Abstract
Background
Recommendations for high risk human papillomavirus (HR-HPV) testing as an adjunct to cytology for cervical cancer screening differ by age group, because HR-HPV tests lack adequate specificity in women aged <30. Here, we assess age-group differences in HPV types and other risk factors for cervical intraepithelial neoplasia (CIN) grade 3 or worse (CIN3+) versus CIN0–2 in women from four colposcopy clinics.
Methods
Women ages 18–69 (n=1658) were enrolled and completed structured interviews to elicit data on behavioral risk factors prior to their examinations. HPV genotyping was performed on exfoliated cervical cell samples. We estimated relative risks (RR) for HPV types and cofactors for CIN3+, overall and stratified by age group.
Results
After 2 years of follow-up, we identified 178 CIN3+, 1305 CIN0–2, and 175 indeterminate outcomes. Non-vaccine HR-HPV types were only associated with CIN3+ among women ≥30 (RR=2.3, 95% CI 1.5–3.4; <30: RR=0.9). Among all HR-HPV positive women, adjusting for age, significant cofactors for CIN3+ included current smoking (RR=1.5), former smoking (RR=1.8), regular Pap screening (RR=0.7), current regular condom use (RR=0.5), and parity ≥5 (RR=1.6, p-trend for increasing parity=.07). However, the parity association differed by age group (≥30: RR=1.8, p-trend=.008; <30: RR=0.9, p-trend=.55).
Conclusions
Subgroup variation by age in the risk of CIN3+ points to the importance of the timing of exposures in relation to CIN3+ detection.
Impact
Future screening strategies need to consider natural history and secular trends in cofactor prevalence in the pursuit of appropriately sensitive and specific screening tools applied to appropriate age groups.
Keywords: Human Papillomavirus, Cervical Intraepithelial Neoplasia, Risk Factors, Early Detection of Cancer, Colposcopy
Introduction
Most high-risk human papillomavirus (HR-HPV) infections clear without treatment(1, 2) or are controlled immunologically and rendered undetectable(3) without clinical consequences. However, some infections persist, and a subset of persistent infections may progress to cervical intraepithelial neoplasia (CIN) or invasive cancer. Because neoplastic changes typically take years to occur, the prevalences of detectable HPV infections, high-grade CIN (e.g. CIN3), and invasive cervical cancer peak at different ages(4). HPV infection is most prevalent near the average age of sexual debut, when CIN3 and cancer are rare. CIN3 is most often detected by screening about a decade later, and invasive cancer is usually diagnosed in women who are 40 years or older. The different clinical implications of a positive HPV test over the life course have led to age differences in recommendations for application of HR-HPV testing in cervical cancer screening. Testing is currently recommended as an adjunct to cytology screening among women who are at least 30 years old; in younger women, it is only recommended for triage of atypical squamous cells of undetermined significance (ASCUS)(5). Among women in their twenties and younger, the specificity and positive predictive value of HR-HPV testing are too low for the test to be clinically useful(4, 6).
Consensus exists that CIN3 should be excised when detected at any age(7, 8). Although many CIN3 lesions might never lead to invasive cancer(9), no markers exist to distinguish those that will invade from those that will not. In practice, CIN2 is the threshold for treatment, even though this is an equivocal diagnosis and often signals active infection or lesions destined to regress(10). Excisional treatments may adversely impact future pregnancies(11, 12), a particular concern for younger patients who often have not completed childbearing. Thus, due to the risks of overtreatment, effectively distinguishing true cancer precursors from transient lesions is a priority, especially among young women. Presence of CIN3 or cervical cancer (CIN3+) in young women often signals an early age at HR-HPV infection(2). In addition, women who present with CIN3+ at young ages might be infected with more aggressively carcinogenic HPV types (e.g. HPV-16) or have more unfavorable HR-HPV cofactor profiles (i.e., more risk factors for progression to neoplasia from HR-HPV infection) than older women presenting with similar diagnoses. A greater understanding of age-group differences among women referred to a colposcopy clinic after abnormal cervical cancer screening could shed light on biological differences in earlier and later-onset cases of CIN3+, and thereby inform future efforts to implement risk stratification strategies aimed at reducing unnecessary referral and possible over-treatment of women without clinically important disease. Using data from women attending colposcopy clinics, we aimed to assess age group differences in (1) the overall distribution of cervical cancer risk factors and CIN grades; (2) the association between HPV types and CIN3+, and (3) cofactors for CIN3+ among HR-HPV positive women.
