Abstract
Three experiments evaluated whether the apparent reflexivity effect reported by Sweeney and Urcuioli (2010) for pigeons might, in fact, be transitivity. In Experiment 1, pigeons learned symmetrically reinforced hue–form (A–B) and form–hue (B–A) successive matching. Those also trained on form–form (B–B) matching responded more to hue comparisons that matched their preceding samples on subsequent hue–hue (A–A) probe trials. By contrast, most pigeons trained on just A–B and B–A matching did not show this effect; but some did—a finding consistent with transitivity. Experiment 2 showed that the latter pigeons also responded more to form comparisons that matched their preceding samples on form–form (B–B) probe trials. Experiment 3 tested the prediction that hue–hue matching versus hue–hue oddity, respectively, should emerge after symmetrically versus asymmetrically reinforced arbitrary matching relations if those relations are truly transitive. For the few pigeons showing an emergent effect, comparison response rates were higher when a probe–trial comparison matched its preceding sample independently of the baseline contingencies. These results indicate neither a reflexivity nor a transitivity effect but, rather, a possible identity bias.
Keywords: transitivity, reflexivity, identity bias, successive matching, symmetrical versus asymmetrical training, stimulus equivalence, stimulus classes, pigeons, key peck
This is the second of two articles examining the origin(s) of an apparent reflexivity effect in pigeons reported by Sweeney and Urcuioli (2010). Reflexivity refers to an untrained ability to match individual stimuli to themselves after explicit training on conditional relations of the form A–B and B–C, where the first letter of each pair designates the sample stimuli of the trained relations and the second letter of each pair designates the comparison stimuli of those relations. If the conditional relations are also equivalence relations, then a new set of relations should emerge from training (Sidman & Tailby, 1982; see also Sidman, 1990). Specifically, subjects should now do the reverse of what they explicitly learned by matching B samples to A comparisons (B–A matching) and C samples to B comparisons (C–B matching), a phenomenon known as symmetry (a.k.a. associative symmetry—Frank & Wasserman, 2005; Lionello-Denolf, 2009; Sidman, Rauzin, Lazar, Cunningham, Tailby, & Carrigan, 1982; Urcuioli, 2008). In addition, they should now match the A samples to the C comparisons (A–C matching)— transitivity (e.g., D'Amato, Salmon, Loukas, & Tomie, 1985; Kuno, Kitadate, & Iwamoto, 1994; Lipkens, Kop, & Matthijs, 1988). Finally, they should match each stimulus to itself (e.g., A samples to A comparisons)—reflexivity (Sidman & Tailby, 1982).
To test for reflexivity and to evaluate the stimulus-class mechanism proposed by Urcuioli (2008) for this and other emergent effects in pigeons, Sweeney and Urcuioli (2010) concurrently trained pigeons on three successive (go/no-go) matching tasks (cf. Wasserman, 1976). Two were symmetrically reinforced (i.e., “mirror-image”) arbitrary matching tasks (A–B and B–A); the third was identity matching involving one set of stimuli appearing in arbitrary matching (B–B). Later, pigeons received periodic, nonreinforced probe trials in which the A samples from the A–B task were followed by the A comparisons from the B–A task. For 5 of the 6 pigeons, comparison response rates were higher on probe trials in which the A comparison matched the preceding A sample (e.g., red comparison after a red sample) than on probe trials in which the A comparison did not match the preceding A sample (e.g., red comparison after a green sample). In short, the three sets of baseline relations yielded emergent A–A matching, a finding consistent with Urcuioli's (2008) theoretical prediction (see below) and interpreted by Sweeney and Urcuioli (2010) as an example of reflexivity.
Readers will undoubtedly notice that the conditional relations used in training by Sweeney and Urcuioli (2010)—A–B, B–A, and B–B matching—differ in a number of ways from the supposedly sufficient A–B and B–C baseline relations mentioned earlier. First, the two arbitrary matching tasks involved exactly the same nominal stimuli, albeit with their roles as samples and comparison reversed from one task to another. Second, including B–B identity matching meant that training involved three rather than two sets of conditional relations. The reason for these differences was entirely theoretical. Specifically, using the same assumptions that Urcuioli (2008) did to account for associative symmetry (Frank & Wasserman, 2005; Urcuioli, 2008, Experiment 3), it was possible to predict the successive matching training contingencies that should yield reflexivity. Those assumptions are that (1) the functional stimuli in successive matching are the nominal stimuli plus their ordinal position within a matching trial (viz., first or second, depending on whether a stimulus appears as a sample or a comparison, respectively); (2) the reinforcement contingencies for successive matching (e.g., Wasserman, 1976) promote the development of stimulus classes containing the elements of the reinforced sample–comparison combinations; and (3) elements common to more than one class cause their respective classes to merge.
To illustrate, if a red (R) sample → triangle (T) comparison combination is reinforced in A–B matching, a triangle sample → red comparison combination is reinforced in B–A matching, and a triangle sample → triangle comparison combination is reinforced in B–B matching, the theory anticipates the development of the following three stimulus classes: [R1, T2], [T1, R2], and [T1, T2]. In this notation, the number after each letter designates the ordinal position of each matching stimulus (viz., as a sample or comparison)1. Note, too, that some classes have an element in common—viz., the [R1, T2] and [T1, T2] classes share T2 (the triangle comparison) and, likewise, the [T1, R2] and [T1, T2] classes share T1 (the triangle sample). If common elements cause their respective classes to merge (Urcuioli, 2008; see also Mackay, Wilkinson, Farrell, & Serna, 2011), the net result should be a [R1, R2, T1, T2] class. This four-member class consists of elements comprising each explicitly reinforced baseline relation (e.g., R1 and T2 of the red sample → triangle comparison relation). In addition, it has the elements of the untrained red sample → red comparison (R1–R2) relation.
A person familiar with stimulus equivalence and who observed pigeons in testing respond more to a hue comparison (e.g., R2) when it matched its preceding hue sample (e.g., R1) than when it did not would likely describe this response-rate difference as reflexivity. Despite appearances, however, this emergent effect is not strictly reflexivity by Urcuioli's (2008) account because “matching each stimulus to itself” entails matching red to red (a R–R relation) and green to green (a G–G relation) and, theoretically speaking, R1–R1 and G1–G1 relations cannot be tested because “1” cannot be used to designate the second stimulus in a sequence. The definition of reflexivity treats the functional and nominal matching stimuli as the same. By contrast, Urcuioli's (2008) theory states that functional matching stimuli in pigeons' successive matching are compounds consisting of a nominal stimulus (e.g., red or green) and its ordinal position in a trial (first or second). Thus, a red comparison (R2), is a different stimulus than a red sample (R1), so matching the former to the latter is not “matching each stimulus to itself.” These distinctions should not, in our view, detract from the finding that certain sets of successive matching contingencies yield novel, untrained behavior not previously observed in nonhuman animals (Frank & Wasserman, 2005; Sweeney & Urcuioli, 2010; Urcuioli, 2008, Experiment 3). Urcuioli's (2008) theory contributes to these noteworthy empirical findings by proposing mechanisms to explain why those contingencies yield such observed emergent effects.
That said, the present article considers the possibility that higher comparison response rates on matching than on nonmatching A–A probe trials following the aforementioned baseline contingencies (Sweeney & Urcuioli, 2010) could arise for reasons other than those specified by Urcuioli (2008). The three experiments described here continue along the lines of Urcuioli (2011) who asked whether such emergent performances might represent generalization of identity matching from the explicitly trained B–B baseline relations to the untrained A–A relations of testing. To evaluate this alternative account, Urcuioli (2011) trained pigeons on symmetrically reinforced arbitrary matching tasks (A–B and B–A) plus an identity matching task with stimuli not appearing in arbitrary matching (viz., C–C). If the explicitly reinforced C–C identity relations generalize to other stimuli, A–A matching should emerge in testing. On the other hand, Urcuioli's (2008) theory clearly predicts that such training should not yield emergent A–A matching. Contrary to theoretical prediction but consistent with generalized identity (Oden, Thompson, & Premack, 1988; Peña, Pitts, & Galizio, 2006), some pigeons did show an emergent A–A effect.
