Abstract
Hepatocellular carcinoma (HCC), the most common primary liver tumor, is notoriously resistant to systemic therapies, and often recurs even after aggressive local therapies. HCCs rely on the formation of new blood vessels for growth, and VEGF is critical in this process. A hallmark of new vessel formation in tumors is their structural and functional abnormality. This leads to an abnormal tumor microenvironment characterized by low oxygen tension. The liver is perfused by both arterial and venous blood and the resulting abnormal microenvironment selects for more-aggressive malignancies. Anti-VEGF therapy with sorafenib was the first systemic therapy to demonstrate improved survival in patients with advanced-stage HCC. This important development in the treatment of HCC raises hope as well as critical questions on the future development of targeted agents including other antiangiogenic agents, which hold promise to further increase survival in this aggressive disease.
Introduction
Despite many treatment options for patients with early-stage hepatocellular carcinoma (HCC), the mortality rate remains high making HCC the third leading cause of cancer-related death worldwide.1 This high mortality rate reflects the poor prognosis for patients with advanced-stage HCC, the pattern of presentation, and the poor outcome associated with cirrhosis. Most patients present with advanced-stage disease, only 30% of patients present with resectable disease, and up to 80% have underlying cirrhosis.2 The treatment options in advanced-stage disease are limited, and the survival rate is dismal. Thus, novel therapeutic approaches are desperately needed.
Primary tumors of the liver can be classified as either benign or malignant and by the cell type of origin (mesenchymal or epithelial). HCC is the most frequently occurring type, accounting for 90% of all primary malignant liver cancers, but others include intrahepatic cholangiocarcinoma, mixed HCC and cholangiocarcinoma, angiosarcoma, hepatoblastoma, and epithelioid hemangioendothelioma.3 The growth of a liver tumor requires the formation of new blood vessels, which has provided a strong rationale for antiangiogenic strategies as therapy.4,5 Indeed, antiangiogenic agents that inhibit the VEGF pathway have been approved for cancer treatment (for example, sorafenib for advanced-stage HCC4 or bevacizumab in combination with chemotherapy for metastatic colorectal cancer7). Unfortunately, less than half of patients with advanced-stage HCC benefit from these therapies, and the benefits are transient.6 Finally, aggressive anti-vascular therapies are available for unresectable HCC—hepatic artery ligation (HAL) and transcatheter arterial chemoembolization (TACE). Unfortunately, aggressive tumor regrowth typically occurs, likely due to exacerbation of tumor hypoxia, surge in VEGF expression, and inflammation.8 However, judicious administration of anti-VEGF or anti-placental growth factor (PlGF) treatments can transiently ‘normalize’ the tumor vasculature,5,8 which could potentially enhance the efficacy of radiation and chemotherapy by alleviating hypoxia and tumor invasiveness.9,10
Two key challenges have hampered progress. First, modeling HCC in mice has been difficult. Ex vivo and subcutaneous in vivo models provide critical cell biology and response data, but do not capture the important interactions occurring between HCC cells and the inflammatory local and ‘distant’ (bone marrow-derived) stroma. Most models do not have underlying cirrhosis—a condition that occurs in 80% of human HCC. Given the critical role that inflammation has in the initiation of HCC—in particular interleukin (IL)-611—establishing novel models that capture the characteristics of human disease will be key for testing future therapies. Second, response assessment has been a challenge. Therapy-induced necrosis or vascular normalization may not lead to tumor shrinkage in HCC and can mask the therapeutic effects of antiangiogenic agents.12,13 Thus, establishing techniques that can measure and/or predict the antitumor effects of antiangiogenics will be critical for testing future therapeutic strategies.
We discuss the current understanding of new blood vessel formation in HCC, and review the cellular and molecular mechanisms involved, the insights that emerged from preclinical and clinical studies of antiangiogenic therapies, and the potential strategies and biomarkers for optimally developing novel antiangiogenic therapies.
Angiogenesis in HCC
Normal liver is organized in lobules segregated by interlobular connective tissue and containing ‘cords’ of hepatic parenchymal cells and hepatocytes, which surround a central vein and are separated by vascular sinusoids. Sinusoidal liver endothelium is fenestrated and lacks a basement membrane. The fenestrations permit blood plasma to surround the exposed surfaces of the hepatocytes through the space between the fenestrated endothelium and the cells—the space of Disse—which contains collagen fibers and fibroblasts. Liver perivascular cells (pericytes) are the hepatic stellate cells localized in the space of Disse. The stellate cells have a major role in liver fibrosis—the formation of scar tissue in response to liver damage. Kupffer cells (liver macrophages that take up and destroy the pathogens that enter the blood in the intestine) are also closely associated with the sinusoids. Blood from the portal vein and hepatic artery mixes together in the hepatic sinusoids, and after ‘filtration’ by hepatocytes drains out of the lobule through the central hepatic vein.
Liver tumors display marked vascular abnormalities. Aberrant microvasculature typically may seem ‘arterialized’ (tight vessels covered by smooth muscle cells) and/or ‘capillarized’ (capillaries without fenestration and with laminin basement membrane deposition),14 and is less dense than normal liver vasculature.15 Liver tumor vessels have an abnormal blood flow and are excessively leaky. In turn, this leads to hypovascular areas and severe hypoxia and/or necrosis—all hallmarks of liver tumors. Although HCC is a highly angiogenic cancer, it is characterized by hypoxia. Hypoxia may promote HCC growth and progression and resistance to therapies.16 Conversely, inducing vessel normalization and alleviating hypoxia delays HCC growth.5
Overexpression of VEGF leads to focal leaks in tumor vessels, causing nonuniform blood flow and heterogeneous delivery of drugs and oxygen.17 VEGF is largely responsible for abnormal structure and function of liver tumor vessels. In addition, VEGF can function as a cytokine and may directly affect the hepatic stellate cells, the Kupffer cells, hepatocytes or the cancer cells themselves if they depend on VEGF receptors for their survival or function.18,19 VEGF expression can be independently regulated by hypoxia and acidosis.20 VEGF expression is regulated by oncogenic gene mutations, hormones, cytokines and various signaling molecules (nitric oxide, MAP kinases).21–23 Moreover, VEGF may be released by stromal cells and from the extracellular matrix, the latter via matrix metalloproteinase (MMP)-9-mediated proteolysis.24,25 High VEGF expression is often seen in chronic liver disease.26
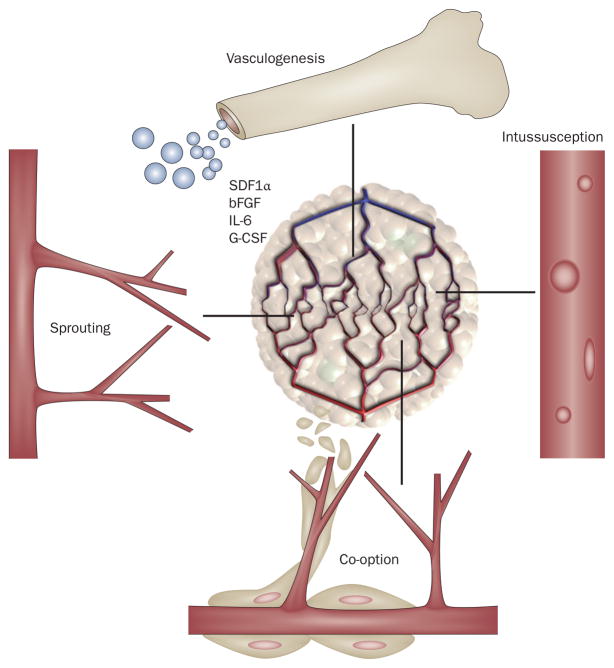
Solid tumors use different mechanisms such as sprouting, intussusception or co-option of local vasculature or incorporation of circulating vascular precursors to acquire new blood vessels (Figure 1).21 Owing to the heterogeneity of tumor endothelial cell phenotypes in HCC and the clear distinction between endothelial cells from the normal and malignant liver, it is conceivable that both local and circulating cells contribute to new vessel formation.8,27 Unfortunately, studying these mechanisms in liver cancer is a major challenge. First, preclinical models often fail to reproduce all features of human disease. Second, tumors have already induced new vessel formation at the time of diagnosis and/or surgery.
