Abstract
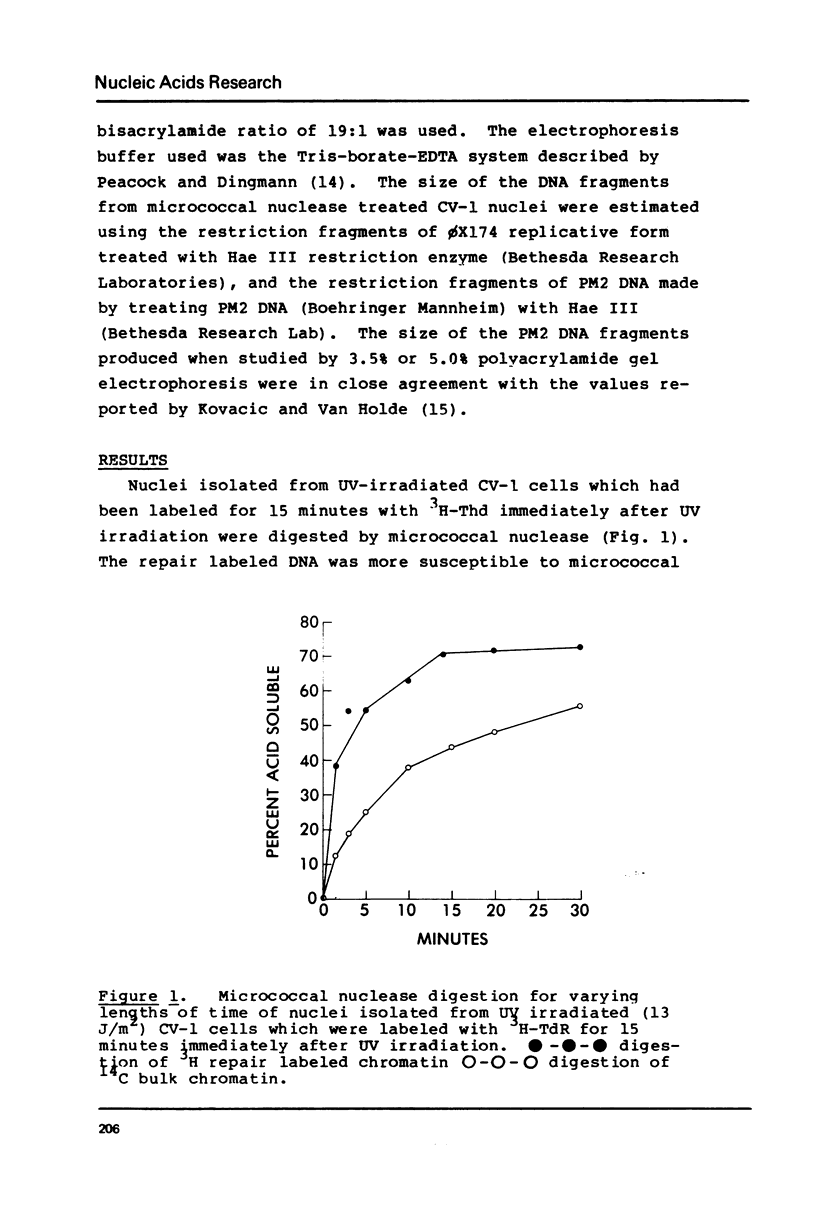
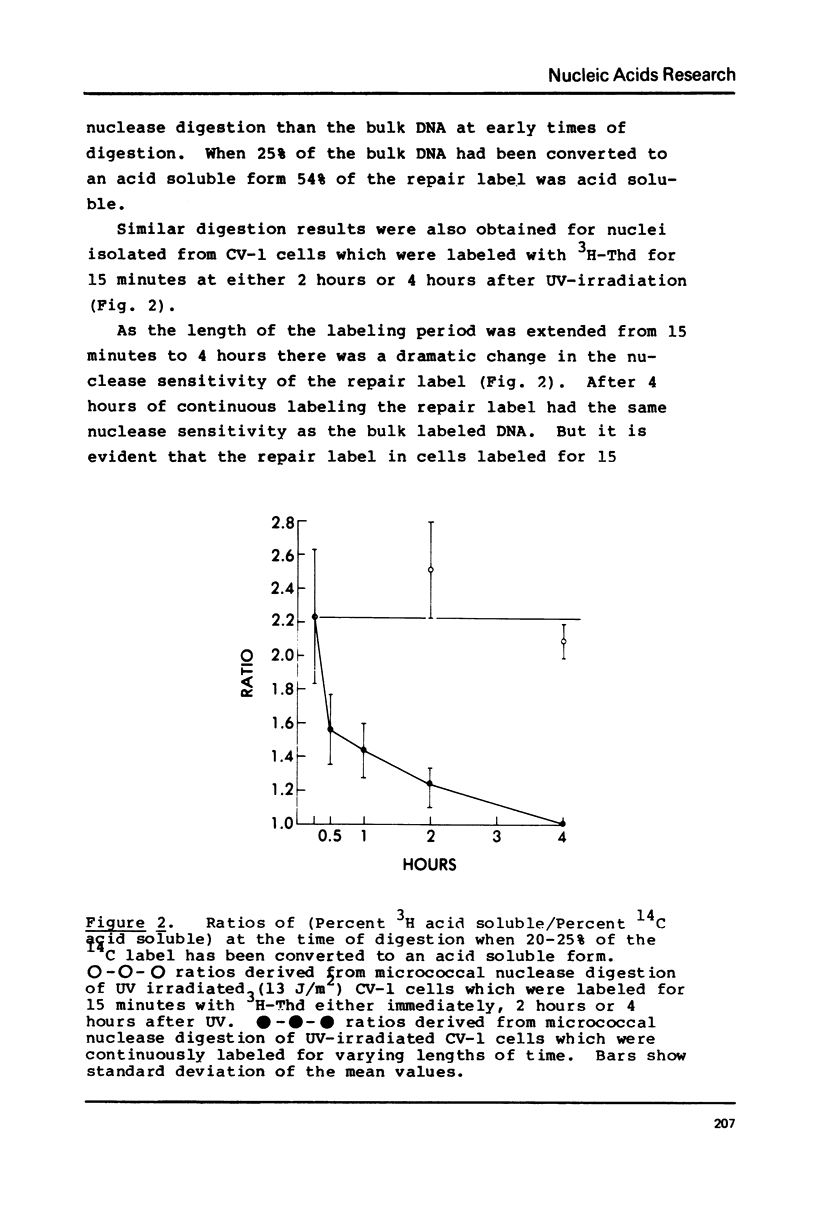
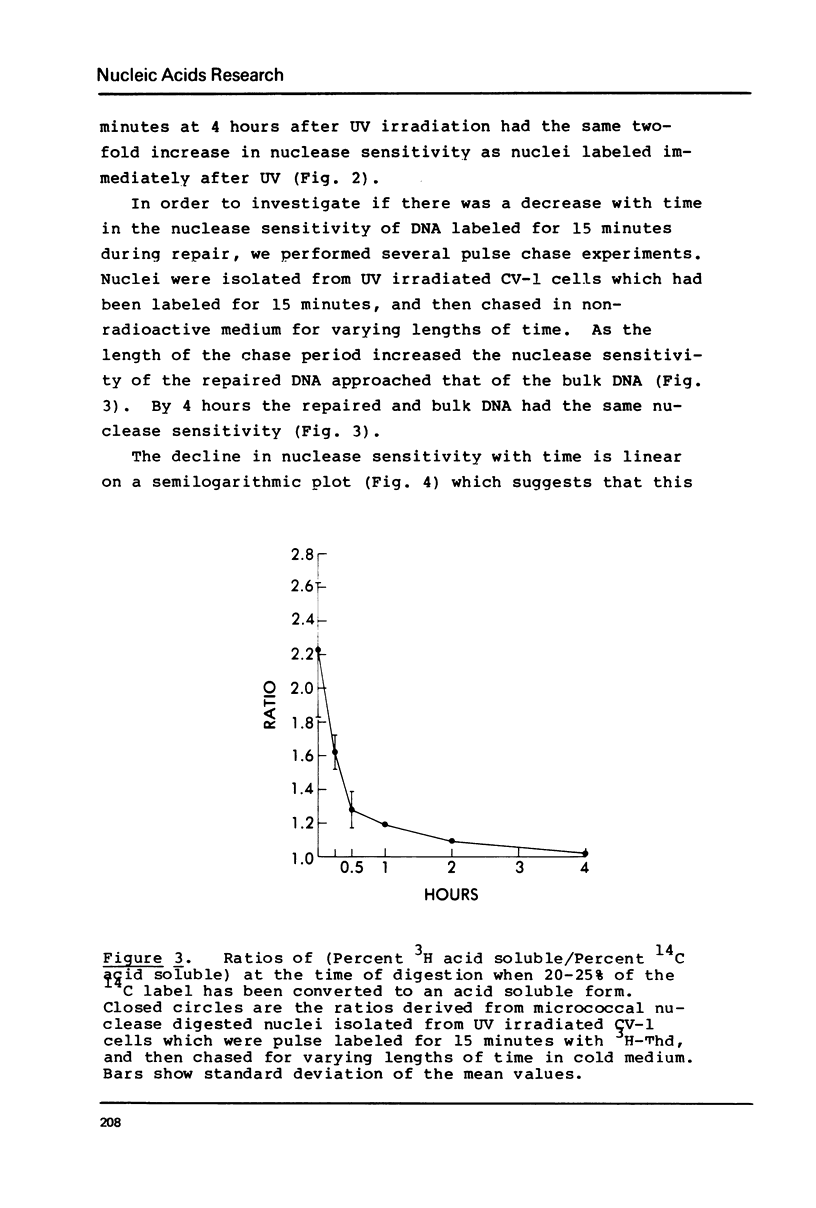
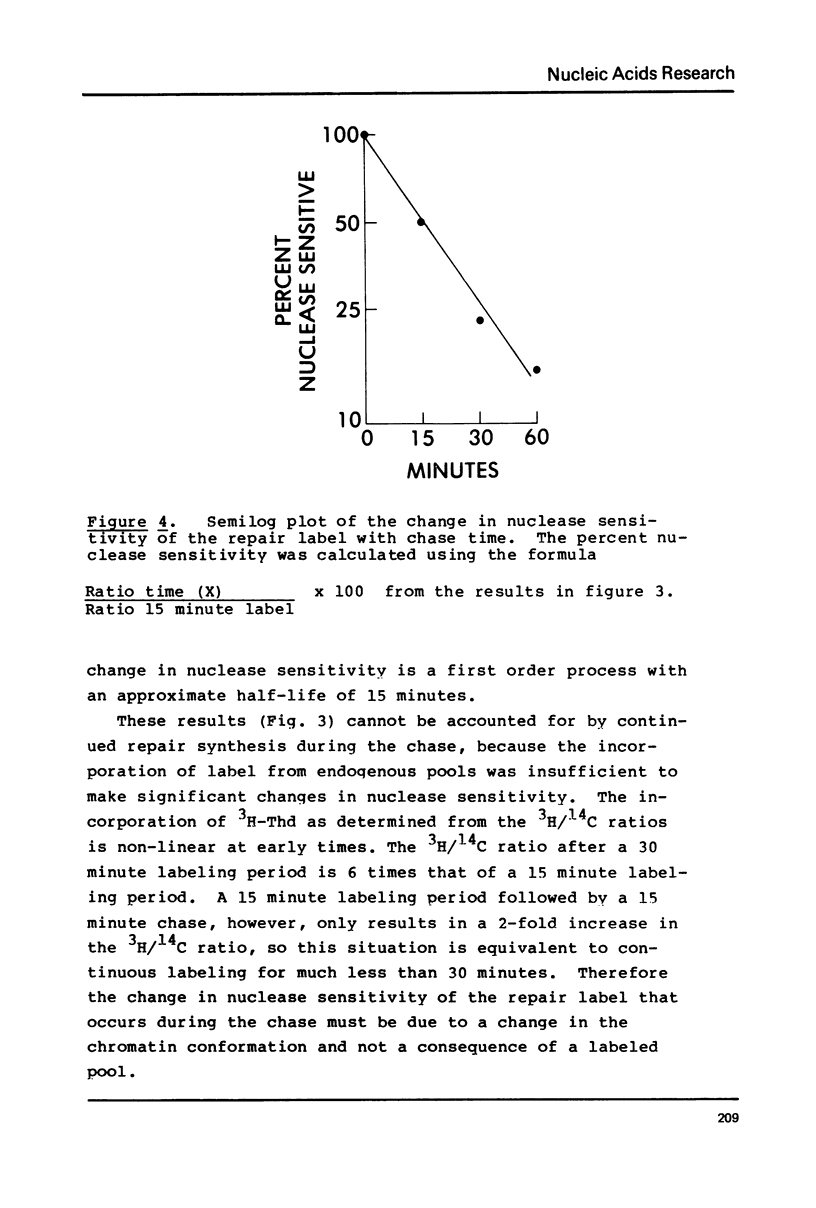
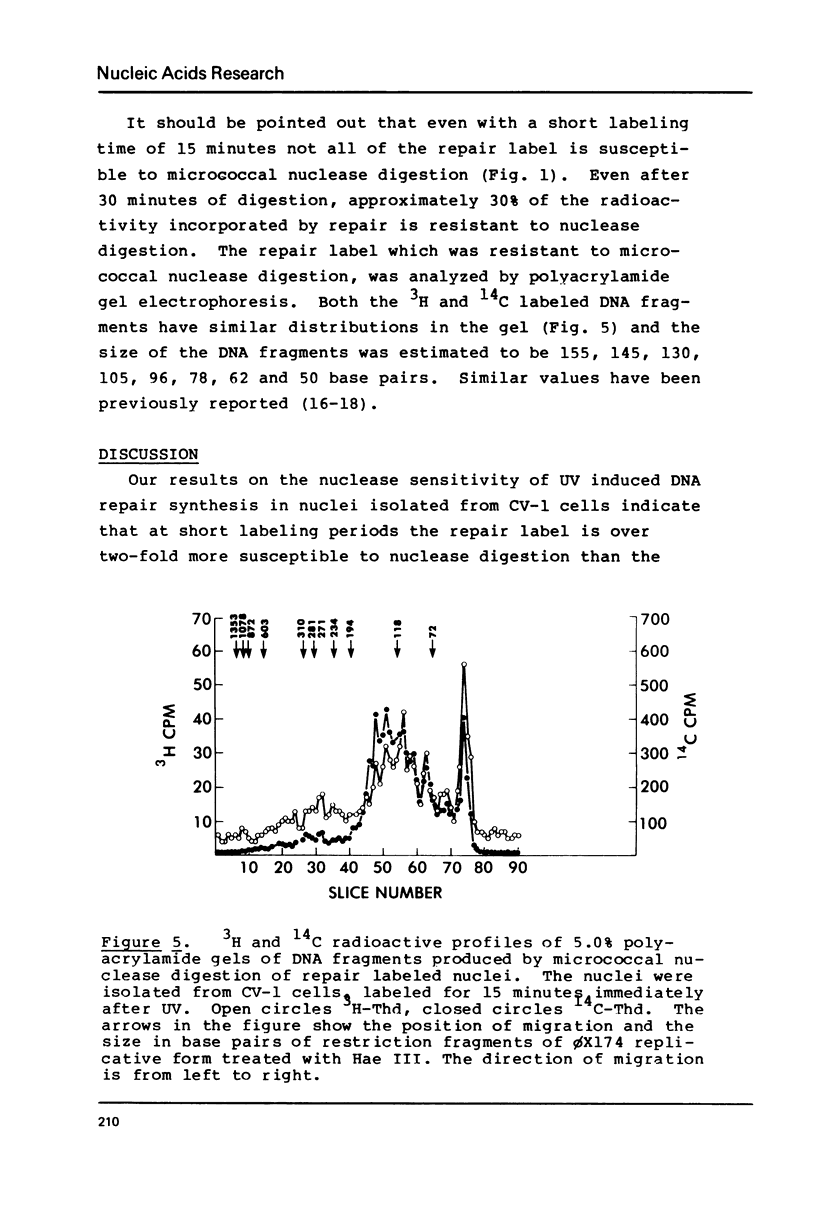
DNA labeled for 15 minutes during UV induced repair synthesis is two-fold more sensitive to micrococcal nuclease than the bulk nuclear DNA. As the length of the labeling period increases from 15 minutes to 4 hours the nuclease sensitivity of repair labeled DNA approaches that of bulk chromatin. Pulse-chase experiments indicate that the nuclease sensitivity of the repaired DNA labeled during a brief pulse decreases with a half-life of about 15 minutes. In contrast to previous interpretations, we consider these results to mean that immediately after synthesis, chromatin labeled during repair has a conformation which renders it more susceptible to nuclease digestion than the bulk chromatin. With time these repaired regions are assembled into a nucleosome structure with normal nuclease sensitivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodell W. J., Banerjee M. R. The influence of chromatin structure on the distribution of DNA repair synthesis studied by nuclease digestion. Nucleic Acids Res. 1979 Jan;6(1):359–370. doi: 10.1093/nar/6.