Abstract
The morphology of healthy podocyte foot processes is necessary for maintaining the characteristics of the kidney filtration barrier. In most forms of glomerular disease, abnormal filter barrier function results when podocytes undergo foot process spreading and retraction by remodeling their cytoskeletal architecture and intercellular junctions during a process known as effacement. The cell adhesion protein nephrin is necessary for establishing the morphology of the kidney podocyte in development by transducing from the specialized podocyte intercellular junction phosphorylation-mediated signals that regulate cytoskeletal dynamics. The present studies extend our understanding of nephrin function by showing that nephrin activation in cultured podocytes induced actin dynamics necessary for lamellipodial protrusion. This process required a PI3K-, Cas-, and Crk1/2-dependent signaling mechanism distinct from the previously described nephrin-Nck1/2 pathway necessary for assembly and polymerization of actin filaments. Our present findings also support the hypothesis that mechanisms governing lamellipodial protrusion in culture are similar to those used in vivo during foot process effacement in a subset of glomerular diseases. In mice, podocyte-specific deletion of Crk1/2 prevented foot process effacement in one model of podocyte injury and attenuated foot process effacement and associated proteinuria in a delayed fashion in a second model. In humans, focal adhesion kinase and Cas phosphorylation — markers of focal adhesion complex–mediated Crk-dependent signaling — was induced in minimal change disease and membranous nephropathy, but not focal segmental glomerulosclerosis. Together, these observations suggest that activation of a Cas-Crk1/2–dependent complex is necessary for foot process effacement observed in distinct subsets of human glomerular diseases.
Introduction
When functioning properly in health, the kidney filtration barrier selectively prevents the passage of macromolecules from the blood compartment into the urinary space. Differentiated podocytes form a remarkable octopus-like morphology, extending numerous interdigitating foot processes defined by a unique 3-dimensional actin cytoskeletal architecture and requiring formation of a specialized intercellular junction. These foot processes adhere to and cover an extracellular matrix interposed between podocytes and an endothelium that creates the glomerular capillary wall. Podocytes undergo cytoskeletal remodeling to alter their morphology in nearly all forms of human glomerular disease, exhibiting what has been described as foot process spreading and retraction or as foot process effacement. This process by which podocytes change their cytoskeletal architecture appears to be a component of a common response of the podocyte to cellular injury, correlating with loss of normal filtration barrier selectivity and predicting the development of proteinuria in human disease and in experimental models (1, 2).
Although the cellular and molecular mechanisms controlling foot process effacement remain incompletely understood, recent studies have identified functional relationships between podocyte intercellular junction–associated proteins and actin cytoskeletal dynamics (3–7). The importance of these relationships is emphasized by experiments of nature, in which human genetic mutations in actin-associated proteins result in altered podocyte morphology and proteinuria (5–10).
Nephrin is a transmembrane protein of the Ig superfamily located at the differentiated podocyte intercellular junction (11). Mutations in the gene encoding nephrin lead to congenital nephrotic syndrome of the Finnish type, which is characterized by developmental failure of foot process morphogenesis and podocyte intercellular junction formation and heavy proteinuria at birth, or delayed-onset disease, presenting as steroid-resistant nephrotic syndrome with variable glomerular histopathology (8, 12). Nephrin and the related Ig superfamily protein Neph1 form hetero-oligomeric receptor complexes that associate via cis- and trans-interactions (13, 14). The activated nephrin-Neph1 receptor complex induces actin filament nucleation and elongation at the plasma membrane by recruiting (in a tyrosine phosphorylation–dependent manner) Src homology domain 2 adaptor proteins, including Nck1/2, the regulatory p85 subunit of PI3K, phospholipase Cγ, and Grb2, and subsequently an actin polymerization complex (3, 4, 15–19). Because genetic deletion of nephrin or Neph1 results in abnormal podocyte process development, it has been hypothesized that beyond solely inducing actin polymerization, nephrin and Neph1 — likely in association with other membrane-associated proteins — participate in determining actin architecture. We reasoned that nephrin regulates enzymes that drive actin cytoskeletal remodeling during podocyte process development or during morphological changes that occur after podocyte injury or recovery. In support of this reasoning, we previously reported that nephrin can regulate the activity of the actin filament–severing protein cofilin in a PI3K-dependent fashion (17).
We recently showed that other SH2-binding domain–containing proteins can associate with nephrin in a tyrosine phosphorylation–dependent fashion. One of these nephrin interaction partners is the cytoskeletal adaptor protein Crk1/2 (4). The ubiquitously expressed, SH2–SH3 domain–containing Crk family of proteins consists of Crk2, its splice isoform Crk1, and the paralog CrkL, whose amino acid composition and domain structure is similar to that of Crk2. Crk-mediated signaling can be initiated by a variety of cellular and extracellular stimuli, such as growth factors, cytokines, or integrin-mediated cell adhesion, and is involved in cell proliferation, differentiation, and cell motility (20). The SH2 domain of Crk interacts with a set of phosphotyrosine-containing proteins such as Cas (also referred to as p130Cas), paxillin, or Cbl (21–23). At the focal adhesion, integrin activation leads to phosphorylation of focal adhesion kinase (FAK) on Y397 and binding of Cas to FAK (22, 24). Concurrently, c-Src kinase is recruited to the auto-phosphorylation site of FAK(Y397) (25), where it efficiently phosphorylates tyrosine residues within the substrate domain of Cas and induces the recruitment of Crk (26). Here, Crk functions as an adaptor via its SH3 domain and binds proline-rich domains of Crk SH3 domain–binding guanine nucleotide exchange factor (C3G), Dock180 (another guanine nucleotide exchange factor), or other effector proteins (27, 28). Therefore, by mobilizing and activating Rho family small GTPases, Crk plays a pivotal role in transducing signals that activate or modulate cytoskeletal dynamics, cell spreading, and motility (29, 30).
Podocyte process formation during glomerular development or remodeling events that occur after podocyte injury or in human glomerular disease all require directed cytoskeletal rearrangement. Given the known function of Crk in other model systems, where it is required for regulating actin dynamics that determine cell spreading, and our finding that Crk interacts with nephrin, we hypothesized that Crk is necessary for mediating nephrin-directed cytoskeletal dynamics in the podocyte. Our present results support this hypothesis and provide evidence for a broader hypothesis that focal adhesion complex–associated Crk-dependent signaling might be a therapeutic target for some forms of human glomerular disease.
Results
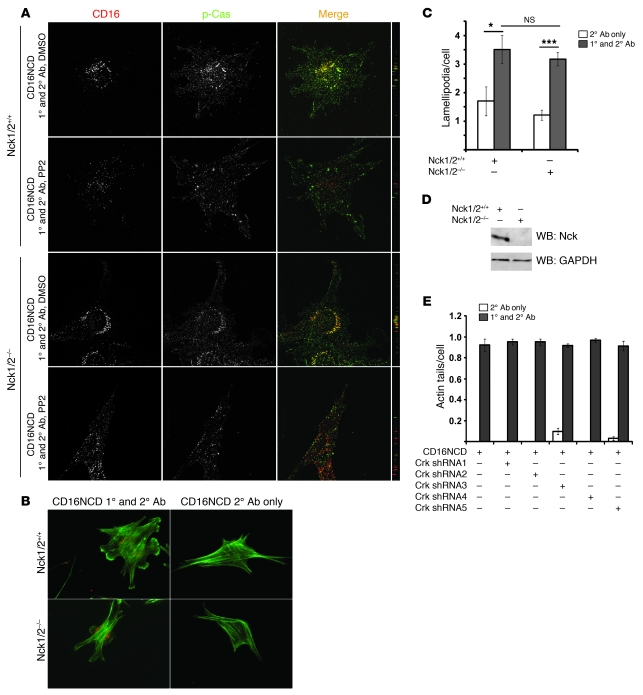
Crk1/2 associates with nephrin in a tyrosine phosphorylation–dependent manner.
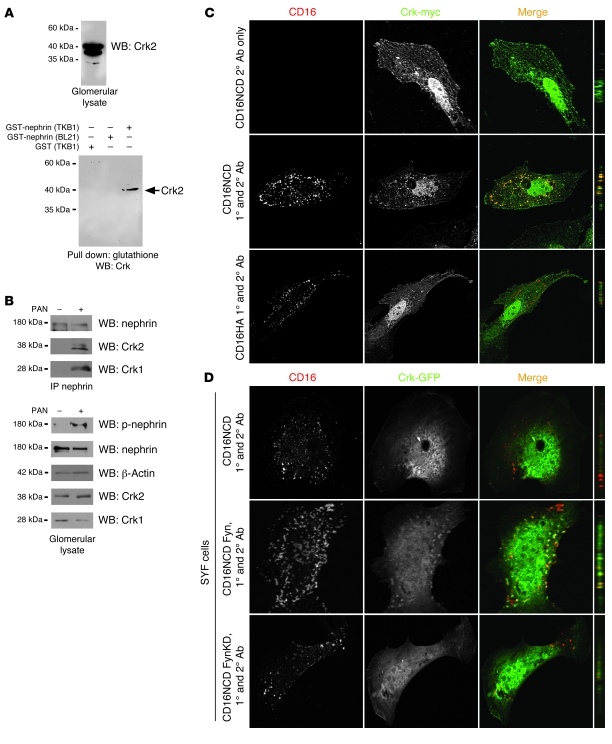
We previously reported an unconfirmed interaction between Crk1/2 and nephrin that appeared to require nephrin tyrosine phosphorylation (4). This interaction was identified in a discovery experiment by pulldown of Crk1/2 from isolated glomerular lysate with recombinant tyrosine-phosphorylated glutathione S-transferase–tagged nephrin cytoplasmic domain (GST-nephrinCD; Figure 1A). To confirm this interaction in an in vivo system, we used a single-dose rat puromycin aminonucleoside (PAN) model of podocyte injury. Rats injected with PAN develop foot process effacement and proteinuria within 3 days of injection (19). Within the same time period, nephrin became tyrosine phosphorylated, and Crk1/2-nephrin complex formation increased compared with vehicle control–injected rats (Figure 1B).
Figure 1. Crk1/2 interacts with nephrin in a tyrosine phosphorylation–dependent fashion.
