Abstract
Mutations in the gene encoding the p110α subunit of PI3K (PIK3CA) that result in enhanced PI3K activity are frequently observed in human cancers. To better understand the role of mutant PIK3CA in the initiation or progression of tumorigenesis, we generated mice in which a PIK3CA mutation commonly detected in human cancers (the H1047R mutation) could be conditionally knocked into the endogenous Pik3ca locus. Activation of this mutation in the mouse ovary revealed that alone, Pik3caH1047R induced premalignant hyperplasia of the ovarian surface epithelium but no tumors. Concomitantly, we analyzed several human ovarian cancers and found PIK3CA mutations coexistent with KRAS and/or PTEN mutations, raising the possibility that a secondary defect in a co-regulator of PI3K activity may be required for mutant PIK3CA to promote transformation. Consistent with this notion, we found that Pik3caH1047R mutation plus Pten deletion in the mouse ovary led to the development of ovarian serous adenocarcinomas and granulosa cell tumors. Both mutational events were required for early, robust Akt activation. Pharmacological inhibition of PI3K/mTOR in these mice delayed tumor growth and prolonged survival. These results demonstrate that the Pik3caH1047R mutation with loss of Pten is enough to promote ovarian cell transformation and that we have developed a model system for studying possible therapies.
Introduction
In human cancers, somatic mutations in PIK3CA commonly target specific hot spots in the helical (E542 and E545) and kinase (H1047) domains (1, 2), resulting in enhanced lipid kinase activity and upregulation of downstream signaling events such as phosphorylation of Akt (3). Activation of Akt is observed in up to 70% of ovarian cancers (4) due to a variety of causes, including PIK3CA mutations (8%–12%) or amplification (3%–8%) and/or PTEN loss (27%) or mutations (3–8%) (1, 5–10). Loss of Pten (11, 12) or overexpression of activated PI3K in the mouse ovarian surface epithelium (OSE) (13) do not lead to tumor formation; however, the ability of mutant Pik3ca to initiate tumorigenesis or drive tumor progression has not been established. Here we describe an animal model that allows us to study the effects of the Pik3caH1047R mutation in the physiologically relevant context of a somatic, heterozygous Pik3ca mutation expressed at endogenous levels in otherwise normal cells and tissues.
Results and Discussion
We analyzed 87 human ovarian cancers for changes in PI3K pathway genes PIK3CA, PIK3R1 (encoding the PI3K p85 regulatory subunit), PTEN, and also in KRAS, which interacts with PI3K to mediate its activity (refs. 14, 15, and Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI59309DS1). We found 37% of samples exhibited genetic aberrations in one of these genes. Notably, 4 (40%) of the 10 patients who had PIK3CA mutations also exhibited PIK3R1, PTEN, and/or KRAS mutations. Analysis of 34 ovarian cancer cell lines found 3 (IGROv1, TOV21G, and MCAS) had coexisting PIK3CA mutation with PTEN and/or KRAS aberrations (Supplemental Table 1). Consistent with this, studies in endometrioid ovarian (11), breast, endometrial, and colon cancers (16) have identified tumors with coexistent PIK3CA and PTEN mutations. Neither the role of endogenous levels of mutant PIK3CA nor the cooperative nature of PIK3CA and PTEN mutations has been evaluated in vivo.
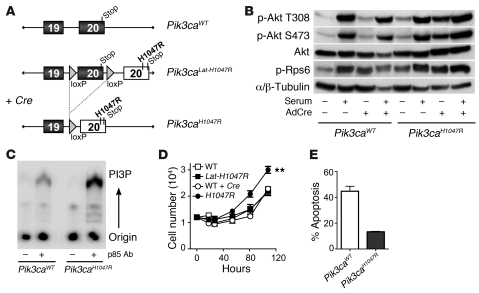
To address this, we used what we believe to be a novel exon-switch strategy to generate a mutant mouse harboring a germline Pik3ca allele with a conditional H1047R mutation. We inserted loxP sites flanking exon 20 of Pik3ca and downstream placed a tandem copy of exon 20 containing a CAT→AGG change in codon 1047 (Figure 1A and Supplemental Figures 1 and 2). Adenoviral Cre–mediated (AdCre-mediated) recombination leads to replacement of wild-type with mutant exon 20, resulting in p110α-H1047R protein expression at endogenous levels in otherwise normal cells, thus accurately reproducing the scenario of a naturally occurring mutation. The presence of the conditional allele did not affect development (Supplemental Table 2). Using murine embryonic fibroblasts (MEFs), we confirmed the knock-in of the Pik3caH1047R mutation after AdCre infection in vitro (Supplemental Figures 3 and 4). Pik3caH1047R resulted in elevated p-Akt and p–ribosomal protein S6 (p-Rps6) levels, increased PI3K activity, enhanced proliferation, and reduced apoptosis (Figure 1, B–E). We have thus generated a model system in which the biological effects of Pik3caH1047R expression can be examined both in vivo and in vitro.