Materials and Methods
Enrollment and baseline study protocol
Women attending colposcopy clinics affiliated with urban public hospitals in Southeastern Michigan (3 clinics) and Atlanta, Georgia (1 clinic) between December 2000 and December 2004 were approached, consented, and enrolled in the study prior to their examinations. These clinics primarily serve urban women who rely on public health clinics for their medical and gynecologic care. Women who were less than 18 years old or older than 69 years old, who had a history of hysterectomy or HIV, or who were pregnant at the time of the visit, were ineligible for the study. Of women approached for the study, 47% were eligible; of these, 69% agreed to participate. At the enrollment visit, women participated in a structured interview with a study nurse to ascertain information on demographics, reproductive history, health behaviors, screening history, health history, and cancer in first-degree relatives(13). During a pelvic examination, ecto- and endocervical cells were collected using a CytoBroom (Cytyc, Marlborough, MA). A conventional Pap smear was prepared if clinically indicated, and the remaining cells were dislodged into PreservCyt collection medium (Cytyc), as previously described(14). During the colposcopy examinations, women underwent biopsy, endocervical curettage, conization, or loop electrosurgical excision procedure (LEEP) at the discretion of the treating physician.
Cervical Intraepithelial Neoplasia (CIN) classification
Tissue specimens were submitted to the respective hospital pathology laboratories and subsequently reviewed by study pathologists. A consistent algorithm was used for combining data on histology, cytology, and colposcopic impression. As would be done clinically, histology findings were followed for classification of those enrolled as having cancer, CIN3, CIN2, CIN1, or no CIN. Women with no pathology results and cytology results within normal limits or indicating benign cellular changes and no lesions at colposcopy were classified as having no CIN. Otherwise, if only cytology results were available, or if findings were conflicting such that further testing would be required clinically to resolve (e.g. biopsy result no CIN and high grade cytology), a classification of indeterminate was used; such cases were excluded from analyses.
Women were passively followed up using review of medical records for up to 2 years to ascertain disease that may have been missed at baseline. If a more severe diagnosis was identified on follow-up than was found at baseline, the patient was classified as having the more severe outcome.
HPV detection and typing
Details of HPV genotyping procedures were described previously(14). Briefly, a total nucleic acid extract for HPV genotyping was prepared from the PreservCyt cells. HPV detection and genotyping were conducted using the prototype Roche line blot assay (reagents provided as a gift from Roche Molecular Systems, Inc., Pleasanton, CA). Samples were screened for the HPV amplicon using gel electrophoresis and positive samples were hybridized to the strips. Hybridized samples that did not yield a positive reaction on the strip were sequenced to determine the HPV type. The assay detected the 14 HR-HPV types targeted by commercial screening tests (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68), 16 low-risk (LR) HPV types (6, 11, 40, 42, 44, 54, 55, 61, 62, 64, 71, 72, 74, 81, 83, 84), and 8 other types referred to here as possible HR types (26, 53, 67, 69, 70, 73, 82, and IS39). Sequencing detected the possible HR-HPV type 85 and LR-HPV types 32 87, 89, 90, 91, and 89CP6108. Samples negative for HPV and the endogenous positive control (beta-globin) were considered inadequate for evaluation and omitted (n=12).
Analytic Strategy
We calculated frequency distributions of demographic, reproductive and sexual history and health behavior characteristics of all women, and evaluated age differences (<30, ≥30) in the distributions of these characteristics using chi-square or Fisher’s exact tests as appropriate. Risk factors were categorized consistently with previous literature when feasible, or using distribution-based cutpoints (e.g. quintiles). We also examined the distributions of age and years since sexual debut (i.e. age at first study visit minus age at sexual debut) among HR-HPV positive women, and tested differences in these distributions by HPV-16 status among cases of CIN 3 and cancer (CIN3+) using the Wilcoxon test.