There is, however, another explanation which could potentially account for these results and those of Sweeney and Urcuioli (2010)—namely, transitivity. After all, pigeons trained on A–B, B–A, and B–B successive matching (Sweeney & Urcuioli, 2010; Urcuioli, 2011) versus A–B, B–A, and C–C successive matching (Urcuioli, 2011) share two potentially consequential baseline relations: A–B and B–A. If the functional matching stimuli in these tasks do not have an ordinal-position component (as assumed by Urcuioli, 2008) and if these baseline relations are transitive (cf. Lipkens et al., 1988; Strasser, Ehrlinger, & Bingman, 2004), then A–A matching should emerge in testing. Colloquially speaking, if A means B and B means A, then A means A (Vasconcelos, 2008). To take a specific example, if comparison responding on red–triangle and triangle–red combinations are both reinforced in training, then pigeons should preferentially respond to red after red (red–red) in testing if the reinforced baseline relations are transitive.
By contrast, Urcuioli's (2008) theory predicts that A–B and B–A training alone are insufficient to produce that effect precisely because of the assumption regarding the ordinal-position component of the functional matching stimuli. Using a specific illustrative example, if reinforced red sample–triangle comparison (R1–T2) trials and reinforced triangle sample–red comparison (T1–R2) trials are components of A–B and B–A arbitrary matching, respectively, these combinations have no functional elements in common. Stated otherwise, R1 ≠ R2 and T1 ≠ T2, so each must be regarded as a different stimulus. Consequently, a class merger in which R1 and R2 join the same stimulus class—the theoretical basis for an emergent A–A effect—cannot occur.
The present experiments contrast the theoretical prediction of no emergent A–A matching following such training with a transitivity account of the reflexivity results reported by Sweeney and Urcuioli (2010) and the generalized identity findings of Urcuioli (2011).
EXPERIMENT 1
In Experiment 1, all pigeons were trained on two symmetrically reinforced (mirror-image) arbitrary matching tasks: hue–form (A–B) and form–hue (B–A) successive matching. For one group (Group TRANS), daily training sessions consisted only of these two arbitrary matching tasks. A second group (Group REFL) received additional, concurrent training on form–form (B–B) successive matching (cf. Group IREF in Sweeney & Urcuioli, 2010). Later, all pigeons were given a series of tests to assess emergent hue–hue (A–A) matching. If the A–B and B–A baseline relations are transitive, both groups should exhibit emergent A–A matching. On the other hand, if the effect reported by Sweeney and Urcuioli (2010) reflects stimulus class formation via the mechanisms proposed by Urcuioli (2008), only Group REFL should show emergent A–A matching in testing.
Method
Subjects
Thirteen White Carneau retired breeders obtained from the Palmetto Pigeon Plant (Sumter, SC) participated in the experiment. Twelve were randomly assigned to two groups of 6, each containing equal numbers of experimentally naïve and experienced pigeons. The experienced pigeons previously served in two-choice experiments unrelated to the present one. The extra (13th) pigeon, also experimentally naïve, was subsequently added to one of the groups as a potential replacement for a pigeon originally scheduled to be removed from the experiment due to long waiting behavior at the start of its sessions. Its waiting behavior eventually diminished, however, so it remained in the experiment.
Pigeons were maintained at 80% of their free-feeding body weights which were established upon arrival in the laboratory by allowing free access to Purina ProGrains for approximately 2 weeks. Water and grit were always available in the stainless steel, wire-mesh home cages that were located in a colony room on a 14hr–10hr light–dark cycle (lights on at 07:00). Pigeons obtained their daily food ration in the experimental sessions and were fed in the home cages on the 1 day/week they were not run.
Apparatus
Two identically configured BRS/LVE (Laurel, MD) chambers (Model PIP-016 three-key response panels inside Model SEC-002 enclosures) were used for this experiment. The three 2.5-cm-diameter response keys were positioned in a row 7.5 cm from the top of the panel and 5.7 cm apart (center to center). A stimulus projector (BRS/LVE Model IC-901-IDD) mounted behind each center key, the only ones used in the experiment, was equipped with films and filters for displaying red, green, and white homogeneous fields, and three white horizontal lines and a solid inverted triangle on black backgrounds (BRS/LVE Pattern No. 692). A 5.8-cm-square opening located 13 cm below the center key permitted access to a rear-mounted food hopper which, when raised, was illuminated by a small miniature bulb (ESB-28) in the metal housing surrounding it. A partially shielded GE #1829 bulb 7.6 cm above the center key provided general chamber illumination with its light directed toward the ceiling. A constantly running blower fan attached to each chamber provided ventilation and masking noise. All experimental events were controlled by a single IBM-compatible computer interfaced to both chambers.
Procedure
Preliminary training
The 7 experimentally naïve pigeons were initially trained to eat from a raised and lit food hopper and then to peck a white center key via the method of shaping by successive approximations. Next, all pigeons learned to peck red and green center-key hues, and the center-key triangle and horizontal lines, for food in separate 60-trial sessions. This was followed by eight 60-trial sessions (the first four with the triangle and horizontal lines, and the second four with red and green) during which center-key pecking was reinforced on a fixed-interval (FI) schedule whose parameter was raised from 2 to 5 s across each four-session block. The two stimuli in each session were presented equally often and in pseudorandom order. Successive trials were separated by a 15-s ITI, the first 14 s of which was spent in darkness. The house light came on for the last 1 s of the ITI and remained on until the end of a trial. Reinforcement duration was constant within a session but varied from 1.8–6.0 s across sessions to maintain a pigeon's 80% body weight.
Successive matching acquisition
Successive matching training began immediately after preliminary training. Each matching trial began with the onset of a sample stimulus on the center key. The first sample key peck initiated a FI 5-s schedule that ended with offset of the sample, a 500-ms blank interval, and the onset of a center-key comparison stimulus. On reinforced trials, the first comparison key peck after a 5-s interval timed from the first peck turned off the comparison and produced food. On nonreinforced trials, the comparison stimulus and the house light went off automatically 5 s after comparison onset. Trials were again separated by a 15-s ITI. The house light came on for the last 1 s of the ITI and remained on throughout the upcoming trial until the end of the reinforcement cycle (reinforced trials) or comparison offset (nonreinforced trials).
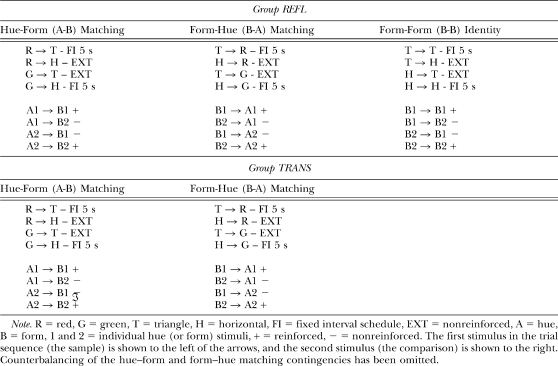
For hue–form (A–B) arbitrary matching (see Table 1), pecking the triangle comparison after the red sample and pecking the horizontal comparison after the green sample were reinforced for one half of the pigeons in each group, whereas the remaining sample–comparison combinations were not reinforced. For the remaining pigeons, the opposite contingencies were in effect. The reinforced and nonreinforced sample–comparison contingencies for form–hue (B–A) arbitrary matching were mirror images of those for hue–form arbitrary matching. Thus, pecking the red comparison after the triangle sample and the green comparison after the horizontal sample in the B–A task were reinforced for those pigeons for which the red sample–triangle comparison and green sample–horizontal comparison combinations were reinforced in the A–B task, etc.
Table 1.
Successive Matching Training Contingencies for the Two Groups in Experiment 1.
Each training session for Group TRANS contained 32 hue–form (A–B) and 32 form–hue (B–A) trials. For Group REFL however, each training session also included 32 form–form (B–B) identity trials in which pecking the triangle comparison after a triangle sample and the horizontal comparison after a horizontal sample (matching trials) were reinforced. Nonmatching trials on which the form comparison differed from the preceding form sample ended without reinforcement (see Table 1).
The four sample–comparison combinations for each successive matching task appeared equally often and in random order in a session with the constraint that no combination occurred more than twice in a row. Acquisition (baseline) performances for each task were assessed by calculating a discrimination ratio (DR) in which the total number of comparison pecks on reinforced trials was divided by the total number of comparison pecks on both reinforced and nonreinforced trials. (Only pecks occurring within the first 5 s of comparison onset entered into these computations). Each pigeon was trained until it achieved a DR of 0.80 or higher on each of its successive matching tasks for 5 of 6 consecutive training sessions (“criterion”). This was followed by a minimum of 10 overtraining sessions, the last 5 of 6 of which had to be at criterion levels. At that point, testing began.
