Abstract
Early onset in bipolar disorder (BPD) has been associated with greater familial risk and unfavorable clinical outcomes. We pooled data from seven international centers to analyze the relationships of family history and symptomatic as well as functional measures of adult morbidity to onset age, or onset in childhood (age <12), adolescence (12-18), or adulthood (19-55 years). In 1,665 adult, DSM-IV BPD-I patients, onset was 5% in childhood, 28% in adolescence, and 53% at peak ages 15-25. Adolescent and adult onset did not differ by symptomatic morbidity (episodes/year, percentage of months ill, co-morbidity, hospitalization, suicide attempts) or family history. Indications of favorable adult functional outcomes (employment, living independently, marriage and children, and a composite measure including education) ranked, by onset: adult > adolescent > child. Onset in childhood versus adolescence had more episodes/year and more psychiatric co-morbidity. Family history was most prevalent with childhood onset, similar over onset ages 12-40 years, and fell sharply thereafter. Multivariate modeling sustained the impression that family history and poor functional, but not symptomatic, outcomes were associated with younger, especially childhood onset. Early onset was more related to poor functional outcomes than greater symptomatic morbidity, with least favorable outcomes and greater family history with childhood onset.
Keywords: Bipolar disorder, adult functional status, morbidity, age at onset, outcome
Age at onset of type-I bipolar disorder (BPD) typically averages 12-24 years, is older among patients with type-II BPD, and oldest in unipolar major depressive disorder 1,2,3. Reported onset ages probably vary by ascertainment methods, and possibly among different countries and cultures 1,2,3,4,5,6. Early onset of BPD in childhood or adolescence is of particular interest as it may help to define subgroups. Juvenile onset generally appears to be common, reportedly ranging from 39% to 65% of samples including patients evaluated as juveniles or adults 2,4,5,6,7. BPD patients with early onset may represent a phenotype of special interest for genetic and other biomedical research, as well as having potential clinical importance 8,9,10,11. Genetic interest arises in part from relatively high rates of familial mood disorders in BPD, generally, and particularly in association with young onset 5,6,7,8,9,10,11. Early onset cases also provide challenges of earlier recognition, prognosis, and treatment of young patients whose early illnesses may differ from typical adult types, and whose diagnosis and treatment are typically delayed for years 7,12,13,14,15.
Several investigators have applied the statistical method of admixture analysis of onset age distributions, which typically yield nodal prevalence in adolescent or early adult years with some skewing to younger ages 16,17,18,19,20,21. When large samples of type-I BPD probands have been evaluated with this method, onset ages typically have yielded three putatively independent, nearly normal Gaussian distributions, with ages averaging 17.1±1.7, 25.3±1.8, and 38.0±4.3 years 16,17,18,19,20,21. Findings in these studies were similar across various geographical regions (including Canada, France, Italy, the US, and Wales), suggesting some consistency despite likely ethnic and clinical heterogeneity. However, the contributions of the three computed onset age subgroups to the total varied widely (36% to 80%, 7% to 39%, and 13% to 25%, respectively). Usually, however, the youngest subgroup predominated and was associated with various adverse clinical outcome measures.
Most previous research has suggested that BPD following pre-adult onset may be particularly severe. Such severity reportedly is indicated by relatively high rates of rapid cycling or even chronic illness, prominent psychotic and anxiety features, substance abuse, and limited response to mood stabilizing treatment, compared to cases with adult onset 4,5,7,22,23,24. Poor outcomes may reflect: a) particularly virulent long-term illness following juvenile onset, b) effects of prolonged delay or refusal of treatment in childhood and adolescence, c) impressions arising from juvenile illnesses that may not be expressed in more clearly episodic, adult form, or d) possible destabilizing effects of antidepressants and stimulants commonly used to treat children and adolescents with behavioral symptoms 7,12,13. Another possibility is that juvenile, and particularly childhood onset disorders may have a severe impact on maturation, particularly to adult functional levels 25. However, not all reports support the hypothesis that early onset BPD follows a more severe course or outcome than adult onset BPD 26,27,28. Moreover, admixture analysis has yielded evidence of discrete subgroups that do not necessarily correspond to developmental periods of clinical interest (childhood, adolescence, young adulthood, middle age, and late years).