We calculated overall and age-stratified (<30, ≥30) risk ratios (RR) for the dichotomous outcome CIN3+ vs. ≤CIN2 (i.e. no CIN, CIN1, and CIN2 combined; indeterminate diagnoses excluded) using log-binomial models(15). We regarded age-stratified models as potentially informative based on a priori clinical grounds, and formally tested for effect measure modification using a likelihood ratio test for an age-group interaction term. We calculated crude (non-hierarchical) RRs for HPV16, HPV18, any vaccine HR-HPV type (16 or 18), non-vaccine HR-HPV types, any clinical HR-HPV type, and by species (i.e. alpha-9 and alpha-7 types(16)). We also calculated RRs for multiple genotypes among HPV-positive women, and recalculated all other RRs after excluding women with multiple HPV types.
Among HR-HPV positive women, we identified demographic, reproductive history, sexual history and health behavior-related cofactors for CIN3+ through multivariable models using backward selection (p<.1 in either age stratum to stay).
We repeated all regression analyses with age stratified at the median of HR-HPV positive women (i.e. age <25 vs. ≥25 years). We also repeated analyses excluding CIN2 cases (i.e. comparing CIN3+ to ≤CIN1). All analyses were performed using SAS v. 9.2 (Statistical Analysis Software, Cary, NC).
Results
Study sample and outcomes
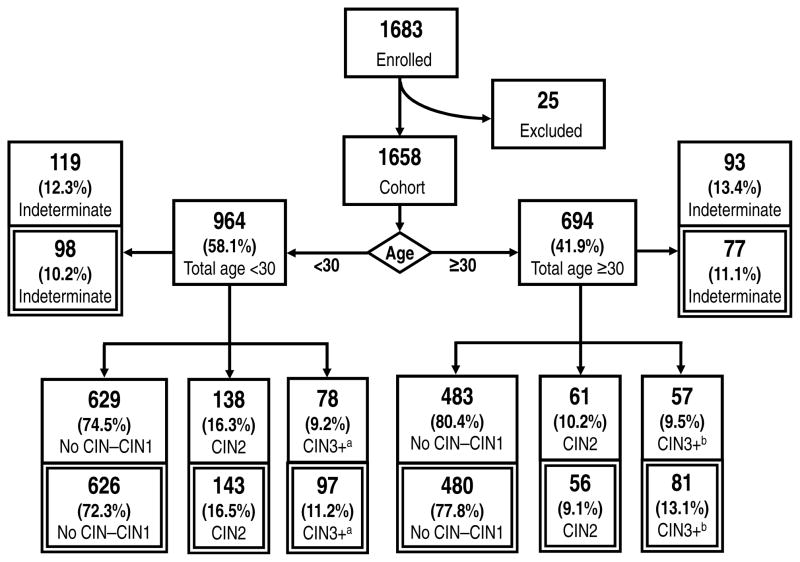
The flow diagram (Figure 1) shows the derivation of the study sample, age groups, and diagnoses, and describes the distribution of outcomes at baseline and after incorporation of follow-up information. Of 1658 women recruited into the study, 1446 (87.2%) had a diagnosis of no CIN through cancer and 212 were indeterminate after their enrollment evaluations. The 6 cancers were included with CIN3 to form the group CIN 3+. There was a significant difference in the distribution of outcomes (i.e. CIN3+, CIN2, no CIN–CIN1) by age group (p=.003), driven by a higher prevalence of CIN2 in younger women (<30, 16.3%; ≥30, 10.2%); prevalence of CIN3+ was similar in both age groups (<30, 9.2%; ≥30, 9.5%).
Figure 1.
Flow diagram showing outcome at baseline (single border) and after 2 years of passive follow-up (double border). Reasons for exclusion from the cohort include missing HPV typing data (n=12), missing diagnosis (n=5), and biopsy of non-cervix tissue (n=8). aIncludes 1 case of cancer. bIncludes 5 cases of cancer.
