenesis | 164 |
| Bcl-2 | Transduction | Rats | Improved cardiac function, cell survival | 72, 73 |
| HSP-20 | Adenoviral | Rats | Improved cardiac function, promotes cell survival | 165 |
| Nuclear targeted Akt | Lentivirus transduction | Mice | Improve cell proliferation, inhibit cardiac commitment | 85 |
Acknowledgments
The authors would like to thank all members of the Sussman laboratory for their helpful discussions and technical support.
Sources of funding Dr. Sussman is supported by NIH grants R21HL102714, R01HL067245, R37HL091102, P01HL085577, RC1HL100891, R21HL102613, R21 HL104544, and R01HL105759.
Nonstandard abbreviations
- CABG
coronary artery bypass graft
- CPC
cardiac progenitor cell
- ESC
embryonic stem cell
- iPS
induced pluripotent stem cell
- MSC
mesenchymal stem cell
- mTOR
mammalian target of rapamycin
- SWOT
strengths, weaknesses, opportunities, threats
Footnotes
Disclosures No conflicts of interest exist.
References
- 1.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short-and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 3.Olivares EL, Ribeiro VP, Werneck de Castro JP, Ribeiro KC, Mattos EC, Goldenberg RC, Mill JG, Dohmann HF, dos Santos RR, de Carvalho AC, Masuda MO. Bone marrow stromal cells improve cardiac performance in healed infarcted rat hearts. Am J Physiol Heart Circ Physiol. 2004;287:H464–470. doi: 10.1152/ajpheart.01141.2003. [DOI] [PubMed] [Google Scholar]
- 4.Xu M, Wani M, Dai YS, Wang J, Yan M, Ayub A, Ashraf M. Differentiation of bone marrow stromal cells into the cardiac phenotype requires intercellular communication with myocytes. Circulation. 2004;110:2658–2665. doi: 10.1161/01.CIR.0000145609.20435.36. [DOI] [PubMed] [Google Scholar]
- 5.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sussman MA, Murry CE. Bones of contention: marrow-derived cells in myocardial regeneration. J Mol Cell Cardiol. 2008;44:950–953. doi: 10.1016/j.yjmcc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rota M, Boni A, Urbanek K, Padin-Iruegas ME, Kajstura TJ, Fiore G, Kubo H, Sonnenblick EH, Musso E, Houser SR, Leri A, Sussman MA, Anversa P. Nuclear targeting of Akt enhances ventricular function and myocyte contractility. Circ Res. 2005;97:1332–1341. doi: 10.1161/01.RES.0000196568.11624.ae. [DOI] [PubMed] [Google Scholar]
- 10.Menasche P. Cardiac cell therapy: lessons from clinical trials. J Mol Cell Cardiol. 50:258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Dawn B, Zuba-Surma EK, Abdel-Latif A, Tiwari S, Bolli R. Cardiac stem cell therapy for myocardial regeneration. A clinical perspective. Minerva Cardioangiol. 2005;53:549–564. [PubMed] [Google Scholar]
- 12.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 13.van der Spoel TI, Jansen Of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, Cramer MJ, Doevendans PA, Chamuleau SA. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 14.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 19.Ke Q, Yang Y, Rana JS, Chen Y, Morgan JP, Xiao YF. Embryonic stem cells cultured in biodegradable scaffold repair infarcted myocardium in mice. Sheng Li Xue Bao. 2005;57:673–681. [PubMed] [Google Scholar]
- 20.Asano T, Sasaki K, Kitano Y, Terao K, Hanazono Y. In vivo tumor formation from primate embryonic stem cells. Methods Mol Biol. 2006;329:459–467. doi: 10.1385/1-59745-037-5:459. [DOI] [PubMed] [Google Scholar]
- 21.Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15:254–259. doi: 10.1089/scd.2006.15.254. [DOI] [PubMed] [Google Scholar]
- 22.Hentze H, Graichen R, Colman A. Cell therapy and the safety of embryonic stem cell-derived grafts. Trends Biotechnol. 2007;25:24–32. doi: 10.1016/j.tibtech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Kooreman NG, Wu JC. Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface. 7(Suppl 6):S753–763. doi: 10.1098/rsif.2010.0353.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature. 471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 30.Smits AM, van Vliet P, Metz CH, Korfage T, Sluijter JP, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–243. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 31.Smits AM, van Laake LW, den Ouden K, Schreurs C, Szuhai K, van Echteld CJ, Mummery CL, Doevendans PA, Goumans MJ. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–535. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 32.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbari M, Krokan HE. Cytotoxicity and mutagenicity of endogenous DNA base lesions as potential cause of human aging. Mech Ageing Dev. 2008;129:353–365. doi: 10.1016/j.mad.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 37.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 193:257–266. doi: 10.1083/jcb.201010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 40.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 41.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. 2009;8:199–213. doi: 10.1016/j.arr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Rattan SI. Theories of biological aging: genes, proteins, and free radicals. Free Radic Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 44.Beltrami AP, Cesselli D, Beltrami CA. At the stem of youth and health. Pharmacol Ther. 129:3–20. doi: 10.1016/j.pharmthera.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Hemberger M, Dean W, Reik W. Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol. 2009;10:526–537. doi: 10.1038/nrm2727. [DOI] [PubMed] [Google Scholar]