We undertook the present study in view of the evident importance of early identification of potentially severe, disabling or even fatal BPD following early onset, and the relative rarity and inconsistency of comparisons of long-term symptomatic and functional outcomes in large numbers of female and male patients meeting standard diagnostic criteria for type-I BPD, followed into adulthood, and across multiple cultural settings. We endeavored to limit effects of potential geographic and cultural variance by pooling demographic and clinical data from 1,665 patients meeting DSM-IV diagnostic criteria as adults, from seven mood disorder centers in Argentina, Italy, Spain, Switzerland, Turkey, and the US. Study hypotheses were that: a) early onset would be followed by greater morbidity by most available measures, and b) family history of affective illness would be inversely and continuously related to onset age. We also planned specifically to consider possible differences between onset in childhood compared to adolescence and adulthood, and to compare measures of symptomatic versus functional outcomes.
METHODS
Subjects
We pooled data from a total of 1,665 patients diagnosed at adult ages with type-I BPD by DSM-IV criteria at seven sites affiliated with an International Consortium for Bipolar Research based at McLean Hospital/Harvard Medical School: Lucio Bini Mood Disorders Center, Cagliari, Italy (n=586); Argentine Network for Bipolar Disorders at Palermo University, Buenos Aires, Argentina (n=328); McLean Hospital, Boston, MA, USA (n=215); University Clinic, Barcelona, Spain (n=204); Viarnetto Psychiatric Clinic, Lugano, Switzerland (n=174); Department of Psychiatry, Dokuz Eylül University, Izmir, Turkey (n=134); and Lucio Bini Juvenile Mood Disorder Center, New York, NY, USA (n=24). The US sites sought to enhance representation of early onset cases by selecting cases with juvenile onset (age ≤18; New York) and first-episode patients (Boston), all followed prospectively into adult years, as at other sites. Patients were evaluated, treated clinically, and followed for at least 3-5 years, using methods detailed previously 29,30,31,32,33,34,35.
Subjects were assessed retrospectively for estimated onset age, based on first clinically appreciable syndromal illness, as indicated by patient history, recollections of family members, and medical records. Onset age was separated into onset groups of clinical interest: childhood (<12 years), adolescence (12-18 years), or adulthood (≥19 years), or onset age was considered as a continuous measure. For assessment of family history rates, all cases were included, with subgroups formed by decades of onset age, as well as during childhood, adolescence, early adult, and advanced ages.
Assessments
As continuous, symptomatic measures of morbidity, we considered the annual rate (episodes/year) of major DSM-IV BPD episodes (mania or hypomania, major depression, mixed states, or psychosis) from illness onset, as well as estimates of percent of months ill per year, both during exposure times limited to ≥2 years. We also considered several categorical clinical measures: presence of any DSM-IV Axis I psychiatric or substance use disorder co-morbidity; ever manifesting psychotic symptoms; ever being psychiatrically hospitalized; and ever having attempted suicide. The following functional or social outcomes were considered: having completed high school or higher education; ever having married; having children; being gainfully employed or a student or homemaker at last follow-up; and living independently, with a composite categorization based on these functional measures. The composite functional measure was based on the sum of weighted ratings of being employed (10 points), living independently (5 points), ever being married (2 points), having children (1 point), and having completed high school (1 point). “Poor functional outcome” was defined by a total score of zero. We also assessed family history of psychiatric illness (affective or substance use) in first-degree relatives.
Data analyses
We compared those with onset in childhood (<12 years), adolescence (12–18 years) or in juvenile years overall (≤18 years) versus adulthood (≥19 years) for the previously defined clinical measures. Family history rates were compared in the same subgroups as well as across decades of onset ages. Outcome assessments were limited to subjects followed-up to age ≥25 years to allow time to attain adult indices of functional accomplishment, as well as being at risk for at least 2 years to avoid exaggerating estimates with very short exposure times 35, and with onset age ≤55 years to avoid cases of secondary mania 36, yielding 1,368 cases (82.2% of the total).