A total of 1095 (66.0%) women had at least 1 follow-up visit. Follow-up was more common among women with more severe baseline diagnoses (CIN3+ 80%, CIN2 79%, no CIN–CIN1 62%, indeterminate 66%, p<.0001), but follow-up status did not differ by study site, race/ethnicity, age group, or smoking. Among women with follow-up, the median number of visits was 2 (range 1–9, IQR 2), and the median duration of follow-up was 341 days (range 1–730).
At follow-up, 43 cases of CIN3 and 0 cancers were diagnosed that were not evident at baseline (Figure 1). More than half of these (n=23) were upgraded from CIN2, 7 were upgraded from no CIN–CIN1, and 13 were resolutions of previously indeterminate outcomes. Among the 43 cases of CIN3 observed only through follow-up, 41 (95.4%) were positive for HR-HPV, and 19 (44.2%) were positive for HPV-16. Only 23 women were upgraded to CIN2 after follow-up, and the majority of these (n=15) had previously been classified as no CIN–CIN1.
A total of 1483 women with a diagnosis of no CIN through cancer (178 CIN3+, 199 CIN2, and 1112 no CIN–CIN1) were identified after 2-year follow-up information was incorporated. Similar to baseline, outcomes differed significantly by age group (p=.0002), driven by a higher prevalence of CIN2 in younger women (<30, 16.5%; ≥30, 9.1%); prevalence of CIN3+ remained similar in both age groups (<30, 11.2%; ≥30, 13.1%). The overall prevalence of CIN3+ increased from 9.3% to 12.0% after follow-up information was incorporated.
Distributions of most sociodemographic, health behavior, reproductive history, and sexual history characteristics differed significantly by age group (Table 1). The overall median age was 27 (range 18–69).
Table 1.
Description of study sample, overall and stratified by age
| Total
|
Age <30
|
Age ≥30
|
p-value
|
||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 1658 | 964 | 694 | ||||
| Sociodemographics | |||||||
| Race/Ethnicity (n=1654) | |||||||
| Non-Hispanic Black | 1216 | 73.5 | 739 | 76.7 | 477 | 69.0 | 0.0002 |
| Non-Hispanic White | 205 | 12.4 | 99 | 10.3 | 106 | 15.3 | |
| Hispanic | 196 | 11.9 | 111 | 11.5 | 85 | 12.3 | |
| Other | 37 | 2.2 | 14 | 1.5 | 23 | 3.3 | |
| Highest education completed (n=1644) | |||||||
| Less than high school | 386 | 23.5 | 232 | 24.3 | 154 | 22.4 | 0.0087 |
| High school | 581 | 35.3 | 339 | 35.5 | 242 | 35.2 | |
| Some college | 418 | 25.4 | 258 | 27.0 | 160 | 23.3 | |
| Completed college | 259 | 15.8 | 127 | 13.3 | 132 | 19.2 | |
| Yearly household income (n=1530) | |||||||
| < $20,000 | 1095 | 71.6 | 659 | 75.7 | 436 | 66.2 | <.0001 |
| $20,000 – 40,000 | 256 | 16.7 | 137 | 15.7 | 119 | 18.1 | |
| > $40,000 | 179 | 11.7 | 75 | 8.6 | 104 | 15.8 | |