- 46.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D’Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, Anversa P. Myocyte turnover in the aging human heart. Circ Res. 107:1374–1386. doi: 10.1161/CIRCRESAHA.110.231498. [DOI] [PubMed] [Google Scholar]
- 48.Imanishi T. Potential mechanisms in angiotensin II-induced EPCs senescence. Hypertens Res. doi: 10.1038/hr.2011.84. [DOI] [PubMed] [Google Scholar]
- 49.Kunieda T, Minamino T, Nishi J, Tateno K, Oyama T, Katsuno T, Miyauchi H, Orimo M, Okada S, Takamura M, Nagai T, Kaneko S, Komuro I. Angiotensin II induces premature senescence of vascular smooth muscle cells and accelerates the development of atherosclerosis via a p21-dependent pathway. Circulation. 2006;114:953–960. doi: 10.1161/CIRCULATIONAHA.106.626606. [DOI] [PubMed] [Google Scholar]
- 50.Fukuda D, Sata M. The renin-Angiotensin system: a potential modulator of endothelial progenitor cells. Hypertens Res. 2007;30:1017–1018. doi: 10.1291/hypres.30.1017. [DOI] [PubMed] [Google Scholar]
- 51.Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132:363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 55.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 56.Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
- 57.Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD. Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant. 2000;9:489–502. doi: 10.1177/096368970000900406. [DOI] [PubMed] [Google Scholar]
- 58.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 59.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 62.Klopsch C, Furlani D, Gabel R, Li W, Pittermann E, Ugurlucan M, Kundt G, Zingler C, Titze U, Wang W, Ong LL, Wagner K, Li RK, Ma N, Steinhoff G. Intracardiac injection of erythropoietin induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J Cell Mol Med. 2009;13:664–679. doi: 10.1111/j.1582-4934.2008.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Eschenhagen T, Stein W, Wu JC, Robbins RC, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 64.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 65.Guo Y, He J, Wu J, Yang L, Dai S, Tan X, Liang L. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res. 2008;39:179–188. doi: 10.1016/j.arcmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 66.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22:1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 67.Chang W, Song BW, Lim S, Song H, Shim CY, Cha MJ, Ahn DH, Jung YG, Lee DH, Chung JH, Choi KD, Lee SK, Chung N, Jang Y, Hwang KC. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27:2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 68.Yang YJ, Qian HY, Huang J, Geng YJ, Gao RL, Dou KF, Yang GS, Li JJ, Shen R, He ZX, Lu MJ, Zhao SH. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J. 2008;29:1578–1590. doi: 10.1093/eurheartj/ehn167. [DOI] [PubMed] [Google Scholar]
- 69.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 71.Haider KH, Idris NM, Kim HW, Ahmed RP, Shujia J, Ashraf M. MicroRNA-21 is a key determinant in IL-11/Stat3 anti-apoptotic signalling pathway in preconditioning of skeletal myoblasts. Cardiovasc Res. 88:168–178. doi: 10.1093/cvr/cvq151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 73.Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]
- 74.Jazwa A, Loboda A, Golda S, Cisowski J, Szelag M, Zagorska A, Sroczynska P, Drukala J, Jozkowicz A, Dulak J. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006;40:1250–1263. doi: 10.1016/j.freeradbiomed.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, Fang J, Huang Z, Lin Y, Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009;11:1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 76.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 78.Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, Liu X, Li Y, Ward CA, Melo LG, Kong D. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 79.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 80.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 81.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 82.