Histographic analysis involved all onset ages observed. We also used contingency table-based (χ²) comparisons of cases following onset in childhood (<12), adolescence 12,13,14,15,16,17,18, or young adult age (19-55 years) for comparison to categorical outcome measures. In addition, continuous measures, including onset ages (log-normalized), episodes/year and proportion of time ill, were compared between onset age categories by ANOVA methods (F). Degrees of freedom (df) are provided. We also used stepwise, multivariate logistic regression modeling for factors associated significantly and independently with the onset age subgroups, as well as multivariate linear regression modeling of representative factors associated with onset age as a continuous measure, in both cases limiting the sample to the 1,368 cases, as defined above. Averages are means ± standard deviation (SD) or medians with interquartile range (IQR), unless stated otherwise.
RESULTS
Overall subject characteristics
The 1,665 type-I BPD subjects included 52.3% women and onset age averaged 25.7±11.3 years. The median (IQR) onset age was 23.0 (13.0) overall. It was 22.0 (12.0) in men vs. 24.0 (14.4) in women (by log-normalized onset-age: F=9.21, p=0.002). Juvenile onset (age ≤18 years) involved 26.6% of subjects (n=477), of whom 83 (5.0% of all cases) were children. Current age averaged 40.8±14.4 years, and exposure time of illness from onset averaged 15.1±11.5 years. Proportions of subjects with juvenile onset (≤18 years) averaged 28.6% (it was 26.9% in Izmir, 24.7% in Buenos Aires, 22.0% in Cagliari, 21.6% in Barcelona, and 17.8% in Lugano based on hospitalized subjects). US patients had higher proportions of juvenile onset cases, owing to selection factors (63.7% in Boston, involving only first-episode patients followed-up prospectively, and 79.2% in New York, at a pediatric mood disorder center), with correspondingly young median (IQR) onset ages: 16.0 (7.8) and 13.5 (10.5), respectively.
Distribution of onset ages
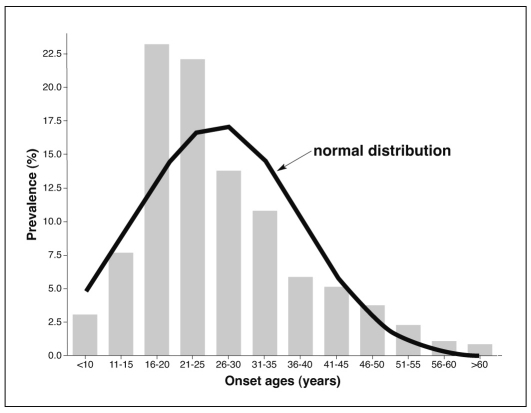
Overall median (IQR) onset age was 24.0 (13.1) years, with moderate skewing toward ages 15-25 years, compared to a normal Gaussian distribution (Figure1). Peak prevalence at ages 15-25 years accounted for a majority (53.0%) of all 1,665 cases, and prevalence was <5% at ages <15, and >45 years.
Figure 1 Histogram of onset ages in 1,665 bipolar-I disorder patients, with superimposed normal Gaussian distribution, indicating moderate skewing toward younger ages.
Characteristics by age subgroups
We compared various demographic and clinical characteristics in patients with onset in childhood (ages <12), adolescence (12-18 years), or adulthood (19-55 years) in a sample limited as defined above (onset age <55, ill ≥2 years, followed to ages ≥25). Statistical comparisons across onset age groups are provided for illustrative purposes and to guide subsequent multivariate modeling, and are unadjusted for multiple comparisons (Table 11). Notable relationships included the following. Family history was strongly associated with younger onset, and significantly greater among childhood than adolescent onset cases (χ² = 11.1, df=1, p=0.004). Episodes/year as a major measure of symptomatic morbidity was highest with childhood and lowest with adolescent onset (F=3.92, p=0.02), whereas the approximate proportion of months/year in a major episode of BPD tended to rise inversely with younger onset, but did not differ significantly between childhood and either adolescent or adult onset. There was also somewhat greater prevalence of psychiatric or substance use co-morbidity among childhood onset than adolescent onset patients (χ²=2.67, p=0.07). Psychosis differed significantly between childhood and adolescent onset patients (χ²=24.8, p<0.0001), but its prevalence decreased with younger onset age. For the composite measure of adult functional status, there was a much greater risk of poor functional outcome with younger onset age, with significantly greater prevalence following onset in childhood than in adolescence (χ²=31.5, p<0.0001; Table 1).