| Health Behaviors and general health status | |||||||
| Smoking (n=1653) | |||||||
| Current | 318 | 19.2 | 147 | 15.3 | 171 | 24.7 | <.0001 |
| Former | 210 | 12.7 | 67 | 7.0 | 143 | 20.6 | |
| Never | 1125 | 68.1 | 746 | 77.7 | 379 | 54.7 | |
| Alcohol, average in last 5 years (n=1654) | |||||||
| Rarely/Never | 764 | 46.2 | 507 | 52.8 | 257 | 37.1 | <.0001 |
| At least once per month but less than once per week | 454 | 27.5 | 269 | 28.0 | 185 | 26.7 | |
| At least once per week | 436 | 26.4 | 185 | 19.3 | 251 | 36.2 | |
| Screening history (n=1571) | |||||||
| 5 or more pap tests in last 5 years | 986 | 62.8 | 566 | 62.1 | 420 | 63.7 | 0.5 |
| Less than 5 pap tests in last 5 years | 585 | 37.2 | 346 | 37.9 | 239 | 36.3 | |
| Currently use condoms | |||||||
| No | 988 | 60.8 | 486 | 51.2 | 502 | 74.3 | <.0001 |
| Sometimes | 236 | 14.5 | 172 | 18.1 | 64 | 9.5 | |
| Regularly | 402 | 24.7 | 292 | 30.7 | 110 | 16.3 | |
| Body Mass Index (kg/m2) (n=1407a) | |||||||
| Underweight (<18.5) | 37 | 2.6 | 27 | 3.3 | 10 | 1.7 | <.0001 |
| Normal weight (18.5 to 24.9) | 455 | 32.3 | 311 | 38.0 | 144 | 24.5 | |
| Overweight (25.0 to 29.9) | 369 | 26.2 | 208 | 25.4 | 161 | 27.4 | |
| Obese (≥30) | 546 | 38.8 | 273 | 33.3 | 273 | 46.4 | |
| Reproductive history | |||||||
| Gravidity (n=1655) | |||||||
| 0 | 254 | 15.4 | 189 | 19.7 | 65 | 9.4 | <.0001 |
| 1 to 2 | 686 | 41.5 | 492 | 51.2 | 194 | 28.0 | |
| 3 to 4 | 438 | 26.5 | 205 | 21.3 | 233 | 33.6 | |
| 5 to 6 | 191 | 11.5 | 60 | 6.2 | 131 | 18.9 | |
| ≥7 | 86 | 5.2 | 15 | 1.6 | 71 | 10.2 | |
| Parity (n=1654) | |||||||
| 0 | 390 | 23.6 | 290 | 30.2 | 100 | 14.4 | <.0001 |
| 1 to 2 | 791 | 47.8 | 529 | 55.1 | 262 | 37.8 | |
| 3 to 4 | 354 | 21.4 | 122 | 12.7 | 232 | 33.5 | |
| ≥5 | 119 | 14.3 | 20 | 2.1 | 99 | 14.3 | |
| Age at menarche (n=1633) | |||||||
| 7–10 | 170 | 10.4 | 106 | 11.1 | 64 | 9.4 | 0.19 |
| 11–12 | 677 | 41.5 | 406 | 42.7 | 271 | 39.8 | |
| 13–14 | 553 | 33.9 | 316 | 33.2 | 237 | 34.8 | |
| ≥15 | 233 | 14.3 | 124 | 16.0 | 109 | 16.0 | |
| Age at first pregnancy (n=1631) | |||||||
| Never pregnant | 254 | 15.6 | 189 | 19.9 | 65 | 9.6 | <.0001 |
| 11–16 | 369 | 22.6 | 236 | 24.8 | 133 | 19.6 | |
| 17–19 | 564 | 34.6 | 342 | 35.9 | 222 | 32.7 | |
| 20–24 | 322 | 19.7 | 169 | 17.8 | 153 | 22.5 | |
| 25+ | 122 | 7.5 | 16 | 1.7 | 106 | 15.6 | |
| Lifetime oral contraceptive use (n=1647) | |||||||
| Never | 436 | 26.5 | 283 | 29.6 | 153 | 22.2 | <.0001 |
| Less than 3 months | 283 | 17.2 | 186 | 19.4 | 97 | 14.1 | |
| 3 to 11 months | 221 | 13.4 | 137 | 14.3 | 84 | 12.2 | |
| 12 to 35 months | 305 | 18.5 | 184 | 19.2 | 121 | 17.5 | |
