Abstract
Purpose
Malignant pleural mesothelioma (MPM) is a highly aggressive tumor. Alternative treatment strategies such as oncolytic viral therapy may offer promising treatment options in the future. In this study, the oncolytic efficacy and induction of tumor remission by a genetically-engineered Newcastle disease virus (NDV(F3aa)-GFP) in MPM is tested and monitored by bioluminescent tumor imaging.
Experimental Design
The efficacy of NDV(F3aa)-GFP was tested against several mesothelioma cell lines in vitro. Firefly luciferase transduced MSTO-211H* orthotopic pleural mesothelioma tumor-bearing animals were treated with either single or multiple doses of NDV(F3aa)-GFP at different time points (days 1 and 10) after tumor implantation. Tumor burden was assessed by bioluminescence imaging.
Results
Mesothelioma cell lines exhibited dose-dependent susceptibility to NDV lysis in the following order of sensitivity: MSTO-211H>MSTO-211H*>H-2452>VAMT>JMN. In vivo studies with MSTO-211H* cells showed complete response to viral therapy in 65% of the animals within 14 days after treatment initiation. Long term survival in all of these animals was > 50 days after tumor installation (control animals <23 days). Multiple compared with single treatment showed a significantly better response (p=0.005).
Conclusions
NDV appears to be an efficient viral oncolytic agent in therapy of MPM in an orthotopic pleural mesothelioma tumor model.
Keywords: Newcastle disease virus, mesothelioma, oncolytic viral therapy
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive tumor that arises from multipotent cells of the pleura. These tumors are related to asbestos exposure, which leads to fiber deposition deep in the lung. Inflammation, free radical production, and direct DNA damage are recognized as the pathogenic features of asbestos exposure (1–3). Tumor formation may also be related to prior simian virus 40 (SV40) infection and possibly to a genetic predisposition.(4–5) A loss of tumor-suppressor gene activity has been shown to cause development of MPM (6).
Currently, this tumor causes about 20,000 deaths a year in the United States. However, due to a latency period of approximately 40 years, it is estimated that the annual incidence will continue to increase through the next decade (7). Unfortunately, this tumor is usually detected at an unresectable stage of disease (8–9). Due to the very limited effect of chemotherapy and radiation therapy, this disease is almost uniformly fatal. Average survival time after diagnosis varies between 10 and 16 months.(4) Alternative therapies such as immunotherapy, gene therapy (including oncolytic viral therapy), and photodynamic therapy are currently under clinical investigation but have no accepted clinical role (10–14).
Oncolytic viral therapy uses the natural life cycle of viruses to infect and lyse cancer cells (15). Such oncolytic viruses can also be used as vectors for local delivery of anticancer agents such as cytokines.(15) Kelly et al. recently reported encouraging results in an orthotopic mesothelioma animal model treated with vaccinia virus (16–17). In the current study, the oncolytic efficacy of Newcastle disease virus (NDV) on MPM is tested in vitro and in vivo using an orthotopic pleural mesothelioma tumor model. NDV has already demonstrated tumor cell infection efficacy in several cancer cell lines (18–23).
NDV contains a single-stranded, negative-sense, nonsegmented RNA genome and belongs to the genus Avulavirus in the family Paramyxoviridae (24). The genomic RNA is 15,186 nucleotides in length and contains six genes that encode at least seven proteins (25–26). The tumor selectivity of NDV has been attributed to the defective activation of type I interferon (IFN) in tumor cells. Additionally, recent studies showed altered response to IFN-β treatment in tumor cells (27).
The aim of the present study was to determine the oncolytic efficacy of NDV against MPM in vitro and in vivo. For this purpose, we established an orthotopic pleural mesothelioma tumor model, which enabled long-term follow-up of NDV treatment in mice using bioluminescence imaging.
Molecular imaging enables the visualization, characterization, and quantification of biologic processes at the cellular and subcellular levels within living subjects (28). Bioluminescence imaging utilizes the light produced by an enzymatic reaction of a luciferase enzyme with its substrate. Firefly (Photinus pyralis) luciferase (FLuc) is the most frequently used enzyme for molecular imaging. This enzyme oxidizes its substrate, luciferin, in a reaction that requires oxygen and adenosine triphosphate (ATP), emitting light with a broad emission spectrum and a peak at 560 nm (29). In the present study, we used a mesothelioma cell line that was stably transduced with the gene for FLuc to image mesothelioma tumors in vivo, and to investigate the efficacy of the oncolytic NDV virus in therapy of MPM.
Materials and Methods
Cell lines
We studied 7 human malignant mesothelioma cancer cell lines of various histologic subtypes, including epithelioid (H-2452, HMESO), sarcomatoid (H-2052, H-2373, and VAMT), and biphasic (MSTO-211H and JMN). The MSTO-211H cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). The JMN and VAMT cell lines were a kind gift from F. Sirotnik (Memorial Sloan-Kettering Cancer Center, New York, NY). Cell lines H-2052, H-2452, and H-2373 were a kind donation from H.I. Pass (Karmanos Cancer Institute, Wayne State University, Detroit, MI). HMESO cell lines were obtained from the National Cancer Institute (NCI, Bethesda, MD). All cells were grown in appropriate media and were maintained at 37°C in a humidified incubator supplied with 5% CO2.
