To survive, all animals must be able to distinguish between sensations resulting from their own actions and sensations coming from outside sources 1. To make this distinction, the sensory areas of the brain are sent an advance copy, or forward model, of the expected sensation resulting from action. This can be thought of as a carbon copy (Cc) from “self” to sensory cortex, saying “It’s just me”. And, so warned, sensory cortex dampens or even cancels the sensation. Importantly, it also notes the sensation as coming from “self”. This forward model system has been labeled the “efference copy” and/or “corollary discharge” system. Although the terms are often used interchangeably, we define efference copy as a copy of a motor plan sent from motor to sensory cortical areas, and corollary discharge as the expected sensory consequences generated by the arrival of the efference copy.
In his seminal paper, Feinberg 2 proposed that auditory hallucinations are caused by a defect in the efference copy and corollary discharge systems of thoughts and ideas. He suggested that an abnormality in these systems could result in an inability to distinguish self-initiated neural activity from neural activity resulting from external stimulation. Thus, patients with schizophrenia might be unable to distinguish ideas or thoughts produced in their own minds from voices or influences coming from the environment. Feinberg noted that Hughlings Jackson, the 19th century British neurologist, considered thinking as the most complex of our motor acts, which “might conserve and utilize the computational and integrative mechanisms evolved for physical movement”. Feinberg suggested that thinking might therefore retain successful control mechanisms present at lower levels of integration. In 1987, Frith 3 expanded this concept and prompted a series of behavioral experiments confirming corollary discharge dysfunction in schizophrenia. Evidence for dysfunction of the corollary discharge system in schizophrenia has been documented in auditory and somatosensory modalities (see 4 for a review).
About 10 years ago, we began to develop electrophysiological tools to address the relationship between auditory hallucinations and biological assays of the efference copy and corollary discharge mechanisms. Taking advantage of the writings of Hughlings Jackson, we decided to link the motor system of thoughts to the motor system of talking. Accordingly, we developed an electroencephalography (EEG)-based event related potential (ERP) method to study the putative action of these mechanisms by recording the response of auditory cortex to the spoken sound as it is being spoken (see 4 for details). Because the N1 component of the ERP, peaking at 100ms after the onset of a sound, is generated in primary and secondary auditory cortices, we have been using it to assess the responsiveness of auditory cortex to onset of the speech sound. Our paradigm and methods are similar to those used by others with human and non-human primates to study the action of the efference copy and corollary discharge mechanisms during vocalization (see 4).
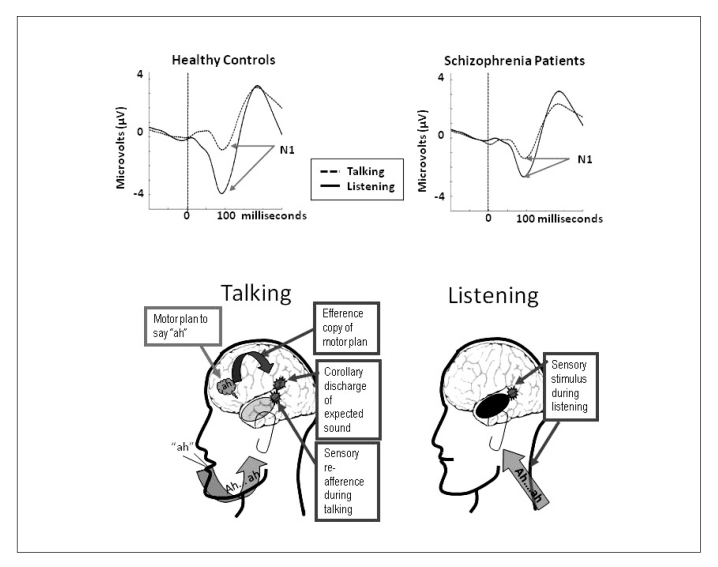
In Figure 1 we illustrate the paradigm using a cartoon profile of a man talking (saying “ah”) and listening (hearing “ah”) to a recording of that speech played back. Above the cartoons, we show ERPs recorded from the vertex of the head, elicited by the onset of the speech sound (at time 0 ms, vertical dotted line) during talking (dashed lines) and listening (solid lines). As can be seen on the left of the Figure, where we show ERP data from 75 healthy control subjects, N1 to the speech sound is suppressed during talking relative to listening 5. ERPs are plotted with amplitude (microvolts, µV) on the y-axis and time (milliseconds, ms) on the x-axis. Negativity recorded at the vertex of the head (Cz) is plotted down. In the cartoon, the intention to say “ah” is indicated as a “thought bubble” over speech production areas in the frontal lobe. The curved arrow pointing to auditory cortex indicates the transmission of the efference copy of the motor plan, which produces a corollary discharge (burst) of the expected sensation in auditory cortex. When the expected sensation (corollary discharge) matches the actual sensation (sensory re-afference) in auditory cortex (gray burst), perception is suppressed. Strong responses in auditory cortex during listening are depicted as black, and weaker responses during talking are depicted as gray.