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, Esposito G, Delucchi F, Arcarese M, Alvarez R, Siddiqi S, Emmanuel GN, Wu W, Fischer K, Martindale JJ, Glembotski CC, Leri A, Kajstura J, Magnuson N, Berns A, Beretta RM, Houser SR, Schaefer EM, Anversa P, Sussman MA. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007;13:1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 83.Borillo GA, Mason M, Quijada P, Volkers M, Cottage C, McGregor M, Din S, Fischer K, Gude N, Avitabile D, Barlow S, Alvarez R, Truffa S, Whittaker R, Glassy MS, Gustafsson AB, Miyamoto S, Glembotski CC, Gottlieb RA, Brown JH, Sussman MA. Pim-1 kinase protects mitochondrial integrity in cardiomyocytes. Circ Res. 2010;106:1265–1274. doi: 10.1161/CIRCRESAHA.109.212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siddiqi S, Gude N, Hosoda T, Muraski J, Rubio M, Emmanuel G, Fransioli J, Vitale S, Parolin C, D’Amario D, Schaefer E, Kajstura J, Leri A, Anversa P, Sussman MA. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ Res. 2008;103:89–97. doi: 10.1161/CIRCRESAHA.107.169334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fischer KM, Din S, Gude N, Konstandin MH, Wu W, Quijada P, Sussman MA. Cardiac progenitor cell commitment is inhibited by nuclear Akt expression. Circ Res. 2011;108:960–970. doi: 10.1161/CIRCRESAHA.110.237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arrell DK, Niederlander NJ, Faustino RS, Behfar A, Terzic A. Cardioinductive network guiding stem cell differentiation revealed by proteomic cartography of tumor necrosis factor alpha-primed endodermal secretome. Stem Cells. 2008;26:387–400. doi: 10.1634/stemcells.2007-0599. [DOI] [PubMed] [Google Scholar]
- 87.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 88.Lim JY, Kim WH, Kim J, Park SI. Involvement of TGF-beta1 signaling in cardiomyocyte differentiation from P19CL6 cells. Mol Cells. 2007;24:431–436. [PubMed] [Google Scholar]
- 89.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye NS, Chen J, Luo GA, Zhang RL, Zhao YF, Wang YM. Proteomic profiling of rat bone marrow mesenchymal stem cells induced by 5-azacytidine. Stem Cells Dev. 2006;15:665–676. doi: 10.1089/scd.2006.15.665. [DOI] [PubMed] [Google Scholar]
- 91.Burlacu A, Rosca AM, Maniu H, Titorencu I, Dragan E, Jinga V, Simionescu M. Promoting effect of 5-azacytidine on the myogenic differentiation of bone marrow stromal cells. Eur J Cell Biol. 2008;87:173–184. doi: 10.1016/j.ejcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 92.van Vliet P, Roccio M, Smits AM, van Oorschot AA, Metz CH, van Veen TA, Sluijter JP, Doevendans PA, Goumans MJ. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–169. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MA, de Kleijn D, Mummery CL, van Veen TA, Sluijter JP, Doevendans PA. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–149. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 94.Bian J, Kiedrowski M, Mal N, Forudi F, Penn MS. Engineered cell therapy for sustained local myocardial delivery of nonsecreted proteins. Cell Transplant. 2006;15:67–74. doi: 10.3727/000000006783982197. [DOI] [PubMed] [Google Scholar]
- 95.Bian J, Popovic ZB, Benejam C, Kiedrowski M, Rodriguez LL, Penn MS. Effect of cell-based intercellular delivery of transcription factor GATA4 on ischemic cardiomyopathy. Circ Res. 2007;100:1626–1633. doi: 10.1161/01.RES.0000269778.75877.68. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AH, Zhang J. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290:H1393–1405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]
- 97.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8:467–474. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 98.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010;299:H1772–1781. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 100.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–15534. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Gude NA, Emmanuel G, Wu W, Cottage CT, Fischer K, Quijada P, Muraski JA, Alvarez R, Rubio M, Schaefer E, Sussman MA. Activation of Notch-mediated protective signaling in the myocardium. Circ Res. 2008;102:1025–1035. doi: 10.1161/CIRCRESAHA.107.164749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grieskamp T, Rudat C, Ludtke TH, Norden J, Kispert A. Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ Res. 2011;108:813–823. doi: 10.1161/CIRCRESAHA.110.228809. [DOI] [PubMed] [Google Scholar]