(A) Purified recombinant GST-nephrinCD expressed in BL21 or TKB1 E. coli (to produce nonphosphorylated or tyrosine-phosphorylated nephrin, respectively) or purified GST alone was mixed with rat glomerular lysate, pulled down with glutathione agarose, and immunoblotted with monoclonal anti-Crk1/2 antibody. (B) Glomerular lysates from rats injected with PBS (control) or PAN were immunoprecipitated and/or immunoblotted using the indicated antibodies. (C) Cultured human podocytes expressing CD16/7-nephrinCD (CD16NCD) or CD16/7-HA (CD16HA) and Crk2-myc were activated by clustering: namely, addition of monoclonal anti-CD16 primary antibody (1°) and/or goat anti-mouse IgG Texas Red–conjugated secondary antibody (2°), as indicated, to the media of live cells. Crk2-myc was detected with rabbit polyclonal anti-myc primary antibody and Alexa Fluor 488–labeled secondary antibody. Cells were analyzed by confocal microscopy. CD16/7-nephrinCD (red) and Crk2-myc (green) colocalized in the plane of the plasma membrane. (D) SYF MEFs transiently expressing CD16/7-nephrinCD and Crk-GFP were activated by clustering. Although colocalization of nephrin and Crk was not observed in SYF MEFs, nephrin-Crk association was rescued by reexpressing Fyn, but not by expressing a kinase-dead variant of Fyn (FynKD). (C and D) Original magnification, ×630. yz plane reconstructions are shown at far right.
The nephrin-Crk1/2 interaction was also examined in an in vitro model previously used by us, which induces nephrin tyrosine phosphorylation and allows for investigation of nephrin activation–mediated signaling events in a simplified model system (4, 17, 19). Plasmid constructs were generated in which the CD16 extracellular domain and the CD7 transmembrane domain are fused either with nephrinCD or with an HA tag as negative control (referred to herein as CD16/7-nephrinCD or CD16/7-HA, respectively). These constructs were individually expressed with myc-tagged Crk2 in a human podocyte cell line. As described previously, addition of mouse anti-CD16 antibody and a fluorescent secondary anti-mouse IgG antibody to the culture media of live cells resulted in “clustering” and tyrosine phosphorylation of CD16/7-nephrinCD fusion proteins in the apical membrane of cultured podocytes (Figure 1C, data not shown, and refs. 4, 19). Concomitantly, this activated CD16/7-nephrinCD recruited and colocalized with Crk2, whereas Crk2 was not recruited to CD16/7-HA.
NephrinCD was previously shown to be tyrosine phosphorylated by the Src family kinase Fyn after nephrin ligation (4, 15, 31). To analyze whether Src kinase activity is necessary for the interaction of nephrin with Crk2 in cells, CD16/7-nephrinCD–expressing human podocytes in culture were treated with the Src kinase inhibitor PP2 or with inactive, related control compound PP3 prior to clustering. Although Crk2 was not recruited to clustered CD16/7-nephrinCD in PP2-treated human podocytes, recruitment was retained after treatment with PP3 (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI60070DS1). The necessity of Fyn for nephrin-Crk2 interaction was confirmed by expressing CD16/7-nephrinCD and Crk2-GFP in mouse embryonic fibroblasts (MEFs) with targeted simultaneous deletions of Src, Yes, and Fyn (referred to herein as SYF cells). In this system, recruitment of Crk2-GFP to activated CD16/7-nephrinCD was not observed. However, Crk2-GFP was recruited to clustered CD16/7-nephrinCD when SYF cells were rescued by reexpressing Fyn, but not after expressing a kinase-dead variant of Fyn (Figure 1D).
Nephrin and Neph1 activation induces lamellipodial protrusions in cultured human podocytes.
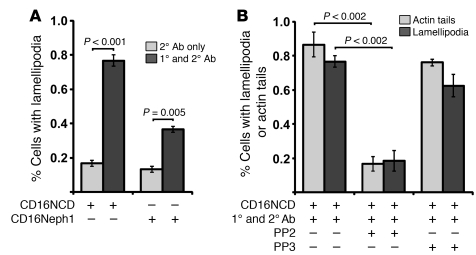
Crk adaptor proteins were shown in other model systems to be necessary for cell spreading and for formation of lamellipodia protrusions at the leading edge (32, 33). For this reason, we hypothesized that nephrin activation transduces a Crk-dependent signal that results in increased lamellipodial protrusion activity in cultured podocytes. Using the CD16 model, we found that nephrin activation induced the formation of lamellipodia in cultured human podocytes (Supplemental Figure 1B and data not shown). Under basal conditions, approximately 20% of cultured podocytes expressing CD16/7-nephrinCD exhibited lamellipodial protrusions. Remarkably, more than 70% of transfected cells showed lamellipodial protrusion formation within 30 minutes of CD16/7-nephrinCD activation (Figure 2A). In similar experiments, clustering and activation of CD16/7-Neph1CD also induced lamellipodial protrusive activity relative to baseline, albeit to a lesser extent than did nephrin (Figure 2A).
Figure 2. Clustered, activated CD16/7-nephrinCD and CD16/7-Neph1CD induce lamellipodia formation in cultured human podocytes.
(A) CD16/7-nephrinCD or CD16/7-Neph1CD was coexpressed with actin-GFP in human podocytes, which were activated by clustering as in Figure 1. Transfected cells with structures typical of lamellipodia on more than 30% of their circumferences were counted as cells with lamellipodial protrusions. (B) Using the same system as in A, cells were treated with PP2 or PP3 before activation. Transfected cells with actin tails longer than 5 μm or lamellipodia extending over more than 30% of the cell circumference were counted positive for these features. 20 transfected cells total were counted per experiment. Data (mean ± SEM) are from single experiments and are representative of 3 unique experiments.
Activated nephrin recruits an actin polymerization complex sufficient to induce actin filament nucleation and elongation (4, 19). Because the experiments above implicated nephrin in transmitting a signal that induces lamellipodial protrusive activity, we asked whether activation of CD16/7-nephrinCD in cultured podocytes induces actin dynamics that result in simultaneous actin filament generation and lamellipodial protrusive activity. Indeed, CD16/7-nephrinCD clustering simultaneously induced actin filaments associated with CD16/7-nephrinCD clusters and lamellipodial protrusive activity; both were inhibited by PP2 (Figure 2B).
Crk1/2 is necessary for nephrin-induced lamellipodia formation.
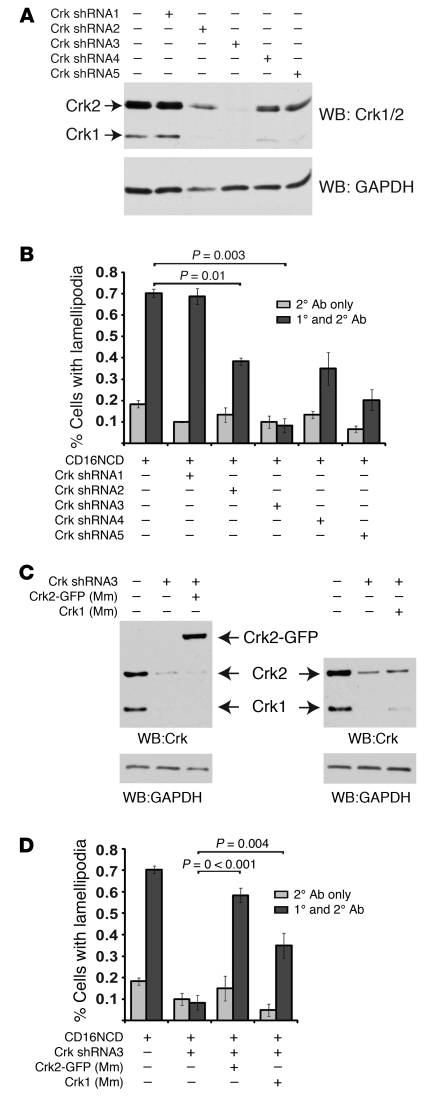
To determine whether nephrin-induced lamellipodial protrusive activity requires Crk1/2, human podocyte cell lines with stable knockdown of Crk1/2 were generated by lentiviral infection using 5 different shRNA templates. Expression of Crk1/2 shRNA3 resulted in efficient knockdown of Crk1/2 (Figure 3A). Upon transient CD16/7-nephrinCD expression and engagement, Crk1/2-deficient podocytes exhibited attenuated induction of lamellipodial activity relative to controls (Figure 3B). Indeed, the extent to which lamellipodial activity was induced after CD16/7-nephrinCD clustering appeared proportional to Crk1/2 abundance. This Crk1/2 knockdown phenotype was rescued by expressing mouse Crk2 in Crk1/2-knockdown human podocytes (Figure 3, C and D). Expression of mouse Crk1 partially rescued nephrin-induced lamellipodial protrusion activation. Taken together, these results demonstrate that Crk1/2 is necessary for induction of nephrin-mediated lamellipodia dynamics in cultured podocytes.
Figure 3. Crk1/2 knockdown attenuates nephrin-induced lamellipodial protrusive activity.
(A) Immunoblot demonstrating attenuation of Crk1/2 expression in human podocyte cell lines stably expressing 1 of 5 different shRNA constructs targeting the Crk1/2 gene. GAPDH protein expression served as loading control. (B) Podocytes stably expressing the indicated Crk1/2 shRNA constructs were transfected with CD16/7-nephrinCD and actin-GFP and were activated by clustering as in Figure 1. The fraction of cells exhibiting lamellipodial protrusions was evaluated after fixation. (C) Immunoblot showing that Crk2-GFP or mouse Crk1 were reexpressed in stably knocked down human Crk shRNA3 podocytes. Mm, Mus musculus. (D) Crk shRNA3 podocytes were transfected with CD16/7-nephrinCD and actin-GFP and activated by clustering. After fixation, the fraction of cells exhibiting lamellipodial protrusions was determined. Results (mean ± SEM) are representative of 3 independent experiments.