Figure 1. Pik3caH1047R results in increased PI3K activity and functional changes in MEFs.
(A) Schematic of the conditional Pik3caH1047R allele. (B) Protein blot in AdCre-treated (+) and untreated (–) Pik3caWT and Pik3caH1047R MEFs with (+) and without (–) 5-minute serum stimulation. (C) MEFs from Pik3caWT or Pik3caLat-H1047R mice were infected with AdCre, and PI3K was immunoprecipitated using an anti-p85 antibody. PI3K activity in the immunoprecipitates was assayed using phosphatidylinositol (PI) as substrate. Phosphorylated lipids were separated by thin-layer chromatography and visualized by phosphoimager. (D) MEFs from Pik3caWT and Pik3caH1047R mice before or after AdCre infection were cultured and cells counted (**P < 0.05 versus WT). (E) Apoptosis was assessed using annexin V after 72 hours serum deprivation. Results are mean ± SEM; 3 independent MEF preparations.
Combining this mouse with a Cre-inducible knockout of Pten (17), we investigated whether two genetic hits affecting PI3K regulation (Pik3caH1047R and Pten loss) are sufficient to promote tumorigenesis in the ovary. We induced the Pik3caH1047R mutation and/or deleted Pten in the left ovary by intrabursal AdCre delivery, which targets recombination to the OSE, granulosa cells of the ovarian follicles, and ovarian bursal cells (refs. 11, 12, 18, 19, and Supplemental Figure 5).
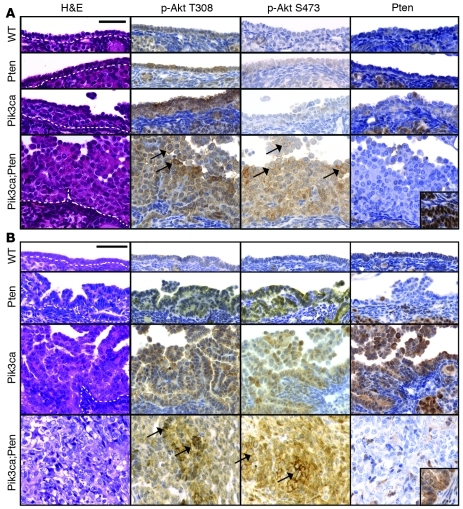
At 8 weeks after AdCre infection, OSE of Pik3caH1047R mice showed mild, focal papillary serous hyperplasia, and Pik3caH1047RPtendel/del mice showed marked hyperplasia (Figure 2A). p-Akt T308 and p-Akt S473 staining was strongest and located at the membrane in the OSE of double Pik3caH1047RPtendel/del mutants (Figure 2A).
Figure 2. Pik3caH1047R and Pten deletion leads to robust p-Akt T308 and p-Akt S473 levels in OSE.
Representative histological images of OSE from Pik3caWTPtenWT (WT, n = 6), Pik3caWTPtendel/del (Pten, n = 6), Pik3caH1047RPtenWT (Pik3ca, n = 6), Pik3caH1047RPtendel/del (Pik3ca;Pten, n = 5) mice at (A) 8 weeks and (B) 12 months after AdCre infection (except for Pik3caH1047RPtendel/del, taken at 6 months); H&E and IHC staining. Dotted lines show the intersection of the OSE and stromal layer. Arrows indicate membranous p-Akt localization. Scale bars: 50 μm. Insets are positively stained area of WT tissue on same section.