Successive matching testing
After completing acquisition and overtraining, each pigeon received eight test sessions during which performances on the untrained hue–hue (A–A) sample–comparison combinations were assessed. Tests were conducted in two-session blocks separated by at least five baseline training sessions at criterion levels of performances. Each test session consisted of either 64 (Group TRANS) or 96 (Group REFL) baseline trials divided equally among each pigeon's baseline tasks and 8 nonreinforced A–A probe trials in which red and green samples were followed by red and green comparisons. The four possible combinations of the red and green stimuli (i.e., R→R, R→G, G→R, and G→G) occurred equally often in each session with successive probes separated by at least six baseline trials. The first probe trial did not occur until at least one of each possible baseline trial had been presented. On all probe trials, the comparison stimulus (and house light) went off automatically 5 s after comparison onset. All other procedural details were identical to those for acquisition.
For all statistical analyses reported in this paper, Type I error rate was set at .05 using the tabled F values reported by Rodger (1975) for controlling error rates on a per decision basis.
Results
Acquisition and baseline performances
The average number of training sessions for the Group REFL pigeons to reach criterion levels of performance on their hue–form (A–B), form–hue (B–A) and form–form (B–B) baseline tasks was 31.8, 38.3, and 43.2 sessions, respectively. Analysis of variance (ANOVA) on these data showed a significant between-task difference in rates of acquisition, F(2, 10) = 4.14. Post-hoc contrasts (Rodger, 1975) indicated that A–B matching was acquired faster than B–B matching, F(2, 10) = 4.11, with the rate for B-A matching falling in between these two extremes, F(2, 10) = 0.03. The average number of training sessions for the Group TRANS pigeons to reach criterion levels of performance on A–B and B–A successive matching was 23.6 and 42.0 sessions respectively. Although this difference was not statistically significant, F(1, 6) = 5.09, the numerically faster acquisition rate for the A–B task corresponded to that observed in Group REFL.
Discriminative performances for both groups over the last five overtraining sessions preceding the first test session were uniformly high across tasks. The average DRs for hue–form (A–B), form–hue (B–A) and form–form (B–B) matching for Group REFL were .95, .92, and .95, respectively, F(2, 10) = 1.12. The DRs for the two corresponding arbitrary matching tasks for Group TRANS were .93 and .92, respectively, F(1, 6) = .09.
Baseline performances during testing were, for the most part, at or above .80. In Group REFL, there were only 8 instances (out of a total of 144) in which the DR for a baseline task dropped below this level, typically into the .75–.79 range. These were confined to 4 pigeons, occurred most frequently on the B–B task, and were scattered haphazardly across test sessions. Baseline performances in testing for Group TRANS were also well maintained with just a few instances of DRs falling below .80. The most notable was DRs of .62 and .67 for the A–B and B–A tasks by 1 pigeon (TRANS5) on its second test session.
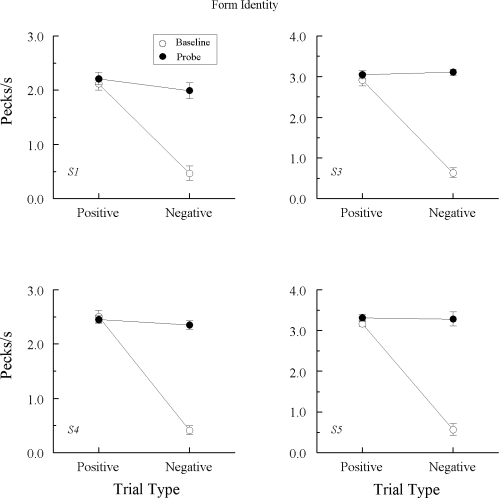
Test performances
Figure 1 shows baseline and probe-trial test performances (open and solid circles, respectively) for each Group REFL pigeon averaged over all eight test sessions. For comparability with Group TRANS, baseline represents performances on the hue–form (A–B) and form–hue (B–A) tasks with “positive” and “negative” referring, in this case, to the reinforced and nonreinforced combinations, respectively. The baseline results represent the average of a randomly selected two trials of each reinforced A–B and B–A combination and each nonreinforced A–B and B–A combination from each test session (total of 32 reinforced and 32 nonreinforced trials). The same selection procedure was followed for Group TRANS whose results are shown in Figures 2 and 3.
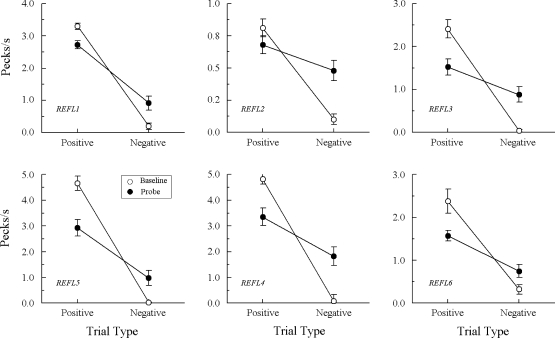
Fig 1.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced hue–hue probe trials (filled circles) averaged over the eight test sessions for each Group REFL pigeon in Experiment 1. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
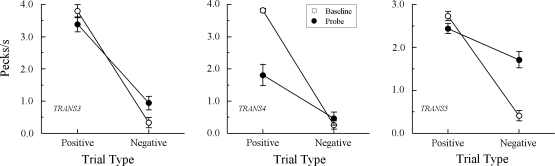
Fig 2.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced hue–hue probe trials (filled circles) averaged over the eight test sessions for the 4 Group TRANS pigeons in Experiment 1 that did not exhibit emergent A–A matching. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
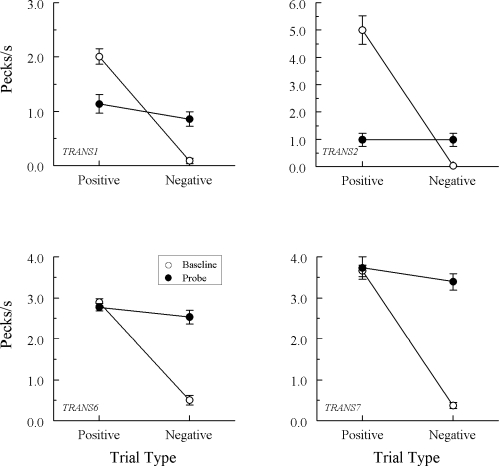
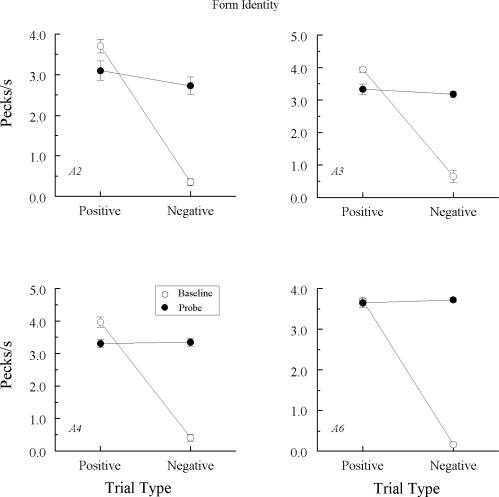
Fig 3.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced hue–hue probe trials (filled circles) averaged over the eight test sessions for the 3 Group TRANS pigeons in Experiment 1 that did exhibit emergent A–A matching. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
Not surprisingly, both groups responded at much higher rates to the comparisons on the positive (reinforced) than on the negative (nonreinforced) arbitrary baseline trials throughout testing. More importantly, every Group REFL pigeon also responded more, on average, to the comparisons on the positive (matching) A–A probe trials than on the negative (nonmatching) A–A probe trials. The difference in probe-trial comparison response rates was statistically significant for all pigeons except REFL2, Fs(1, 64) = 54.55, 3.69, 5.98, 9.57, 20.33, and 15.24 for pigeons REFL 1–6, respectively2. Interestingly, overall comparison response rates for REFL2 on its baseline and probe trials were considerably lower than for any other pigeons in this group.