MSTO-211H transduction
Transduction was done using the vector SFG-tdRFP-cmvFLucSFG-tdRFP-cmvFLuc, in which tandem repeat red fluorescent protein (tdRFP) (30) and firefly luciferase (FLuc)-encoding cDNAs were placed under constitutive promoters, LTR and CMV (31). RFP-positive cells were sorted by FACS.
Virus cloning and rescue
The fusogenic NDV mutant viruses with modified F cleavage site (NDV(F3aa)) were previously described.(32) To generate NDV(F3aa) virus expressing green fluorescent protein (GFP), a DNA fragment encoding GFP flanked by the appropriate NDV-specific RNA transcriptional signals was inserted into the XbaI site created between the P and M genes of pT7NDV/F3aa. Viruses were rescued from cDNA using methods described previously (33) and sequenced by reverse transcription polymerase chain reaction (RT-PCR) for insert fidelity.
Cytotoxicity assay
Cells were plated at 4e4 per well in 12-well plates in 1 mL of appropriate media per well. After incubation for 6 hours, cells were infected with NDV(F3aa)-GFP at multiplicities of infection (MOI) of 1.00, 0.10, 0.01, and 0 (control wells). Viral cytotoxicity was measured every other day for 7 days. Cells were washed with phosphate buffered saline (PBS) and lysed in 1.35% Triton X (200 μL per well; Sigma-Aldrich, St. Louis, MO) to release intracellular lactate dehydrogenase, which was quantified utilizing a CytoTox 96 kit (Promega, Madison, WI) and a spectrophotometer (EL321e; Bio-Tek Instruments, Winooski, VT) at 490 nm. Results are expressed as the percentage of surviving cells. This percentage was determined by comparing the measured lactate dehydrogenase of each infected sample with that in uninfected, control cells. All samples were analyzed in triplicate.
GFP microscopy
The status of viral infection in cell culture was monitored by GFP microscopy (Nikon Eclipse TE 2000, Melville, NY) every 12 hours during the first 48 hours of virus infection, and every 24 hours afterward.
Establishment of an animal model of MPM
Athymic female mice were purchased from the National Cancer Institute (Bethesda, MD) and were provided with food and water ad libitum. All animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at MSKCC. Anesthesia was induced with a mixture of isoflurane (2 L/minute) and oxygen (4 L/minute) in an induction chamber and was maintained with a nasal cone. Mice were placed in the left lateral position. The right chest was prepared with 10% povidone–iodine solution. A 3–5-mm incision was made over the fourth to fifth intercostal space. Sharp dissection was carried out, exposing but not breaching the parietal pleura. The underlying lung was thereby easily visualized through the thin membrane. Slowly, a transduced MSTO-211H malignant mesothelioma cellular suspension (1e7 cells in 100 μL PBS) was injected into the pleura with a 27-gauge needle. After injection, the skin was closed with surgical staples. Recovery was observed for 15 minutes before mice were returned to their cages.
Treatment of MPM
Intrapleural treatment with virus was performed in a fashion similar to the technique previously described for cancer cell injection. Viral treatment was administered intrapleurally at a dose of 1e7 plaque-forming units (PFU) suspended in 100 μL PBS. After injection, animals were gently rotated from side to side to help distribute the virus throughout the pleural cavity.
The following study groups were established (each group originally consisting of 5 animals):
Single treatment group:
Group S1 (T1S): single viral dose injection 1 day after tumor implantation.
Group S10 (T10S): single viral dose injection 10 days after tumor implantation.
Multiple treatment group:
Group M1 (T1M): 4 viral dose injections every other day starting 1 day after tumor implantation.
Group M10 (T10M): 4 viral dose injections every other day starting 10 days after tumor implantation.
The control group consisted of 10 mice who received an intrapleural injection of 100 μL PBS either 1 day (5 animals) or 10 days (5 animals) after tumor implantation. Animals were regularly assessed for weight loss and tachypnea throughout the experimental period. Tumor burden was assessed with bioluminescent imaging every other day for the first 20 days after tumor instillation and every 5th day thereafter. Animals suffering from end-stage tumor burden were sacrificed by CO2 narcosis.
In vivo bioluminescent imaging
The IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA) was used for bioluminescence image acquisition and analysis. Firefly D-luciferin potassium salt was purchased from Xenogen (Caliper Life Sciences), diluted to 30-mg/mL stock in PBS, and filtered through a 0.22-μm filter before use. After initial anesthesia with a mixture of isoflurane (2 L/minute) and oxygen (4 L/minute) in an induction chamber mice were injected with 100 μL of the D-luciferin solution (150 mg/kg body weight) intraperitoneally. Ten minutes after injection animals were again anesthetized in an induction chamber and placed in the bioluminescence chamber in a standardized way. Images were acquired for 1 second under general anesthesia maintained over a nasal cone. Each treatment group of 5 mice was placed in the specimen chamber mounted with the charge-coupled device (CCD) camera cooled to −120 °C, with a field of view (FOV) set at 25 cm above the sample shelf. Photon emission transmitted from cell samples and mice was measured in prone and supine position. The gray scale photographic images and bioluminescence color images were superimposed using LIVINGIMAGE V. 2.11 software overlay (Caliper Life Sciences) and IGOR image analysis software (V. 4.02 A, WaveMetrics, Lake Oswego, OR). A region of interest (ROI) was manually selected over the signal intensity. Values were expressed in photons/second/cm2/steradian (p/s/cm2/sr) and represent the mean values from the prone and supine positions of each animal.