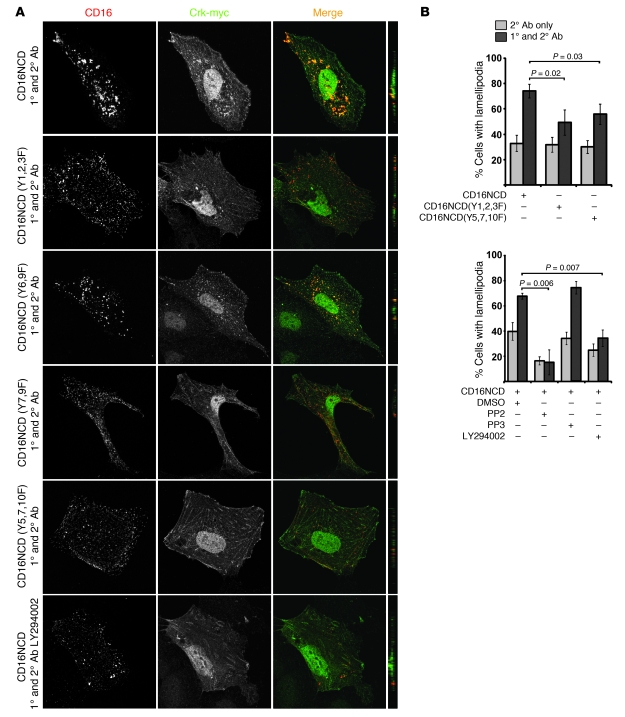
Nephrin-induced Crk recruitment and lamellipodial activity requires 2 sets of nephrin tyrosine residues.
Because the nephrin-Crk interaction described above was Src family kinase dependent, we used the CD16/7-nephrinCD model to analyze whether nephrin tyrosine residues are necessary for nephrin-Crk association. Nephrin tyrosine residues Y1128, Y1153, Y1154 (referred to herein as Y1,2,3; see Supplemental Table 2) were previously shown to be necessary for activating PI3K by mediating recruitment of the regulatory p85 subunit of PI3K to nephrin (17). These residues are also required for nephrin-mediated cofilin dephosphorylation and/or activation (17). Residues Y5,7,10 are necessary for direct SH2 domain–mediated interaction of Nck1/2 with nephrin and for initiation of nephrin-mediated actin filament polymerization (3, 4). As both subsets of nephrin tyrosine residues mediate known nephrin activation–dependent effects, we examined whether these residues are necessary for recruitment of Crk1/2 to activated CD16/7-nephrinCD. Upon engagement, Crk2 again colocalized with CD16/7-nephrinCD in the plane of the membrane. This recruitment was abrogated in cells expressing clustered mutant CD16/7-nephrinCD(Y5,7,10F) or CD16/7-nephrinCD(Y7,9F) (Figure 4A). Additionally, nephrin-mediated PI3K activation appeared necessary for recruitment of Crk2 in this model, since clustered mutant CD16/7-nephrinCD(Y1,2,3F) failed to recruit Crk2, and the PI3K inhibitor LY294002 inhibited the same (Figure 4A). Mutant CD16/7-nephrinCD(Y6,9F) did not disrupt recruitment of Crk2 to activated clusters.
Figure 4. Nephrin-Crk association and nephrin-induced lamellipodial protrusions rely on nephrin tyrosine residues.
(A) CD16/7-nephrinCD plasmids expressed in cultured podocytes were activated by clustering as in Figure 1 and analyzed by confocal microscopy. Podocytes pretreated with LY294002 before clustering are also shown. Crk was stained with anti-myc antibody and Alexa Fluor 488–conjugated secondary antibody. Original magnification, ×630. yz plane reconstructions are shown at far right. (B) Podocytes expressing CD16/7-nephrinCD or the indicated mutants and actin-GFP were activated with or without PP2, PP3, or LY294002, and the fraction of cells with lamellipodia was determined. Cells treated without primary CD16 antibody served as controls. Data (mean ± SEM) are representative of 3 experiments.
To extend these observations, we investigated whether tyrosine residues necessary for nephrin-Crk association are also necessary for nephrin-induced lamellipodia formation. Using CD16/7-nephrinCD or its mutants, we observed that clustered CD16/7-nephrinCD(Y1,2,3F) or cells pretreated with LY294002 prior to clustering induced significantly less lamellipodial protrusive activity than did activated control CD16/7-nephrinCD (Figure 4B). Similarly, activation of CD16/7-nephrinCD(Y5,7,10F) exhibited attenuated lamellipodial activity relative to control. Therefore, nephrinCD tyrosine residues necessary for PI3K activation or tyrosine residues previously identified as necessary for Nck1/2 recruitment and actin filament nucleation and elongation (3, 4) appeared to be necessary for Crk1/2 recruitment and induction of lamellipodial activity in the CD16/7-nephrinCD model.
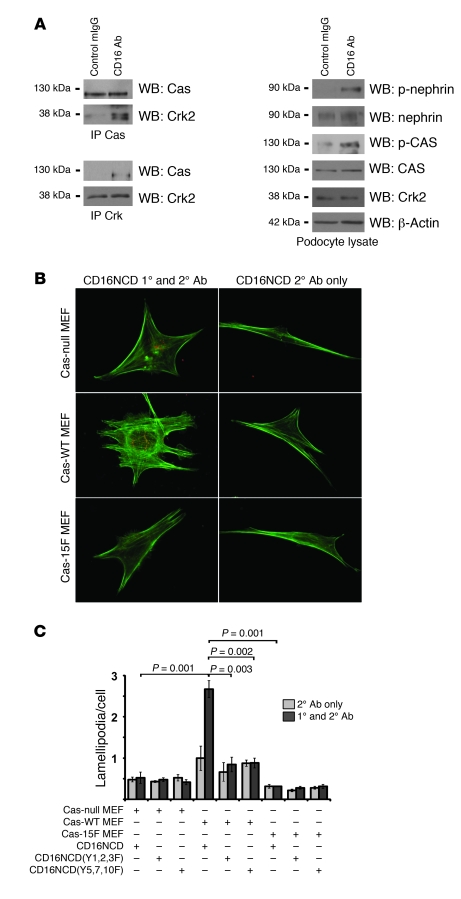
Nephrin engagement leads to phosphorylation of Cas, induces Cas-Crk complex formation, and results in Cas-Crk interaction–dependent lamellipodia formation.
Crk1/2 in other model systems functions in complex with the focal adhesion complex protein Cas to transduce signals necessary for lamellipodial protrusive activity (34). For this reason, we considered the possibility that recruitment of Crk1/2 to nephrin observed in the above-described models occurs via intermediary proteins, rather than by direct interaction. When tyrosine phosphorylated, the Cas substrate domain exhibits increased affinity for the SH2 domain of Crk1/2 (29). Therefore, we examined whether nephrin activation similarly induces Cas phosphorylation and recruitment of Crk to Cas and found that CD16/7-nephrinCD engagement in cultured podocytes resulted in robust phosphorylation of Cas and induction of Cas-Crk complex formation relative to baseline (Figure 5A).
Figure 5. Nephrin engagement leads to phosphorylation of Cas, induces Cas-Crk complex formation, and results in Cas-Crk interaction–dependent lamellipodia formation.
(A) Coimmunoprecipitation and immunoblots using indicated antibodies demonstrated tyrosine phosphorylation of Cas and increased Cas-Crk complex formation after CD16/7-nephrinCD clustering in cultured podocytes. (B and C) Cas-null, Cas-WT, and Cas-15F MEFs were stably transduced to express CD16/7-nephrinCD or the indicated mutants. Cells were activated by clustering as in Figure 1. Actin was stained with Alexa Fluor 488–labeled phalloidin. (B) Cells imaged by fluorescence microscopy. Original magnification, ×630. (C) Lamellipodia per cell were counted. Data (mean ± SEM) are representative of 3 independent experiments.
Increased Cas-Crk complex formation after nephrin engagement suggested that nephrin-induced, Crk-dependent lamellipodia formation requires Cas as an intermediary. In support of this hypothesis, activation of CD16/7-nephrinCD in Cas-null MEFs did not induce lamellipodial protrusive activity, whereas reexpression of WT Cas in these null cells (referred to herein as Cas-WT MEFs) rescued nephrin-induced lamellipodia formation (Figure 5, B and C). To test the specificity of the Cas-Crk interaction in mediating nephrin-induced lamellipodia formation, we used Cas-null MEFs reexpressing Cas mutated in its Crk-interacting domain such that all tyrosine residues of the Cas substrate domain are substituted by phenylalanine (referred to herein as Cas-15F MEFs; ref. 29). Activation of CD16/7-nephrinCD in Cas-15F MEFs failed to induce lamellipodia (Figure 5, B and C), which suggests that the interaction of Cas with Crk is necessary for nephrin-induced lamellipodia formation. Recapitulating the results in human podocytes reported above, lamellipodia protrusive activity was induced in Cas-WT MEFs, but lamellipodia induction was not observed in these cells after induction of CD16/7-nephrinCD(Y1,2,3F) or CD16/7-nephrinCD(Y5,7,10F) (Figure 5C).
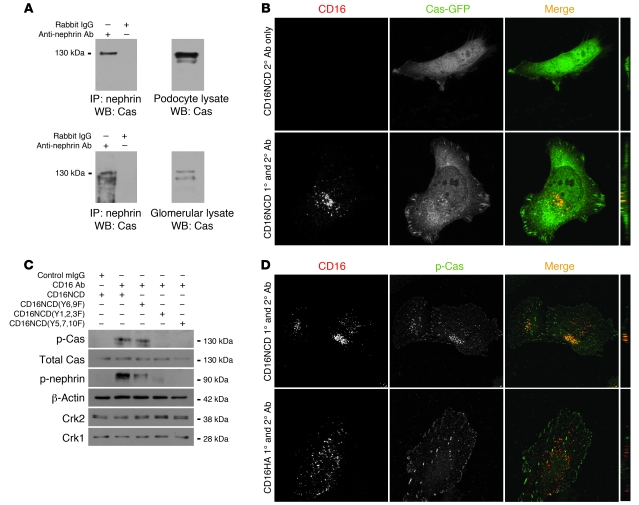
Cas is recruited to clustered nephrin in a PI3K-dependent fashion, while its phosphorylation requires Y5,7,10.
