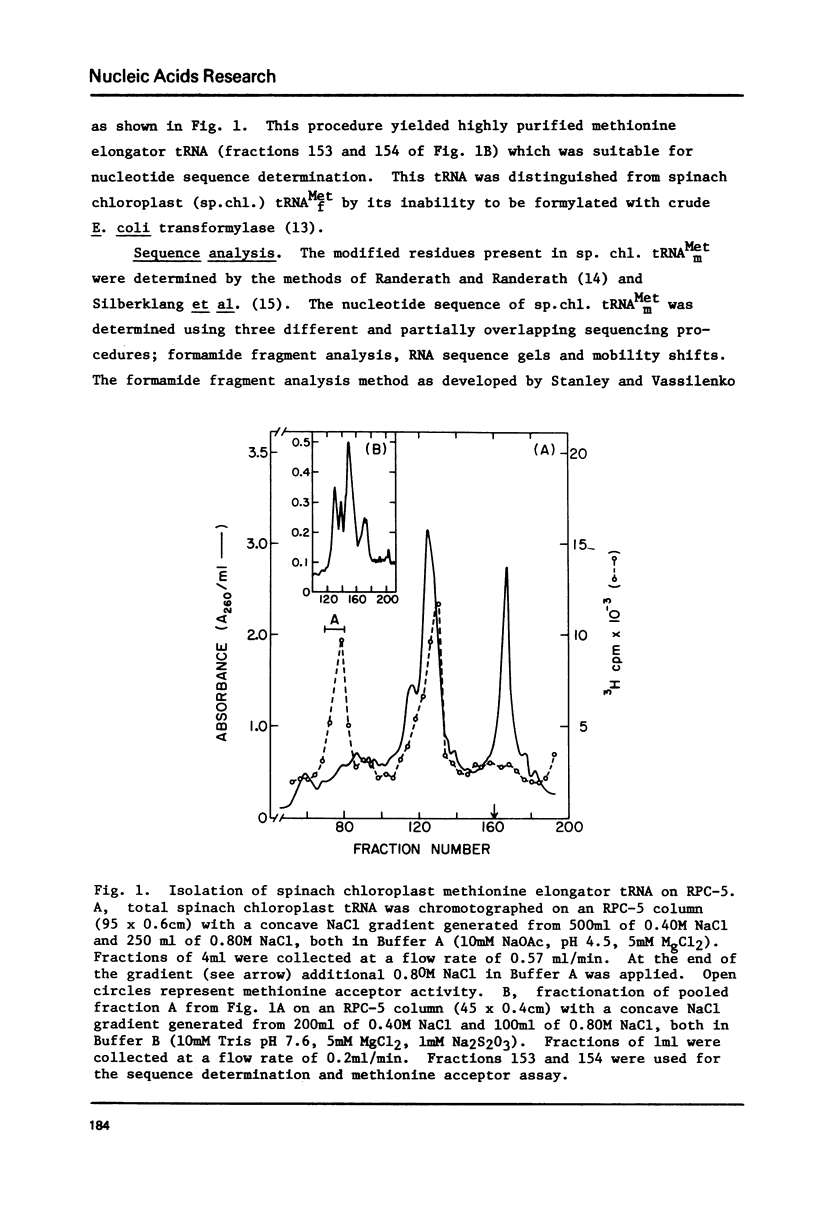
Abstract
The nucleotide sequence of spinach chloroplast methionine elongator tRNA (sp. chl. tRNAm Met) has been determined. This tRNA is considerably more homologous to E. coli tRNAm Met (67% homology) than to the three known eukaryotic tRNAm Met (50-55% homology). Sp. chl. tRNAm Met, like the eight other chloroplast tRNAs sequenced, contains a methylated GG sequence in the dihydrouridine loop and lacks unusual structural features which have been found in several mitochondrial tRNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Osinga K. A., Van der Horst G., Borst P. Nucleotide sequence of the mitochondrial structural genes for cysteine-tRNA and histidine-tRNA of yeast. Nucleic Acids Res. 1979 Jul 25;6(10):3255–3266. doi: 10.1093/nar/6.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calagan J. L., Pirtle R. M., Pirtle I. L., Kashdan M. A., Vreman H. J., Dudock B. S. Homology between chloroplast and prokaryotic initiator tRNA. Nucleotide sequence of spinach chloroplast methionine initiator tRNA. J Biol Chem. 1980 Oct 25;255(20):9981–9984. [PubMed] [Google Scholar]
- Canaday J., Guillemaut P., Weil J. H. The nucleotide sequences of the initiator transfer RNAs from bean cytoplasm and chloroplasts. Nucleic Acids Res. 1980 Mar 11;8(5):999–1008. doi: 10.1093/nar/8.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. H., Brum C. K., Siberklang M., RajBhandary U. L., Hecker L. I., Barnett W. E. The first nucleotide sequence of an organelle transfer RNA: chloroplastic tRNAphe. Cell. 1976 Dec;9(4 Pt 2):717–723. doi: 10.1016/0092-8674(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Cory S., Marcker K. A. The nucleotide sequence of methionine transfer RNA-M. Eur J Biochem. 1970 Jan;12(1):177–194. doi: 10.1111/j.1432-1033.1970.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesel A. J., Crouse E. J., Gordon K., Bohnert H. J., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Fractionation and identification of spinach chloroplast transfer RNAs and mapping of their genes on the restriction map of chloroplast DNA. Gene. 1979 Aug;6(4):285–306. doi: 10.1016/0378-1119(79)90070-2. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhl H., Feldmann H. The primary structure of a non-initiating methionine-specific tRNA from brewer's yeast. Eur J Biochem. 1976 Sep;68(1):209–217. doi: 10.1111/j.1432-1033.1976.tb10780.x. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Keith G. Primary structure of bean chloroplastic tRNAPhe. Comparison with Euglena chloroplastic tRNAPhe. FEBS Lett. 1977 Dec 15;84(2):351–356. doi: 10.1016/0014-5793(77)80723-0. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Alzner-Deweerd B., RajBhandary U. L. Interesting and unusual features in the sequence of Neurospora crassa mitochondrial tyrosine transfer RNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):717–721. doi: 10.1073/pnas.76.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Hecker L. I., Schwartzbach S. D., Barnett W. E., Baumstark B., RajBhandary U. L. Structure and function of initiator methionine tRNA from the mitochondria of Neurospora crassa. Cell. 1978 Jan;13(1):83–95. doi: 10.1016/0092-8674(78)90140-x. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., RajBhandary U. L. Organization of tRNA and rRNA genes in N. crassa mitochondria: intervening sequence in the large rRNA gene and strand distribution of the RNA genes. Cell. 1979 Jul;17(3):583–595. doi: 10.1016/0092-8674(79)90266-6. [DOI] [PubMed] [Google Scholar]