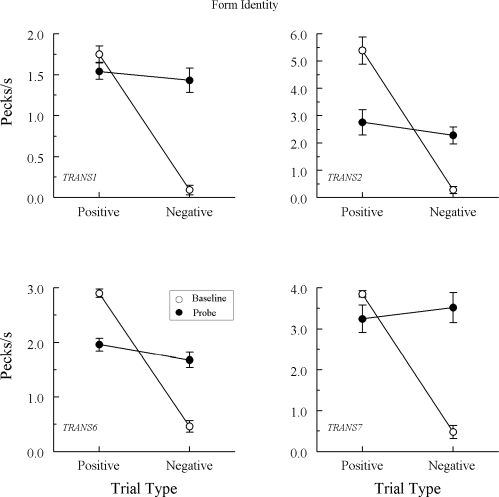
Figure 2 shows the test results from 4 of the 7 Group TRANS pigeons that responded nondifferentially to the comparisons on the A–A probe trials. Indeed, ANOVA showed no significant difference between each pigeon's rate on positive (matching) versus negative (nonmatching) A–A probes, Fs(1, 64) = 1.73, 0.00, 1.59, and 1.33 for pigeons TRANS1, TRANS2, TRANS6, and TRANS7, respectively.
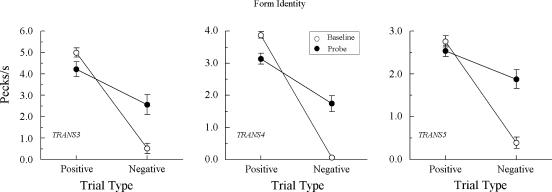
Figure 3 shows the test results from the 3 Group TRANS pigeons demonstrating a clear difference in their probe-trial comparison response rates. Specifically, each responded at higher rates on positive (matching) than on negative (nonmatching) A–A probes, Fs(1, 64) = 62.13, 12.23, and 10.43 for pigeons TRANS3, TRANS4, and TRANS5, respectively. The response rate difference for TRANS5 may have been even larger than that depicted in Figure 3 had its baseline performances on the second test session not dropped considerably below criterion.
Discussion
Urcuioli's (2008) theory of pigeons' stimulus-class formation predicts that concurrent training on A–B, B–A, and B–B matching should yield emergent A–A matching, and it did for 5 of the 6 pigeons in Group REFL. Moreover, this group's results replicate the A–A test results reported by Sweeney and Urcuioli (2010) and Urcuioli (2011) for pigeons trained and tested in exactly the same fashion. There seems to be no question, then, that this is a reproducible emergent effect following concurrent baseline training on these sample–comparison relations.
Urcuioli's (2008) theory also predicts, however, that pigeons trained just on A–B and B–A arbitrary successive matching will not show this effect because the classes containing both the A samples and A comparisons (necessary for emergent A–A matching) cannot develop. The theory considers additional B–B training, which Group TRANS did not receive, crucial because such training provides common elements for class merger, resulting in classes containing both A elements. The test results from 4 of the 7 Group TRANS pigeons confirm this theoretical prediction: They responded at virtually the same rate to the comparisons on positive and negative A–A probe trials.
However, the theoretical prediction is clearly disconfirmed by the test results from the other 3 Group TRANS pigeons. They responded with higher comparison response rates on positive than on negative A–A probes, mimicking the pattern of results observed in Group REFL. Apparently, then, the explicitly reinforced arbitrary baseline relations for these pigeons were transitive: Training A–B and B–A yielded A–A.
If learning mirror-image A–B and B–A arbitrary tasks is sufficient to yield differential responding on positive versus negative A–A probe trials (at least for some pigeons), the results of the Group REFL pigeons may also be explicable in the same manner. If so, the stimulus-class mechanisms proposed by Urcuioli (2008) would be unnecessary to account for the Group REFL results and the corresponding results reported by Urcuioli (2011) and Sweeney and Urcuioli (2010). In view of this and the theoretically unexpected results from Group TRANS, the next experiment sought additional verification for the apparent transitivity effect of Experiment 1.
EXPERIMENT 2
If the A–B and B–A arbitrary baseline relations are transitive, B–B (as well as A–A) matching should emerge in testing. Simply put, B–A plus A–B yields B–B. This prediction was tested in Experiment 2 by testing the Group TRANS pigeons on probe trials involving the form samples and form comparisons (i.e., the B stimuli).
Method
Subjects and Apparatus
The 7 Group TRANS pigeons from Experiment 1 participated in this experiment. The same apparatuses were used.
Procedure
Baseline retraining
After completing Experiment 1, each Group TRANS pigeon was returned to baseline training on hue–form (A–B) and form–hue (B–A) arbitrary matching (cf. Table 1) for a minimum of five sessions and until it met the baseline performance criterion. Procedural details for these baseline sessions were identical to those in Experiment 1.
Form identity (B–B) testing
Eight test sessions then followed during which nonreinforced form–form (B–B) probe trials were interspersed among the A–B and B–A baseline trials. Each session consisted of 64 baseline trials (32 each of A–B and B–A arbitrary matching) and 8 nonreinforced B–B probe trials, 2 each of the following sample–comparison combinations: T→T, T→H, H→T, and H→H. Test sessions were again conducted in two-session blocks with a minimum of five baseline sessions at criterion levels of performance separating successive blocks. Procedural details were identical to those for the A–A tests in Experiment 1.
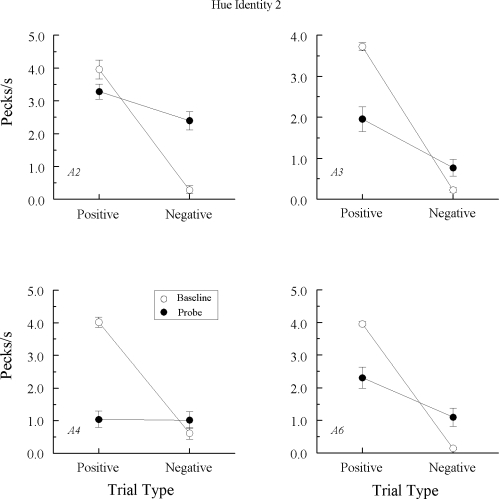
Hue identity (A–A) testing 2
Eight additional hue–hue (A–A) test sessions were conducted after the form identity (B–B) tests and reestablishment of baseline levels of performance in order to evaluate the reproducibility of the findings from Experiment 1. All details regarding the grouping of these sessions, intervening baseline training, etc. were identical to those previously described.
Results and Discussion
Baseline performances
DRs averaged over the last five retraining sessions preceding the first form–identity (B–B) test were .93 and .92 for A–B and B–A arbitrary matching, respectively. The baseline DRs during the test sessions themselves were consistently above .80 (range: .80–1.00) for 5 of the 7 pigeons. Performance by the other 2 pigeons dropped below .80 on multiple occasions on B–A matching but generally remained at or above .75.
DRs for the last five baseline sessions preceding the first hue–hue (A–A) test session averaged .93 and .92 for A–B and B–A matching, respectively. Across those eight test sessions, there were only 7 instances (out of a possible 144) in which a baseline DR fell below .80, 5 of those occurring for pigeon TRANS7. Indeed, this pigeon inexplicably lost its performance baseline on the last two A–A test sessions: DRs for its arbitrary tasks fell into the .57–.68 range on these sessions.
Test performances
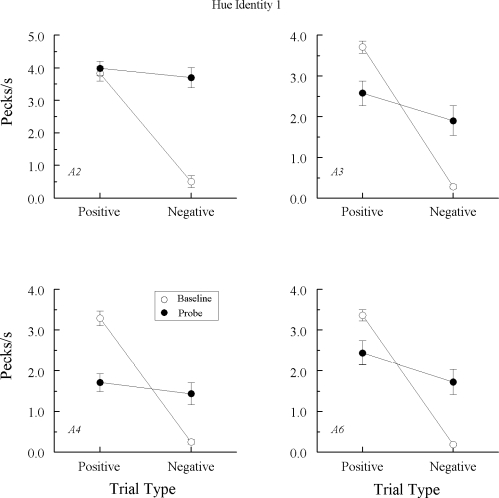
Figures 4 and 5 depict the average results of the eight form–identity (B–B) tests. Figure 4 presents test data from the 4 pigeons that, in Experiment 1, showed no evidence of emergent A–A matching; Figure 5 presents test data from the 3 pigeons that showed an emergent A–A effect in Experiment 1.
Fig 4.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight form–form (B–B) test sessions in Experiment 2 for the 4 Group TRANS pigeons that did not exhibit emergent A–A matching in Experiment 1. Positive = reinforced arbitrary baseline trials and test trials on which the form comparison matched the preceding form sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the form comparison did not match the preceding form sample. Note that ordinates differ across pigeons.
Fig 5.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight form–form (B–B) test sessions in Experiment 2 for the 3 Group TRANS pigeons that did exhibit emergent A–A matching in Experiment 1. Positive = reinforced arbitrary baseline trials and test trials on which the form comparison matched the preceding form sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the form comparison did not match the preceding form sample. Note that ordinates differ across pigeons.