Histologic work-up
From all animals representative tissue samples were taken before sacrifice. In tumor bearing animals tumor samples from the chest wall were harvested. In healed animals at the final sacrifice several representative tissue samples from several locations within the chest wall were harvested. Tissue samples were frozen in Tissue Tek embedding medium (Sakura Finetek, Torrance, CA) and sectioned by cryotome for histologic examination. Slides were fixed with paraformaldehyde and stained with hematoxylin and eosin (H&E).
Statistical analysis
In the animal experiments, tumor signal was represented graphically as the mean value of back and front imaging at each time point for each treatment group. Each animal’s time-tumor signal curve was represented using the area under the curve (AUC), which is interpreted as the total tumor burden of the animal. A logarithmic transformation to normalize the AUC was followed by an analysis of variance for group comparisons with an adjustment for multiple comparisons using resampling. All significance testing was done at the p <0.05 level, protecting the family-wise error rate.
Results
Recombinant NDV exhibits strong oncolytic activity against mesothelioma cell lines
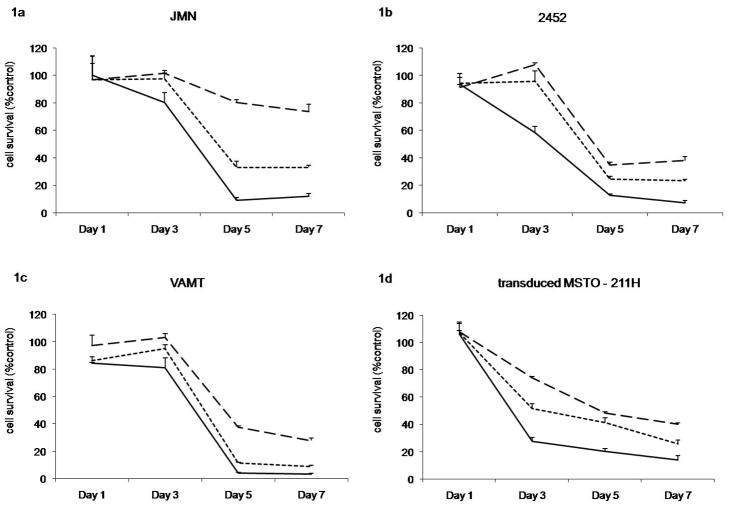
We employed several existing human MPM cell lines to assess their sensitivity to NDV oncolysis. Several cell lines demonstrated significant sensitivity to NDV(F3aa)-GFP. JMN (Figure 1a), H-2452 (Figure 1b), MSTO-211H (not shown), and VAMT (Figure 1c) showed significant cell kill at MOIs of 0.1 and 1 by day 7 after infection (67/88%; 77/93%; 90/93%; 91/97%, respectively). Significant cytotoxicity was even produced with MOI as low as 0.01 by day 7 after infection (JMN 27% cell kill; H-2452 64%, MSTO-211H 80%, and VAMT 73%). The transduced MSTO-211H* cell line showed comparable results to MSTO-211H (40%/75%/87% with MOI 0.01/0.1/1, respectively, by day 7; Figure 1d).
Figure 1. Cytotoxicity of NDV(F3aa)GFP against mesothelioma cell lines in vitro.
LDH assays of mesothelioma cell lines of (a) JMN, (b) H-2452, (c) VAMT, and transduced (d) MSTO-211H* at different MOIs (0.01=dashed line, 0.1=dotted line, 1=solid line) at day 1, 3, 5, 7. Data are expressed as the ratio of surviving cells determined by comparing the LDH-concentration of infected sample relative to control untreated cells.
Recombinant NDV enhances survival in a murine orthotopic mesothelioma model
We proceeded to test the oncolytic efficacy of genetically-engineered NDV in an orthotopic murine mesothelioma model. Intrapleural treatment with NDV(F3aa)-GFP proved to be a safe procedure, even in animals that received several treatments every other day. Based on a standard animal care facility–defined dietary regimen, it was observed that all animals continued to exhibit normal activity, feeding, and grooming, and were able to maintain their body weights within the first 2 weeks after intrapleural virus injection. This was comparable to our previous experience with NDV, where intravenous, intraperitoneal, or intratumoral injection of the virus was demonstrated to be safe and did not result in significant change in weight or activity (34;35). Significant weight loss was observed only in animals with severe tumor burden. All animals with a weight loss >15% of their initial weight were sacrificed within 5 days due to overwhelming tumor burden.