Table 1.
Table 1 Comparisons of adult bipolar-I disorder patients with onset in childhood, adolescence or adulthood
| Onset age group | |||||
| Child (<12) | Adolescent (12-18) | Adult (19-55) | χ² or F | p | |
| Cases (n) | 53 | 335 | 980 | - | - |
| % women | 34.0 | 53.1 | 57.4 | 11.6 | 0.003 |
| Onset age (years) | |||||
| Mean ± SD | 7.94±2.03 | 16.9±1.78 | 30.2±10.2 | - | <0.0001 |
| Median (IQR) | 8.00 (4.00) | 16.0 (2.00) | 28.0 (13.1) | - | - |
| Current age (years, mean ± SD) | 34.1±9.83 | 38.4±11.1 | 45.7±12.8 | 59.7 | <0.0001 |
| Years of illness (mean ± SD) | 26.2±9.54 | 22.0±11.4 | 15.5±10.4 | 64.9 | <0.0001 |
| Family affective history (%)* | 88.6 | 66.3 | 61.6 | 11.1 | 0.004 |
| Episodes/year (mean ± SD)* | 1.06±1.71 | 0.68±0.72 | 0.79±0.89 | 3.92 | 0.02 |
| % of months/year ill (mean ± SD) | 46.9±41.1 | 39.7±35.4 | 33.7±32.7 | 2.69 | 0.07 |
| Ever hospitalized (%) | 82.9 | 86.2 | 81.3 | 2.35 | 0.31 |
| Ever psychotic (%)* | 16.2 | 38.9 | 53.5 | 24.8 | <0.0001 |
| Ever attempted suicide (%) | 27.3 | 27.8 | 20.4 | 4.45 | 0.11 |
| Any psychiatric co-morbidity (%)* | 90.9 | 54.1 | 57.4 | 5.71 | 0.06 |
| Education ≥ high school (%) | 53.8 | 61.6 | 63.1 | 1.05 | 0.59 |
| Ever married (%) | 37.0 | 44.7 | 58.4 | 24.2 | <0.0001 |
| With children (%) | 10.0 | 29.2 | 38.1 | 6.52 | 0.04 |
| Employed (%) | 37.5 | 62.0 | 71.8 | 28.2 | <0.0001 |
| Living independently (%) | 0.00 | 43.9 | 75.5 | 16.9 | 0.0002 |
| Functionally impaired (%)* | 60.9 | 42.7 | 30.2 | 31.5 | <0.0001 |
| * Significantly different in patients with onset in childhood versus adolescence | |||||
Since childhood onset (ages <12 years) represented a minority of cases (5.0%), we also compared adult outcomes among all subjects with juvenile onset (age ≤18 years; 33.0% of the total) and those with older onset ages in the restricted sample of 1,368 patients. Adult functional outcome measures again differed much more between juvenile and adult onset cases than did symptomatic measures. For example, the pooled index of successful functional outcome was 41% lower following younger onset (χ²=17.0, p<0.0001), with similar differences for employment, independent living, and marital status. In contrast, among symptomatic measures, episodes/year, any co-morbidity, hospitalization, and psychotic features did not differ significantly between these onset age groups, although, with younger onset, percentage time ill/year was 31% greater (F=5.65, p=0.02) and suicide attempts 33% more frequent (χ²=4.47, p=0.04). Moreover, the effect of onset age on adult functional outcomes was even greater among men than women (relative risk by juvenile/adult onset age = 1.68 versus 1.17).
The preceding findings were similar across geographic regions sampled. Notably, employment, a measure of functional outcome, was consistently lower among juvenile onset cases (by an average of 1.37±0.16 times) in both US and other centers.