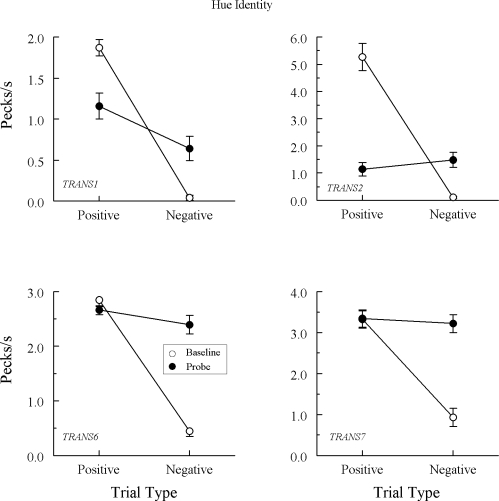
Baseline performances (open circles) remained intact during testing for each pigeon: Comparison response rates were much higher on the positive (reinforced) than on the negative (nonreinforced) arbitrary baseline trials. More importantly, the test profiles (filled circles) were remarkably similar to those in Experiment 1. Specifically, Figure 4 shows that the comparison response rates for the 4 pigeons that responded nondifferentially on the A–A probe trials in Experiment 1 were very much the same on the positive (matching) and negative (nonmatching) B–B probe trials in this experiment. By contrast, Figure 5 shows that the 3 pigeons that responded differentially on the A–A probe trials in Experiment 1 also did so here in their B–B probe tests. Their comparison response rates were higher on positive (matching) probe trials than on negative (nonmatching) probe trials.
The results from ANOVA on each pigeon's data confirm these statements. Comparison response rates on the positive versus negative probe trials for pigeons TRANS1, TRANS2, TRANS6, and TRANS7 did not differ statistically from one another, Fs(1, 64) = 0.38, 0.74, 2.34, and 0.44, respectively (cf. Figure 4). Those rates did differ significantly, however, for pigeons TRANS3, TRANS4, and TRANS5, Fs(1, 64) = 8.25, 22.65, and 6.45, respectively (cf. Figure 5).
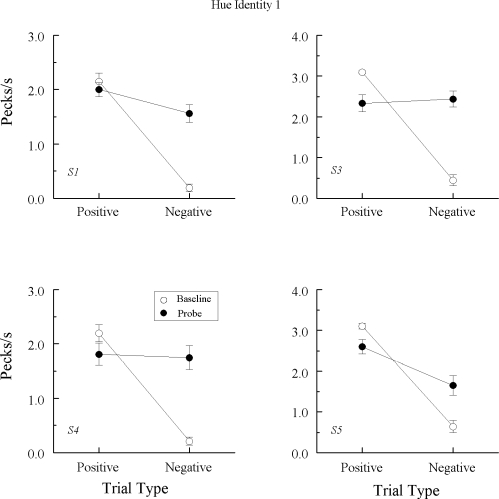
Figures 6 and 7 plot the average results from the subsequent eight hue–identity (A–A) tests. To be consistent, Figure 6 shows the results from each pigeon that showed no evidence of emergent A–A matching in Experiment 1, whereas Figure 7 shows the results from each pigeon that previously showed an emergent A–A effect.
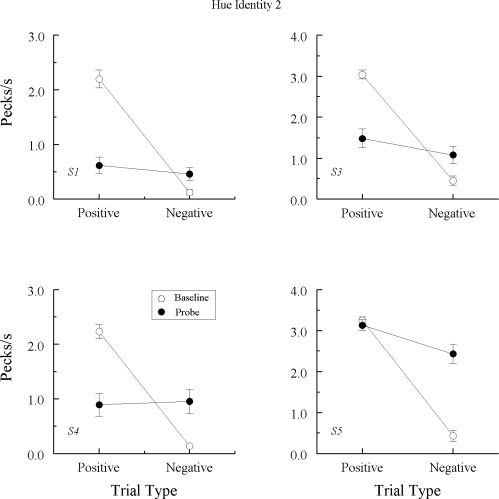
Fig 6.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight hue–hue (A–A) test sessions in Experiment 2 for the 4 Group TRANS pigeons that did not exhibit emergent A–A matching in Experiment 1. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
Fig 7.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight hue–hue (A–A) test sessions in Experiment 2 for the 3 Group TRANS pigeons that did exhibit emergent A–A matching in Experiment 1. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
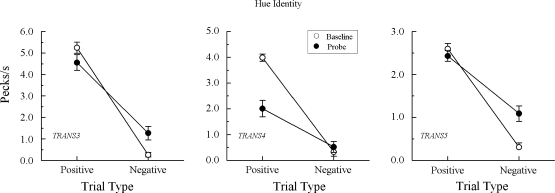
Baseline performances were again well-maintained during testing. (Despite the loss of baseline, data from pigeon TRANS7's last two test sessions were included in its averages because their exclusion made no difference.) Of the 4 pigeons not showing an emergent A–A effect in Experiment 1 nor emergent B–B matching in this experiment (cf. Figure 4), 3 pigeons continued to respond nondifferentially on the A–A probe trials, Fs (1, 64) = 0.78, 2.02, and 0.13 for TRANS2, TRANS6, and TRANS7, respectively. Pigeon TRANS1, however, now responded at higher rates to the comparisons on the positive (matching) than on the negative (nonmatching) probes, F(1, 64) = 5.14. Of the 3 pigeons previously exhibiting emergent A–A matching in Experiment 1 and emergent B–B matching in this experiment (cf. Figure 5), each again responded differentially on the A–A probe trials, Fs (1, 64) = 47.94, 14.91, and 37.40, respectively, for pigeons TRANS3, TRANS4, and TRANS5.
Together, the data from this experiment provide solid evidence that the emergent A–A effect shown by some of the Group TRANS pigeons in Experiment 1 was a reliable effect, one consistent with transitivity. These pigeons showed an emergent B–B effect and reproduced the A–A test performances they previously exhibited. Most of the remaining Group TRANS pigeons, the ones that responded nondifferentially on the A–A probes in Experiment 1, behaved similarly in the B–B and A–A probes in this experiment. Together, their test results indicate that, for whatever reason, their baseline A–B and B–A relations were not transitive.
EXPERIMENT 3
The next experiment provided an independent test of transitivity in successive matching with pigeons different from those participating in the preceding experiment(s). If the A–B and B–A relations are truly transitive, then the baseline contingencies of these arbitrary tasks can be structured to produce either emergent A–A matching (higher rates of comparison responding on novel matching combinations) or emergent A–A oddity (higher rates of comparison responding on novel nonmatching combinations). Emergent matching is predicted if, as in Experiments 1 and 2, baseline training consists of symmetrically reinforced (mirror-image) arbitrary matching contingencies (see Table 2). For example, if the red sample–triangle comparison relation is reinforced in A–B matching and the triangle sample–red comparison relation is reinforced in B–A matching, pigeons should subsequently respond more frequently to a red comparison following a red sample (i.e., R → T and T → R should yield R → R in testing). On the other hand, emergent oddity is predicted if baseline training consists of asymmetrical arbitrary matching contingencies. Thus, if the red sample–triangle comparison relation is reinforced in A–B matching but the triangle sample–green comparison relation is reinforced in B–A matching, pigeons should subsequently respond more frequently to a green comparison following a red sample (i.e., R → T and T → G should yield R → G in testing). These predictions, which again assume that the nominal stimuli are the functional matching stimuli (i.e., that ordinal position is not a component of the latter), were tested in Experiment 3.
Table 2.
Successive Matching Training Contingencies for the Two Groups in Experiment 3.
Method
Subjects and Apparatus
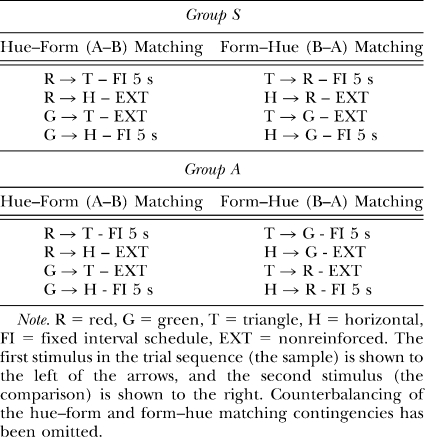
Eight experimentally naïve White Carneau pigeons obtained from the Double “T” Farm (Glenwood, IA) and approximately 1–2 years old at the start of the experiment participated. Their free-feeding body weights were established upon arrival in the lab and they were gradually reduced to 80% of these weights prior to the experiment. Four pigeons were randomly assigned to Group S and the other 4 to Group A, where the group labels indicate symmetrically versus asymmetrically reinforced arbitrary matching contingencies, respectively.