To analyze whether nephrin activation induced Cas-Crk complex formation while Cas was associated with nephrin, coimmunoprecipitation experiments were performed from isolated rat glomerular lysate or cultured podocyte lysate (Figure 6A). In both systems, Cas coimmunoprecipitated with nephrin. Consistent with these results, Cas-GFP was recruited to activated CD16/7-nephrinCD clusters in transiently transfected cultured podocytes (Figure 6B). The observed Cas-GFP recruitment required Src family kinase activity, since recruitment was abolished in the presence of PP2, but not PP3 (Table 1, Supplemental Figure 2A, and data not shown). Distinct from the above-described results obtained for nephrin-dependent Crk recruitment, cultured podocytes expressing CD16/7-nephrinCD(Y6,9F), CD16/7-nephrinCD(Y7,9F), or CD16/7-nephrinCD(Y5,7,10F) recruited Cas to nephrin, whereas cells expressing the CD16/7-nephrinCD(Y1,2,3F) mutant did not (Table 1 and Supplemental Figure 2B). Like Crk recruitment, Cas recruitment to nephrin required both the nephrin p85-binding domain and PI3K activity, since recruitment of Cas was also inhibited in the presence of LY294002 (Table 1 and Supplemental Figure 2A). In similar experiments, endogenous Cas recruited to CD16/7-nephrinCD clusters was tyrosine phosphorylated (Figure 6D). We anticipated that we would not detect Cas phosphorylation in cells expressing CD16/7-nephrinCD(Y1,2,3F) or in cells treated with LY294002 prior to nephrin engagement, because, as described above, total Cas was not observed at these mutant clusters (Table 1 and Supplemental Figure 3, A and B). Surprisingly, although total Cas was recruited to clustered CD16/7-nephrinCD(Y7,9F) or CD16/7-nephrinCD(Y5,7,10F), Cas phosphorylation was not associated with these clustered mutants (Table 1 and Supplemental Figure 3B). This phosphorylation pattern was confirmed by probing protein lysate of NIH3T3 cells expressing clustered CD16/7-nephrinCD or the clustered CD16/7-nephrinCD(Y1,2,3F), CD16/7-nephrinCD(Y6,9F), or CD16/7-nephrinCD(Y5,7,10F) mutants with polyclonal p-Cas antibody (Figure 6C). These results suggested that Cas is recruited to activated nephrin in a PI3K-dependent fashion and that Cas phosphorylation in this complex is regulated by a distinct mechanism requiring Y5,7,10. Because the recruitment and phosphorylation pattern of Cas was similar to the recruitment pattern of Crk to nephrin (Table 1), we suspected that Crk is recruited to nephrin via Cas.
Figure 6. Cas is recruited to clustered nephrin in a PI3K-dependent fashion, while its phosphorylation depends on Y5,7,10.
(A) Nephrin coimmunoprecipitated with Cas from cultured podocytes transiently transfected with plasmid encoding CD16/7-nephrinCD (top) and from isolated rat glomerular cell lysate (bottom). (B) Human podocytes expressing CD16/7-nephrinCD (red) and Cas-GFP (green) were activated by clustering as in Figure 1, fixed, and imaged by confocal microscopy. (C) Cultured NIH3T3 cells expressing CD16/7-nephrinCD from the indicated plasmids were activated as indicated. Immunoblots shown are representative of 3 independent experiments. (D) Cultured human podocytes were transfected with CD16/7-nephrinCD or CD16/7-HA (red) and activated, and endogenous p-Cas was identified using a specific p-Cas antibody and Alexa Fluor 488–conjugated secondary antibody. Cells were analyzed by confocal microscopy. (B and D) Original magnification, ×630. yz plane reconstructions are shown at far right.
Table 1 .
Nephrin tyrosine residues necessary for Cas/Crk recruitment and phosphorylation of recruited Cas and FAK
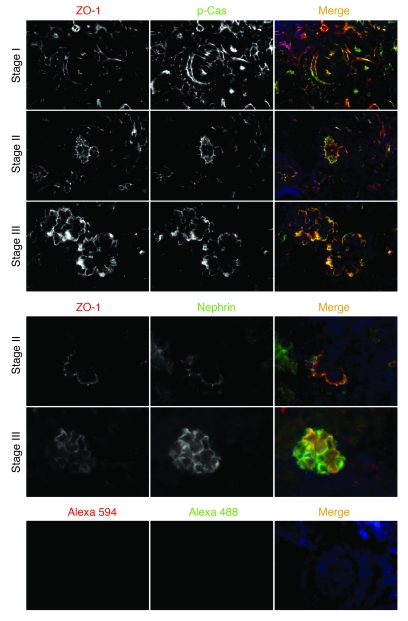
Nephrin and p-Cas colocalize at the podocyte precursor intercellular junction in vivo.
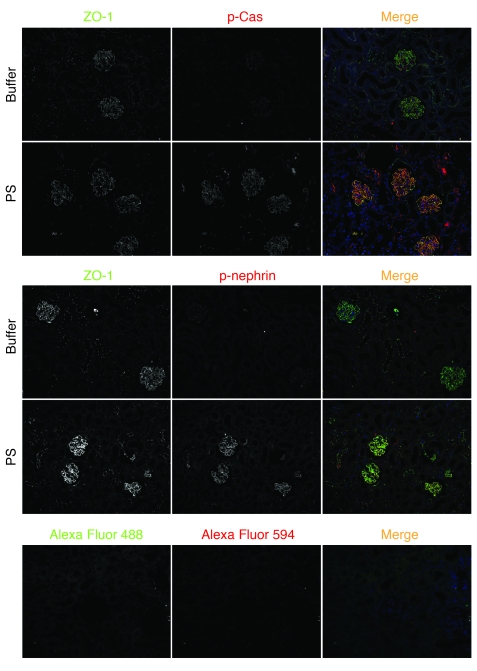
Using indirect immunofluorescence, we investigated whether Cas is located and phosphorylated at the podocyte intercellular junction in vivo. During glomerular maturation, podocyte precursors undergo a transformation by which the intercellular junction moves from an apical-lateral membrane position (s-shape body) to the basolateral aspect of the cell (capillary loop stage), while changing its molecular composition to become a specialized adherens junction known as the slit diaphragm in the mature podocyte. Nephrin is first expressed at the late capillary loop stage, when foot processes begin to develop (35). Because the podocyte precursor intercellular junction at the s-shaped and capillary loop stages of kidney development is easily distinguishable from the basal aspect of the cell, where focal adhesions might be anticipated to be targeted, we used this developmental transition to analyze whether Cas is located at the podocyte precursor adherens junction. Since all developmental stages can be observed in single newborn mouse kidney sections, these sections were examined by indirect immunofluorescence for nephrin or ZO-1 (as a marker of the adherens junction) as well as with p-Cas. p-Cas was detected in podocyte precursors of the s-shape body, where it colocalized with ZO-1 (Figure 7). During the early capillary loop stage, when the adherens junction had descended toward the basal aspect of the lateral cell wall, p-Cas remained with ZO-1. At later stages, p-Cas staining showed a pattern typical of other slit diaphragm proteins (Figure 7). We confirmed that nephrin was first expressed during the late capillary loop stage and was visualized at the podocyte precursor adherens junction, where — like p-Cas — it colocalized with ZO-1 (Figure 7). Nephrin was targeted to the podocyte intercellular junction later than p-Cas.
Figure 7. p-Cas is targeted to podocyte precursor intercellular junctions in newborn mice.
Mouse newborn kidney sections were stained with anti–p-Cas (green) or anti-nephrin antibody (green) as indicated. Note that p-Cas was detected at the podocyte precursor intercellular junction as early as the s-shape body stage (stage I), whereas nephrin expression was first seen at the capillary loop stage (stage II). ZO-1 staining was used as a junction marker (red). Secondary antibody controls are shown below. Stage III, maturing glomerulus. Original magnification, ×630.
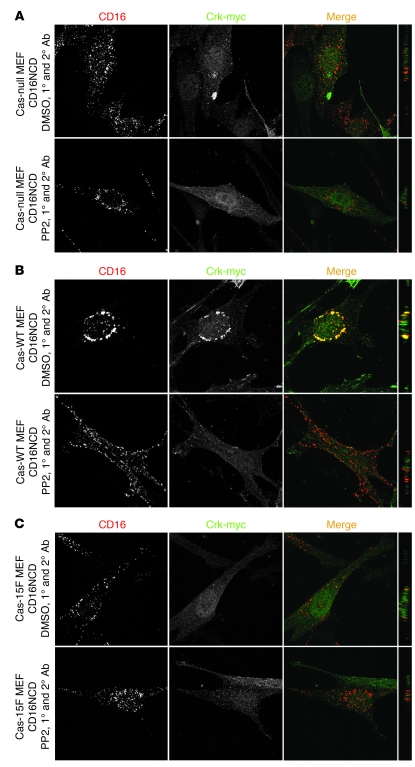
Confirmation that Crk is recruited to nephrin indirectly via Cas.
Despite the results reported above, we could not exclude that nephrin and Crk1/2 interact directly. In fact, in in vitro pulldown experiments, purified recombinant His-tagged Crk2 and tyrosine-phosphorylated GST-nephrinCD bound directly (Supplemental Figure 4A). An overlay experiment confirmed this result and suggested that the Crk1/2 SH2 domain can interact with nephrin oligopeptides containing Y7,9,10, but not other nephrinCD tyrosine residues, in a phosphorylation-dependent manner (Supplemental Figure 4B). Because these results were inconsistent with observations reported above, which suggested that activated nephrin recruits Crk via an intermediary complex requiring PI3K activity and Cas, we asked whether Crk1/2 could be recruited to nephrin in Cas-null MEFs. In CD16/7-nephrinCD–expressing Cas-null or Cas-15F MEFs, activation of CD16/7-nephrinCD did not result in Crk2 recruitment to nephrin; in contrast, Crk2 was recruited to CD16/7-nephrinCD clusters in Cas-WT MEFs (Figure 8). In summary, although direct interaction of activated nephrin and Crk could not be entirely ruled out, indirect interaction of Crk and nephrin via Cas prevailed in cells.
Figure 8. Crk is recruited to nephrin indirectly via Cas.
Cas-null (A), Cas-WT (B), and Cas-15F (C) MEFs were transfected with CD16/7-nephrinCD and Crk-myc as indicated. Cells were activated by clustering as in Figure 1 and analyzed by confocal microscopy. Crk-myc was stained with myc antibody and labeled with secondary antibody (Alexa Fluor 488). Cells treated with PP2 prior to clustering are also shown. (A–C) Original magnification, ×630. yz plane reconstructions are shown at far right.
FAK is present at CD16/7-nephrinCD clusters and at the podocyte precursor intercellular junction in vivo.