Family history
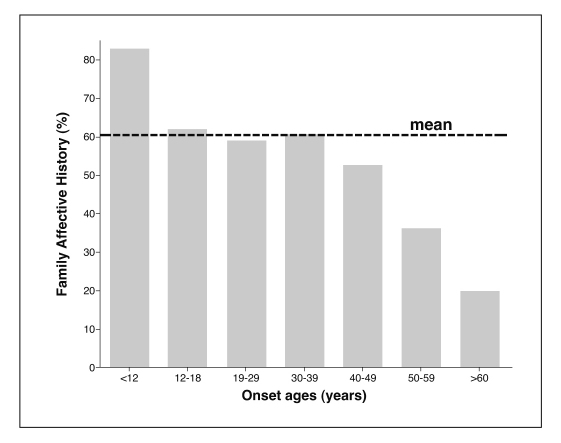
The frequency of identified affective illness or substance abuse among first-degree relatives was strongly related to onset age, overall (Figure 2). Prevalence of family history was highest with childhood onset (83.1%), similar from ages 12 through 39 (averaging 60.7±1.4%) years, and declined with higher onset ages, to 52.8% at 40-49, 39.4% at 50–59, and only 20.0% at ≥60 years.
Figure 2 Prevalence (%) of family history of affective illness versus onset ages among 1,665 bipolar-I disorder patients.
Multivariate analyses of factors associated with onset age
We used both linear (actual onset age) and logistic (juvenile vs. adult, and childhood vs. adolescent onset) multivariate regression modeling to test for significant and independent associations of selected factors with onset age (Table 2). We were particularly interested in testing the hypothesis that functional outcome and family history, but not a measure of symptomatic morbidity (episodes/year), would be associated with earlier onset age. This prediction was sustained with both models. The impression that outcomes were generally even less favorable, and family history greater, with onset in childhood versus adolescence was also supported (Table 2).
Table 2.
Table 2 Multivariate regression modeling: factors associated with onset age
| Factors associated with juvenile onset: logistic regression | |||
| Factors | Odds ratio (95%CI) | χ² | p |
| Poor functional outcome | 2.00 (1.34 to 2.95) | 8.82 | 0.003 |
| More family history | 1.71 (1.20 to 2.43) | 5.86 | 0.015 |
| More episodes/year | 1.08 (0.89 to 1.33) | 0.63 | 0.43 |
| Factors associated with onset in childhood vs. adolescence: logistic regression | |||
| Factors | Odds ratio (95%CI) | χ² | p |
| Poor functional outcome | 2.70 (1.08 to 6.77) | 4.51 | 0.03 |
| More family history | 4.65 (1.04 to 20.8) | 4.04 | 0.04 |
| More episodes/year | 4.85 (1.05 to 22.4) | 0.63 | 0.04 |
| Factors associated with younger onset age: linear regression | |||
| Factors | β coefficient (95% CI) | 1 | p |
| More family history | 2.78 (1.05 to 4.41) | 3.35 | 0.001 |
| Poor functional outcome | 2.61 (1.01 to 4.21) | 3.20 | 0.001 |
| Episodes/year | 0.62 (-1.90 to 1.43) | 1.50 | 0.13 |
DISCUSSION
The present findings indicate that several demographic, clinical, and functional outcome factors were associated variably with onset age in a large, international sample of DSM-IV, type-I BPD patients. Outcome analyses were limited to patients with onset age below 55 years and followed for at least 2 years into adult years (≥25) to limit potential confounding effects of immaturity, of very short exposure times 36, and risk of secondary mania 37.
Generally, measures of symptomatic morbidity were surprisingly similar following onset in adolescent versus adult years, whereas functional outcomes were more favorable with older onset, sometimes differing significantly between childhood and adolescent onset. Family history was more prevalent with childhood onset, similar from onset ages of 12-40, and fell sharply at later onset ages. The general tendency for family history to be greater with younger onset is well known 4,5,6,7,8,9,10,11, but the striking differences among cases of childhood, adolescent to middle aged, and older onset ages appear to be a novel finding. Multivariate modeling sustained the impression that family history and poor functional, but not symptomatic, outcomes were associated with juvenile onset, with somewhat greater morbidity as well as familial risk with onset in childhood versus adolescence.
It is particularly noteworthy that the present findings indicate that cases of BPD with juvenile onset may have different outcomes, in that childhood onset appears to differ from both adolescent and adult onset and to be a particularly virulent form of the illness. Moreover, there was evidence that adult functional outcomes were even more impaired than symptomatic and other clinical measures following juvenile onset. If these hypothesis-generating findings are valid, they may be particularly important clinically in suggesting that developmental or maturational effects of early, and especially childhood onset BPD, may be especially great.