Figure 1.
Schematic representation of potential escape mechanisms from anti-VEGF therapy. HCCs might use four potential mechanisms to acquire new blood vessels for their growth and after VEGF blockade: co-option, angiogenesis (sprouting), vasculogenesis (bone-marrow-derived endothelial progenitor cell recruitment to increase the tumor vascular supply) and intussusception. SDF1α, bFGF, IL-6 and G-CSF are increased in the circulation of patients with HCC treated with anti-VEGF agents. These molecules may potentially contribute to HCC neovascularization during VEGF-pathway inhibition. Permission obtained from Nature Publishing Group © Carmeliet, P. & Jain, R. K. Nature 407, 249–257 (2000). Abbreviations: bFGF, basic fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; HCC, hepatocellular carcinoma; IL, interleukin; SDF1α, stromal-cell-derived factor 1α.
The molecular pathways involved in liver tumor angiogenesis are incompletely characterized. Currently, the main targets for the antiangiogenic agents in development for liver cancer therapy are VEGF and its receptors VEGFR1 and VEGFR2. However, an increasing number of molecular pathways involved in blood vessel formation have been identified. We discuss the key proangiogenic growth factors and inflammatory molecules identified in liver tumors (Boxes 1 and 2 and Supplementary Table 1 online).
Box 1. Molecular mechanisms of angiogenesis in liver.
The effects of VEGF are primarily mediated via VEGFR2 in endothelial cells.21,22,113 Tumor vessels dilate and become leaky in response to VEGF. MMPs, Ang2 and VEGF mediate the dissolution of the vascular basement membrane and the interstitial matrix. A variety of molecules promote endothelial proliferation, migration and assembly into vascular networks, including VEGF, Ang1, Ang2 and bFGF.21 Endothelial cell migration and spreading in response to growth factor signaling is mediated by αvβ5, αvβ3, and α5β1 integrins.114 Quiescent endothelial cells may survive for several years in the vessels of normal adult tissues. Soluble receptors for VEGF (VEGFR1 or NRP1115) sequester the ligands and reduce angiogenic activity. bFGF is a potent mitogen implicated in angiogenesis, but its role in liver cancer remains to be clarified. Other molecules involved in tumor angiogenesis are PlGF, IGF-I, PAI-1, NOS, COX2, TSP2, PDGF isoforms, and EGF.21,28,116 The Dll4/Notch pathway is a negative mediator of angiogenesis.117 Dll4 decreased the expression of VEGFR2 and its co-receptor NRP1.118,119 An anti-Dll4 antibody decreased endothelial cell proliferation and caused defective cell fate specification or differentiation, and led to tumor growth inhibition in several tumor models.120 Dll4/Notch1 signaling regulates the number of tip cells that control vessel sprouting and branching by restricting tip-cell formation in response to VEGF.121 Dll4 might have a role in the progression of liver tumors122 and may serve as a potential target. PI3K/Akt that is activated in endothelial cells123 is being explored as a target for HCC treatment. The levels of angiogenic molecules (VEGF, soluble VEGFR1, PlGF and bFGF) in circulating blood from cancer patients significantly change in response to anti-VEGF treatment.102
Abbreviations: Ang, angiopoietin; bFGF, basic fibroblast growth factor; COX2, cyclooxygenase-2; Dll4, delta-like protein 4; IGF-I, insulin-like growth factor 1; MMP, matrix metalloproteinase; NOS, nitric oxide synthase; NRP, neuropilin; PAI-1, plasminogen activator inhibitor 1; PDGF, platelet-derived growth factor; PIGF, placental growth factor; TSP, thrombospondin.
Box 2. Inflammatory molecules and their potential role in liver cancer angiogenesis.
Chronic inflammation is a potential precursor of liver carcinogenesis.11,124 In liver cancer, NFκB is involved in tumor initiation and progression mediated via STAT3 activation.125–127 Inflammatory cytokines induced by NFκB might affect angiogenesis directly via endothelial cells, or indirectly by cancer cells or recruitment and/or activation of inflammatory cells.8,128 IL-1α has a critical role129 by recruitment of inflammatory cells.130 TNF-α can also promote tumor progression by different pathways: direct effect on tumor cells, induction of CXCR4 and stimulation of epithelial–mesenchymal transition.131 TNF-α promotes cell survival and angiogenesis or induces endothelial cell apoptosis, and vascular disruption and increased permeability. IL-6 is induced by NFκB and other transcription factors (C/EPBb and AP-1), and modulates inflammation via IL-6R and gp130. Smooth muscle cells, T cells and macrophages secrete IL-6 to stimulate immune responses and promote inflammation. IL-6 may also have anti-inflammatory effects by inhibition of TNF-α and IL-1, and activation of IL-1Ra and IL-10. The proliferative and survival effects of IL-6 are mediated by STAT3.11 In HCC, IL-8 may have a role in cell invasion.132,133 IL-8 can promote tumorigenesis and angiogenesis through CXCR1 and CXCR2, and the Duffy antigen receptor for cytokines, which has no defined intracellular signaling capabilities.134 Overexpression of VEGF induces SDF1α expression, and SDF1α and CXCR4135 may drive cell migration and angiogenesis by VEGF-independent mechanisms.136 SCF is the ligand for c-KIT, primarily expressed by early hematopoietic precursors. While c-KIT expression is rarely detectable in HCC, both SCF and c-KIT are expressed during cholangiocarcinogenesis.137
Abbreviations: AP-1, activator protein 1; C/EPB, CAAT/enhancer binding-protein; CXCR, CXC-chemokine receptor; HCC, hepatocellular carcinoma; IL, interleukin; NFκB, nuclear factor κB; SCF, stem cell factor; SDF1α, stromal-cell-derived factor 1α; STAT, signal transducers and activators of transcription; TNF, tumor necrosis factor.
Angiogenesis and clinical outcomes
Angiogenesis is initiated by destabilization of existing microvasculature, which leads to vascular hyper-permeability, remodeling of the extracellular matrix, and endothelial cell activation. Upon activation, the endothelial cells proliferate, migrate, and undergo cord formation to form new vessels. Subsequent activation and recruitment of pericytes stabilize the new blood vessels.22,28,27 During angiogenesis, the expression of proangiogenic factors is balanced by release of antiangiogenic molecules.30 In HCC, a net excess of angiogenic factors produced by tumor cells, vascular endothelial cells, immune cells and pericytes tips this balance leading to the activation and recruitment of endothelial cells and pericytes.4,31 The plasma concentration of proangiogenic growth factors VEGF, angiopoietin-2 (Ang2), and platelet-derived growth factor (PDGF)-B is increased in patients with HCC compared with cirrhotic patients.32 Other angiogenic factors potentially involved in liver cancer are PlGFs, basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-α, TGF-β, hepatoctye growth factor (HGF), EGF, IL-4, IL-6 and IL-8 (Boxes 2 and 3).30
Box 3. Sorafenib in HCC.