- 103.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3beta-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, Conboy I. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- 106.Chen C, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 108.Allsopp RC, Cheshier S, Weissman IL. Telomerase activation and rejuvenation of telomere length in stimulated T cells derived from serially transplanted hematopoietic stem cells. J Exp Med. 2002;196:1427–1433. doi: 10.1084/jem.20021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sengbusch JK, He W, Pinco KA, Yang JT. Dual functions of [alpha]4[beta]1 integrin in epicardial development: initial migration and long-term attachment. J Cell Biol. 2002;157:873–882. doi: 10.1083/jcb.200203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, Chung JW, Hur J, Yoon CH, Park KW, Oh BH, Park YB, Kim HS. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–159. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 112.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 113.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 114.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 115.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Suzuki G, Iyer V, Lee TC, Canty JM., Jr Autologous Mesenchymal Stem Cells Mobilize cKit+ and CD133+ Bone Marrow Progenitor Cells and Improve Regional Function in Hibernating Myocardium. Circ Res. 2011;109:1044–1054. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 117.Wu Y, Ip JE, Huang J, Zhang L, Matsushita K, Liew CC, Pratt RE, Dzau VJ. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ Res. 2006;99:315–322. doi: 10.1161/01.RES.0000235986.35957.a3. [DOI] [PubMed] [Google Scholar]
- 118.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 119.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, Eaton L, Masuda H, Asahara T, Losordo DW. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow-derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase-mediated activation of matrix metalloproteinase-9. Circulation. 2006;113:1605–1614. doi: 10.1161/CIRCULATIONAHA.105.553925. [DOI] [PubMed] [Google Scholar]
- 120.Fischer KM, Cottage CT, Konstandin MH, Volkers M, Khan M, Sussman MA. Pim-1 kinase inhibits pathological injury by promoting cardioprotective signaling. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer KM, Cottage CT, Konstandin MH, Volkers M, Khan M, Sussman MA. Pim-1 kinase inhibits pathological injury by promoting cardioprotective signaling. J Mol Cell Cardiol. doi: 10.1016/j.yjmcc.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deng L, Li G, Xi L, Yin A, Gao Y, You W, Wang X, Sun B. Hepatitis B virus inhibition in mice by lentiviral vector mediated short hairpin RNA. BMC Gastroenterol. 2009;9:73. doi: 10.1186/1471-230X-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–796. doi: 10.1161/01.cir.101.7.790. [DOI] [PubMed] [Google Scholar]
- 124.Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, Chen C, Wang DW, Li J, Xiao X. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005;112:2650–2659. doi: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]
- 125.Remy S, Nguyen TH, Menoret S, Tesson L, Usal C, Anegon I. The use of lentiviral vectors to obtain transgenic rats. Methods Mol Biol. 597:109–125. doi: 10.1007/978-1-60327-389-3_8. [DOI] [PubMed] [Google Scholar]
- 126.Hockemeyer D, Wang H, Kiani S, Lai CS, Gao Q, Cassady JP, Cost GJ, Zhang L, Santiago Y, Miller JC, Zeitler B, Cherone JM, Meng X, Hinkley SJ, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Genetic engineering of human pluripotent cells using TALE nucleases. Nat Biotechnol. doi: 10.1038/nbt.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hockemeyer D, Soldner F, Beard C, Gao Q, Mitalipova M, DeKelver RC, Katibah GE, Amora R, Boydston EA, Zeitler B, Meng X, Miller JC, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jaenisch R. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat Biotechnol. 2009;27:851–857. doi: 10.1038/nbt.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 129.Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat Biotechnol. 29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Njeim MT, Hajjar RJ. Gene therapy for heart failure. Arch Cardiovasc Dis. 103:477–485. doi: 10.1016/j.acvd.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calabrese F, Thiene G. Myocarditis and inflammatory cardiomyopathy: microbiological and molecular biological aspects. Cardiovasc Res. 2003;60:11–25. doi: 10.1016/s0008-6363(03)00475-9. [DOI] [PubMed] [Google Scholar]
- 133.Vorburger SA, Hunt KK. Adenoviral gene therapy. Oncologist. 2002;7:46–59. doi: 10.1634/theoncologist.7-1-46. [DOI] [PubMed] [Google Scholar]
- 134.Samulski RJ. Adeno-associated virus: integration at a specific chromosomal locus. Curr Opin Genet Dev. 1993;3:74–80. doi: 10.1016/s0959-437x(05)80344-2. [DOI] [PubMed] [Google Scholar]
- 135.Lu Y. Recombinant adeno-associated virus as delivery vector for gene therapy--a review. Stem Cells Dev. 2004;13:133–145. doi: 10.1089/154732804773099335. [DOI] [PubMed] [Google Scholar]