In other model systems, FAK is associated with Cas and coordinates Src kinase–mediated phosphorylation of the Cas substrate domain and subsequent recruitment of Crk1/2 (26). For this reason, it was not surprising that FAK was associated with nephrin and was phosphorylated after its activation in the CD16/7-nephrinCD model (Supplemental Figure 5A and Supplemental Figure 6). Like Cas recruitment and phosphorylation, FAK activation required Src kinase activity (Supplemental Figure 5A), nephrin-induced PI3K activity, and Y7,9 (Supplemental Figure 5A, Supplemental Figure 6, and Table 1). In a pattern similar to that observed with p-Cas, p-FAK was identified at intercellular junctions on the lateral aspect of podocyte precursor cells during the capillary loop stage of glomerular development. FAK expression appeared to occur initially at a developmental stage slightly later than p-Cas and more typical of the previously described expression pattern of nephrin (ref. 4 and Supplemental Figure 5B).
Distinct signaling pathways mediate nephrin-induced lamellipodia and actin filament formation.
The SH2-SH3 adapter protein Nck binds to nephrin in a tyrosine phosphorylation–dependent fashion requiring Y5,7,10, an interaction necessary for nephrin-induced actin filament polymerization (3, 4). As Cas phosphorylation and subsequent recruitment of Crk1/2 was also dependent on Y5,7,10, we examined whether Nck is necessary for phosphorylation of Cas and recruitment of Crk. Using MEFs deleted of Nck1 and Nck2 (referred to herein as Nck1/2–/–) and stably expressing CD16/7-nephrinCD, we found that Crk-GFP — or, in separate experiments, endogenous p-Cas — was recruited to CD16/7-nephrinCD clusters (Figure 9A and Supplemental Figure 7). Thus, Nck1/2 was not necessary for Cas phosphorylation and subsequent binding of Crk to Cas. Consistent with this observation, nephrin clustering induced lamellipodial activity to a similar extent when performed in Nck1/2–/– and Nck1/2+/+ MEFs (Figure 9, B and C). Reciprocally, Crk1/2 was required for CD16/7-nephrinCD–induced lamellipodial activity, but was not necessary for actin filament polymerization at CD16/7-nephrinCD clusters. Human podocytes in which Crk1/2 was stably knocked down were examined for induction of cluster-associated actin tails after CD16/7-nephrinCD activation (19). Actin tails were readily induced in Crk1/2 knockdown cells at the site of nephrin aggregates (Figure 9E), which indicates that nephrin-induced local actin tail formation is mediated via a pathway requiring Nck1/2 as an intermediary, whereas nephrin-induced lamellipodia formation is transduced by a distinct signal requiring Cas and Crk.
Figure 9. Nephrin-induced actin filament polymerization and lamellipodia formation are mediated by distinct signaling pathways.
(A) Nck1/2+/+ and Nck1/2–/– MEFs stably expressing CD16/7-nephrinCD were activated as in Figure 1, and endogenous p-Cas was stained with p-Cas antibody and Alexa Fluor 488–labeled secondary IgG antibody and imaged by confocal microscopy. Cells treated with PP2 prior to clustering are also shown. (B and C) Nephrin-induced lamellipodia formation was independent of Nck. Nck1/2+/+ and Nck1/2–/– MEFs expressing CD16/7-nephrinCD were evaluated for lamellipodial protrusion activity 30 minutes after clustering. Actin was stained with green-labeled phalloidin. (D) Cell lysates from Nck1/2+/+ and Nck1/2–/– MEFs were analyzed by immunoblotting with Nck antibody or GAPDH antibody as loading control. (E) Nephrin-induced local actin filament polymerization was independent of Crk1/2. Human podocyte cell lines stably expressing 1 of 5 different shRNA targeting human Crk1/2 were transfected with CD16/7-nephrinCD. Actin tails per podocyte were counted after activation by clustering. (A and B) Original magnification, ×630. (C and E) Data (mean ± SEM) are from 3 independent experiments. *P < 0.05; ***P < 0.0005.
Podocyte-specific deletion of Crk1/2 in the mouse blocks protamine sulfate–induced foot process spreading.
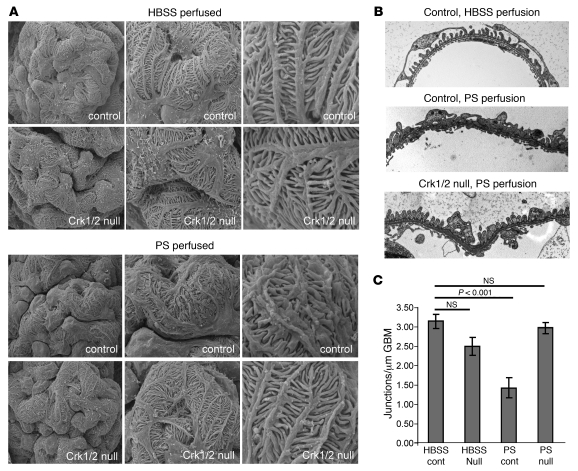
We speculated that Crk1/2-dependent lamellipodia protrusion in culture might be a surrogate for foot process spreading observed after podocyte injury in situ. To analyze the function of Crk1/2 in podocytes in vivo, we used a strategy in which Crk1/2 was selectively deleted from kidney podocytes in mice (referred to herein as Crk1/2fl/flpodocin-CreTg/+ mice, homozygous for the floxed Crk1/2 allele and heterozygous for podocin-Cre; Figure 10A and refs. 36, 37). Podocyte-specific knockout of Crk1/2 protein was confirmed by indirect immunofluorescence staining (Figure 10B). At birth, mice were obtained at the expected Mendelian frequency, and Crk1/2fl/flpodocin-CreTg/+ mice behaved and aged normally. Relative to control mice, mutants did not have increased proteinuria over a 20-month observational period (data not shown). Because Crk1/2 is necessary for cell spreading in culture, we speculated that functional loss of Crk1/2 might become evident when challenging these mice using the protamine sulfate model of podocyte injury. This model resulted in rapid foot process spreading, concomitant with nephrin tyrosine phosphorylation and phosphorylation of Cas (Figure 11 and ref. 4). Crk1/2fl/flpodocin-CreTg/+ mice and their control littermates (2–3 months old) were perfused via the renal artery with either protamine sulfate or control buffer. Kidneys were fixed and analyzed by scanning and transmission EM. Whereas perfusion of kidneys of control littermates with protamine sulfate resulted in foot process spreading, foot process morphology was remarkably preserved in protamine sulfate–perfused Crk1/2fl/flpodocin-CreTg/+ mice (Figure 12, A and B). Evaluation of podocyte intercellular junction frequency by transmission EM confirmed that control mice had a significantly reduced junction frequency after injury, whereas foot process architecture of protamine sulfate–perfused Crk1/2fl/flpodocin-CreTg/+ mice remained similar to that of buffer-perfused control mice (Figure 12C). These results are consistent with the conclusion that Crk1/2 is necessary for protamine sulfate–induced foot process spreading.
Figure 10. Selective deletion of Crk1/2 in mouse podocytes.
(A) Crk1/2fl/fl mice with loxP sites flanking exon 1 were crossed with podocin-CreTg/+ mice to generate Crk1/2fl/flpodocin-CreTg/+ mice (deleted of Crk1/2 in podocytes). (B) Paraffin-embedded mouse kidney sections of Crk1/2fl/fl (control) and Crk1/2fl/flpodocin-CreTg/+ (Crk1/2 null) mice were double stained for Crk1/2 (green) and podocin as a podocyte marker (red). Higher-magnification images of portions of a glomerulus are shown below. Note the absence of Crk1/2 staining in podocytes (arrows) of Crk1/2fl/flpodocin-CreTg/+, whereas Crk1/2 was still expressed in mesangial cells. Original magnification, ×600 (top); ×4,200 (bottom).
Figure 11. Enhanced nephrin and Cas phosphorylation after protamine sulfate–induced foot process spreading.
WT mice were perfused with either buffer or protamine sulfate (PS), and paraffin-embedded kidney sections were stained with either anti–p-nephrin (red) or anti–p-Cas (red) and anti–ZO-1 (green) antibodies. Cas and nephrin phosphorylation was enhanced after protamine sulfate–induced podocyte injury. Original magnification, ×200.
Figure 12. Foot process spreading in Crk1/2fl/flpodocin-CreTg/+ mice is prevented by protamine sulfate perfusion.
(A and B) Scanning EM (A) and transmission EM (B) of Crk1/2fl/fl and Crk1/2fl/flpodocin-CreTg/+ mice perfused with HBSS or protamine sulfate. Results are representative of 3–5 mice per group. Original magnification, ×3,000 (A, left); ×7,000 (A, middle); ×20,000 (A, right); ×15,000 (B). (C) Number of junctions per micron glomerular basement membrane (GBM), as seen by transmission EM. Data are mean ± SEM.
Podocyte-specific deletion of Crk1/2 attenuates the glomerular phenotype induced by nephrotoxic serum.
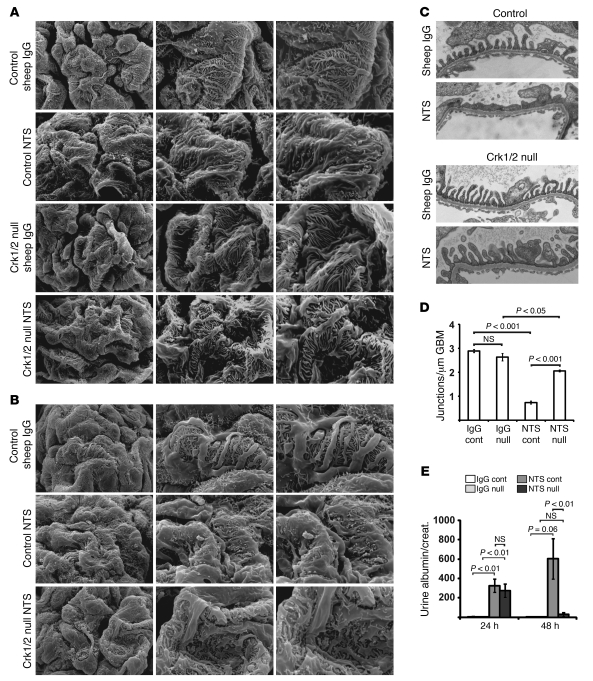
To test whether the observations made in the protamine sulfate model could be extended to another mouse model of podocyte foot process spreading, we used the previously described nephrotoxic serum (NTS) model, in which mice are injected with sheep anti-rat glomerular lysate antiserum (38–43). Intravenous injection of NTS into WT mice resulted in a dramatic increase in proteinuria within 24 hours that persisted for more than 48 hours, whereas injection of control sheep IgG had no apparent effect. This was accompanied by severe foot process effacement, as evaluated by both scanning and transmission EM (Figure 13). Remarkably, in Crk1/2fl/flpodocin-CreTg/+ mice, while proteinuria of similar magnitude was obtained at 24 hours after NTS injection, proteinuria appeared to decline rapidly by 48 hours. In parallel with these results, there was no significant difference in podocyte morphology at 24 hours between NTS-treated mice on WT and Crk1/2fl/flpodocin-CreTg/+ backgrounds, whereas there was partial recovery of normal podocyte morphology by 72 hours in Crk1/2fl/flpodocin-CreTg/+ mice, but not WT mice.