- Hensgens L. A., Grivell L. A., Borst P., Bos J. L. Nucleotide sequence of the mitochondrial structural gene for subunit 9 of yeast ATPase complex. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1663–1667. doi: 10.1073/pnas.76.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan M. A., Pirtle R. M., Pirtle I. L., Calagan J. L., Vreman H. J., Dudock B. S. Nucleotide sequence of a spinach chloroplast threonine tRNA. J Biol Chem. 1980 Sep 25;255(18):8831–8835. [PubMed] [Google Scholar]
- Koiwai O., Miyazaki M. The primary structure of non-initiator methionine transfer ribonucleic acid from Bakers' yeast. II. Partial digestion with ribonuclease T1 and derivation of the complete sequence. J Biochem. 1976 Nov;80(5):951–959. doi: 10.1093/oxfordjournals.jbchem.a131382. [DOI] [PubMed] [Google Scholar]
- Li M., Tzagoloff A. Assembly of the mitochondrial membrane system: sequences of yeast mitochondrial valine and an unusual threonine tRNA gene. Cell. 1979 Sep;18(1):47–53. doi: 10.1016/0092-8674(79)90352-0. [DOI] [PubMed] [Google Scholar]
- Macino G., Coruzzi G., Nobrega F. G., Li M., Tzagoloff A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3784–3785. doi: 10.1073/pnas.76.8.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Schneller J. M., Keith G., Stahl A. J., Dirheimer G. Primary structure of yeast mitochondrial DNA-coded phenylalanine-tRNA. Nucleic Acids Res. 1978 Dec;5(12):4579–4592. doi: 10.1093/nar/5.12.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Almeida M. L., Guillemaut P., Keith G., Canaday J., Weil J. H. Primary structure of three leucine transfer RNAs from bean chloroplast. Biochem Biophys Res Commun. 1980 Jan 15;92(1):102–108. doi: 10.1016/0006-291x(80)91525-9. [DOI] [PubMed] [Google Scholar]
- Piper P. W. The nucleotide sequence of a methionine tRNA which functions in protein elongation in mouse myeloma cells. Eur J Biochem. 1975 Feb 3;51(1):283–293. doi: 10.1111/j.1432-1033.1975.tb03928.x. [DOI] [PubMed] [Google Scholar]
- Pirtle R., Kashdan M., Pirtle I., Dudock B. The nucleotide sequence of a major species of leucine tRNA from bovine liver. Nucleic Acids Res. 1980 Feb 25;8(4):805–815. [PMC free article] [PubMed] [Google Scholar]
- Pétrissant G., Boisnard M. Particularités structurales du méthionin-tRNAmMet de foie de lapin. Biochimie. 1974;56(5):787–790. doi: 10.1016/s0300-9084(74)80053-2. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. R., Herrmann R. G., Bottomley W. Mapping of the ribosomal RNA genes on spinach chloroplast DNA. Nucleic Acids Res. 1978 Jun;5(6):1741–1751. doi: 10.1093/nar/5.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]