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodell W. J. Nonuniform distribution of DNA repair in chromatin after treatment with methyl methanesulfonate. Nucleic Acids Res. 1977 Aug;4(8):2619–2628. doi: 10.1093/nar/4.8.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Sollner-Webb B., Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell. 1976 Jul;8(3):333–347. doi: 10.1016/0092-8674(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. DNA repair and its coupling to DNA replication in eukaryotic cells. Biochim Biophys Acta. 1978 Dec 11;516(4):489–516. doi: 10.1016/0304-419x(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Nucleosome structure controls rates of excision repair in DNA of human cells. Nature. 1977 Dec 1;270(5636):451–453. doi: 10.1038/270451a0. [DOI] [PubMed] [Google Scholar]
- Cox R. Differences in the removal of N-methyl-N-nitrosourea-methylated products in DNase I-sensitive and -resistant regions of rat brain DNA. Cancer Res. 1979 Jul;39(7 Pt 1):2675–2678. [PubMed] [Google Scholar]
- Feldman G., Remsen J., Wang T. V., Cerutti P. Formation and excision of covalent deoxyribonucleic acid adducts of benzo[a]pyrene 4,5-epoxide and benzo[a]pyrenediol epoxide I in human lung cells A549. Biochemistry. 1980 Mar 18;19(6):1095–1101. doi: 10.1021/bi00547a008. [DOI] [PubMed] [Google Scholar]