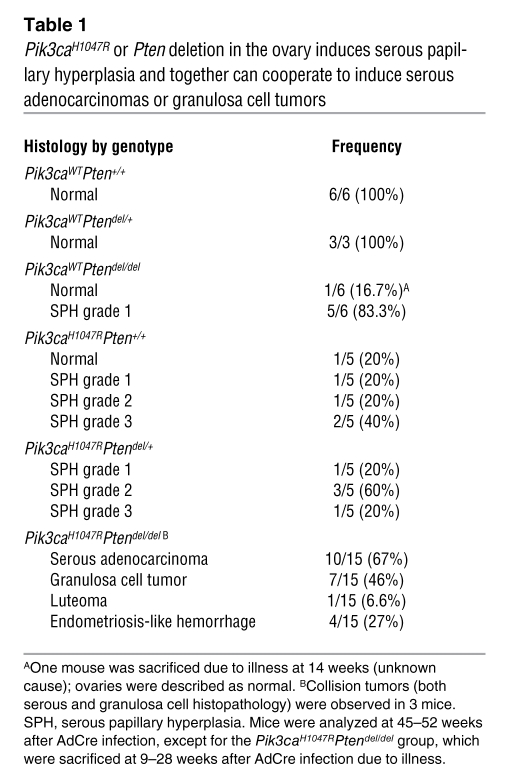
Over 1 year of observation, no wild-type or single mutant mice developed tumors. Pik3caH1047R, Pik3caH1047RPtendel/WT, and Ptendel/del mice developed serous papillary hyperplasia of the OSE (Figure 2B, Supplemental Figure 6, and Table 1), coincident with an increase in p-Akt T308 and p-Akt S473 compared with equivalent OSE at 8 weeks after AdCre infection. The degree of the hyperplasia varied between mice and was highest in the Pik3caH1047R and Pik3caH1047RPtendel/WT compared with the Ptendel/del mice, suggesting that alone, Pik3caH1047R more potently promotes proliferative changes compared with Pten loss. In some Pik3caH1047R mice, the hyperplasia resembled epithelial proliferation seen in the borderline (low malignant potential) subtype of human ovarian serous carcinoma. In 2 mice, we observed microinvasion of the ovarian stroma, indicating that the epithelial proliferation was neoplastic despite the absence of definitive cytological evidence of malignancy. The inability of Pik3caH1047R to induce ovarian tumorigenesis differs from transgenic mouse models of Pik3caH1047R inducing lung and breast (20–23) tumors, but these models are driven by overexpressed Pik3caH1047R rather than mutation of the endogenous gene, as occurs in human tumors. Possibly, the ovary could be less susceptible to transformation via a single mutation compared with other tissue types. Studies are underway to examine the role of this mutation in the lung, breast, and gastrointestinal tract.
Table 1 .
Pik3caH1047R or Pten deletion in the ovary induces serous papillary hyperplasia and together can cooperate to induce serous adenocarcinomas or granulosa cell tumors
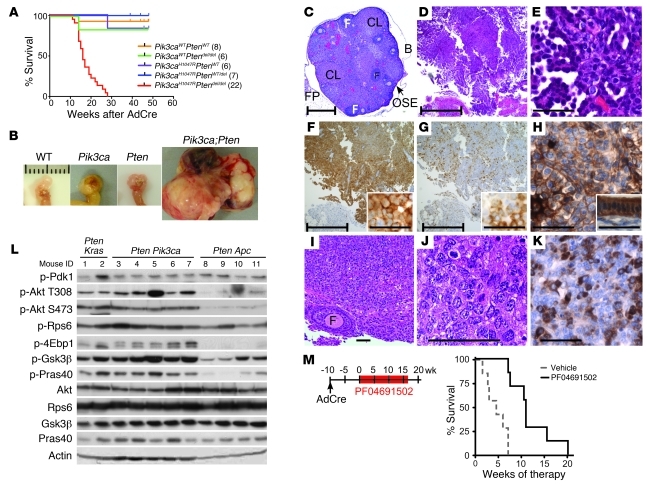
In contrast to the single mutant mice, all Pik3caH1047RPtendel/del mice developed ovarian masses, necessitating sacrifice with a median latency of 16 weeks (Figure 2B and Figure 3, A and B). Macroscopic analyses at autopsy revealed that many Pik3caH1047RPtendel/del mice developed hemorrhagic ascites, and some showed intraperitoneal masses on the peritoneal wall and diaphragm. No distant metastases were evident macroscopically. Histological examination confirmed malignancy in 15 of 18 ovarian masses, consisting of ovarian serous adenocarcinomas (Figure 3, D and E), ovarian granulosa cell tumors (Figure 3, I–K), and a single ovarian luteoma (Table 1). Three mice had collision tumors composed of different histological cancer types within the same mass. Serous carcinomas exhibited high abundance and membrane localization of p-Akt S473, p-Akt T308 (Figure 2B and Figure 3F), cytoplasmic p-Rps6 (Figure 3G), and keratin (Figure 3H). Granulosa cell tumors were positive for inhibin (Figure 3K). Expression of the Pik3caH1047R mutant and loss of PTEN protein were confirmed in tumors (Figure 2 and Supplemental Figure 7). Consistent with these findings, recent genomic analysis of high-grade serous ovarian cancers shows that while PIK3CA amplification and mutations are rare events in this subtype, upregulation of the pathway is seen in 45% of cases (24).