Sorafenib is an oral multikinase inhibitor that inhibits VEGFR1, VEGFR2, VEGFR3 and PDGFR-α, PDGFR-β, c-KIT, Raf-1 and BRAF. Early evidence of antitumor activity was observed from a phase II study of 137 patients with advanced HCC: TTP was 4.2 months and overall survival 9.2 months.13 An international phase III trial (SHARP) subsequently demonstrated improved overall survival and TTP. Median survival was 10.7 months in the sorafenib arm versus 7.9 months in the placebo group. Median TTP was 5.5 months in the sorafenib arm versus 2.8 months in the placebo group.6 The magnitude of this benefit was similar in another phase III study conducted in Asia in patients with advanced-stage HCC. Overall survival was 6.5 months in the sorafenib group versus 4.2 months in the placebo group.57 The typical response rates for sorafenib in advanced-stage HCC are extremely low (2–3% as evaluated by RECIST). However, tumor necrosis has been reported in those treated with sorafenib, indicating that RECIST may not be an appropriate end point for antiangiogenics in HCC. Toxic effects associated with sorafenib are generally manageable. Grade 3 adverse events included hand–foot skin reactions, diarrhea, and fatigue. No prospective data are available regarding the efficacy and toxicity of sorafenib in patients with HCC with worsening underlying hepatic dysfunction. No validated biomarker is available to predict the clinical benefits from sorafenib. The efficacy of sorafenib in the adjuvant setting or in combination with molecularly targeted agents or chemotherapy remains unknown. Ongoing phase III studies (NCT01004978, NCT00692770, NCT00901901, and NCT01075555) will hopefully provide insight into these critical issues.
Abbreviations: PDGFR, platelet-derived growth factor receptor; HCC, hepatocellular carcinoma; TTP, time to tumor progression
The expression of VEGF and its receptors, which include VEGFR1, VEGFR2, and VEGFR3, is elevated in HCC cell lines and tissues, as well as in the blood circulation in patients with HCC.32–35 The increase in VEGF expression is seen in cirrhotic and dysplastic liver tissues, suggesting a possible role for VEGF-driven angiogenesis in hepatocarcinogenesis.36 One study found that VEGF levels were progressively increased through the successive steps of low-grade dysplasia, high-grade dysplasia, and early-stage HCC.37 In addition, elevated VEGF expression is linked with high HCC tumor grade, vascular invasion, and portal vein invasion.38–41
A poor prognosis for patients with HCC is correlated with elevated circulating VEGF levels after surgery, radiofrequency ablation (RFA) or TACE.42–49 Similarly, high levels of VEGF in HCC tissues correlated with rapid tumor recurrence in patients with HCC.50–54 There are limited studies on other angiogenic factors as prognostic biomarkers. For example, rapid recurrence after therapy has been linked with higher PlGF, platelet-derived endothelial cell growth factor (PD-ECGF), MMP-2, Ang2 and hypoxia-inducible factor (HIF)-1α levels.50,51,55,56
VEGF is a critical player in liver cancer angiogenesis, and its elevation in tumor tissue or in circulation correlates with more-aggressive disease. Thus, future studies should identify and characterize these pathways, with the goal of targeting inherent or acquired resistance to anti-VEGF therapies.
Antiangiogenic therapy of liver cancer
A large number of antiangiogenic agents are currently being tested for the treatment of HCC. We discuss the experience with agents that have reached more advanced phases of development (Table 1).
Table 1.
Antiangiogenic agents in development for HCC*
| Agent and manufacturer | Drug targets | Stage of development (NCI trial identifier) |
|---|---|---|
| Sorafenib (Nexavar, Bayer and Onyx)7,57 | Oral multikinase inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR-α, PDGFR-β, Raf-1, p38MAPK, Flt-3, c-KIT, RET | Approved for the treatment of HCC |
| Brivanib (BMS-582664, Bristol-Myers Squibb)91 | Oral TKI against VEGFR2 and FGFR-1 | Phase II–III (NCT00858871, NCT00825955, NCT01108705) |
| Linifanib (ABT-869, Abbot)107 | Oral selective TKI against VEGFR, PDGFR | Phase II–III (NCT01009593) |
| Pazopanib (GW786034)84 | Oral TKI targeting VEGFR, PDGFR, and c-KIT | Phase I |
| Vatalanib‡ (PTK787, Novartis)87,88 | Oral tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, c-KIT | Phase I–II |
| Cediranib (AZD2171, Recentin, AstraZeneca)83 | Oral tyrosine kinase inhibitor of VEGFR1, VEGFR2, VEGFR3 | Phase II (NCT00427973) |
| Ramucirumab (IMC-1121B, ImClone Systems and Eli Lilly)71 | Recombinant human monoclonal antibody that binds to the extracellular domain of the VEGFR2 | Phase II–III (NCT00627042) |
| TSU-68 (SU6668, Taiho)92 | Oral angiogenesis inhibitor targeting VEGFR, PDGFR, and FGFR | Phase I–II (NCT00784290) |
| Vandetanib (ZD6474, Zactima, AstraZeneca) | Oral dual inhibitor of VEGFR and EGFR | Phase II (NCT00508001) |
| Foretinib (GSK1363089; XL-880, GlaxoSmithKline) | Oral dual inhibitor of VEGFR and c-Met | Phase II (NCT00920192) |
See Supplementary Tables 2 and 3 online for sunitinib and bevacizumab studies.
Discontinued. Abbreviations: FGFR, fibroblast growth factor receptor; HCC, hepatocellular carcinoma; NCI, National Cancer Institute; PDGFR, platelet-derived growth factor receptor; TKI, tyrosine kinase inhibitor.
Sorafenib and sunitinib
Sorafenib, a multi-targeted tyrosine kinase inhibitor (TKI) approved by the FDA for patients with advanced-stage renal cell carcinoma, is the first systemic therapy to improve survival in phase III trials of patients with advanced-stage HCC (Box 3).6,57 The exact mechanism by which sorafenib benefits patients with advanced-stage HCC remains unknown.