| At least 36 months | 235 | 34.1 | 167 | 17.5 | 235 | 34.1 | |
| Injectable contraceptive use (ever, N=1642) | |||||||
| Yes, regularly | 517 | 31.5 | 408 | 42.7 | 109 9 |
15.9 | <.0001 |
| Yes, sometimes | 213 | 13.0 | 145 | 15.2 | 68 | 9.9 | |
| Never | 912 | 55.5 | 403 | 42.2 | 509 | 74.2 | |
| Any hormonal contraceptive use | |||||||
| Yes | 1407 | 84.9 | 839 | 87.0 | 568 | 81.8 | 0.004 |
| No | 251 | 15.1 | 125 | 13.0 | 126 | 18.2 | |
| Periods stopped (n=1652) | |||||||
| Yes, natural menopause | 99 | 6.0 | 0 | 0.0 | 99 | 14.4 | <.0001b |
| Yes, other reason | 30 | 1.8 | 4 | 0.4 | 26 | 3.8 | |
| No | 1523 | 92.2 | 960 | 99.6 | 563 | 81.8 | |
| Sexual history | |||||||
| Lifetime number of male sexual partners (n=1567) | |||||||
| 0 to 1 (1 woman claims 0) | 158 | 10.1 | 98 | 10.5 | 60 | 9.4 | 0.15 |
| 2 to 5 | 802 | 51.2 | 490 | 52.7 | 312 | 49.0 | |
| ≥6 | 607 | 38.7 | 342 | 36.8 | 365 | 41.6 | |
| Age at first sexual intercourse | |||||||
| 5 to 14 | 339 | 21 | 220 | 23.1 | 119 | 17.9 | <.0001 |
| 15 to 16 | 614 | 38 | 414 | 43.5 | 200 | 30.1 | |
| 17 to 18 | 418 | 25.9 | 225 | 23.7 | 193 | 29.0 | |
| 19 to 20 | 143 | 8.9 | 66 | 6.9 | 77 | 11.6 | |
| 21 or older | 102 | 6.3 | 26 | 2.7 | 76 | 11.4 | |
231 women enrolled prior to 08/27/2009 were not asked about height and weight, and 20 other women did not answer.
P-value for comparison between Yes and No.
HPV types
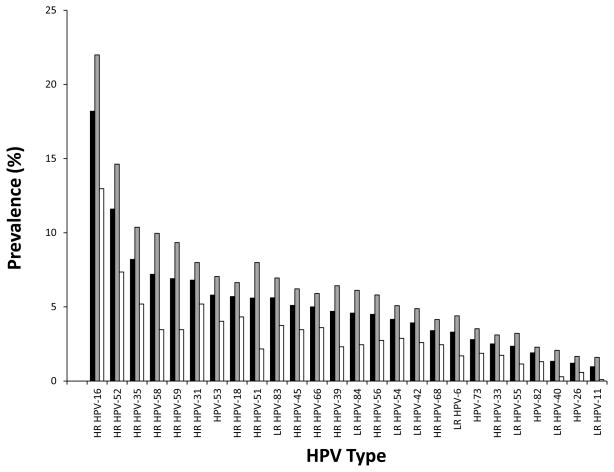
Overall, 1189 (71.7%) women tested positive for HPV, and 982 (59.2%) tested positive for HR-HPV. HPV and HR-HPV were more often detected in younger than older women (any HPV: <30, 81.6%, ≥30 57.6%, p<.0001; HR-HPV: <30, 70.3%, ≥30 43.8%, p<.0001). The 17 most prevalent HPV types had significantly higher prevalence in the younger age group (Figure 1). A total of 596 women (36.0%) had multiple HPV types, and women aged <30 were more than twice as likely as women aged ≥30 to have multiple HPV types (47.6% vs. 19.7%, p<.0001). Stratified by outcome (CIN3+ and ≤CIN2), most HR-HPV types were still more common in the <30 age group, except for HPV-31, HPV18 and HPV45, which were non-significantly more common in older women among CIN3+ (Supplemental Figure I).
Among HR-HPV positive women, the median age was 25 [IQR 21–32]. In women with CIN3+, those positive for HPV-16 had a slightly younger median age than those positive only for other types (median (IQR) 28 (24–34) vs 30 (25–40), p=.097). The median number of years since first sexual intercourse among HR-HPV positive women was 9 [IQR 5–15]. In the subset with CIN3+, those positive for HPV-16 had fewer years since first intercourse than those positive only for other types (median (IQR) 11 (7–17) vs 14 (8–22), p=.044).