It is tempting to speculate that the observed effects on functional disability may be associated with the depressive-dysphoric components of BPD, which remain highly prevalent and a major therapeutic challenge 32,38. However, both depression 7 and mania/hypomania 39 have been reported to occur in excess with early onset BPD. Cognitive impairment in BPD also can occur and may contribute to the observed adverse functional outcomes associated with early onset 40. Whatever the bases of poor outcomes with very young onset may be, we propose that it is likely that morbidity-associated developmental delays are involved.
The observed lack of evidence of more severe symptomatic morbidity among juvenile onset cases of BPD followed-up for ≥2 years into adult years seems inconsistent with much 4,7,22,23,24, but not all 26,27,28, of the literature reviewed above. Inconsistencies may reflect differences in inclusion of cases with onset in childhood versus adolescence. Another possible factor is that most studies involving onset of putative BPD in childhood or adolescence considered diagnosis and morbidity in young ages with variable later verification of diagnosis in adult life, and few have included direct and systematic comparisons of cases involving juvenile versus adult onset, or childhood versus adolescent onset 4,7,23. Patients with BPD of juvenile, and especially childhood onset, continue to present severe diagnostic uncertainties, and tend to differ from adult onset forms of BPD, by lacking clear episodes or following a rapidly fluctuating, chaotic, or chronic course, and having high rates of psychotic features and co-morbidities that include anxiety, attentional, conduct, and substance-related disorders 2,3,12,19,23,24. These characteristics of juvenile onset BPD patients may contribute to the impression that such illnesses may be more severe than in adults. Whether that hypothesis is valid or not, it seems clear that earlier recognition, diagnosis, and improved treatments for early onset BPD are required 7,13,14,15,16. Our observations suggest that BPD starting in childhood may differ in its clinical implications not only from adult onset cases, but possibly also from adolescent onset patients – another hypothesis that requires further testing.
Limitations of this study include potential differences among study sites in methods of case and morbidity ascertainment, although onset age (except for samples selected for early onset in the US sites) and representative outcome measures were similar across geographic regions. Another limitation was that estimates of morbidity factors as well as onset ages were largely retrospective, including the need for recall by patients or families over many years; such recall might differentially impact cases with earlier onset. Also, some measures (suicidal acts, co-morbidity, psychosis) were present in relatively low prevalence, which may limit the reliability of their estimates. In addition, case selection biases may be involved, in that patients who were followed for long times may not be representative of others who were less accessible to, or cooperative with long-term follow-up and treatment.
Despite efforts to limit their impact, there may still be effects of years-at-risk, and years of adult life. Since duration of illness was longer with earlier onset, it may be that longer exposure times increased risk of some clinical outcomes (such as hospitalization, suicide attempts, psychosis, anxiety disorder, or other co-morbid psychiatric or substance use disorders). However, such an effect would tend to limit the observed lack of association of more of such outcomes with earlier onset. In addition, longer exposure times with younger onset would likely have limited, rather than increased, measures of morbidity-per-time (such as episodes/year) 36. Treatment was clinical and uncontrolled, and may have modified long-term morbidity, though presumably randomly. A noteworthy aspect of this study was that DSM-IV diagnostic criteria for type-I BPD were met in adult life, to avoid the diagnostic complexity and uncertainties of pediatric diagnoses 41. Despite their potential limitations, reported findings were strikingly consistent across several methods of analysis and among geographic regions.
In conclusion, the findings presented raise the intriguing possibility that particular illness-related factors and outcomes may be associated differentially with onset of type-I BPD in childhood versus both adolescence and adulthood. We found especially strong relationships of juvenile onset with adverse social and functional outcome measures, including not being employed, not living independently, being unmarried and not having children, whereas most measures of symptomatic morbidity were much less related to onset age. We also found especially high prevalence of family history among childhood onset cases. If these observations are valid and replicable, they may suggest a particularly important impact of juvenile, and especially childhood, onset of BPD at the level of maturation and functional success in attaining major adult roles of later life, with lesser impact on the symptomatic expression of BPD. The particularly strong association of childhood-onset with high rates of reported family history further supports efforts to identify specific phenotypic subgroups of interest for genetic and other biomedical investigations. Early versus late onset has also been proposed as a course specifier for DSM-5 42. Finally, the present findings further encourage earlier diagnosis and development of interventions aimed particularly at limiting maturational-functional impairments among young persons with BPD.