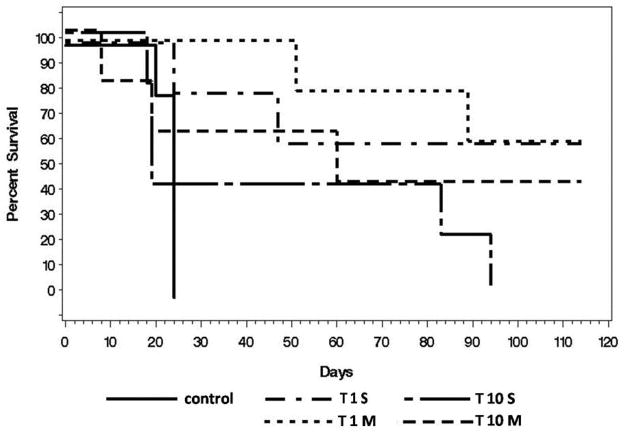
All control animals that received only PBS instead of virus were sacrificed within 24 days after tumor injection due to disease burden. 60% of animals with either single or multiple early treatments (T1S, T1M) survived the whole follow-up period of 4 months. In the T1 treatment groups there was only a 60% survival rate in animals treated once (T1S) compared to 100% in animals receiving multiple treatments (T1M) 50 days after tumor injection. Even in the late treatment groups (T10S, T10M) 40% of animals survived longer than 80 days after tumor instillation. After 100 days survival rates of 0% for T10S vs. 40% for T10M have to be reported. Survival curves for all treatment groups are shown in Figure 2.
Figure 2. Overall survival of animals stratified by treatment groups.
All control animals (control; solid line) were sacrificed due to tumor burden within 24 days , while the majority of treated animals exhibited survival to at least 90 days after tumor instillation. Animals receiving either single (T1S) or multiple (T1M) early treatment (Day 1) showed a 60% survival after 115 days. Animals out of the late treatment group (Day 10) showed 40% survival receiving multiple treatments (T10M) or had to be sacrificed within 95 days receiving single treatment (T10S).
Recombinant NDV decreases tumor burden in treated animals
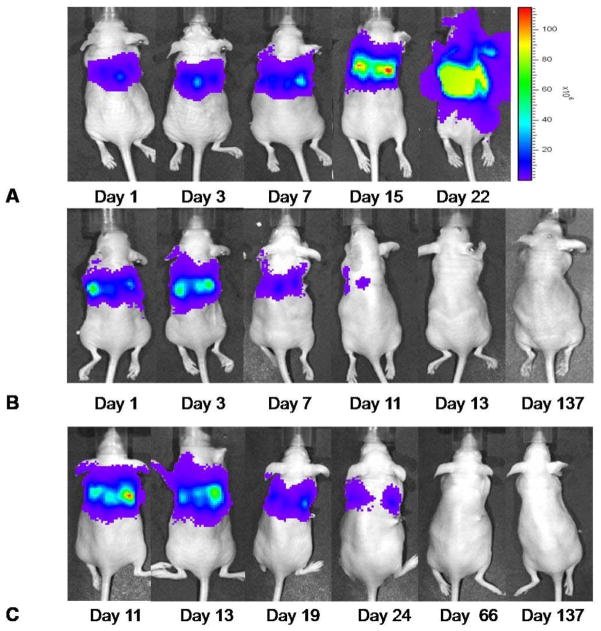
To assess for tumor burden, we employed bioluminescent imaging of the transduced MSTO-211H* cells with luciferase substrate. Animals were injected intraperitoneally with luciferin substrate and the pleural signal was visualized using the IVIS Imaging System, as outlined in the Materials and Methods section. In vivo bioluminescence images of a representative animal from the control group at several follow-up time points are shown in Figure 3A. Images of a representative animal from treatment group TS1 at several follow-up time points are shown in Figure 3B and for an animal from treatment group T10M in Figure 3C.
Figure 3. Representative bioluminescence images demonstrate treatment effect of NDV(F3aa)GFP over time.
Images were received 10 min after intraabdominal Luciferin injection with an acquisition time of 1sec. The control animals demonstrate an increasing bioluminescent signal after tumor instillation and had to be sacrificed due to tumor burden at the latest by day 24. Bioluminescent images of a representative animal of the control group are shown in Figure 3A. Animals receiving a single treatment on day 1 showed significant decrease of tumor signal within ten days after treatment. In Figure 3B a representative animal shows complete tumor signal elimination until day 13 after treatment start. Comparable results were investigated in animals receiving multiple treatments starting 10 days after tumor instillation (Figure 3C).