Sorafenib targets VEGF receptors, and is now thought to exert its effect primarily by blocking VEGF signaling, as its efficacy against BRAF is questionable.58 However, sorafenib has a moderate anti-VEGFR2 activity. Since sorafenib has demonstrated improved overall survival benefits in patients with advanced-stage HCC, its potential value in early-stage disease is being assessed. One such setting is after TACE, to counteract the surge in VEGF,46,47 and sorafenib is being tested either concurrently or after TACE in clinical trials.59 An ongoing randomized phase III trial of adjuvant sorafenib will test if this agent reduces the high recurrence rates of HCC after surgical resection. However, it should be noted that anti-VEGF therapy has failed to show benefit in the adjuvant setting in colorectal cancer, despite its efficacy in metastatic disease.60,61
Sunitinib is an oral multi-targeted TKI with more potent activity against VEGFR1 and VEGFR2 compared with sorafenib. It also targets PDGFR-α, PDGFR-β, c-KIT, FLT3, RET and other kinases.62–64 Currently, clinical data of sunitinib efficacy in HCC are based on four single-arm phase II studies that used three different dose schedules (Supplementary Table 2 online).12,65–67 Three of the studies used the standard 4-weeks-on, 2-weeks-off regimen (6 weeks per cycle), which was efficacious in patients with renal cell carcinoma and gastrointestinal stromal tumors.68,69 The studies showed activity for sunitinib in advanced-stage HCC, but indicated that the higher 50 mg dose may not be well tolerated in this patient population.12,65,66 Koeberle and colleagues reported that continuous 37.5 mg daily dosing has comparable safety and efficacy profiles to the intermittent regimens (Supplementary Table 2 online).67 To date, no randomized study has compared directly the intermittent versus continuous schedule for efficacy and tolerability. Nevertheless, a randomized phase III study comparing sunitinib with sorafenib in advanced-stage HCC (SUN 1170) used the continuous daily dosing of 37.5 mg of sunitinib. This was based on preclinical results and anecdotal clinical evidence that intermittent regimens may promote tumor progression during treatment breaks.70 While these observations await confirmation in controlled clinical trials, the SUN 1170 trial was stopped early because of a higher incidence of serious adverse events in the sunitinib arm, and because sunitinib did not demonstrate superiority or non-inferiority to sorafenib. Since the full dataset from this trial are not available, it remains unknown if the toxicity associated with this dose schedule and study design contributed to the failure of sunitinib in this study. However, further development of sunitinib in HCC is unlikely. This failure raises important questions regarding the mechanism of action and predictive biomarkers for antiangiogenic agents in this tumor type. Answering these questions will be critical for the development of other anti-VEGF agents.
Specific or selective VEGFR blockers
Ramucirumab (IMC-1121B, ImClone Systems and Eli Lilly, NJ, USA) is a recombinant human monoclonal antibody that binds to the extracellular domain of VEGFR2. Intravenous ramucirumab given biweekly at a dose of 8 mg/kg in patients with advanced-stage HCC showed a median progression-free survival (PFS) of 4.0 months and median overall survival of 12 months with limited toxic effects in a single-arm phase II study.71 A phase III study of best supportive care plus ramucirumab or placebo in patients with advanced-stage HCC who failed to respond to sorafenib (REACH trial) is planned (Table 1).
Bevacizumab is a recombinant, humanized mono-clonal antibody that targets VEGF, and is approved by the FDA for the treatment of advanced-stage colorectal, lung, breast, renal and brain cancers. In addition to its direct antiangiogenic effects, bevacizumab may enhance chemotherapy administration by ‘normalizing’ tumor vasculature and decreasing the elevated interstitial pressure in tumors.9,10,72,73 Several studies have explored the use of bevacizumab either as a single agent or in combination with cytotoxic or molecular-targeted agents in patients with advanced-stage HCC (Supplementary Table 3 online).74–79 As a single agent, bevacizumab administered intravenously once every 2 weeks at 5 mg/kg or 10 mg/kg produced a median PFS of 6.9 months and median overall survival of 12.4 months in patients with HCC.74 Bevacizumab combined with gemcitabine and oxaliplatin (GEMOX-B) produced a median PFS of 5.3 months and overall survival of 9.6 months in advanced-stage HCC.75 Bevacizumab and erlotinib produced a median PFS of 9 months and overall survival of 15 months in patients with advanced-stage HCC.79 Despite the early evidence of activity, no registration study is currently planned for bevacizumab in patients with HCC.
Linifanib (ABT-869, Abbott Laboratories, IL, USA) is a TKI that has potent activity against VEGFR and PDGFR.80 Preliminary data from an open-label, multicenter phase II study of linifanib given at 0.25 mg/kg daily in patients with advanced-stage HCC showed a median time to tumor progression (TTP) of 3.7 months and overall survival of 9.7 months, with a tolerable safety profile.81 This finding has encouraged further development of linifanib in HCC, and a phase III study comparing linifanib with sorafenib is ongoing (Table 1).
Cediranib (AZD2171, AstraZeneca, Cheshire, UK) is an oral pan-VEGFR TKI with activity against PDGFR and c-KIT. Cediranib is a potent inhibitor of both VEGFR2 and VEGFR1.82 A small phase II trial of daily cediranib at a dose of 45 mg showed a high rate of grade 3 adverse effects (primarily fatigue), which frequently lead to treatment discontinuation.83 Another phase II study of cediranib at 30 mg daily in patients with HCC conducted at our institution is ongoing, and the results are pending (Table 1).
Pazopanib (GW786034, GlaxoSmithKline, Brentford, UK) is an oral TKI that targets VEGFRs, PDGFRs, and c-KIT, and was recently approved by the FDA for advanced-stage renal cell carcinoma. A phase I study determined the maximum tolerated dose (MTD) of 600 mg once daily for pazopanib in advanced-stage HCC. The median TTP was 137.5 days.84
Vatalanib (PTK787, Novartis, Basel, Switzerland) is an oral TKI which targets VEGFRs, PDGFRs, and c-KIT.85,86 A phase I study of single-agent vatalanib given at 750 mg or 1,250 mg per day induced stable disease in nine of 18 patients with unresectable HCC who were evaluable for response.87 A phase I–II study of daily vatalanib with doxorubicin (60 mg/m2 every 3 weeks) in advanced-stage HCC showed that patients treated at the MTD for vatalanib had a median PFS of 5.4 months and overall survival of 7.3 months.88 Vatalanib development has been discontinued due to an industry decision.
Dual blockers of VEGF and bFGF pathways
Brivanib alaninate (Bristol-Myers Squibb, NJ, USA) and TSU-68 (SU6668, Taiho Pharmaceutical, Tokyo, Japan) are dual inhibitors of VEGF and FGF receptors. Preclinical reports showed that brivanib treatment can inhibit HCC growth and that TSU-68 can normalize tumor vasculature in mouse xenograft models.89,90 A phase II study was conducted to assess the efficacy and safety of daily brivanib (800 mg) in patients with advanced-stage HCC. In patients who had received no prior systemic therapy, a median overall survival of 10 months, TTP of 2.8 months and manageable adverse effects were reported.91 A phase I–II trial of TSU-68 in heavily pretreated patients with advanced-stage HCC established the MTD at 200 mg twice daily and showed a median TTP of 2.1 months and survival of 13.1 months.92 Currently, brivanib is being evaluated in phase III studies in the first-line setting versus sorafenib, and in the second-line setting in patients with sorafenib-refractory advanced-stage HCC (Table 1).
Multitargeted inhibitors of VEGFR
Vandetanib (ZD6474, AstraZeneca, Cheshire, UK) is a TKI with activity against VEGFR2, EGFR and RET. A randomized phase II study of vandetanib in advanced-stage HCC is ongoing (Table 1). Foretinib (GSK1363089, XL-880, GlaxoSmithKline, Brentford, UK) is an oral TKI that selectively inhibits c-Met and VEGFR2. A phase I study of foretinib has established the MTD at 240 mg, given on the first 5 days of a 14-day cycle.93 A phase I–II study of foretinib in advanced-stage HCC is ongoing (Table 1).
Toxic effects of antiangiogenic therapy
With the increasing use of antiangiogenic therapy, certain ‘class’ toxicity profiles have emerged, which include hypertension, bleeding, thromboembolic events and proteinuria. Other toxic effects are more specific for TKIs, such as hand–foot skin reaction and rash. Whether any of these adverse effects are associated with clinical outcome remains to be determined in future trials.