Acknowledgements
This study was supported in part by a grant from the Bruce J. Anderson Foundation and by the McLean Private Donors Research Fund for Bipolar Disorders, and by the Lucio Bini Private Donors Research Fund.
References
- 1.Baldessarini RJ, Bolzani L, Cruz N. Onset-age of bipolar disorders at six international sites. J Affect Dis. 2010;121:143–146. doi: 10.1016/j.jad.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Larsson S, Lorentzen S, Mork E. Age at onset of bipolar disorder in a Norwegian catchment area sample. J Affect Disord. 2010;124:174–177. doi: 10.1016/j.jad.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Tondo L, Lepri B, Cruz N. Age at onset in 3014 Sardinian bipolar and major depressive disorder patients. Acta Psychiatr Scand. 2010;121:446–452. doi: 10.1111/j.1600-0447.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- 4.Perlis RH, Miyahara S, Marangell LB. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BP) Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin RK, Jamison KR, editors. Manic-depressive illness, 2nd ed. New York: Oxford University Press; 2007. [Google Scholar]
- 6.Beesdo K, Höfler M, Leibenluft E. Mood episodes and mood disorders: patterns of incidence and conversion in the first three decades of life. Bipolar Disord. 2009;11:637–649. doi: 10.1111/j.1399-5618.2009.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Post RM, Leverich GS, Kupka RW. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71:864–872. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 8.Leboyer M, Henry C, Paillere-Martinot ML. Age at onset of bipolar affective disorders: a review. Bipolar Disord. 2005;7:111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 9.Pavuluri MN. Effects of early intervention on the course of bipolar disorder: theories and realities. Curr Psychiatry Rep. 2010;12:490–498. doi: 10.1007/s11920-010-0155-1. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Glatt SJ, Su J. Three potential susceptibility loci shown by a genome-wide scan for regions influencing the age at onset of mania. Am J Psychiatry. 2004;161:625–630. doi: 10.1176/appi.ajp.161.4.625. [DOI] [PubMed] [Google Scholar]
- 11.Priebe L, Degenhardt FA, Herms S. Genome-wide survey implicates the influence of copy number variants (CNVs) in the development of early-onset bipolar disorder. Mol Psychiatry. doi: 10.1038/mp.2011.8. in press. [DOI] [PubMed] [Google Scholar]
- 12.Faedda GL, Baldessarini RJ, Glovinsky IP. Pediatric bipolar disorder: phenomenology and course of illness. Bipolar Disord. 2004;6:305–313. doi: 10.1111/j.1399-5618.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 13.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: review of the existing literature. Dev Psychopathol. 2006;18:1023–1035. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- 14.Leverich GS, Post RM, Keck PE. The poor prognosis of childhood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 15.Suominen K, Mantere O, Valtonen H. Early age at onset of bipolar disorder is associated with more severe clinical features but delayed treatment-seeking. Bipolar Disord. 2007;9:698–705. doi: 10.1111/j.1399-5618.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 16.Bellivier F, Golmard JL, Rietschel M. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160:999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 17.Lin PI, McInnis MG, Potash JB. Clinical correlates and familial aggregation of age at onset in bipolar disorder. Am J Psychiatry. 2006;163:240–246. doi: 10.1176/appi.ajp.163.2.240. [DOI] [PubMed] [Google Scholar]
- 18.Manchia M, Lampus S, Chillotti C. Age at onset in Sardinian bipolar I patients: evidence for three subgroups. Bipolar Disord. 2008;10:443–446. doi: 10.1111/j.1399-5618.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamshere ML, Gordon-Smith K, Forty L. Age-at-onset in bipolar-I disorder: mixture analysis of 1369 cases identifies three distinct clinical sub-groups. J Affect Disord. 2009;116:23–29. doi: 10.1016/j.jad.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Ortiz A, Bradler K, Slaney C. An admixture analysis of the age at index episodes in bipolar disorder. Psychiatry Res. 2011;188:34–39. doi: 10.1016/j.psychres.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Tozzi F, Manchia M, Galwey NW. Admixture analysis of age at onset in bipolar disorder. Psychiatry Res. 2011;185:27–32. doi: 10.1016/j.psychres.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Schürhoff F, Bellivier F, Jouvent R. Early and late onset bipolar disorders: two different forms of manic-depressive illness? J Affect Disord. 2000;58:215–221. doi: 10.1016/s0165-0327(99)00111-1. [DOI] [PubMed] [Google Scholar]