Figure 3. Pik3caH1047R and Ptendel/del cooperate to promote tumorigenesis in mouse ovary.
(A) Kaplan-Meier curve of Pik3caH1047RPtendel/del mice (red; median survival, 16 weeks) compared with control mice (n in parentheses) exposed to AdCre in the ovarian bursa. (B) Ovarian tumors from Pik3caH1047RPtendel/del mice (20–25 weeks after AdCre infection) and non-tumor-bearing control mice 1 year after AdCre infection. Scale bars: 1 cm. (C–K) Representative histopathology of tumors from Pik3caH1047RPtendel/del mice. Non-AdCre-exposed ovary (C), serous adenocarcinoma (D–H), and granulosa cell tumor (I–K). Staining with H&E (C–E, I, and J), p-Akt S473 (F, high-power inset), p-Rps6 (G, high power inset), pan-keratin (H, adjacent uterus inset), or inhibin (K) is shown. Scale bars: bracketed, 500 mm; unbracketed, 50 mm. B, bursa; CL, corpus luteum; F, follicle; FP, fat pad. (L) Protein blots from KrasG12DPtendel/del (Pten Kras), Pik3caH1047RPtende/del (Pten Pik3ca), and ApcS580/S580Ptendel/del (Pten Apc) ovarian tumors. (M) Tumor-bearing Pik3caH1047RPtendel/del mice were treated with vehicle or PF04691502 daily. Mice receiving vehicle exhibited 4.5 weeks median survival compared with 11 weeks in PF04691502-treated mice; log-rank (Mantel-Cox) test, P = 0.0006.
Additionally, 13 of the 15 (86.7%) Pik3caH1047RPtendel/del mice showed variable degrees of fibrous and/or smooth muscle hyperplasia, which formed irregular or solid masses of desmoplastic stroma or resulted in bursal wall thickening by actin-positive smooth muscle (data not shown). This phenotypic heterogeneity demonstrates that combined Pik3caH1047R mutation and Pten loss can cooperate to potently promote proliferation or transformation in several cell types, including OSE, ovarian granulosa cells, fibroblasts, and smooth muscle cells following AdCre infection.
We compared PI3K/Akt/mTOR pathway signaling in Pik3caH1047RPtendel/del tumor lysates with that in other mouse ovarian cancer models: KrasG12DPtendel/del and ApcS580/S580Ptendel/del (refs. 11, 12, and Figure 3L). Pik3caH1047RPtendel/del and KrasG12DPtendel/del tumors exhibited equivalent pathway signaling to multiple substrate proteins. While ApcS580/S580Ptendel/del tumors showed equivalent p-Pdk1 and p-Rps6 levels, they showed relatively low levels of p-Akt and its substrates. This suggests that cooperating mutations — Pten loss and Kras or Pik3ca mutation, but not Apc mutation — are required to robustly activate Akt and its direct targets.
These mutations may cooperate because both Pik3caH1047R kinase activity and loss of PTEN’s phosphatase activity are necessary to reach a threshold of PIP3 levels required for transformation. Alternatively, since PTEN has additional, PI3K-independent tumor-suppressive functions (25), its loss may promote tumorigenesis via these additional mechanisms. Finally, both mutations may be required to overcome negative feedback loops that could be activated as a result of only one of these genetic events.