Biomarkers: progress and challenges
Antiangiogenic therapies have brought new promise for HCC therapy, but have also changed the needs and expectations of how imaging modalities can be used to determine the efficacy of these treatments. This is because the mechanisms of action of these new agents are inconsistent with the assessment of response by RECIST.94–96 For example, if these therapies cause tumor necrosis this effect may induce no shrinkage or even an apparent enlargement of the tumor due to cystic changes and edema.97 Therefore, the European Association for the Study of the Liver (EASL) guidelines recommended that assessment of tumor response should incorporate the reduction in viable tumor burden.98 However, whether the current imaging techniques allow consistent quantification of tumor necrosis and if this is a meaningful end point after antiangiogenic therapy in HCC remains unclear.
The structural and functional abnormalities of tumor vessels may be reversed by antiangiogenic therapies.9 Detecting these responses requires functional, ‘vascular’ imaging. Functional imaging has conventionally been the domain of nuclear medicine. However, the high spatial resolution, easy availability and technologic innovations in imaging have opened the doors for establishing techniques such as dynamic contrast-enhanced (DCE) MRI, perfusion CT, and DCE ultrasonography to evaluate treatment response. On contrast-enhanced CT and MRI, tumor enhancement characteristics are influenced by several parameters such as blood flow, blood volume fraction, blood vessel permeability and distribution volume fraction. However, the tumor physiologic features can be quantified by applying appropriate mathematic modeling (Box 4).99
Box 4. Functional imaging of tumor vasculature in hepatocellular carcinoma.
Perfusion CT (CTp) is being increasingly used for quantification of tumor vascular density and angiogenesis, and may permit evaluation of tumor response to antiangiogenic agents.138–140 In advanced disease, CTp after bevacizumab has shown significant decreases in tumor blood flow, blood volume, and permeability-surface area and an increase in mean transit time (MTT). Moreover, baseline MTT values and the change after bevacizumab correlated with a better clinical outcome.141 These changes are tumor specific as the HCCs exhibit substantial changes in their perfusion parameters such as Ktrans and blood volume after bevacizumab treatment without any significant changes in these parameters in vessels of the caudate lobe and spleen.142 Similarly, the contrast-enhancement patterns in HCC obtained by dynamic contrast-enhanced (DCE) MRI are influenced by tumor angiogenesis and correlate with tumor microvascular density and VEGF expression.143 Thus, suppression of tumor vascular permeability induced by antiangiogenic agents can be reliably detected and quantified by DCE MRI. For example, sunitinib treatment in patients with advanced-stage HCC led to rapid and significant decreases in Ktrans.12 The extent of decrease in Ktrans was substantially higher in patients who experienced partial response or stable disease compared with that in patients with progressive disease or who died during the first two cycles of therapy.12 This is consistent with the effects of antiangiogenic agents in recurrent glioblastoma and the potential predictive biomarker value of the rapid decrease in Ktrans.144,145 Data are emerging to support DCE ultrasonography (DCE US) as a valuable and less expensive second level imaging modality.146 Quantitative functional evaluation by DCE US performed at day 3 and 14 was able to predict response at 2 months in patients with HCC treated by bevacizumab.147
Despite this progress, important challenges remain with the use of these imaging biomarkers. First, there is no consensus on how to use CT to assess response to antiangiogenic therapies in liver tumors.100 Estimates of viable tumor volume or extent of tumor necrosis in HCC to predict the outcome of patients after antiangiogenic treatment are promising.101 The process of estimation of tumor volume-—although feasible on all commercially available image-processing workstations—is not fully automated and demands expertise and dedicated personnel. Therefore, it is not currently integrated into routine oncologic imaging workflow. The novel antiangiogenic agents currently in clinical development vary in their ability to induce tumor necrosis, which adds to the complexity of obtaining total liver tumor volume as a surrogate end point.94 Likewise, imaging of tumor angiogenesis and vascular responses to antiangiogenic therapies will require routine availability of state-of-the-art dynamic imaging technologies and local expertise, robust and reliable analysis of results and a mandatory customization of imaging protocols in clinical trials. Finally, due to the inherent complexity of these novel imaging modalities and high costs, it remains a challenge how to integrate these methods in large phase III studies to prospectively validate some of these potentially useful imaging end points and biomarkers.
Future directions
Future research needs to improve our understanding of antiangiogenic therapy for HCC. While most pharmaceutical companies are developing selective or potent anti-VEGF agents, it is likely that progress will come from the use of agents targeting multiple proangiogenic factors (for example, bFGF, c-Met, Ang2, PlGF, stromal-cell-derived factor 1α [SDF1α]). The paucity of data from preclinical models limits our understanding of the relevance of these targets in HCC. Nevertheless, several trials with agents targeting VEGF and FGFR or c-Met are underway. Novel strategies combining antiangiogenic agents with chemotherapy or other molecular-targeted agents are urgently needed. However, neither sorafenib nor any of the other anti-VEGFR TKIs under development in HCC has shown an increase in survival when combined with chemotherapy. Predictive biomarkers are urgently needed for antiangiogenic therapy.102 Circulating biomarkers show promise in identifying patients most likely to benefit from antiangiogenic therapies: changes in α-fetoprotein (AFP), IL-6, SDF1α, soluble c-KIT, soluble VEGFR1, VEGF-C, IL-8, TNF-α, Ang2, soluble VEGFR2, collagen IV and in circulating monocytes and circulating progenitor cells have been shown in exploratory studies to associate with outcome of treatment in HCC (Table 2). These biomarker candidates need to be validated in large prospective studies.
Table 2.
Blood-based biomarkers for antiangiogenic therapy in advanced HCC
| Biomarker candidate | Agent | Correlation with outcome |
|---|---|---|
| AFP12,108 | Sorafenib, bevacizumab, sunitinib | Greater decrease in serum AFP; better PFS and OS |
| IL-612 | Sunitinib | Greater increase correlates with lower survival |
| SDF1α12 | Sunitinib | Greater increase correlates with lower survival |
| Soluble c-KIT12 | Sunitinib | Greater decrease on treatment improved PFS and OS |
| Soluble VEGFR112 | Sunitinib | Elevated levels at progression |
| VEGF-C109 | Sunitinib | Elevated levels in responders |
| IL-812,110 | Sunitinib | Elevated levels correlate with a shorter PFS and OS |
| TNF-α12 | Sunitinib | Elevated levels correlate with a shorter OS |
| Ang2111 | Bevacizumab | Higher Ang2 and decreased OS |
| Soluble VEGFR2111 | Bevacizumab | Higher sVEGFR2 and decreased PFS |
| Collagen IV91 | Brivanib | Greater decrease in collagen IV correlates with PFS and OS |
| Circulating monocytes105 | Sunitinib | Greater decrease; longer PFS and OS |
| Circulating progenitor cells12,112 | Sunitinib | High values on treatment correlate with a low OS; high baseline correlates with a low OS and PFS |
Abbreviations: AFP; α-fetoprotein; Ang2, angiopoietin-2; HCC, hepatocellular carcinoma; IL, interleukin; OS, overall survival; PFS, progression-free survival; SDF1α, stromal-cell-derived factor 1α; TNF, tumor necrosis factor.
The critical importance of biomarker discovery and validation for antiangiogenic agents in advanced-stage HCC is exemplified by the following: first, our poor understanding of the mechanism by which sorafenib benefits patients; second, the recent failure of sunitinib; third, the largely equivalent and modest efficacy observed in all phase II trials of other anti-VEGF agents conducted to date; and finally, the serious toxic effects and the high costs of these therapies. Unfortunately, the limited resources continue to be a challenge for conducting clinical trials incorporating biomarker studies in HCC.