Figure 13. Podocyte-specific Crk1/2 deletion attenuates the glomerular phenotype induced by NTS.
(A–C) Scanning EM (A and B) and transmission EM (C) of Crk1/2fl/fl and Crk1/2fl/flpodocin-CreTg/+ mouse glomeruli, either 72 (A and C) or 24 (B) hours after injection of NTS or control sheep IgG. Original magnification, ×3,000 (A and B, left); ×7,000 (A and B, middle); ×10,000 (A and B, right); ×25,000 (C). (D) Number of junctions per micron glomerular basement membrane, as seen by transmission EM 72 hours after injection (mean ± SEM). (E) Albumin/creatinine ratios of Crk1/2fl/fl and Crk1/2fl/flpodocin-CreTg/+ mouse urines 24 and 48 hours after injection with NTS or sheep IgG. Data (mean ± SEM) are representative of 3 (A–D) or 3–9 (E) mice per group.
Cas and FAK phosphorylation is enhanced in human proteinuric glomerulopathies, such as membranous nephropathy and minimal change disease.
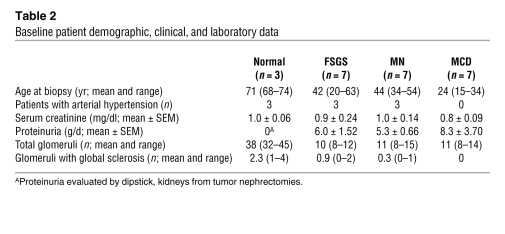
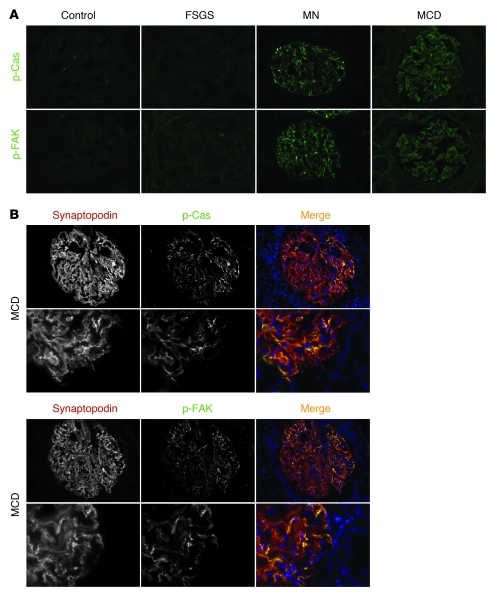
To analyze whether proteins of the FAK-Cas-Crk complex show altered activity in human proteinuric kidney disease, snap-frozen kidney biopsy sections of patients with focal segmental glomerulosclerosis, membranous nephropathy, or minimal change disease or tissue of healthy control subjects were examined by indirect immunofluorescence with p-Cas or p-FAK antibodies. We evaluated 7 cases per diagnosis. The clinical characteristics associated with tissue samples are summarized in Table 2. Phosphorylation of Cas and FAK was considerably and reproducibly enhanced in glomeruli of patients with membranous nephropathy and minimal change disease relative to healthy controls (Figure 14A). The degree of Cas and FAK phosphorylation in glomeruli of patients with FSGS was similar to that of healthy controls. Tyrosine-phosphorylated Cas or FAK colocalized with the podocyte marker synaptopodin (Figure 14B and data not shown). Together with the above-described results obtained in cell culture and in rodent models, these observations suggest that FAK-Cas-Crk signaling is invoked in some forms of human glomerulopathy.
Table 2 .
Baseline patient demographic, clinical, and laboratory data
Figure 14. Enhanced Cas and FAK phosphorylation in membranous nephropathy and minimal change disease.
(A) Representative images showing anti–p-Cas (green) and anti–p-FAK (green) antibody staining of frozen kidney sections from control subjects and from patients with focal segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), or minimal change disease (MCD). (B) Coimmunofluorescence study of frozen kidney sections of patients with minimal change disease with anti–p-Cas (green) or anti–p-FAK antibody (green) and the podocyte slit diaphragm marker synaptopodin (red). Higher-magnification images are shown below. Note that p-Cas and p-FAK almost completely colocalized with synaptopodin. Original magnification, ×400 (A and B, top); ×1,000 (B, bottom).
Discussion
Podocyte cytoskeletal remodeling that results in foot process spreading is a common response of the podocyte to injury and is found universally in chronic forms of human glomerular disease. Because these changes are associated with altered glomerular permselectivity and progressive loss of renal function, and because therapeutic interventions are limited, identifying the cellular mechanisms that govern podocyte cytoskeletal remodeling has been a priority for research in this area. We have approached this problem by focusing on the broad hypothesis that nephrin assembles a signaling complex that regulates actin polymerization and architecture. Earlier studies established that the nephrin-Neph1 complex transduces signals that induce polymerization of linear actin filaments (3, 4, 17, 19). The present studies extend our understanding of the function of nephrin by demonstrating that nephrin activation in cultured cells induced actin dynamics necessary for lamellipodial protrusion. This required a PI3K, Cas, and Crk1/2-dependent signaling mechanism that was distinct from the nephrin-Nck1/2 pathway required for assembly of an actin polymerization complex. Therefore, at least in cultured cells, nephrin integrates a signaling network that both initiates actin polymerization and, by a second and presumably integrated mechanism, determines its dynamics.
In other cell culture model systems, Crk1/2 forms a complex with FAK and Cas at readily identifiable, basally located focal adhesions at which signals transduced by epidermal growth factor receptors or platelet-derived growth factor receptors and integrins are integrated to regulate lamellipodial dynamics and cell motility (24). Our results demonstrated that nephrin activated this complex in a manner similar to that of growth factor receptors. We were surprised to observe that in addition to apparent basolateral membrane targeting, p-Cas and p-FAK were targeted to the intercellular junction of differentiating podocyte precursors in vivo, where they colocalized late in podocyte differentiation with nephrin. While not commonly appreciated, published precedence exists for the targeting of FAK and its associated complex at the adherens junction, with several reports that FAK and Cas coimmunoprecipitate or colocalize with adherens junction proteins in cell culture models (44, 45). An additional report describes FAK targeting to the specialized adherens junction of the seminiferous epithelium, where it participates in adherens junction remodeling during spermatogenesis (46). Our results in the PAN model indicated that the nephrin-Cas-Crk1/2 complex reassembled and became activated during actin remodeling that occurred with podocyte injury when foot process spreading was observed. In the protamine sulfate model, in which Crk1/2 was required for foot process spreading, nephrin phosphorylation and Cas phosphorylation were rapidly induced. Together, these results tempt speculation that nephrin participates in regulating podocyte actin dynamics in response to injury or in some forms of disease. Testing this hypothesis will require development of tools that will allow direct examination of the effects of activating nephrin on mature podocyte morphology.
There is little contention that nephrin functions during podocyte development, since nephrin mutations in humans or genetic deletion of nephrin in mice result in abnormal formation of podocyte tertiary processes and intercellular junctions. Therefore, given the relationship established in culture between nephrin and Crk1/2, it was surprising that podocytes in vivo deleted of Crk1/2 developed normally and did not display a primary phenotype, even with aging. Although this result might suggest that Crk1/2 is not necessary for podocyte differentiation and morphogenesis, one cannot exclude the possibility that normal podocyte development occurred as a result of functional genetic complementation of Crk1/2 by the paralog CrkL, which shows an amino acid composition and domain structure similar to that of Crk2. Germline Crk1/2- and CrkL-null mice exhibit distinct developmental phenotypes, even though CrkL and Crk2 have highly conserved primary sequences. Crk1/2-null mice exhibit cardiovascular and craniofacial developmental defects and do not survive embryogenesis, whereas CrkL-null embryos show lethal defects in cardiac and neural crest development (47, 48). Although this difference in phenotype suggests that CrkL and Crk1/2 mediate distinct functions during embryogenesis, several reports suggest that CrkL and Crk1/2 can exhibit context-specific overlapping functional roles (49, 50). In the Reelin pathway, Crk1/2 and CrkL fulfill essential overlapping functions to control neuronal positioning in the developing brain, as mutation of either gene by itself in mice does not compromise Reelin signaling (44). Whether CrkL can functionally complement Crk1/2 in the podocyte will require additional study.
Protamine sulfate perfusion of the kidney induces foot process spreading, which occurs within minutes and can be reversed within minutes by further perfusion with heparin sulfate. It was previously argued that protamine sulfate causes alteration in podocyte structure by dissipating the repulsive negative charges on the apical surface of podocytes thought to maintain separation between processes (51). The rapid alteration in podocyte cytoskeletal architecture observed in this model argues that protamine sulfate induces changes that are dependent on regulation of podocyte cytoskeletal dynamics. Our observation that genetic deletion of Crk1/2 prevented detectable foot process spreading after perfusion of protamine sulfate demonstrated that protamine sulfate induces a signal that results in regulated podocyte cytoskeleton remodeling.
The observation that deletion of Crk1/2 prevented foot process effacement in the protamine sulfate model suggests that disrupting Crk1/2-dependent signaling attenuates foot process spreading in human glomerular disease. This conclusion is likely too simplistic, given our results from the NTS nephritis model and several human glomerular diseases. Unlike the attenuation of foot process effacement observed in the Crk1/2fl/flpodocin-CreTg/+ mouse after treatment with protamine sulfate, treatment of these mice with NTS resulted in initial foot process effacement and proteinuria relative to control at 24 hours, followed by dramatic attenuation in proteinuria and effacement by 48 hours. These results suggest that podocyte injury by NTS is mediated by at least 2 independent mechanisms: one mechanism that is Crk1/2 independent and occurs early and transiently, and another delayed mechanism that relies on Crk1/2. Indeed, the NTS model is known to have independent complement- and inflammation-mediated effects as well as direct effects on glomerular podocytes by binding of IgG to aminopeptidase A and β1 integrin (52, 53). These results also suggest that Crk1/2-dependent foot process effacement induced by protamine sulfate occurs by a mechanism distinct from effacement observed early after NTS-induced podocyte injury. The possibility that there are at least 2 mechanisms that result in foot process effacement in different settings is emphasized by the additional observation that proteins of the FAK/Cas protein complex were considerably more phosphorylated in podocytes of patients presenting with minimal change disease and membranous nephropathy than in those presenting with FSGS or in healthy control tissue.