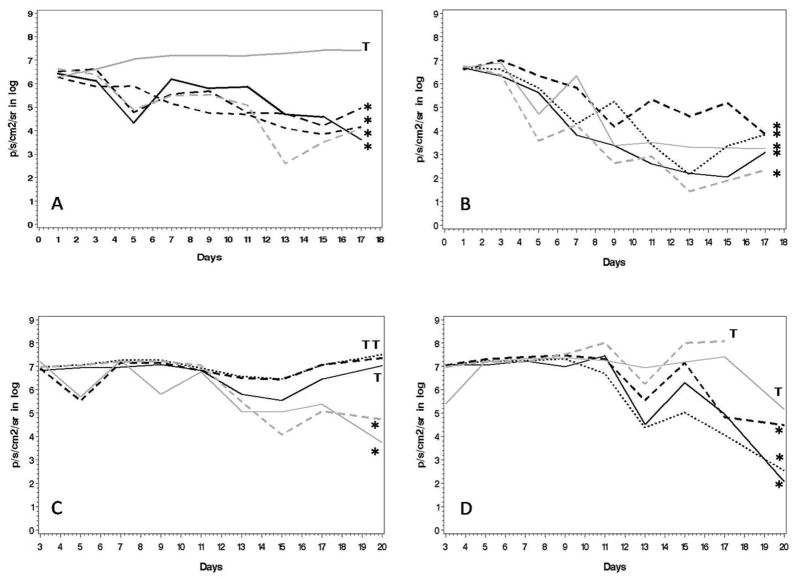
Progression of the bioluminescence signal during follow-up of each treatment group is shown in Figure 4. Each graph represents the mean bioluminescent values of a single animal calculated using the mean value of prone and supine position. The threshold of signal intensity to provide a visible image by bioluminescence was 7e4 p/s/cm2/sr.
Figure 4. Values of thoracic bioluminescence signal decrease in all treatment groups.
Each graph represents a single animal. Graphs labeled with * represent animals with no bioluminescent sign of tumor 14 days after treatment initiation. Graphs labeled with T represent animals with persistent tumor burden 14 days after treatment start. Bioluminescent values (p/s/cm2/sr) were calculated as following: (prone + spine value)/2. In the upper row animals are shown who received treatment starting one day after tumor injection. In the lower row animals are presented with treatment start on day 10 after tumor injection. A and C show animals who received single treatment, B and D represent animals with multiple treatments. The mean bioluminescent signal values decreased significantly more in animals receiving multiple treatments (day 1: p <0.01; day 10: p <0.01).
All control animals showed a continuous increase in tumor signal until sacrificed. For the rest of the animals, the following results were observed:
-
Group TS1 (single treatment day 1 after cancer cells injection, Figure 4a):
Four out of 5 animals lost the bioluminescent tumor signal during the first 2 weeks after treatment and showed no bioluminescent signal 13 days after treatment. Three out of 5 animals survived the entire 4-month follow-up period without showing a tumor signal with bioluminescence. No histologic signs of tumor were found in these animals at the final follow-up time point, when these animals were sacrificed.
-
Group TM1 (several treatments every other day, Figure 4b):
The bioluminescent tumor signal was eradicated in all animals within 12 days and 80% of these animals survived 90 days of follow-up. All animals surviving the complete follow-up period showed no histological signs of tumor when sacrificed.
-
Group TS10 (single injection day 10, Figure 4c):
Two nonresponders were observed. One animal showed significant tumor signal decrease after 10 days of treatment (reduction of signal by >e2). The other 2 animals showed no tumor signal 10 days after treatment. Sixty percent of animals survived 60 days after tumor instillation.
-
Group M10 (multiple treatments day 10, Figure 4d):
Two animals showed a significant decrease of tumor signal within 10 days (minus log 0.5); 1 of these animals progressed to complete tumor signal disappearance 30 days after the start of treatment. Two other animals showed tumor signal regression within the first 3 weeks after the start of treatment. One animal showed no decrease in the tumor signal. Two animals (40%; both showed complete tumor signal extinction 30 days after treatment) survived the whole follow-up period and did not show any histological signs of tumor when they were finally sacrificed after 137 days of tumor follow-up.
Animals receiving multiple treatments starting at days 1 and 10 (groups M1 and M10) showed a significantly higher decrease of tumor signal within the first 10 days after the start of treatment (day 1: p <0.01; day 10: p <0.01) compared with animals with only single treatment (groups S1 and S10). Absolute log-difference decrease of tumor signal within the first 10 days after viral treatment initiation was 0.3 for single versus multiple treated animals in the early treatment groups (T1S vs. T1M) and 0.32 for the late treatment groups (T10S vs T10M).
Overall, multiple-dose treatment with NDV(F3aa)-GFP demonstrated significant survival benefit when compared with single treatment (p = 0.005). Interestingly, comparing the different treatment groups according to treatment start time showed no significant difference in survival (1 vs. 10, p = 0.84). Nevertheless survival was significantly correlated to tumor burden detectable by bioluminescence. Animals with tumor signal either at day 5, 10, 20 or 30 had a significant poorer survival compared to “cured” animals (all p<0.005).
Discussion
MPM is a highly malignant cancer resulting in poor long-term survival. There is great hope for novel therapeutic options due to the limited effectiveness of currently established chemotherapeutics and radiotherapy (36–37). Gene therapy strategies have been investigated in several studies with varying benefit (38). Several replication-competent oncolytic viruses have already been designed and tested for targeted cancer therapy. Examples include herpes simplex virus, adenovirus, vesicular stomatitis virus, myxoma virus, lentivirus, reovirus, and vaccinia virus (39–41).