There is an urgent need to identify ‘druggable’ primary and acquired resistance and/or escape pathways in relevant preclinical models of HCC, in order to guide the design of improved treatment strategies. HCC etiology is inextricably linked to inflammation, as a result of focal hypoxia and necrosis inside these tumors and by enhanced expression of VEGF and other cytokines.103 Cytokines may be important in recruiting circulating progenitor cells to tumor tissue.104 Indeed, VEGF blockade by sunitinib affected both the tumor vasculature and the ‘distant stroma’, that is, bone marrow-derived progenitor cells and their progeny in advanced HCC (Figure 1).12,105
The time-dependent changes in the number of circulating progenitor cells in the blood, and the plasma concentration of IL-6 and SDF1α after sunitinib significantly correlated with outcome.12 Circulating progenitor cells were considerably decreased by sunitinib, probably due to additional inhibition of c-KIT and FLT3 in hematopoietic progenitor or stem cells.106 Hematologic toxic effects are frequent side effects of anti-VEGF agents. Indeed, sunitinib significantly and rapidly decreased all myeloid and lymphoid circulating cell populations.106 The extent of the early decrease in neutrophils, platelets and monocytes, as well as the development of nonhematologic toxic effects (skin toxicities), was significantly associated with improved survival outcomes.106 These observations suggest that the effects of these types of agents on the hematopoietic system are rapid, may be directly related to their activity in advanced-stage HCC, and could potentially be used to predict survival outcomes in advanced-stage HCC. This paradigm has been proposed for other toxic effects such as hypertension or skin toxicity, and deserves further investigation given the role of inflammation in liver cancer. In particular, mechanistic preclinical and clinical studies should determine how this information could be used therapeutically. For example, should anti-VEGF therapy be combined with anti-inflammatory agents or anti-SDF1α or anti-CXCR4 agents to go beyond what is achievable with anti-VEGF agents alone?
Conclusions
Approval of sorafenib for HCC has opened a new era for antiangiogenic therapies in this disease, which is notoriously resistant to systemic therapies. However, the initial enthusiasm has been tempered by recent failures or modest efficacy of other antiangiogenic agents. This underscores the need for thorough, mechanistic investigations in relevant preclinical models and well-designed, randomized studies of this highly heterogeneous disease. These approaches should lead to a better selection of patients for antiangiogenic therapy based on biomarkers, and should provide critical insight into the mechanisms of resistance, thus facilitating the discovery of new targets. In turn, this may finally allow us optimize the current therapies for this dreadful disease.
Supplementary Material
Key points.
Hepatocellular carcinoma (HCC) is a heterogeneous disease with multiple etiologies that is uniformly fatal when unresectable; other malignant liver tumors include cholangiocarcinoma, angiosarcoma, hemangioendothelioma and hepatoblastoma
The growth of HCCs depends on their ability to recruit blood vessels by forming new vessels through sprouting (angiogenesis) and potentially by recruiting proangiogenic bone marrow-derived cells
Tumor neovasculature is highly abnormal, both structurally and functionally because of overexpression of VEGF; other molecules are also involved and may be important therapeutic targets
Sorafenib was developed as a VEGFR2, VEGFR3, PDGFR-β, and Raf/MEK/ERK signaling inhibitor; despite being standard of care in advanced-stage HCC, its mechanism of action remains unknown
Antiangiogeneic agents can transiently prune and normalize the tumor vasculature and improve the outcome of other treatments (chemotherapy, radiation) given during the normalization window
Circulating and imaging markers may be useful as pharmacodynamic end points, and may hold promise as potential surrogate and predictive markers for antiangiogenic therapy
Review criteria.
Information on antiangiogenesis in hepatocellular carcinoma (available from the NIH databases) and the publications related to clinical studies were retrieved from the NIH website (www.clinicaltrials.gov). PubMed was searched for studies of angiogenesis and antiangiogenic agents published before 6 January 2011, including early-release publications. Search terms included “hepatocellular carcinoma”, “clinical trial”, “biomarker”, “anti-angiogenesis”, “anti-vascular”, “imaging”, and “tyrosine kinase inhibitor”. Full articles were checked for additional material when appropriate. Data published in abstract form from ASCO meetings from 2006 to 2010 and the 2009 Radiological Society of North America (RSNA) meeting were also included.
Acknowledgments
The authors acknowledge support by the National Institutes of Health (P01CA80124, R01CA115767, R21CA139168, M01RR01066, Federal Share/NCI Proton Beam Program Income Grants); by a Department of Defense Breast Cancer Research Innovator award (W81XWH-10-1-0016) and by an American Cancer Society Research Grant (RSG-11-073-01-TBG).
Footnotes
Competing interests
A. X. Zhu declares associations with the following companies: Bayer, Novartis, Pfizer, Sanofi-Aventis. R. K. Jain declares associations with the following companies: Astellas, AstraZeneca, Dyax, Fibrogen, Genzyme, MedImmune, MorphoSys, Noxxon, Regeneron, SynDevRx. See the article online for full details of the relationships. The other authors declare no competing interests.
Author contributions
A. X. Zhu and R. K. Jain contributed equally to the preparation of this manuscript. All authors contributed to researching data for the article, discussions of content, writing of the manuscript and to review and editing of the article before submission.