The apparatuses were the same as before.
Procedure
Preliminary training
Preliminary training to shape the key peck response and to establish responding to the stimuli used as samples and comparisons in successive matching was identical to that described for Experiment 1.
Successive matching acquisition
All pigeons were concurrently trained on hue–form (A–B) and form–hue (B–A) matching to the same performance criterion previously described. For Group S (see Table 2), the baseline contingencies for its two arbitrary matching tasks were symmetrically reinforced whereas for Group A, the contingencies were asymmetrically reinforced. In other words, for Group S, the reverse of the reinforced hue sample–form comparison combinations in A–B matching were also reinforced in B–A matching, and likewise for the nonreinforced combinations. By contrast, for Group A, the reverse of the reinforced hue sample–form comparison combinations in A–B matching were nonreinforced in B–A matching, and vice versa for the nonreinforced A–B matching combinations. All other training details were identical to those described for Group TRANS in Experiment 1.
Successive matching testing
After reaching criterion levels of acquisition performance and completing a minimum of 10 overtraining sessions, each pigeon received eight test sessions during which nonreinforced hue sample–hue comparison probe trials (two each of R→R, R→G, G→R, and G→G) were intermixed among 32 A–B and 32 B–A baseline trials. Procedural details for these hue identity (A–A) test sessions, run as before in blocks of two, were identical to those described for Group TRANS in Experiments 1 and 2.
Successive matching retraining and testing
After completing the hue identity tests, each pigeon was retrained on its arbitrary matching tasks until the baseline performance criteria were met. It then received eight form–identity (B–B) tests during which nonreinforced form sample–form comparison probe trials (two each of T→T, T→H, H→T, and H→H) were intermixed among 64 baseline training trials split equally across the A–B and B–A tasks. These tests were conducted in the same fashion as the A–A tests that preceded them.
Finally, each pigeon received eight additional hue identity tests after baseline performance levels were reestablished. This second set of A–A tests were conducted as before.
Predictions
If the A–B and B–A baseline relations are transitive, pigeons in Group S should respond more to the comparisons on matching probe trials than to the comparisons on nonmatching probe trials. By contrast, pigeons in Group A should respond more on nonmatching than on matching probe trials.
Results and Discussion
Acquisition and baseline performances
The average number of sessions to criterion on hue–form (A–B) and form–hue (B–A) matching for Group S was 33.8 and 55.2, respectively. The corresponding averages for Group A were 24.0 and 30.0, respectively. Neither difference was statistically significant in ANOVA, Fs(1, 3) = 2.62 and 1.70, for Groups S and A, respectively. Average DRs for A–B and B–A matching over the last five baseline sessions preceding the first test session were .90 and .89, respectively, for Group S and .87 and .90, respectively, for Group A. Again, neither difference was statistically significant, Fs(1, 3) = 5.40 and 0.30, respectively.
Baseline DRs during the first eight hue identity (A–A) test sessions mostly remained at or above criterion levels. There were a few instances in Group S in which baseline DRs fell below .80, but these were primarily into the .75–.79 range, and the same was true in Group A. The one exception was pigeon A2 whose B–A DRs for test sessions 7 and 8 fell to .59 and .50, respectively. Because of the clear loss of baseline, those two test sessions were excluded from its data analysis. During the second set of hue identity (A–A) tests, baseline DRs in both groups were consistently at or above criterion with just a few minor exceptions.
During the form identity (B–B) tests, baseline DRs were well maintained throughout most of testing. The one exception in Group S was pigeon S5 whose B–A DR fell below .80 on six of its eight test sessions (averaging .74 for those six sessions) and whose A–B DR fell below .80 on three test sessions (averaging .76 for those sessions). In Group A, pigeon A3's baseline DRs dropped noticeably on its fourth session (.61 and .75 for A–B and B–A matching, respectively), but this did not appear to adversely affect its overall probe-trial performances.
Test performances
Figures 8 and 9 show individual performances in Groups S and A, respectively, averaged over their first eight hue identity (A–A) test sessions. Figures 10 and 11 show the corresponding performances for the form identity (B–B) tests, and Figures 12 and 13 show the results for the second block of set identity (A–A) tests. Average baseline performances (open circles) throughout all of these tests were well maintained in both groups.
Fig 8.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the first eight hue identity (A–A) test sessions for each Group S pigeon in Experiment 3. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
Fig 9.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the first eight hue identity (A–A) test sessions for each Group A pigeon in Experiment 3 (first six sessions for pigeon A2). Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
Fig 10.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight form identity (B–B) test sessions for each Group S pigeon in Experiment 3. Positive = reinforced arbitrary baseline trials and test trials on which the form comparison matched the preceding form sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the form comparison did not match the preceding form sample. Note that ordinates differ across pigeons.
Fig 11.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the eight form identity (B–B) test sessions for each Group A pigeon in Experiment 3. Positive = reinforced arbitrary baseline trials and test trials on which the form comparison matched the preceding form sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the form comparison did not match the preceding form sample. Note that ordinates differ across pigeons.
Fig 12.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the second eight hue identity (A–A) test sessions for each Group S pigeon in Experiment 3. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
Fig 13.
Comparison pecks/sec (± 1 SEM) on the baseline arbitrary matching trials (open circles) and nonreinforced probe trials (filled circles) averaged over the second eight hue identity (A–A) test sessions for each Group A pigeon in Experiment 3. Positive = reinforced arbitrary baseline trials and test trials on which the hue comparison matched the preceding hue sample. Negative = nonreinforced arbitrary baseline trials and test trials on which the hue comparison did not match the preceding hue sample. Note that ordinates differ across pigeons.
For the initial A–A tests, pigeons S1 and S5 responded significantly more, on average, to the hue comparisons on positive (matching) than on negative (nonmatching) probe trials, Fs(1, 62) = 4.56 and 10.33. The other two Group S pigeons (S3 and S4) responded nondifferentially on these trials, Fs(1, 62) = 0.12 and 0.04, respectively. In Group A, all 4 pigeons responded with roughly equal frequency on the positive (matching) and negative (nonmatching) probe trials, F(1, 46) = .55 for pigeon A2; all other Fs(1, 62) < 2.84.
On the form identity (B–B) tests (cf. Figures 10 and 11), no pigeon showed any evidence of differential comparison responding on positive versus negative probes: Fs(1, 62) < 1.43 in Group S and Fs(1, 62) < 1.36 in Group A.
For the second set of hue identity (A–A) tests (Figures 12 and 13), pigeon S5 again pecked more frequently to the comparisons on positive (matching) than on negative (nonmatching) probe trials, F(1, 62) = 6.38, but none of the other Group S pigeons did, Fs(1, 62) = 0.62, 1.64, and 0.04 for S1, S3, and S4, respectively. Interestingly, despite their nondifferential probe-trial responding during the initial A–A tests, 3 of the Group A pigeons (A2, A3, and A6) responded significantly more to the comparisons on positive (matching) than on negative (nonmatching) probes in the second set of A–A tests, Fs(1, 62) = 6.15, 10.62, and 7.89, respectively.
As in Experiment 1, baseline training on symmetrically reinforced A–B and B–A matching yielded emergent hue–hue (A–A) matching in some pigeons, a finding consistent with transitivity. However, this same training did not produce emergent form–form (B–B) matching, as transitivity also predicts. In fact, the latter results are at odds with the finding from Experiments 1 and 2 which showed that pigeons exhibiting emergent A–A matching after symmetrically reinforced A–B and B–A baseline training also exhibited emergent B–B matching. The reason(s) for the discrepancy is (are) unclear. Certainly more noteworthy, however, was the finding that asymmetrically reinforced A–B and B–A training did not yield higher rates of comparison responding on nonmatching (“negative”) than on matching (“positive”) probe trials. In fact, the few instances of differential probe-trial responding that were observed in Group A (namely, during the second set of A–A tests—cf. Figure 13) were opposite in direction to that predicted by the hypothesized transitive baseline relations. Specifically, 3 Group A pigeons responded significantly more to matching than to nonmatching probe-trial comparisons in these A–A tests. Transitivity predicts the reverse pattern.