- Galbraith A. I., Barker M., Itzhaki R. F. Methylation of DNAase-digestible DNA and of RNA in chromatin from rats treated with dimethylnitrosamine. Biochim Biophys Acta. 1979 Feb 27;561(2):334–344. doi: 10.1016/0005-2787(79)90142-4. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Hewish D. Features of the structure of replicating and non-replicating chromatin in chicken erythroblasts. Nucleic Acids Res. 1977 Jun;4(6):1881–1890. doi: 10.1093/nar/4.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C. E., Walters R. A. Rapid assembly of newly synthesized DNA into chromatin subunits prior to joining to small DNA replication intermediates. Biochem Biophys Res Commun. 1976 Nov 8;73(1):157–163. doi: 10.1016/0006-291x(76)90510-6. [DOI] [PubMed] [Google Scholar]
- Ishiwata K., Oikawa A. Actions of human DNA glycosylases on uracil-containing DNA, methylated DNA and their reconstituted chromatins. Biochim Biophys Acta. 1979 Jul 26;563(2):375–384. doi: 10.1016/0005-2787(79)90056-x. [DOI] [PubMed] [Google Scholar]
- Kovacic R. T., van Holde K. E. Sedimentation of homogeneous double-strand DNA molecules. Biochemistry. 1977 Apr 5;16(7):1490–1498. doi: 10.1021/bi00626a038. [DOI] [PubMed] [Google Scholar]
- Lohr D., Corden J., Tatchell K., Kovacic R. T., Van Holde K. E. Comparative subunit structure of HeLa, yeast, and chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):79–83. doi: 10.1073/pnas.74.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Seale R. L. Assembly of DNA and protein during replication in HeLa cells. Nature. 1975 May 15;255(5505):247–249. doi: 10.1038/255247a0. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Kastan M. B., Lieberman M. W. Distribution of repair-incorporated nucleotides and nucleosome rearrangement in the chromatin of normal and xeroderma pigmentosum human fibroblasts. Biochemistry. 1979 Aug 21;18(17):3732–3739. doi: 10.1021/bi00584a014. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Lieberman M. W. Nucleosome rearrangement in human chromatin during UV-induced DNA- reapir synthesis. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4238–4241. doi: 10.1073/pnas.75.9.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerdon M. J., Tlsty T. D., Lieberman M. W. Distribution of ultraviolet-induced DNA repair synthesis in nuclease sensitive and resistant regions of human chromatin. Biochemistry. 