We have collected data from several samples of patients and controls with this paradigm and showed that auditory cortical responsiveness to sounds is dampened during talking in healthy controls but less so in patients with schizophrenia 5,6,7,8,9. Data from 75 patients are shown on the right of the Figure, where a relative failure to suppress N1 during talking is seen in the ERP 5.
Counter to our expectations, the amount of suppression of the N1 to the speech sounds during talking was not related to auditory hallucinations 6,7,8. However, neural synchrony in the EEG, 100ms before speech onset, was related to them. Because pre-speech neural synchrony was related to subsequent suppression of the N1 during talking in controls, we suggested EEG synchrony preceding speech may reflect the action of the efference copy of the motor command to speak 7. Perhaps, deficits in tagging the thought or idea as coming from self, and not deficits in suppressing the experience, map onto auditory hallucinations.
Initially, our studies were focused on whether dysfunction of the efference copy and corollary discharge mechanisms could explain auditory verbal hallucinations. However, in doing a “control experiment” with simple button pressing, we found that dysfunction of the efference copy of the motor command, preceding the button press, can also be observed in the somatosensory system in patients with schizophrenia, and its neurobiological manifestation was shown to selectively map onto symptoms in the motor domain, such as avolition and apathy 10. Schizophrenia is a pan-cerebral illness, affecting almost every modality, function, and brain region studied. While each symptom and function might have its own failed mechanism, parsimony encourages us to find an elemental mechanism that could be at the root of at least some of the dysfunctions observed. We suggest that dysfunction of the forward model system may reflect an elementary deficit in schizophrenia patients.
If our measures of these elementary mechanisms are reliable, valid, and not affected by antipsychotic medications, they might represent a major new domain of electrophysiological measurement sensitive to a fundamental and ubiquitous pathophysiological process in schizophrenia. These electrophysiological signals, reflecting abnormal feed-forward motor-sensory circuitry, could generate aberrant identification and experience of the sensory consequences of self-generated actions in schizophrenia. As such, they may underlie the aberrant experiences that may give rise to the variety of symptoms characteristic of patients with schizophrenia, from auditory verbal hallucinations to avolition and apathy. Accordingly, these electrophysiological measures may serve as novel neurophysiological outcome measures for developing and testing novel treatments in schizophrenia. They may also represent a novel endophenotype for studies of risk for schizophrenia, as we have shown that people at clinical high risk for the illness show N1 suppression during talking that is intermediate between healthy controls and patients with a confirmed diagnosis 5. To the extent that they precede the onset of psychosis itself, they may enhance the prediction of psychosis onset in individuals with prodromal symptoms.
Most important, mechanistic studies such as these offer translation to bench neuroscience and translation to other species, and hence they can open the door to invasive manipulations that are not possible with in vivo human studies. For example, studies of the efference copy mechanism, like the ones reviewed above in humans, can be applied to animals that make social calls, such as song-birds and non-human primates. In such experiments, perturbations of the neurotransmitters implicated in schizophrenia might produce a pattern in the neural signature of the mechanism that resembles the pattern seen in schizophrenia patients who hallucinate.
Figure 1 Schema of Talk/Listen Paradigm and resulting event related potentials from healthy controls and patients with schizophrenia.
Acknowledgements
This work was supported by VA Merit Review (J.M. Ford) and National Institute of Mental Health grants T32 MH089920 (V. Perez), K02 MH067967 and R01 MH58262 (J.M. Ford), and R01 MH076989 (D.H. Mathalon).
Permission to reproduce elements of Figure 1 was granted by Schizophrenia Bulletin.
References
- 1.Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophr Bull. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- 3.Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- 4.Ford JM, Roach BJ, Mathalon DH. How to assess the corollary discharge in humans using non-invasive neurophysiological methods. Nature Protocols. 2010;5:1160–1168. doi: 10.1038/nprot.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez VB, Ford JM, Roach BJ. Auditory cortex responsiveness during talking and listening: early illness schizophrenia and patients at clinical high-risk for psychosis. Schizophr Bull. doi: 10.1093/schbul/sbr124. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ford JM, Mathalon DH, Heinks T. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. Am J Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- 7.Ford JM, Roach BJ, Faustman WO. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 8.Ford JM, Gray M, Faustman WO. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 9.Heinks-Maldonado TH, Mathalon DH, Houde JF. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- 10.Ford JM, Roach BJ, Faustman WO. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]