In particular, herpes simplex and vaccinia have already shown promising efficacy in the detection and treatment of MPM (16–17, 42). Newcastle disease virus is another promising agent that recently re-emerged in the field of oncolytic virotherapy. The oncolytic effects of NDV were first described in the mid 1950s by Sinkovics and Flanagan (43–44). Numerous characteristics make NDV an attractive oncolytic vector and several clinical trials recently demonstrated its safety and therapeutic efficacy (45–47). Several cellular mechanisms have been proposed for NDV anti-neoplastic properties: First of all, oncolytic viral strains may kill tumor cells directly by inducing apoptosis (48–49). Secondly, replication of NDV occurs in the cytoplasm and is associated with the production of single- and double-stranded viral RNA. NDV infection of tumor cells introduces danger signals that can be recognized by RIG-I and PKR in the cytoplasm and by Toll-like receptors in endosomes, leading to induction of an antiviral state and apoptosis (50–51). Thirdly, application of NDV may stimulate the host to produce cytokines such as interferons (IFNs) or tumor necrosis factor (TNF), which in turn leads to the activation of natural killer (NK) cells, monocytes, macrophages, and sensitized T cells, which are supportive in tumor clearance (52–53).
Additionally, NDV was shown to be an effective vaccine vector capable of eliciting a potent immune response targeted to the encoded vaccine antigens (20). Infection with NDV induces a strong immune response within the tumor, helping the host immune system overcome tumor-induced immunologic barriers. The ability of oncolytic NDV to induce tumor-specific immune responses has been shown in clinical trials (54).
Using NDV as a vector for tumor gene therapy offers several advantages over other viral expression systems. First of all, it is an avian virus and the majority of humans have no pre-existing immunity to the virus. Second, it is an RNA virus replicating in the cytoplasm without a DNA stage, thus limiting the possibility for genetic recombination with host cell DNA (52).
In this study for the first time NDV (F3aa)-GFP was successfully used to treat MPM in an orthotopic mouse model. Viral efficacy was monitored with bioluminescence in a long term follow up setting for more than 4 months.
During in vitro testing, mesothelioma cell lines proved to be susceptible to NDV oncolysis and showed encouraging results even with low MOIs. Nevertheless differences were found in the grade of efficacy to viral treatment in different cell lines. Still the exact mechanism of susceptibility of cancer cells to NDV is not clearly understood and is part of intensive investigation. Schirrmacher and Fournier described that the induction of an antiviral state depends on the expression of IFN-stimulated factors such as OAS and PKR (55). In support of the latter, our previous studies demonstrated that type I IFN response plays a strong role in inhibiting NDV replication and spread (34;56).
On the other hand, Puhlmann et al. showed that H-Ras was essential for viral replication and the GTPase Rac1 for viral susceptibility (57). We speculate that the factors above may be responsible for the differential sensitivity to the virus, however further studies need to be done.
To confirm these findings in vivo, a firefly luciferase-transduced MSTO-211H cell line was incorporated into an orthotopic pleural mesothelioma tumor model and treated with locally administered NDV (F3aa)-GFP. Bioluminescence was used as long term follow up investigation tool to detect tumor progression in live animals. Additionally, bioluminescence imaging enabled indirect evaluation of the efficiency of tumor lysis in the pleural cavity. Consistent with the in vitro results, tumor signal decrease was noted starting with day 1 to 3 after viral injection and was obvious within the first 10 days after treatment. This suggested that the inoculated viruses continued to replicate in the tumor cells for several cycles. Interestingly, a higher oncolytic potency of the virus was detectable within the early compared with the late treatment groups, which did not reach statistical significance.
Survival rates of approximately 40% or higher could be achieved in all treatment groups after 80 days of tumor follow-up, compared with sacrifice of all control animals within 24 days. Earlier treatment resulted in higher survival, likely secondary to lower tumor burden during the earlier stages. These data may provide prognostic speculation regarding response to NDV treatment in more advanced stages of malignant mesothelioma. Animals surviving until day 137 after tumor injection showed no signs of tumor in their pleural cavity after final sacrifice. NDV proved to be safe even after multiple intrapleural virus applications. No signs of viral toxicity were observed, and all animals maintained normal food intake and normal activity.
Virotherapy with either single or multiple treatments showed significant response to oncolytic therapy with NDV(F3aa)-GFP. Nevertheless, overall survival was significantly better in the multiple-treatment groups. Similar results were found in previous studies with human neuroblastoma xenografts and melanoma cell lines (58–59). Multiple dosing likely allows the delivery of higher virus titers, which may improve viral diffusion to cells deeper within the tumor.
This is especially true in the case of Newcastle disease virus, which has been previously shown to be highly susceptible to the effects of mammalian type I interferon response (59).
In conclusion, NDV showed promising results as a cytotoxic agent against MPM. The virus produced no signs of toxicity and offered prolonged survival in animals. These findings prompt further investigation of NDV as a therapeutic agent against this highly malignant tumor.