The recognition that distinct mechanisms result in foot process spreading might be clinically useful for defining subsets of glomerular disease. Phosphospecific antibodies against FAK and Cas might aid in clinical classification or to identify subsets of proteinuric glomerular disease that may be amenable to specific drug treatment. For example, the observation that Crk1/2 deletion prevented protamine sulfate–induced foot process effacement was consistent with previous in vivo observations in which genetic or pharmacological inactivation of FAK attenuated foot process effacement and proteinuria in disease models (54). Thus, FAK-Cas-Crk complex inhibitors, or inhibitors that specifically interfere with Cas-Crk association, might be useful to prevent foot process effacement in asynchronous glomerular disease in which not all glomeruli are involved at the time of diagnosis, or in recurrent foot process effacement that might occur in relapsing disease. In particular, future studies should be considered to evaluate such inhibitors in specific susceptible subsets of human glomerulopathy in which podocyte FAK and Cas are phosphorylated.
Methods
Antibodies.
Rabbit polyclonal antibodies against nephrin, p-nephrin, and podocin were described previously (4, 35). Monoclonal antibodies against Cas (BD Transduction), Crk (BD Transduction), CD16 clone 3G8 (BD Pharmingen), β-actin (Sigma-Aldrich), ZO-1 (Zymed), GST (BD Transduction), GAPDH (abcam), synaptopodin (GenWay), and phosphotyrosine clone 4G10 (Millipore) and polyclonal antibodies against p-Cas (Cell Signaling Technology), p-FAK (Cell Signaling Technology), c-myc (Sigma-Aldrich), and Nck (BD Transduction) were obtained commercially.
Plasmids.
Plasmid encoding Cas-GFP was a gift from G. Longmore (Washington University, St. Louis, Missouri, USA); Crk2 DNA was amplified from a mouse brain library and cloned into pcDNA3.1MycHis using BamHI and EcoRV restriction sites. pEGFPCrk2 (mouse) was a gift from K. Vuori (Burnham Institute of Medical Research, La Jolla, California, USA). pEBBCD16/CD7-nephrinCD, pEBBCD16/CD7-Neph1CD, and pEBBCD16/CD7-HA (gift from B. Mayer, University of Connecticut, Farmington, Connecticut, USA) were described previously and include the entire mouse nephrinCD or Neph1CD (4, 19). Various nephrin tyrosine-to-phenylalanine point mutations were introduced using PCR-based standard techniques. CD16/7-nephrinCD was cloned into pBABE vector using EcoRI and SalI restriction sites (gift from M. Lazzara, University of Pennsylvania, Philadelphia, Pennsylvania, USA). Nephrin tyrosine-to-phenylalanine mutations were generated using standard PCR techniques. Furthermore, CD16/7-nephrinCD was cloned into a modified pLL3.7 vector using NheI and PciI restriction sites. Expression plasmids for Fyn and FynKD (K295M) were described previously (55). Plasmid encoding GST-nephrinCD (mouse) was used as a template to introduce various tyrosine-to-phenylalanine mutations.
Phosphopeptide mapping by peptide array.
Nephrin oligopeptides (13- to 15-mers) with or without phosphorylated tyrosines encompassing each tyrosine residue in nephrinCD were synthesized (Sigma-Aldrich Genosys PEPScreen). Tyrosine residues in the oligopeptides were flanked by 6 or 7 amino acid residues. Oligopeptides were blotted onto PVDF membranes using a dot blot apparatus. Membranes were overlaid with recombinant GST-Crk full-length, GST-Crk SH2 domain, or GST-Crk SH3 domain protein in PBS containing 2% BSA and 5% milk. HRP-conjugated anti-GST antibody (Sigma-Aldrich) was added, and signals were detected using regular ECL (Thermo Scientific).
Pulldown.
GST or GST-nephrinCD was purified from BL21 or TKB1 E. coli, and pulldown assays were carried out as described previously (4). In the initial screening experiment, GST or GST-nephrinCD was incubated with rat glomerular lysates. In further experiments, GST-nephrinCD was mixed with recombinant his-Crk1/2 prepared in BL21 E. coli (4).
Cell culture.
Conditionally immortalized human podocytes were a gift from M. Saleem (University of Bristol, Bristol, United Kingdom) and were cultured under permissive conditions as described previously (56). Nck1/2+/+ and Nck1/2–/– MEFs were a gift from T. Pawson (University of Toronto, Toronto, Ontario, Canada). Cas-null, Cas-WT, and Cas-15F MEFs were described previously. Nck1/2+/+, Nck1/2–/–, Cas-null, Cas-15F, and Cas-WT MEFs were grown in DMEM with high glucose supplemented with 10% FBS and 200 U/ml penicillin/streptomycin. NIH3T3 cells were cultured as previously described (4). Transient transfections were performed using Lipofectamine 2000 (Invitrogen) or electroporation (Amaxa MEF 1 Nucleofecter Kit; Lonza) according to the manufacturer’s instructions. In experiments using the PI3K inhibitor LY294002 (Calbiochem), the Src kinase inhibitor PP2, or the inactive control compound PP3 (Calbiochem), cells were treated with these substances for 1 hour prior to clustering, and inhibitors were present when adding the secondary clustering antibody.
Generation of stable cell lines.
Phoenix HEK 293 retrovirus packaging cells (2.5 × 106/10 cm dish) were plated before transfection with pBABECD16/CD7-nephrinCD or -nephrin mutant plasmids using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Retroviruses were produced for 18 hours, culture supernatant was filtered and transferred to target cells plated at subconfluency. Viral supernatant was refreshed twice after 6 hours. Stable human podocyte cell lines with knockdown of Crk1/2 were generated by using 5 different prepackaged lentivirus-based shRNA oligonucleotides (Mission shRNA Lentiviral Transduction Particles; Sigma-Aldrich; see Supplemental Table 1 for sequences). Puromycin was added to the target cells 24 hours after infection at a concentration of 2.5 μg/ml.
CD16 chimera, immunoprecipitation, and immunoblotting.
Human podocytes were infected with pLL3.7.2CD16/CD7-nephrinCD at a cell confluency of 70%, cells were split to maintain this density and podocytes were infected again. This maneuver led to a transduction efficiency greater than 95%. On day 6 after the first infection, human podocytes were used for a CD16 clustering experiment and subsequent immunoprecipitation studies as previously described (4, 17, 19).
Indirect immunofluorescence of tissue sections.
Adult kidneys were perfusion fixed and newborn kidneys drop fixed in 4% paraformaldehyde, after which kidneys were paraffin embedded and sectioned (4 μm). Indirect immunofluorescence was performed as previously described (4). Antigen retrieval was achieved by heating sections to 95°C for 30 minutes in Tris-EDTA buffer (pH 9.0).
CD16 chimera Crk, Cas recruitment, and phosphorylation of Cas and FAK.
Human podocytes were transiently transfected with CD16/7-nephrinCD, CD16/7-HA, or mutants, or stable MEF lines were used for these experiments (see above). For recruitment assays of Crk and Cas, myc-Crk2, Crk2-GFP, or Cas-GFP plasmids were cotransfected according to the manufacturer’s guidelines (Lipofectamine 2000; Invitrogen). Activation assays using the CD16 model were performed as previously described (4). When evaluating Crk2-myc recruitment to CD16/7-nephrinCD or phosphorylation of recruited Cas or FAK, indirect immunofluorescence was performed as described previously (57). Anti-myc antibody was diluted at 1:7,500, and polyclonal anti–p-Cas and polyclonal anti–p-FAK397 antibodies were diluted at 1:100 in 2% BSA in PBS with 0.1% TX-100. Samples were analyzed by confocal fluorescence microscopy using a confocal Leica SP5 microscope with an ×63 oil immersion objective lens and LAS AF software (Leica) at the Penn Veterinary Medicine Imaging Facility, University of Pennsylvania.
Lamellipodia and actin tail assays.
For lamellipodia assays, human podocytes were transiently transfected with CD16/7-nephrinCD plasmid or mutants and actin-GFP. Cells were activated as previously described (4). MEFs stably expressing CD16/7-nephrinCD plasmid or mutants were stained with phalloidin Alexa Fluor 488 (Invitrogen) after clustering. Images of 20 cells per group were taken using an Olympus IX70 microscope with a ×60 oil immersion objective lens and MetaMorph software. Transfected podocytes with protrusions containing actin cytoskeletal architecture typical of lamellipodia on more than 30% of their circumferences were counted as positive for with lamellipodia. By this definition, the fraction of transfected cells exhibiting lamellipodia was ascertained. When evaluating lamellipodia formation of MEFs, the number of lamellipodia per cell was counted for at least 20 MEFs per group. The number of cells with actin tails was determined as described previously (4, 19).
PAN nephrosis.
Female Sprague-Dawley rats weighing 200–250 g were injected intraperitoneally with PAN (Sigma-Aldrich) at a concentration of 15 mg/100 g body weight or with PBS (vehicle). Rats were sacrificed 72 hours after injection, and glomeruli were isolated using graded sieving as described previously (17, 58).
Crk1/2-floxed mice.
Crk1/2-floxed mice were previously described and maintained on a mixed C57BL/6 and 129SvEv background (50). Crk1/2fl/fl mice were crossed with Cre mice in which Cre recombinase was driven by the podocyte-specific podocin promoter NPHS2, as previously described (36, 37). For experiments, Crk1/2fl/flpodocin-CreTg/+ mice (homozygous for the floxed Crk1/2 allele and heterozygous for podocin-Cre) were used. Littermate Crk1/2fl/fl controls (homozygous for the floxed Crk1/2 allele but lacking podocin-Cre) were carried along. Urine albumin was determined by separating proteins using SDS-PAGE and staining with Coomassie dye.
NTS nephritis model.