GENERAL DISCUSSION
The three experiments reported in this paper were designed to provide further clarity regarding the origin(s) of the ostensible reflexivity effect in pigeons reported by Sweeney and Urcuioli (2010) and they followed other experiments (Urcuioli, 2011) designed for the same purpose. To reiterate what has been said throughout this paper, Sweeney and Urcuioli found that pigeons concurrently trained on A–B, B–A, and B–B successive matching later showed emergent A–A matching. Their interpretation of this finding was grounded in terms of Urcuioli's (2008) theory of stimulus class formation which predicts that the net effect of such training are two 4-member stimulus classes containing the A samples and A comparisons, elements of the untrained reflexive relations.
As we have explained in the Introduction, the term “reflexive” is used to label the pigeons' behavior on the derived A–A tests: Despite no explicit training to do so, pigeons pecked a hue comparison more frequently after a matching than after a nonmatching hue sample on A–A test trials. Group REFL in Experiment 1 provides another demonstration of this emergent effect. Although the effect appears to an observer to be “matching each stimulus to itself”, the theory states that the matching stimuli in question—for example, a red comparison (R2) following a red sample (R1)—are functionally different stimuli given that each has an assumed ordinal-position component. This should not diminish the importance of the behavioral result but, instead, should be regarded as one means by which such a result can be obtained.
Besides the empirical analyses provided by the present experiments and those of Urcuioli (2011), they also bear directly on the stimulus-class mechanisms proposed in Urcuioli (2008). For instance, Urcuioli (2011) found that most pigeons concurrently trained on A–B, B–A, and C–C successive matching also showed emergent A–A matching in testing. These results suggest that the A–A effect may reflect generalized identity matching (Barros, Galvão, & McIlvane, 2002; Oden et al., 1988; Peña et al., 2006): Reinforced identity training with one set of stimuli (that is, the C stimuli) may, for some pigeons, generalize to other, familiar stimuli (namely, the A stimuli). More important, this result is not predicted by Urcuioli's (2008) theory which views C–C baseline training in the context of A–B and B–A arbitrary matching as insufficient to yield emergent A–A matching. Consequently, the emergent effect following such training questions the generality of the stimulus-class formation processes the theory proposes for pigeons.
The design of Experiment 1 can be viewed as incorporating a simpler control group for the effects of A–B, B–A, and B–B baseline training than that of Urcuioli (2011). Here, the control group (Group TRANS) learned just symmetrically reinforced A–B and B–A matching. Would this be sufficient to yield emergent A–A matching like that observed after training which also includes B–B matching? According to Urcuioli's (2008) theory, the answer is “no”: Without concurrent B–B training, four-member stimulus classes containing the elements necessary for A–A matching cannot develop. On the other hand, if the functional stimuli in the A–B and B–A tasks are the nominal stimuli themselves (as opposed to compounds consisting of those stimuli plus their ordinal positions within a trial), the answer is “yes” if the baseline relations are transitive. Although 4 of the 7 Group TRANS pigeons did not show an emergent A–A effect in testing, the other 3 most certainly did. Furthermore, the latter results do not appear to be random error given that those same 3 pigeons also exhibited emergent B–B matching in Experiment 2. This, too, is expected if their baseline B–A and A–B relations were transitive. Their data, then, also disconfirm the prediction derived from Urcuioli's (2008) stimulus-class analysis of successive matching.
Previous studies of transitivity following conditional discrimination training on A–B and B–C relations have met with mixed success. For example, Lipkens et al. (1988) and D'Amato et al. (1985, Experiment 3) found no evidence for transitivity in two-alternative matching by pigeons, although D'Amato et al. (1985, Experiment 2) reported evidence for the effect in monkeys. Kuno et al. (1994) reported that A–C matching for 3 of 4 pigeons was significantly above chance after two-alternative A–B and B–C training in which the spatial locations of samples and comparisons varied across trials (cf. Lionello & Urcuioli, 1998; Lionello-DeNolf & Urcuioli, 2000) and 10 responses were required to both sample and comparison stimuli (cf. Urcuioli, 2008, Experiment 2). It is unclear, however, if the Kuno et al. results truly represent transitivity in view of some apparent similarities between the samples in B–C training and those in the A–C test. Specifically, in BC matching, an unfilled or “open” triangle sample cued a reinforced red comparison choice and a cross sample cued a reinforced green comparison choice. In A–C testing, pigeons preferentially chose the red comparison following an unfilled or “open” circle sample and the green comparison following a single vertical line sample. An alternative (nontransitivity) explanation for this preference is that the red versus green choices had been cued throughout training and testing by the presence versus absence of an enclosed, unfilled area in the sample stimulus.
Steirn, Jackson-Smith, and Zentall (1991, Experiment 2) reported a weak transitivity effect in pigeons receiving discriminative autoshaping training (A–B) in which red and green center-key stimuli were followed by food or an empty food hopper (“no food”), respectively, and training on two-alternative matching in which food and no-food samples cued vertical- and horizontal-line choices (B–C). In A–C testing, red and green preferentially cued the vertical and horizontal choices, respectively. These preferences may not, however, represent a derived A–C relation if they were mediated by the behavior of pecking versus not pecking prior to a line choice (i.e., by learned pecking → vertical and not-pecking → horizontal choice relations; see, for example, Urcuioli & DeMarse, 1994).
Holland and Forbes (1982) reported a transitivity-like effect in rats. Their A–B task involved Pavlovian discriminative conditioning in which different visual CSs signaled whether or not sucrose would be delivered. In subsequent B–C training, sucrose presentation served as a feature indicating whether a forthcoming tone would be followed by food (feature-positive group) or not (feature-negative group). In A–C testing, it was found that the visual CS+ from A–B training effectively substituted for sucrose in either the B–C feature-positive or feature-negative discriminations.
In a study procedurally similar to the present one, Strasser et al. (2004) trained pigeons on A–B and B–C go/no-go matching with stimuli of different shapes and colors. On reinforced (positive) baseline trials, seven pecks within 10 s of comparison onset produced food whereas on nonreinforced (negative) baseline trials, seven pecks within 10 s produced a 5-s timeout. (Any trial on which seven pecks were not completed within the 10-s interval ended with entry into the ITI.) In testing, four nonreinforced and nonpunished A–C probes were randomly inserted among the various baseline trials. Average seven-peck completion times were found to be significantly shorter on positive than on negative A–C probes, a difference that mirrored completion times on positive versus negative baseline trials.
In all of these transitivity studies, the tested relations were arbitrary ones (A–C). Here, the nonreinforced probes consisted of matching versus nonmatching sample–comparison combinations (A–A). Could such identity relations have affected the test outcomes? After all, in Experiment 3 we observed higher rates of comparison responding on matching than nonmatching A–A trials even when the baseline A–B and B–A relations were asymmetrically reinforced (see Figure 13). Transitivity predicts that such asymmetrical training will yield precisely the opposite pattern—higher rates on nonmatching probes.
The results, then, indicate that pigeons' test performances may reflect an identity bias (Hogan & Zentall, 1981; Zentall, Edwards, Moore, & Hogan, 1981) following successive matching training of the sort provided here. In other words, after A–B and B–A training (Groups TRANS, S, and A), A–B, B–A, and B–B training (Group REFL; see also Sweeney & Urcuioli, 2010), A–B, B–A, or C–C training (Urcuioli, 2011), pigeons may preferentially respond at higher rates to matching than to nonmatching A comparisons that follow A samples. Indeed, Experiment 3 showed that it did not matter whether the A–B and B–A baseline relations were symmetrically or asymmetrically reinforced. Note that the baseline relations also insure that pigeons are highly familiar with the A stimuli as samples and as comparisons, and this may enhance the probability of observing the hypothesized identity bias in testing.
At first glance, an identity bias seems to be contradicted by Group REFL's acquisition results in Experiment 1. These pigeons took longer, on average, to reach criterion levels of performance on form–form (B–B) identity matching than on either of the two arbitrary tasks (A–B and B–A). However, responding at higher rates to matching comparisons in novel A–A combinations after extensive baseline training with the A stimuli does not necessarily imply a similar preferential pattern as pigeons initially learn successive matching contingencies that include identity matching (see, for example, Sweeney & Urcuioli, 2010, Table 2). More important, comparing the rates of acquisition of B–B identity versus A–B and B–A arbitrary matching confounds the nature of the tasks with the discriminability of the sample and comparison stimuli comprising them. Indeed, acquisition of two-alternative B–B matching with matching stimuli like those used here takes longer to acquire than comparable A–B and B–A tasks in which red and green hues serve either as A samples or A comparisons, respectively (Carter & Eckerman, 1975, see also Urcuioli & Zentall, 1986). Indeed, Carter and Eckerman showed that these acquisition differences could be predicted from the rates at which pigeons learn simple successive and simultaneous discriminations between the stimuli serving as samples and comparisons, respectively, in the conditional discriminations. A proper comparison, then, must somehow equate discriminability profiles across tasks.