Supplementary information is linked to the online version of the paper at www.nature.com/nrclinonc
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892–2899. doi: 10.1200/JCO.2005.03.196. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Kemeny N, Lawrence TS. In: Cancer, Principles and Practice of Oncology. 6. DeVita VT, Hellman S, Rosenberg SA, editors. Lippincott Williams and Wilkins; Philadelphia, PA, USA: 2001. pp. 1162–1203. [Google Scholar]
- 4.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 5.Van de Veire S, et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. doi: 10.1016/j.cell.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Sun HC, Tang ZY. Angiogenesis in hepatocellular carcinoma: the retrospectives and perspectives. J Cancer Res Clin Oncol. 2004;130:307–319. doi: 10.1007/s00432-003-0530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 10.Jain RK. Taming vessels to treat cancer. Sci Am. 2008;298:56–63. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
- 11.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhu AX, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou-Alfa GK, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 14.Yang ZF, Poon RT. Vascular changes in hepatocellular carcinoma. Anat Rec (Hoboken) 2008;291:721–734. doi: 10.1002/ar.20668. [DOI] [PubMed] [Google Scholar]
- 15.Fukumura D, Yuan F, Monsky WL, Chen Y, Jain RK. Effect of host microenvironment on the microcirculation of human colon adenocarcinoma. Am J Pathol. 1997;151:679–688. [PMC free article] [PubMed] [Google Scholar]
- 16.Wu XZ, Xie GR, Chen D. Hypoxia and hepatocellular carcinoma: the therapeutic target for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1178–1182. doi: 10.1111/j.1440-1746.2007.04997.x. [DOI] [PubMed] [Google Scholar]
- 17.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeCouter J, et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenberger BM, et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 20.Fukumura D, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 21.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 22.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 23.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 24.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 25.Bergers G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amarapurkar AD, Amarapurkar DN, Vibhav S, Patel ND. Angiogenesis in chronic liver disease. Ann Hepatol. 2007;6:170–173. [PubMed] [Google Scholar]
- 27.Ho JW, et al. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836–843. doi: 10.1002/hep.21353. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 30.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Roberts LR, Gores GJ. Emerging drugs for hepatocellular carcinoma. Expert Opin Emerg Drugs. 2006;11:469–487. doi: 10.1517/14728214.11.3.469. [DOI] [PubMed] [Google Scholar]
- 32.Mas VR, Maluf DG, Archer KJ, Yanek KC, Fisher RA. Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation. 2007;84:1262–1271. doi: 10.1097/01.tp.0000287596.91520.1a. [DOI] [PubMed] [Google Scholar]
- 33.Poon RT, et al. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298–304. doi: 10.1016/s0002-9610(01)00708-5. [DOI] [PubMed] [Google Scholar]
- 34.Poon RT, et al. Serum vascular endothelial growth factor predicts venous invasion in hepatocellular carcinoma: a prospective study. Ann Surg. 2001;233:227–235. doi: 10.1097/00000658-200102000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar DK, et al. Requisite role of VEGF receptors in angiogenesis of hepatocellular carcinoma: a comparison with angiopoietin/Tie pathway. Anticancer Res. 2002;22:379–386. [PubMed] [Google Scholar]
- 36.El-Assal ON, et al. Clinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liver. Hepatology. 1998;27:1554–1562. doi: 10.1002/hep.510270613. [DOI] [PubMed] [Google Scholar]
- 37.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi R, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- 39.Li XM, Tang ZY, Zhou G, Lui YK, Ye SL. Significance of vascular endothelial growth factor mRNA expression in invasion and metastasis of hepatocellular carcinoma. J Exp Clin Cancer Res. 1998;17:13–17. [PubMed] [Google Scholar]
- 40.Yao DF, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:220–226. [PubMed] [Google Scholar]
- 41.Zhou J, et al. Expression of platelet-derived endothelial cell growth factor and vascular endothelial growth factor in hepatocellular carcinoma and portal vein tumor thrombus. J Cancer Res Clin Oncol. 2000;126:57–61. doi: 10.1007/s004320050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon RT, et al. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 2004;91:1354–1360. doi: 10.1002/bjs.4594. [DOI] [PubMed] [Google Scholar]
- 43.Chao Y, et al. Prognostic significance of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin in patients with resectable hepatocellular carcinoma after surgery. Ann Surg Oncol. 2003;10:355–362. doi: 10.1245/aso.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Tamesa T, et al. High serum levels of vascular endothelial growth factor after hepatectomy are associated with poor prognosis in hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1122–1126. [PubMed] [Google Scholar]
- 45.Xiong HQ, et al. A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs. 2004;22:459–466. doi: 10.1023/B:DRUG.0000036688.96453.8d. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–2882. doi: 10.3748/wjg.v10.i19.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shim JH, et al. Association between increment of serum VEGF level and prognosis after transcatheter arterial chemoembolization in hepatocellular carcinoma patients. Cancer Sci. 2008;99:2037–2044. doi: 10.1111/j.1349-7006.2008.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sergio A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914–921. doi: 10.1111/j.1572-0241.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 49.Poon RT, et al. High serum vascular endothelial growth factor levels predict poor prognosis after radiofrequency ablation of hepatocellular carcinoma: importance of tumor biomarker in ablative therapies. Ann Surg Oncol. 2007;14:1835–1845. doi: 10.1245/s10434-007-9366-z. [DOI] [PubMed] [Google Scholar]
- 50.Cui J, Dong BW, Liang P, Yu XL, Yu DJ. Effect of c-myc, Ki-67, MMP-2 and VEGF expression on prognosis of hepatocellular carcinoma patients undergoing tumor resection. World J Gastroenterol. 2004;10:1533–1536. doi: 10.3748/wjg.v10.i10.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu J, et al. High expressions of vascular endothelial growth factor and platelet-derived endothelial cell growth factor predict poor prognosis in alpha-fetoprotein-negative hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol. 2009;135:1359–1367. doi: 10.1007/s00432-009-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon JI, et al. Expression of vascular endothelial growth factor (VEGF) family members and prognosis after hepatic resection in HBV-related hepatocellular carcinoma [Korean] Korean J Hepatol. 2008;14:185–196. doi: 10.3350/kjhep.2008.14.2.185. [DOI] [PubMed] [Google Scholar]
- 53.Jeng KS, et al. Prognostic significance of preoperative circulating vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma: a prospective study. World J Gastroenterol. 2004;10:643–648. doi: 10.3748/wjg.v10.i5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeng KS, et al. Is the vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma of prognostic value after resection? World J Gastroenterol. 2004;10:676–681. doi: 10.3748/wjg.v10.i5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho MC, et al. Placenta growth factor not vascular endothelial growth factor A or C can predict the early recurrence after radical resection of hepatocellular carcinoma. Cancer Lett. 2007;250:237–249. doi: 10.1016/j.canlet.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Wada H, et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int. 2006;26:414–423. doi: 10.1111/j.1478-3231.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 57.Cheng AL, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 58.McDermott U, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci USA. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dufour JF, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist. 2010;15:1198–1204. doi: 10.1634/theoncologist.2010-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allegra CJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2010;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Cutsem E, Lambrechts D, Prenen H, Jain RK, Carmeliet P. Lessons from the adjuvant bevacizumab trial in colon cancer: what next? J Clin Oncol. 2010;29:1–4. doi: 10.1200/JCO.2010.32.2701. [DOI] [PubMed] [Google Scholar]
- 62.Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 63.Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 64.Pawson T. Regulation and targets of receptor tyrosine kinases. Eur J Cancer. 2002;38 (Suppl 5):S3–S10. doi: 10.1016/s0959-8049(02)80597-4. [DOI] [PubMed] [Google Scholar]
- 65.Faivre S, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 66.Hoda D, et al. Phase II study of sunitinib malate in adult patients with metastatic or surgically unresectable hepatocellular carcinoma (HCC) [abstract] Proc 2008 Gastrointestinal Cancers Symp. 2008:a267. [Google Scholar]
- 67.Koeberle D, et al. Continuous sunitinib treatment in patients with advanced hepatocellular carcinoma: a Swiss Group for Clinical Cancer Research (SAKK) and Swiss Association for the Study of the Liver (SASL) multicenter phase II trial (SAKK 77/06) Oncologist. 2010;15:285–292. doi: 10.1634/theoncologist.2009-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demetri GD, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 69.Motzer RJ, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu AX, et al. A phase II study of ramucirumab as first-line monotherapy in patients with advanced hepatocellular carcinoma [abstract] J Clin Oncol. 2010;28 (15 Suppl):a4083. [Google Scholar]