To examine whether established Pik3caH1047RPtendel/del tumors remained dependant on PI3K pathway activity for tumor maintenance, we utilized an ATP-competitive PI3K/mTOR inhibitor, PF04691502, currently in phase I clinical trials (26, 27). Treatment of Pik3caH1047RPtendel/del mice commenced 10 weeks following AdCre exposure (Figure 3M), and tumor progression was monitored by ultrasound (Supplemental Figure 8). Early response was characterized by cytostasis, which significantly lengthened median survival time (Figure 3M), indicating a continued dependence on PI3K and/or mTOR for tumorigenesis in this model. Interestingly, tumors eventually regrew despite continuous PI3K/mTOR inhibition with PF04691502. This suggests that patients with PIK3CAH1047R mutations and loss of PTEN could be initially responsive to PI3K/mTOR inhibition, but that resistance is likely to develop. This model has the potential to provide a crucial new system for examining resistance mechanisms to PI3K pathway inhibitors and for testing novel therapeutics targeting the PI3K pathway alone or in combination with other therapies.
In summary, study of the Pik3caH1047R mouse model described herein has demonstrated that Pik3ca mutation requires a second hit to initiate tumorigenesis in the ovary. We found that mutations in PI3K regulatory proteins co-occur in human ovarian tumors with PIK3CA mutations and demonstrate that in vivo, Pik3caH1047R and loss of Pten is sufficient to promote ovarian cell transformation.
Methods
Generation of Pik3caLat-H1047R mice.
Pik3caLat-H1047R mice were generated by homologous recombination in embryonic stem cells (Ozgene). The targeting construct, detailed in Supplemental Figure 1, was assembled from C57BL/6 genomic DNA and cloned into the Ozgene F_PacI_Neo vector containing a PGK-neomycin cassette flanked by FLP recombinase target (FRT) sequences. The targeting vector was electroporated into 129S1/Sv-derived W9.5 ES cells. Targeted ES cells were injected into C57BL/6 blastocysts and chimeras crossed with C57BL/6 mice. The PGK-neomycin cassette was deleted by crossing with C57BL/6 ACTB-FLPe mice.
AdCre recombination in the ovary.
Cells in the ovarian bursa were infected with AdCre, a gift from Walter Thomas (Baker IDI Heart and Diabetes Institute, Melbourne, Victoria, Australia) as described previously (18); details are provided in Supplemental Methods. Endpoints were excessive tumor burden (>1.5 cm3), severe abdominal distension, or signs of severe illness (hunching, ruffled fur, or loss of responsiveness).
Mouse cohorts.
Ptendel/del mice (c;129S4-Ptentm1Hwu/J) were from The Jackson Laboratory. All Pik3caH1047RPtendel/del mice were maintained on a mixed (C57BL/6; BALB/c; 129S4) background, and littermates were used where possible. Pik3caH1047R refers to heterozygotes. KrasG12DPtendel/del and APC580S/580SPtendel/del ovarian tumor samples were generated using APC580S (a gift from Tetsuo Noda, Department of Cell Biology, Cancer Institute, Toshima-ku, Tokyo, Japan; ref. 28) and KrasG12D (MMHCC, ref. 29) mice.
Histology and protein analysis.
Tumors were formalin fixed, and immunohistochemistry was performed as described previously (27). For Western blotting, tumors were snap frozen and lysed in RIPA buffer. Antibodies are detailed in Supplemental Methods.
In vivo therapy.
Mice received vehicle (0.5% methylcellulose) or 10 mg/kg PF04691502 orally, daily. Ovary volume was monitored by ultrasound imaging (Vevo 770, Visualsonics). Endpoints were as above or weight loss of greater than 20%.
Statistics.
Statistical analyses were performed using a 2-tailed Student’s t test or log-rank (Mantel-Cox) Kaplan-Meier survival test. P values less than 0.05 were considered statistically significant.
Study approval.
Animal experiments followed the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the PMCC ethics committee.