References
- 1.Mossman BT, Kamp DW, Weitzman SA. Mechanisms of carcinogenesis and clinical features of asbestos-associated cancers. Cancer Invest. 1996;14:466–80. doi: 10.3109/07357909609018904. [DOI] [PubMed] [Google Scholar]
- 2.Sluis-Cremer GK. Asbestos disease at low exposure after long residence time in amphibole miners. Toxicol Ind Health. 1991;7:89–95. doi: 10.1177/074823379100700106. [DOI] [PubMed] [Google Scholar]
- 3.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–71. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zervos MD, Bizekis C, Pass HI. Malignant mesothelioma 2008. Curr Opin Pulm Med. 2008;14:303–9. doi: 10.1097/MCP.0b013e328302851d. [DOI] [PubMed] [Google Scholar]
- 5.Robinson BW, Musk AW, Lake RA. Malignant mesothelioma. Lancet. 2005;366:397–408. doi: 10.1016/S0140-6736(05)67025-0. [DOI] [PubMed] [Google Scholar]
- 6.Nowak AK, Lake RA, Kindler HL, Robinson BW. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82–96. doi: 10.1053/sonc.2002.30234. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379–87. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- 8.Stewart DJ, Martin-Ucar A, Pilling JE, Edwards JG, O'Byrne KJ, Waller DA. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg. 2004;78:245–52. doi: 10.1016/j.athoracsur.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg. 2004;128:138–46. doi: 10.1016/j.jtcvs.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Robinson C, Callow M, Stevenson S, Scott B, Robinson BW, Lake RA. Serologic responses in patients with malignant mesothelioma: evidence for both public and private specificities. Am J Respir Cell Mol Biol. 2000;22:550–6. doi: 10.1165/ajrcmb.22.5.3930. [DOI] [PubMed] [Google Scholar]
- 11.Castagneto B, Zai S, Mutti L, et al. Palliative and therapeutic activity of IL-2 immunotherapy in unresectable malignant pleural mesothelioma with pleural effusion: Results of a phase II study on 31 consecutive patients. Lung Cancer. 2001;31:303–10. doi: 10.1016/s0169-5002(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 12.Davidson JA, Musk AW, Wood BR, et al. Intralesional cytokine therapy in cancer: a pilot study of GM-CSF infusion in mesothelioma. J Immunother. 1998;21:389–98. doi: 10.1097/00002371-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Sterman DH, Treat J, Litzky LA, et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther. 1998;9:1083–92. doi: 10.1089/hum.1998.9.7-1083. [DOI] [PubMed] [Google Scholar]
- 14.Pass HI. Photodynamic therapy (PDT) and pleural mesothelioma. In: Robinson BWS, Chahinian AP, editors. Mesothelioma Martin Dunitz. Vol. 13. 2002. pp. 235–51. [Google Scholar]
- 15.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly KJ, Woo Y, Brader P, et al. Novel oncolytic agent GLV-1h68 is effective against malignant pleural mesothelioma. Hum Gene Ther. 2008;19:774–82. doi: 10.1089/hum.2008.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brader P, Kelly KJ, Chen N, et al. Imaging a Genetically Engineered Oncolytic Vaccinia Virus (GLV-1h99) Using a Human Norepinephrine Transporter Reporter Gene. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schirrmacher V, Haas C, Bonifer R, Ertel C. Virus potentiation of tumor vaccine T-cell stimulatory capacity requires cell surface binding but not infection. Clin Cancer Res. 1997;3:1135–48. [PubMed] [Google Scholar]
- 19.Fabian Z, Csatary CM, Szeberenyi J, Csatary LK. p53-independent endoplasmic reticulum stress-mediated cytotoxicity of a Newcastle disease virus strain in tumor cell lines. J Virol. 2007;81:2817–30. doi: 10.1128/JVI.02490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vigil A, Martinez O, Chua MA, Garcia-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16:1883–90. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vigil A, Park MS, Martinez O, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–92. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 22.Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009;58:61–9. doi: 10.1007/s00262-008-0526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiola C, Peeters B, Fournier P, Arnold A, Bucur M, Schirrmacher V. Tumor selective replication of Newcastle disease virus: association with defects of tumor cells in antiviral defence. Int J Cancer. 2006;119:328–38. doi: 10.1002/ijc.21821. [DOI] [PubMed] [Google Scholar]
- 24.Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147:1655–63. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamurthy S, Samal SK. Nucleotide sequences of the trailer, nucleocapsid protein gene and intergenic regions of Newcastle disease virus strain Beaudette C and completion of the entire genome sequence. J Gen Virol. 1998;79 ( Pt 10):2419–24. doi: 10.1099/0022-1317-79-10-2419. [DOI] [PubMed] [Google Scholar]
- 26.Steward M, Vipond IB, Millar NS, Emmerson PT. RNA editing in Newcastle disease virus. J Gen Virol. 1993;74 ( Pt 12):2539–47. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamurthy S, Takimoto T, Scroggs RA, Portner A. Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol. 2006 Jun;80(11):5145–55. doi: 10.1128/JVI.02618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasberg RG, Gelovani-Tjuvajev J. In vivo molecular-genetic imaging. J Cell Biochem Suppl. 2002;39:172–83. doi: 10.1002/jcb.10433. [DOI] [PubMed] [Google Scholar]