- 72.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 73.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel AB, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu AX, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 76.Hsu CH, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–986. doi: 10.1038/sj.bjc.6605580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun W, et al. Combination of capecitabine, oxaliplatin with bevacizumab in treatment of advanced hepatocellular carcinoma: a phase II study [abstract] J Clin Oncol. 2007;25 (18 Suppl):a4574. [Google Scholar]
- 78.Malka D, et al. Bevacizumab in patients with advanced hepatocellular carcinoma: preliminary results of a phase II study with circulating endothelial cell monitoring [abstract] J Clin Oncol. 2007;25 (18 Suppl):a4570. [Google Scholar]
- 79.Thomas MB, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 80.Albert DH, et al. Preclinical activity of ABT-869, a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006;5:995–1006. doi: 10.1158/1535-7163.MCT-05-0410. [DOI] [PubMed] [Google Scholar]
- 81.Toh H, et al. Linifanib phase II trial in patients with advanced hepatocellular carcinoma [abstract] J Clin Oncol. 2010;28 (15 Suppl):a4038. [Google Scholar]
- 82.Wedge SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 83.Alberts SR, et al. NCCTG phase II trial (N044J) of AZD2171 for patients with hepatocellular carcinoma—interim review of toxicity [abstract] Proc 2007 Gastrointestinal Cancers Symp. 2007:a186. [Google Scholar]
- 84.Yau CC, et al. A phase I study of pazopanib in patients with advanced hepatocellular carcinoma [abstract] J Clin Oncol. 2009;27 (15 Suppl):a3561. [Google Scholar]
- 85.Wood JM, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–89. [PubMed] [Google Scholar]
- 86.Drevs J, et al. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- 87.Koch I, et al. Influence of hepatic dysfunction on safety, tolerability, and pharmacokinetics of PTK787/ZK 222584 in patients with unresectable hepatocellular carcinoma [abstract] J Clin Oncol. 2005;23 (16 Suppl):a4134. [Google Scholar]
- 88.Yau T, et al. Phase 1–2 trial of PTK787/ZK222584 combined with intravenous doxorubicin for treatment of patients with advanced hepatocellular carcinoma: implication for antiangiogenic approach to hepatocellular carcinoma. Cancer. 2010;116:5022–5029. doi: 10.1002/cncr.25372. [DOI] [PubMed] [Google Scholar]
- 89.Huynh H, et al. Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:6146–6153. doi: 10.1158/1078-0432.CCR-08-0509. [DOI] [PubMed] [Google Scholar]
- 90.Ohta M, et al. TSU68, an antiangiogenic receptor tyrosine kinase inhibitor, induces tumor vascular normalization in a human cancer xenograft nude mouse model. Surg Today. 2009;39:1046–1053. doi: 10.1007/s00595-009-4020-y. [DOI] [PubMed] [Google Scholar]
- 91.Raoul JL, et al. An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma [abstract] J Clin Oncol. 2009;27 (15 Suppl):a4577. [Google Scholar]
- 92.Kanai F, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011;67:315–324. doi: 10.1007/s00280-010-1320-2. [DOI] [PubMed] [Google Scholar]
- 93.Eder JP, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 94.Llovet JM, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 95.Zhu AX, Duda DG, Sahani DV, Jain RK. Development of sunitinib in hepatocellular carcinoma: rationale, early clinical experience and correlative studies. Cancer J. 2009;15:263–268. doi: 10.1097/PPO.0b013e3181af5e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 97.Faivre SJ, Bouattour M, Dreyer C, Raymond E. Sunitinib in hepatocellular carcinoma: redefining appropriate dosing, schedule, and activity end points. J Clin Oncol. 2009;27:e248–e250. doi: 10.1200/JCO.2009.25.0670. [DOI] [PubMed] [Google Scholar]
- 98.Bruix J, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 99.Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97:172–187. doi: 10.1093/jnci/dji023. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki C, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 101.Forner A, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115:616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 102.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–687. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 104.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 105.Zhu AX, et al. Exploratory analysis of early toxicity of sunitinib in advanced hepatocellular carcinoma patients: Kinetics and potential biomarker value. Clin Cancer Res. 2011;17:918–927. doi: 10.1158/1078-0432.CCR-10-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar R, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi: 10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Toh H, Chen P, Carr B, et al. A phase II study of ABT-869 in hepatocellular carcinoma (HCC): interim analysis [abstract] J Clin Oncol. 2009;27 (15 Suppl):a4581. [Google Scholar]
- 108.Shao YY, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 109.DePrimo SE, et al. Circulating biomarkers of sunitinib in patients with unresectable hepatocellular carcinoma (HCC): analysis of correlations with outcome and tumor imaging parameters [abstact] J Clin Oncol. 2008;26 (Suppl):a4593. [Google Scholar]
- 110.Boige V, et al. Circulating endothelial cells (CECs) and angiogenic proteins monitoring in patients (pts) with advanced hepatocellular carcinoma (HCC) treated with bevacizumab [abstract] J Clin Oncol. 2009;27 (15 Suppl):a4597. [Google Scholar]
- 111.Kaseb AO, et al. Molecular predictors of response to antiangiogenic therapy in HCC: Data from bevacizumab and erlotinib phase II study [abstract] J Clin Oncol. 2010;28 (15 Suppl):a4046. [Google Scholar]
- 112.Shao Y, et al. Prognostic values of baseline circulating endothelial progenitor level for advanced hepatocellular carcinoma (HCC) patients under antiangiogenic therapy [abstract] J Clin Oncol. 2010;28 (15 Suppl):a4063. [Google Scholar]
- 113.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 114.Hood JD, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 115.Gagnon ML, et al. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci USA. 2000;97:2573–2578. doi: 10.1073/pnas.040337597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 117.Gridley T. Vascular biology: vessel guidance. Nature. 2007;445:722–723. doi: 10.1038/445722a. [DOI] [PubMed] [Google Scholar]
- 118.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leslie JD, et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 120.Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 121.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 122.Vincent F, et al. Angiotensinogen delays angiogenesis and tumor growth of hepatocarcinoma in transgenic mice. Cancer Res. 2009;69:2853–2860. doi: 10.1158/0008-5472.CAN-08-2484. [DOI] [PubMed] [Google Scholar]
- 123.Phung TL, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 125.Pikarsky E, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 126.He G, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang W, et al. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res. 2010;16:3420–3430. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 129.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carmi Y, et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol. 2009;183:4705–4714. doi: 10.4049/jimmunol.0901511. [DOI] [PubMed] [Google Scholar]
- 131.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 132.Mizukami Y, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 133.Kubo F, et al. Interleukin 8 in human hepatocellular carcinoma correlates with cancer cell invasion of vessels but not with tumor angiogenesis. Ann Surg Oncol. 2005;12:800–807. doi: 10.1245/ASO.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 134.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. [PubMed] [Google Scholar]
- 136.Grunewald M, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 137.Mansuroglu T, et al. Expression of stem cell factor and its receptor c-Kit during the development of intrahepatic cholangiocarcinoma. Lab Invest. 2009;89:562–574. doi: 10.1038/labinvest.2009.15. [DOI] [PubMed] [Google Scholar]
- 138.Wang B, Gao ZQ, Yan X. Correlative study of angiogenesis and dynamic contrast-enhanced magnetic resonance imaging features of hepatocellular carcinoma. Acta Radiol. 2005;46:353–358. doi: 10.1080/02841850510021247. [DOI] [PubMed] [Google Scholar]
- 139.d’Assignies G, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407–416. doi: 10.1148/radiol.2501080291. [DOI] [PubMed] [Google Scholar]
- 140.Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology. 2007;243:736–743. doi: 10.1148/radiol.2433052020. [DOI] [PubMed] [Google Scholar]
- 141.Zhu AX, Holalkere NS, Muzikansky A, Horgan K, Sahani DV. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 142.Liaw JV, et al. Tumor vascularity assessment in hepatocellular carcinoma and disease free caudate and spleen before and after targeted therapy using a distributed parameter model [abstract] Presented at RSNA. 2009 [Google Scholar]
- 143.Jarnagin WR, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20:1589–1595. doi: 10.1093/annonc/mdp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Battistella M, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199–203. doi: 10.1111/j.1468-3083.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 147.Lassau N, et al. Dynamic contrast-enhanced ultrasonography (DCEUS): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53–58. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.