The hypothesized identity bias may explain other test results reported by Sweeney and Urcuioli (2010) for pigeons trained on A–B and B–A arbitrary matching plus B–B oddity (viz., comparison responding reinforced only when a form comparison differs from a form sample). Specifically, comparison responding on the explicitly trained form–form relations in this group was reinforced only when a form comparison differed from a form sample. The prediction (Urcuioli, 2008) was that these pigeons would exhibit emergent A–A oddity in testing. One of 5 pigeons did, but another (OREF2) curiously showed emergent A–A matching. The latter result is not only a clear theoretical disconfirmation but, in the present light, might represent another example of a preference for pecking matching comparisons after successive matching training similar or identical to that used here. Indeed, it is equally tempting to speculate that the other 3 pigeons trained with B–B oddity contingencies responded nondifferentially on A–A probes because explicit reinforcement for responding to nonmatching comparisons (and nonreinforcement for responding to matching comparisons) in the B–B baseline task counteracted the chances of observing an identity bias when pigeons were confronted with novel matching and nonmatching stimulus combinations in testing.
It is also the case, however, that a higher percentage of pigeons trained on A–B, B–A, and B–B identity (e.g., Group IREF in Sweeney & Urcuioli, 2010, Group REFL in Experiment 1, and Group RF in Urcuioli, 2011) show emergent A–A matching than after A–B and B–A training (Group TRANS in Experiment 1; Group S in Experiment 3) or A–B, B–A, and C–C training (Group GI in Urcuioli, 2011). Could the higher percentage reflect the stimulus-class mechanism proposed by Urcuioli (2008)? The answer is uncertain and experimentally separating these different processes would be challenging at the very least. Indeed, it may be impossible with any successive matching training that includes identity matching.
Still, the anti-symmetry result reported by Urcuioli (2008, Experiment 4) appears to require functional stimuli with an ordinal position component and class merger via common elements. For obvious reasons, then, that result is well worth replicating. Another way to establish such hypothetical processes is to arrange successive matching training contingencies that predict one pattern of test results given the assumptions of the theory but the opposite pattern if those assumptions are relinquished. An example of such a strong inference test would be to train pigeons on asymmetrically reinforced A–B and B–A arbitrary matching plus B–B oddity. The theoretical prediction based on Urcuioli (2008) is that testing should reveal emergent A–A matching. On the other hand, transitive relations involving the nominal A and B stimuli predict emergent A–A oddity.
On a final note, demonstrating transitivity in successive matching that cannot be attributed to an identity bias requires baseline arbitrary matching tasks in which the comparisons of one task are nominally different from the samples of the other (i.e., A–B and B–C). Strasser et al. (2004) have already provided such a demonstration using baseline reinforcement contingencies similar, albeit not identical, to those described here. Their results are also well worth replicating in part because of their theoretical implications. Indeed, Urcuioli's (2008) theory predicts that A–B and B–C successive matching training alone should not yield emergent A–C matching in testing. It also predicts that transitivity will be evident in testing if C–C successive matching is trained concurrently with those two arbitrary tasks. Note that if the A–C effect were obtained after A–B, B–C and C–C training, it, by definition, cannot reflect an identity bias (i.e., there are no identity relations in testing). These types of future experiments promise to enhance our understanding of the conditions yielding emergent relations in pigeons and will aid in identifying the explanatory processes underlying them.
Acknowledgments
This research was supported by NICHD Grant R01 HD061322. The author thanks Blake Polak, Dana Harkins, and Cody Neal for their assistance in conducting this research.
Footnotes
These numerical designations differ from what is common in the equivalence literature where number is used to identify stimulus class. That is not the case here. Instead, “1” or “2” following a particular matching stimulus, such as red (R), denotes the stimulus’ position within a matching trial (first = sample, second = comparison).
Comparison response rates by individual pigeons on the 32 “positive” and 32 “negative” probe trials over the eight test sessions (four each per session) were compared using one-way analyses of variance with positive versus negative as the factor.
REFERENCES
- Barros R, Galvão O, McIlvane W.J. Generalized identity matching-to-sample in Cebus Apella. The Psychological Record. 2002;52:441–460. doi: 10.1007/s40732-014-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D.E, Eckerman D.A. Symbolic matching by pigeons: Rate of learning complex discriminations predicted from simple discriminations. Science. 1975;187:662–664. doi: 10.1126/science.1114318. [DOI] [PubMed] [Google Scholar]
- D'Amato M.R, Salmon D.P, Loukas E, Tomie A. Symmetry and transitivity in the conditional relations in monkeys (Cebus apella) and pigeons (Columba livia) Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A.J, Wasserman E.A. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan D.E, Zentall T.R. The role of identity in the learning and memory of a matching-to-sample problem by pigeons. Bird Behaviour. 1981;3:27–36. [Google Scholar]
- Holland P.C, Forbes D.T. Control of conditional discrimination performance by CS-evoked event representations. Animal Learning & Behavior. 1982;10:249–256. [Google Scholar]
- Kuno H, Kitadate T, Iwamoto T. Formation of transitivity in conditional matching to sample by pigeons. Journal of the Experimental Analysis of Behavior. 1994;62:399–408. doi: 10.1901/jeab.1994.62-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello K.M, Urcuioli P.J. Control by sample location in pigeons' matching-to-sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Transfer of pigeons' matching to sample to novel sample locations. Journal of the Experimental Analysis of Behavior. 2000;73:141–161. doi: 10.1901/jeab.2000.73-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop P.F.M, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay H.A, Wilkinson K.M, Farrell C, Serna R.W. Evaluating merger and intersection of equivalence classes with one member in common. Journal of the Experimental Analysis of Behavior. 2011;96:87–105. doi: 10.1901/jeab.2011.96-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oden D.L, Thompson R.K.R, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. [PubMed] [Google Scholar]
- Peña T, Pitts R.C, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85:203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger R.S. The number of non-zero, post hoc contrasts from ANOVA and error rate. I. British Journal of Mathematical and Statistical Psychology. 1975;28:71–78. [Google Scholar]
- Sidman M. Equivalence relations: Where do they come from. In: Lejeune H, Blackman D, editors. Behavior analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 93–114. (Eds.) [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discriminations of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;22:261–273. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching-to-sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steirn J.N, Jackson-Smith P, Zentall T.R. Mediational use of internal representations of food and no-food events by pigeons. Learning and Motivation. 1991;22:353–365. [Google Scholar]
- Strasser R, Ehrlinger J.M, Bingman V.P. Transitive behavior in hippocampal-lesioned pigeons. Brain, Behavior, and Evolution. 2004;63:181–188. doi: 10.1159/000076442. [DOI] [PubMed] [Google Scholar]
- Sweeney M.M, Urcuioli P.J. A reflexivity effect in pigeons. Journal of the Experimental Analysis of Behavior. 2010;94:267–282. doi: 10.1901/jeab.2010.94-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J. Associative symmetry, “anti-symmetry”, and a theory of pigeons' equivalence-class formation. Journal of the Experimental Analysis of Behavior. 2008;90:257–282. doi: 10.1901/jeab.2008.90-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J. Emergent identity matching after successive matching training: Reflexivity or generalized identity. Journal of the Experimental Analysis of Behavior. 2011;96:329–314. doi: 10.1901/jeab.2011.96-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, DeMarse T. On the relationship between differential outcomes and differential sample responding in matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:249–263. doi: 10.1037//0097-7403.20.3.249. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J, Zentall T.R. Retrospective coding in pigeons' delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:69–77. [PubMed] [Google Scholar]
- Vasconcelos M. Transitive inference in non-human animals: An empirical and theoretical analysis. Behavioural Processes. 2008;78:313–334. doi: 10.1016/j.beproc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Wasserman E.A. Successive matching-to-sample in the pigeon: Variation on a theme by Konorski. Behavior Research Methods & Instrumentation. 1976;8:278–282. [Google Scholar]
- Zentall T.R, Edwards C.A, Moore B.S, Hogan D.E. Identity: The basis for both matching and oddity learning in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:70–86. [PubMed] [Google Scholar]