8-week-old male Crk1/2fl/flpodocin-CreTg/+ mice and control Crk1/2fl/fl littermates were injected retro-orbitally with sheep anti-rat glomerular lysate antiserum or sheep IgG (control) at a concentration of 1.5 mg per mouse (approximately 25 g BW). The characteristics of whole NTS were described previously (42, 59). Urine was collected at 24 and 48 hours after injection. Urine albumin/creatinine ratios were obtained by enzyme-linked immunosorbent assay using a mouse Albuwell and corresponding creatinine kit (Exocell) and a Beckman Coulter DTX 880 plate reader. For transmission and scanning EM analysis, mice were perfusion fixed either 24 or 72 hours after injection as previously described (17).
Mouse kidney perfusion.
Perfusion of 8-week-old Crk1/2fl/flpodocin-CreTg/+ and control Crk1/2fl/fl littermate mouse kidneys with protamine sulfate was carried out as described previously (4, 17).
EM and slit diaphragm frequency analysis.
Preparation of mouse kidneys for scanning and transmission EM were performed by standard methods using pieces of diced kidney perfused with 4% paraformaldehyde. For scanning EM, 20 glomeruli of each sample were analyzed with a Philips XL 20 Scanning Electron Microscope at the Cell and Developmental Biology Core, University of Pennsylvania School of Medicine. For transmission EM, samples were examined with a JEOL 1010 electron microscope at the Electron Microscopy Resource Laboratory, University of Pennsylvania School of Medicine. At least 10 glomeruli per sample were analyzed, and slit diaphragm frequency was assessed quantitatively by counting the number of junctions per micron of basement membrane.
Statistics.
Results are presented as mean ± SEM. To analyze the difference between 2 groups, 2-tailed Student t test was used. A P value less than 0.05 was considered statistically significant.
Study approval.
All animal studies were approved by the University Committee on the Use and Care of Animals Institutional Review Board at the University of Michigan Medical School and the Institutional Animal Care and Use Committee at the University of Pennsylvania. Human kidney biopsy material was collected after receipt of informed consent from the patients, and the protocol was approved by the Scientific-Ethical Committee of the Hospital of Milan (Ospedale S. Carlo Borrome, Milan, Italy). Control kidneys were from the unaffected pole of tumor nephrectomies.
Supplementary Material
Acknowledgments
This work was supported by grants to L.B. Holzman from the NIDDK (DK080751) and the Department of Veterans Affairs, and to B. George from the Deutsche Forschungsgemeinschaft (GE 2158/1-1). This work was also supported by a Pennsylvania Department of Health Cure Formulary grant to T. Curran (SAP#410047628) and a grant to D.J. Salant (DK30932).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(2):674–692. doi:10.1172/JCI60070.
References
- 1.Johnstone DB, Holzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol. 2006;2(5):271–282. doi: 10.1038/ncpneph0180. [DOI] [PubMed] [Google Scholar]
- 2.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17(9):428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Jones N, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440(7085):818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 4.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116(5):1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan JM, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24(3):251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 6.Winn MP, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308(5729):1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300(5623):1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 8.Kestilä M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 9.Brown EJ, et al. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat Genet. 2010;42(1):72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak MR. The genetic basis of FSGS and steroid-resistant nephrosis. Semin Nephrol. 2003;23(2):141–146. doi: 10.1053/snep.2003.50014. [DOI] [PubMed] [Google Scholar]
- 11.Ruotsalainen V, et al. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96(14):7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Philippe A, et al. Nephrin mutations can cause childhood-onset steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2008;19(10):1871–1878. doi: 10.1681/ASN.2008010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem. 2003;278(21):19266–19271. doi: 10.1074/jbc.M301279200. [DOI] [PubMed] [Google Scholar]
- 14.Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J Am Soc Nephrol. 2003;14(4):918–926. doi: 10.1097/01.ASN.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- 15.Verma R, et al. Fyn binds to and phosphorylates the kidney slit diaphragm component Nephrin. J Biol Chem. 2003;278(23):20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 16.Zhu J, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 17.Garg P, et al. Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem. 2010;285(29):22676–22688. doi: 10.1074/jbc.M110.122929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harita Y, et al. Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-{gamma}1. J Biol Chem. 2009;284(13):8951–8962. doi: 10.1074/jbc.M806851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27(24):8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birge RB, Kalodimos C, Inagaki F, Tanaka S. Crk and CrkL adaptor proteins: networks for physiological and pathological signaling. Cell Commun Signal. 2009;7:13. doi: 10.1186/1478-811X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birge RB, et al. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13(8):4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271(23):13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 23.Tsygankov AY, Teckchandani AM, Feshchenko EA, Swaminathan G. Beyond the RING: CBL proteins as multivalent adapters. Oncogene. 2001;20(44):6382–6402. doi: 10.1038/sj.onc.1204781. [DOI] [PubMed] [Google Scholar]
- 24.Sieg DJ, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 25.Xing Z, Chen HC, Nowlen JK, Taylor SJ, Shalloway D, Guan JL. Direct interaction of v-Src with the focal adhesion kinase mediated by the Src SH2 domain. Mol Biol Cell. 1994;5(4):413–421. doi: 10.1091/mbc.5.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol Cell Biol. 2001;21(22):7641–7652. doi: 10.1128/MCB.21.22.7641-7652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, et al. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci U S A. 1994;91(8):3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda M, et al. Interaction between the amino-terminal SH3 domain of CRK and its natural target proteins. J Biol Chem. 1996;271(24):14468–14472. doi: 10.1074/jbc.271.24.14468. [DOI] [PubMed] [Google Scholar]
- 29.Shin NY, Dise RS, Schneider-Mergener J, Ritchie MD, Kilkenny DM, Hanks SK. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J Biol Chem. 2004;279(37):38331–38337. doi: 10.1074/jbc.M404675200. [DOI] [PubMed] [Google Scholar]
- 30.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol. 2004;15(12):3006–3015. doi: 10.1097/01.ASN.0000146689.88078.80. [DOI] [PubMed] [Google Scholar]
- 32.Altun-Gultekin ZF, et al. Activation of Rho-dependent cell spreading and focal adhesion biogenesis by the v-Crk adaptor protein. Mol Cell Biol. 1998;18(5):3044–3058. doi: 10.1128/mcb.18.5.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antoku S, Saksela K, Rivera GM, Mayer BJ. A crucial role in cell spreading for the interaction of Abl PxxP motifs with Crk and Nck adaptors. J Cell Sci. 2008;121(pt 18):3071–3082. doi: 10.1242/jcs.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mielenz D, Hapke S, Poschl E, von Der Mark H, von Der Mark K. The integrin alpha 7 cytoplasmic domain regulates cell migration, lamellipodia formation, and p130CAS/Crk coupling. J Biol Chem. 2001;276(16):13417–13426. doi: 10.1074/jbc.M011481200. [DOI] [PubMed] [Google Scholar]
- 35.Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56(4):1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- 36.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Two gene fragments that direct podocyte-specific expression in transgenic mice. J Am Soc Nephrol. 2002;13(6):1561–1567. doi: 10.1097/01.ASN.0000015614.68893.0B. [DOI] [PubMed] [Google Scholar]
- 37.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB. Podocyte-specific expression of cre recombinase in transgenic mice. Genesis. 2003;35(1):39–42. doi: 10.1002/gene.10164. [DOI] [PubMed] [Google Scholar]
- 38.Clement LC, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17(1):117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin F, Salant DJ, Meyerson H, Emancipator S, Morgan BP, Medof ME. Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic serum nephritis. . J Immunol. 2004;172(4):2636–2642. doi: 10.4049/jimmunol.172.4.2636. [DOI] [PubMed] [Google Scholar]
- 40.Lin F, Emancipator SN, Salant DJ, Medof ME. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab Invest. 2002;82(5):563–569. doi: 10.1038/labinvest.3780451. [DOI] [PubMed] [Google Scholar]
- 41.Quigg RJ, et al. Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J Immunol. 1998;160(9):4553–4560. [PubMed] [Google Scholar]
- 42.Hebert MJ, et al. Acute nephrotoxic serum nephritis in complement knockout mice: relative roles of the classical and alternate pathways in neutrophil recruitment and proteinuria. Nephrol Dial Transplant. 1998;13(11):2799–2803. doi: 10.1093/ndt/13.11.2799. [DOI] [PubMed] [Google Scholar]
- 43.Yanagita M, et al. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest. 2002;110(2):239–246. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun X, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77(3):304–313. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donaldson JC, Dempsey PJ, Reddy S, Bouton AH, Coffey RJ, Hanks SK. Crk-associated substrate p130(Cas) interacts with nephrocystin and both proteins localize to cell-cell contacts of polarized epithelial cells. Exp Cell Res. 2000;256(1):168–178. doi: 10.1006/excr.2000.4822. [DOI] [PubMed] [Google Scholar]
- 46.Siu MK, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of beta 1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144(5):2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 47.Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nat Genet. 2001;27(3):293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- 48.Park TJ, Boyd K, Curran T. Cardiovascular and craniofacial defects in Crk-null mice. Mol Cell Biol. 2006;26(16):6272–6282. doi: 10.1128/MCB.00472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallock PT, Xu CF, Park TJ, Neubert TA, Curran T, Burden SJ. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010;24(21):2451–2461. doi: 10.1101/gad.1977710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park TJ, Curran T. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci. 2008;28(50):13551–13562. doi: 10.1523/JNEUROSCI.4323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189(4200):390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- 52.O’Meara YM, Natori Y, Minto AW, Goldstein DJ, Manning EC, Salant DJ. Nephrotoxic antiserum identifies a beta 1-integrin on rat glomerular epithelial cells. Am J Physiol. 1992;262(6 pt 2):F1083–F1091. doi: 10.1152/ajprenal.1992.262.6.F1083. [DOI] [PubMed] [Google Scholar]
- 53.Chugh S, et al. Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int. 2001;59(2):601–613. doi: 10.1046/j.1523-1755.2001.059002601.x. [DOI] [PubMed] [Google Scholar]
- 54.Ma H, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. . J Am Soc Nephrol. 2010;21(7):1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94(5):625–634. doi: 10.1016/S0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 56.Saleem MA, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 57.Arif E, et al. Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol Cell Biol. 2011;31(10):2134–2150. doi: 10.1128/MCB.05051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and alpha-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci U S A. 2005;102(28):9814–9819. doi: 10.1073/pnas.0504166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Topham PS, et al. Lack of chemokine receptor CCR1 enhances Th1 responses and glomerular injury during nephrotoxic nephritis. J Clin Invest. 1999;104(11):1549–1557. doi: 10.1172/JCI7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.