- 29.Luker GD, Luker KE. Optical imaging: current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- 30.Campbell RE, Tour O, Palmer AE, et al. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99:7877–82. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang Y, He W, Tulley S, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A. 2005;102:13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc Natl Acad Sci U S A. 2006;103:8203–8. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakaya T, Cros J, Park MS, et al. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001;75:11868–73. doi: 10.1128/JVI.75.23.11868-11873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigil A, Park MS, Martinez O, Chua MA, Xiao S, Cros JF, et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007 Sep 1;67(17):8285–92. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 35.Song KY, Wong J, Gonzalez L, Sheng G, Zamarin D, Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J Mol Med. 2010 Apr 15;88(6):589–96. doi: 10.1007/s00109-010-0605-6. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman AJ, Pass HI. Current concepts in malignant pleural mesothelioma. Expert Rev Anticancer Ther. 2008;8:293–303. doi: 10.1586/14737140.8.2.293. [DOI] [PubMed] [Google Scholar]
- 37.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 38.van der Most RG, Robinson BW, Nelson DJ. Gene therapy for malignant mesothelioma: beyond the infant years. Cancer Gene Ther. 2006;13:897–904. doi: 10.1038/sj.cgt.7700935. [DOI] [PubMed] [Google Scholar]
- 39.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–8. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu TC, Kirn D. Systemic efficacy with oncolytic virus therapeutics: clinical proof-of-concept and future directions. Cancer Res. 2007;67:429–32. doi: 10.1158/0008-5472.CAN-06-2871. [DOI] [PubMed] [Google Scholar]
- 42.Adusumilli PS, Stiles BM, Chan MK, et al. Imaging and therapy of malignant pleural mesothelioma using replication-competent herpes simplex viruses. J Gene Med. 2006;8:603–15. doi: 10.1002/jgm.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinkovics J. Enhancement of carcinostatic activity of Newcastle disease virus (NDV) associated with adaptation to suckling mouse brain. Bacteriol Proc. 1957a;96:M108. [Google Scholar]
- 44.Flanagan AD, Love R, Tesar W. Propagation of Newcastle disease virus in Ehrlich ascites cells in vitro and in vivo. Proc Soc Exp Biol Med. 1955;90:82–6. doi: 10.3181/00379727-90-21945. [DOI] [PubMed] [Google Scholar]
- 45.Lorence RM, Pecora AL, Major PP, et al. Overview of phase I studies of intravenous administration of PV701, an oncolytic virus. Curr Opin Mol Ther. 2003;5:618–24. [PubMed] [Google Scholar]
- 46.Karcher J, Dyckhoff G, Beckhove P, et al. Antitumor vaccination in patients with head and neck squamous cell carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004;64:8057–61. doi: 10.1158/0008-5472.CAN-04-1545. [DOI] [PubMed] [Google Scholar]
- 47.Steiner HH, Bonsanto MM, Beckhove P, et al. Antitumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22:4272–81. doi: 10.1200/JCO.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 48.Sinkovics JG, Horvath JC. Newcastle disease virus (NDV): brief history of its oncolytic strains. J Clin Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 49.Lam KM, Vasconcelos AC, Bickford AA. Apoptosis as a cause of death in chicken embryos inoculated with Newcastle disease virus. Microb Pathog. 1995;19:169–74. doi: 10.1006/mpat.1995.0055. [DOI] [PubMed] [Google Scholar]
- 50.Fournier P, Zeng J, Schirrmacher V. Two ways to induce innate immune responses in human PBMCs: paracrine stimulation of IFN-alpha responses by viral protein or dsRNA. Int J Oncol. 2003;23:673–80. [PubMed] [Google Scholar]
- 51.Zeng J, Fournier P, Schirrmacher V. Induction of interferon-alpha and tumor necrosis factor-related apoptosis-inducing ligand in human blood mononuclear cells by hemagglutinin-neuraminidase but not F protein of Newcastle disease virus. Virology. 2002;297:19–30. doi: 10.1006/viro.2002.1413. [DOI] [PubMed] [Google Scholar]
- 52.Janke M, Peeters B, de Leeuw O, et al. Recombinant Newcastle disease virus (NDV) with inserted gene coding for GM-CSF as a new vector for cancer immunogene therapy. Gene Ther. 2007;14:1639–49. doi: 10.1038/sj.gt.3303026. [DOI] [PubMed] [Google Scholar]
- 53.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 54.Batliwalla FM, Bateman BA, Serrano D, et al. A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med. 1998;4:783–94. [PMC free article] [PubMed] [Google Scholar]
- 55.Schirrmacher V, Fournier P. Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol. 2009;542:565–605. doi: 10.1007/978-1-59745-561-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamarin D, Martinez-Sobrido L, Kelly K, Mansour M, Sheng G, Vigil A, et al. Enhancement of Oncolytic Properties of Recombinant Newcastle Disease Virus Through Antagonism of Cellular Innate Immune Responses. Mol Ther. 2009 Feb 10; doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puhlmann J, Puehler F, Mumberg D, Boukamp P, Beier R. Rac1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene. 2010 Apr 15;29(15):2205–16. doi: 10.1038/onc.2009.507. [DOI] [PubMed] [Google Scholar]
- 58.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 59.Zamarin D, Martinez-Sobrido L, Kelly K, et al. Enhancement of Oncolytic Properties of Recombinant Newcastle Disease Virus Through Antagonism of Cellular Innate Immune Responses. Mol Ther. 2009 doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]