Supplementary Material
Acknowledgments
We thank Kerry Ardley for animal experiments and the Peter Mac core histology and pathology facilities. This work was supported by the NHMRC (grant no. 628620 to W.A. Phillips and no. 566876 to G.A. McArthur and R.B. Pearson); the Association of International Cancer Research (no. 10-0052 to W.A. Phillips); Pfizer (grant to G.A. McArthur and R.J. Hicks); the Cancer Council Victoria and the Victorian Cancer Agency (grants to R.W. Johnstone); and the Victorian Breast Cancer Research Consortium, Australia (to I.G. Campbell and K.L. Gorringe). Researchers were funded by a Sir Edward Weary Dunlop Fellowship, Cancer Council of Victoria (to G.A. McArthur); research fellowships from the NHMRC (to R.B. Pearson and R.W. Johnstone); Cure Cancer Australia Foundation and Cancer Australia (to I. Ivetac); the Egyptian government and NHMRC (to M. Saad); Australian Postgraduate Research Awards (to L. Hare and G.L. Ryland); and the Swiss National Science Foundation (to V. Roh).
Footnotes
Conflict of interest: James G. Christensen is a Pfizer employee and shareholder.
Citation for this article: J Clin Invest. 2012;122(2):553–557. doi:10.1172/JCI59309.
References
- 1.Campbell IG, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64(21):7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 2.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 3.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27(41):5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altomare DA, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23(34):5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 5.Bast RC, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9(6):415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Despierre E, Lambrechts D, Neven P, Amant F, Lambrechts S, Vergote I. The molecular genetic basis of ovarian cancer and its roadmap towards a better treatment. Gynecol Oncol. 2010;117(2):358–365. doi: 10.1016/j.ygyno.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Gorringe KL, Campbell IG. Large-scale genomic analysis of ovarian carcinomas. Mol Oncol. 2009;3(2):157–164. doi: 10.1016/j.molonc.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shayesteh L, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21(1):99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 9.Hashiguchi Y, Tsuda H, Inoue T, Berkowitz RS, Mok SC. PTEN expression in clear cell adenocarcinoma of the ovary. Gynecol Oncol. 2006;101(1):71–75. doi: 10.1016/j.ygyno.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishna M, et al. Identification of candidate growth promoting genes in ovarian cancer through integrated copy number and expression analysis. PLoS One. 2010;5(4):e9983. doi: 10.1371/journal.pone.0009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11(4):321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11(1):63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 13.Liang S, et al. Expression of activated PIK3CA in ovarian surface epithelium results in hyperplasia but not tumor formation. PLoS One. 2009;4(1):e4295. doi: 10.1371/journal.pone.0004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Viciana P, et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89(3):457–467. doi: 10.1016/S0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129(5):957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 16.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groszer M, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294(5549):2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 18.Flesken-Nikitin A, Choi K-C, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63(13):3459–3463. [PubMed] [Google Scholar]
- 19.Clark-Knowles KV, Senterman MK, Collins O, Vanderhyden BC. Conditional inactivation of Brca1, p53 and Rb in mouse ovaries results in the development of leiomyosarcomas. PLoS One. 2009;4(12):e8534. doi: 10.1371/journal.pone.0008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelman JA, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14(12):1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams JR, et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res. 2011;71(7):2706–2717. doi: 10.1158/0008-5472.CAN-10-0738. [DOI] [PubMed] [Google Scholar]
- 22.Meyer DS, Brinkhaus H, Muller U, Muller M, Cardiff RD, Bentires-Alj M. Luminal expression of PIK3CA mutant H1047R in the mammary gland induces heterogeneous tumors. Cancer Res. 2011;71(13):4344–4351. doi: 10.1158/0008-5472.CAN-10-3827. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17(9):1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Yu D. PI(3)King apart PTEN’s role in cancer. Clin Cancer Res. 2010;16(17):4325–4330. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 26.Cheng H, et al. Discovery of the highly potent PI3K-mTOR dual inhibitor PF-04691502 through structure based drug design. MedChemComm. 2010;1:139–144. doi: 10.1039/c0md00072h. [DOI] [Google Scholar]
- 27.Kinross KM, et al. In vivo activity of combined PI3K/mTOR and MEK inhibition in a Kras(G12D);Pten deletion mouse model of ovarian cancer. Mol Cancer Ther. 2011;10(8):1440–1449. doi: 10.1158/1535-7163.MCT-11-0240. [DOI] [PubMed] [Google Scholar]
- 28.Shibata H, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. . Science. 1997;278(5335):120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 29.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.