Relative Risk of CIN3+ by HPV Types
Among all study participants, significantly increased risk of CIN3+ was observed for women with any HR-HPV, HPV-16, and any alpha-9 genotype (Table 2). There were significant interactions with age group (p<.05) for non-vaccine HR-HPV types and for alpha-7 HPV, and a nearly significant interaction for HPV-18 (p=.06): in stratified analyses, these types or groups were only associated with CIN3+ among women aged ≥30. Excluding women with multiple HPV types increased several RRs, and suggested that the HPV16 association was stronger in younger vs. older women, although confidence intervals were wide (p-interaction=.32).
Table 2.
Relative Risk (RR) of CIN3+ vs. ≤CIN2 by HPV Type Categories, overall and stratified by age group
| All Ages | Age <30 | Age ≥30 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| CIN3+ | ≤CIN2 | RR | 95% CI | CIN3+ | ≤CIN2 | RR | 95% CI | CIN3+ | ≤CIN2 | RR | 95% CI | |
|
|
|
|
|
|
|
|
|
|||||
| All women (n=1483) | 178 | 1305 | 97 | 769 | 83 | 534 | ||||||
| HR-HPVa | 173 | 700 | 24.2 | 10.0–58.5 | 95 | 506 | 20.9 | 5.2–84.3 | 78 | 194 | 32.9 | 10.5–103.3 |
| HPV-16 | 105 | 164 | 6.5 | 5.0–8.5 | 61 | 125 | 6.2 | 4.2–9.0 | 44 | 39 | 7.7 | 5.3–11.1 |
| HPV-18 | 10 | 73 | 1.0 | 0.6–1.8 | 4 | 54 | 0.6 | 0.2–1.6 | 6 | 19 | 1.9 | 0.9–3.9 |
| Vaccine HR-HPV typesb | 114 | 222 | 6.1 | 4.6–8.1 | 64 | 166 | 5.4 | 3.6–7.9 | 50 | 53 | 7.8 | 5.2–11.6 |
| Non-vaccine HR-HPV typesc | 95 | 595 | 1.3 | 1.0–1.7 | 53 | 439 | 0.9 | 0.6–1.3 | 42 | 156 | 2.3 | 1.5–3.4 |
| Any alpha-9 HPV typed | 155 | 476 | 9.1 | 5.9–13.9 | 89 | 349 | 10.9 | 5.3–22.1 | 66 | 127 | 9.7 | 5.7–16.5 |
| Any alpha-7 HPV typee | 34 | 144 | 0.9 | 0.69–1.2 | 17 | 213 | 0.6 | 0.4–1.0 | 17 | 73 | 1.6 | 1.0–2.5 |
| Multiple (vs. single) HPV typesf | 175 | 881 | 0.9 | 0.7–1.1 | 52 | 356 | 0.9 | 0.6–1.2 | 30 | 93 | 1.1 | 0.8–1.7 |
| Women without multiple HPV types (n=952) | ||||||||||||
| Total | 96 | 856 | 45 | 413 | 51 | 443 | ||||||
| HR-HPVa | 91 | 276 | 29.0 | 11.9–70.7 | 43 | 170 | 24.7 | 6.1–100.9 | 48 | 106 | 35.3 | 11.2–111.6 |
| HPV-16 | 61 | 57 | 12.3 | 8.5–17.8 | 32 | 30 | 15.7 | 8.7–28.3 | 29 | 27 | 10.3 | 6.4–16.6 |
| HPV-18 | 4 | 19 | 1.8 | 0.7–4.4 | 1 | 12 | 0.8 | 0.1–5.2 | 3 | 7 | 3.0 | 1.1–8.1 |
| Vaccine HR-HPV typesb | 65 | 76 | 12.1 | 8.2–17.8 | 33 | 42 | 14.0 | 7.6–25.9 | 32 | 34 | 10.9 | 6.6–18.1 |
| Non-vaccine HR-HPV typesc | 26 | 200 | 1.2 | 0.8–1.8 | 10 | 128 | 0.7 | 0.3–1.3 | 16 | 72 | 2.1 | 1.2–3.6 |
| Any alpha-9 HPV typed | 80 | 161 | 14.8 | 8.8–24.7 | 40 | 349 | 19.8 | 8.0–49.0 | 40 | 69 | 12.8 | 6.8–24.2 |
| Any alpha-7 HPV typee | 8 | 72 | 1.0 | 0.5–2.0 | 1 | 213 | 0.2 | 0.03–1.4 | 7 | 26 | 2.2 | 1.1–4.5 |
Includes types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
Includes types 16 and 18.