- 23.Craney JL, Geller B. A prepubertal and early adolescent bipolar disorder-I phenotype: review of phenomenology and longitudinal course. Bipolar Disord. 2003;5:243–256. doi: 10.1034/j.1399-5618.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 24.Geller B, Tillman R, Bolhofner K. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldstein TR, Birmaher B, Axelson D. Psychosocial functioning among bipolar youth. J Affect Disord. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGlashan TH. Adolescent versus adult onset of mania. Am J Psychiatry. 1988;145:221–223. doi: 10.1176/ajp.145.2.221. [DOI] [PubMed] [Google Scholar]
- 27.Jairam R, Srinath S, Girimaji SC. Prospective 4–5-year follow-up of juvenile onset bipolar disorder. Bipolar Disord. 2004;6:386–394. doi: 10.1111/j.1399-5618.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 28.Birmaher B, Axelson D, Strober M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tohen M, Zarate CA Jr, Hennen J. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 30.Tondo L, Lepri B, Baldessarini RJ. Risks of suicidal ideation, attempts and suicides among 2826 men and women with types I and II bipolar, and recurrent major depressive disorders. Acta Psychiatr Scand. 2007;116:419–428. doi: 10.1111/j.1600-0447.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- 31.Yildiz A, Guleryuz S, Ankerst DP. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65:255–263. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]
- 32.Baldessarini RJ, Salvatore P, Khalsa HM. Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord. 2010;12:264–270. doi: 10.1111/j.1399-5618.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 33.Tondo L, Lepri B, Baldessarini RJ. Reproduction in women and men with major affective disorders. Acta Psychiatr Scand. 2011;123:283–289. doi: 10.1111/j.1600-0447.2010.01660.x. [DOI] [PubMed] [Google Scholar]
- 34.Vázquez GH, Lolich M, Leiderman EA. Age-at-onset in 648 patients with major affective disorders: clinical and prognostic implications. Mind Brain J Psychiatry. in press. [Google Scholar]
- 35.Vieta E. Bipolar disorder units and programs: are they really needed? World Psychiatry. 2011;10:152–152. doi: 10.1002/j.2051-5545.2011.tb00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldessarini RJ, Tondo L, Baethge C. Effects of treatment latency on response to maintenance treatment in manic-depressive disorders. Bipolar Disord. 2007;9:386–393. doi: 10.1111/j.1399-5618.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 37.Shulman KI, Tohen M, Kutcher S, editors. Mood disorders across the life span. New York: Wiley; 1996. [Google Scholar]
- 38.Baldessarini RJ, Vieta E, Calabrese JR. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18:143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- 39.Bauer M, Glenn T, Rasgon N. Association between age of onset and mood in bipolar disorder: comparison of subgroups identified by cluster analysis and clinical observation. J Psychiatr Res. 2010;44:1170–1175. doi: 10.1016/j.jpsychires.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Harvey PD, Wingo AP, Burdick K. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12:364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 41.Faedda GL, Baldessarini RJ, Suppes T. Pediatric-onset bipolar disorder: a neglected clinical and public-health problem. Harv Rev Psychiatry. 1995;3:171–195. doi: 10.3109/10673229509017185. [DOI] [PubMed] [Google Scholar]
- 42.Colom F, Vieta E. The road to DSM-V: bipolar disorder episode and course specifiers. Psychopathology. 2009;42:209–218. doi: 10.1159/000218518. [DOI] [PubMed] [Google Scholar]