- 136.Flotte TR, Afione SA, Conrad C, McGrath SA, Solow R, Oka H, Zeitlin PL, Guggino WB, Carter BJ. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, Brown BW, Desch JK, Norbash AM, Conrad CK, Guggino WB, Flotte TR, Wine JJ, Carter BJ, Reynolds TC, Moss RB, Gardner P. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther. 2002;13:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- 138.Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, High KA. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.During MJ, Leone P. Adeno-associated virus vectors for gene therapy of neurodegenerative disorders. Clin Neurosci. 1995;3:292–300. [PubMed] [Google Scholar]
- 140.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, Borow K, Dittrich H, Zsebo KM, Hajjar RJ. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15:171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick PM, Wittenberg BA, Cipriani L, Leinwand LA. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993;90:11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res. 1993;73:1202–1207. doi: 10.1161/01.res.73.6.1202. [DOI] [PubMed] [Google Scholar]
- 143.Huard J, Lochmuller H, Acsadi G, Jani A, Massie B, Karpati G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 144.Kass-Eisler A, Falck-Pedersen E, Elfenbein DH, Alvira M, Buttrick PM, Leinwand LA. The impact of developmental stage, route of administration and the immune system on adenovirus-mediated gene transfer. Gene Ther. 1994;1:395–402. [PubMed] [Google Scholar]
- 145.Gollan TJ, Green MR. Redirecting retroviral tropism by insertion of short, nondisruptive peptide ligands into envelope. J Virol. 2002;76:3558–3563. doi: 10.1128/JVI.76.7.3558-3563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fielding AK, Chapel-Fernandes S, Chadwick MP, Bullough FJ, Cosset FL, Russell SJ. A hyperfusogenic gibbon ape leukemia envelope glycoprotein: targeting of a cytotoxic gene by ligand display. Hum Gene Ther. 2000;11:817–826. doi: 10.1089/10430340050015437. [DOI] [PubMed] [Google Scholar]
- 147.Gennari F, Lopes L, Verhoeyen E, Marasco W, Collins MK. Single-chain antibodies that target lentiviral vectors to MHC class II on antigen-presenting cells. Hum Gene Ther. 2009;20:554–562. doi: 10.1089/hum.2008.189. [DOI] [PubMed] [Google Scholar]
- 148.Lee CJ, Fan X, Guo X, Medin JA. Promoter-specific lentivectors for long-term, cardiac-directed therapy of Fabry disease. J Cardiol. 57:115–122. doi: 10.1016/j.jjcc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 149.Franz WM, Breves D, Klingel K, Brem G, Hofschneider PH, Kandolf R. Heart-specific targeting of firefly luciferase by the myosin light chain-2 promoter and developmental regulation in transgenic mice. Circ Res. 1993;73:629–638. doi: 10.1161/01.res.73.4.629. [DOI] [PubMed] [Google Scholar]
- 150.Franz WM, Brem G, Katus HA, Klingel K, Hofschneider PH, Kandolf R. Characterization of a cardiac-selective and developmentally upregulated promoter in transgenic mice. Cardioscience. 1994;5:235–243. [PubMed] [Google Scholar]
- 151.Franz WM, Rothmann T, Frey N, Katus HA. Analysis of tissue-specific gene delivery by recombinant adenoviruses containing cardiac-specific promoters. Cardiovasc Res. 1997;35:560–566. doi: 10.1016/s0008-6363(97)00154-5. [DOI] [PubMed] [Google Scholar]
- 152.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 153.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Song MB, Yu XJ, Zhu GX, Chen JF, Zhao G, Huang L. Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol. 2009;51:205–213. doi: 10.1016/j.vph.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Ahmad N, Wani MA, Ashraf M. Hepatocyte growth factor prevents ventricular remodeling and dysfunction in mice via Akt pathway and angiogenesis. J Mol Cell Cardiol. 2004;37:1041–1052. doi: 10.1016/j.yjmcc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 157.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 158.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khan M, Akhtar S, Mohsin S, S NK, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011;20:67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 160.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 161.Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, Kangtao M, Chen F, Zhou C. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 163.Huang W, Wang T, Zhang D, Zhao T, Dai B, Ashraf A, Wang X, Xu M, Millard RW, Fan GC, Ashraf M, Yu XY, Wang Y. Mesenchymal Stem Cells Overexpressing CXCR4 Attenuate Remodeling of Postmyocardial Infarction by Releasing Matrix Metalloproteinase-9. Stem Cells Dev. 2011 doi: 10.1089/scd.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zeng B, Lin G, Ren X, Zhang Y, Chen H. Over-expression of HO-1 on mesenchymal stem cells promotes angiogenesis and improves myocardial function in infarcted myocardium. J Biomed Sci. 2010;17:80. doi: 10.1186/1423-0127-17-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27:3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]