1978 Jun 13;17(12):2377–2386. doi: 10.1021/bi00605a020. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Oudet P., Chambon P. Rearrangement of chromatin structure induced by increasing ionic strength and temperature. Eur J Biochem. 1979 Oct;100(1):225–235. doi: 10.1111/j.1432-1033.1979.tb02053.x. [DOI] [PubMed] [Google Scholar]
- Weischet W. O. On the de novo formation of compact oligonucleosomes at high ionic strength. Evidence for nucleosomal sliding in high salt. Nucleic Acids Res. 1979 Sep 25;7(2):291–304. doi: 10.1093/nar/7.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Staphylococcal nuclease and pancreatic DNase cleave the DNA within the chromatin core particle at different sites. J Biol Chem. 1977 Nov 10;252(21):7635–7639. [PubMed] [Google Scholar]
- Wilkins R. J., Hart R. W. Preferential DNA repair in human cells. Nature. 1974 Jan 4;247(5435):35–36. doi: 10.1038/247035a0. [DOI] [PubMed] [Google Scholar]
- Williams J. I., Friedberg E. C. Deoxyribonucleic acid excision repair in chromatin after ultraviolet irradiation of human fibroblasts in culture. Biochemistry. 1979 Sep 4;18(18):3965–3972. doi: 10.1021/bi00585a019. [DOI] [PubMed] [Google Scholar]