Includes types 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68.
Includes types 16, 31, 33, 35, 52, 58 and 67
Includes types 18, 39, 45, 59, 68, 70 and 85.
Excludes 427 HPV-negative women.
Stratifying age at 25 did not meaningfully change RRs for most HPV types (not shown), except to suggest an age-group interaction for multiple types (≥ 25: RR=1.4, 95% CI 1.0–1.8; <25: RR=0.8, 95% CI 0.5–3.1; p-interaction=.09). Excluding CIN2 cases resulted in slightly stronger associations (e.g. RR for HR-HPV increased from 24.2 to 29.0).
HPV cofactors
Adjusted RRs of CIN3+ among HR-HPV positive women are shown in Table 3 (unadjusted RRs and absolute numbers shown in Supplemental Table I). Tests of age interactions with dichotomous forms of all variables (smoking ever vs. never, current condom use ever vs. never, annual screening yes vs. no, parity 3+ vs. 0–2), revealed a statistically significant interaction with parity (p=.048). Increasing parity was only associated with CIN3+ among women ≥30 (p-trend=.008). Additional adjustment for race/ethnicity did not meaningfully change the cofactors models (not shown).
Table 3.
Overall and age-stratified Relative Risks (RR) for CIN3+ vs. ≤CIN2 by HPV cofactors, among HR-HPV positive women, from adjusted log-binomial regression models
| All ages (n=812)
|
Age <30 (n=556)
|
Age ≥30 (n=256)
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
|
|
|
|
||||
| Age | ||||||
| 18–21 | 1.0 | 1.0 | ||||
| 22–24 | 1.3 | 0.8, 2.4 | 1.4 | 0.8, 2.5 | ||
| 25–29 | 2.1 | 1.3, 3.4 | 2.4 | 1.4, 4.0 | ||
| 30–39 | 2.3 | 1.4, 3.8 | 1.0 | |||
| >=40 | 1.7 | 1.0, 3.0 | 0.7 | 0.5, 1.7 | ||
| Smoking | ||||||
| Current | 1.5 | 1.1, 2.1 | 1.6 | 1.0, 2.5 | 1.5 | 1.0, 2.4 |
| Former | 1.8 | 1.2, 2.5 | 1.3 | 0.7, 2.5 | 1.9 | 1.3, 3.0 |
| Never | 1.0 | 1.0 | 1.0 | |||
| Screening history | ||||||
| 5 or more pap tests in last 5 years | 0.7 | 0.6, 0.9 | 0.8 | 0.5, 1.1 | 0.7 | 0.5, 1.0 |
| Less than 5 pap tests in last 5 years | 1.0 | 1.0 | 1.0 | |||
| Parity | ||||||
| 0 | 1.0 | 1.0 | 1.0 | |||
| 1 to 2 | 1.0 | 0.7, 1.5 | 1.1 | 0.7, 1.7 | 0.9 | 0.5, 1.7 |
| 3 to 4 | 1.2 | 0.8, 1.8 | 0.7 | 0.3, 1.5 | 1.4 | 0.8, 2.5 |
| ≥5 | 1.6 | 1.0, 2.5 | 0.9 | 0.3, 1.5 | 1.8 | 1.0, 3.3 |
| P-value for trend | p=.07 | p=.55 | p=.008 | |||
| Currently use condoms | ||||||
| Never | 1.0 | 1.0 | 1.0 | |||
| Sometimes | 0.8 | 0.6, 1.1 | 0.9 | 0.6, 1.4 | 0.6 | 0.2, 1.4 |
| Regularly | 0.5 | 0.3, 0.9 | 0.6 | 0.3, 1.1 | 0.6 | 0.3, 1.1 |
Stratifying age at 25 did not change the cofactors analysis much, although the RR for parity >=5 in the older group declined from 1.8 to 1.4 and an interaction with screening history was suggested (>=25: RR=0.6, 95% CI 0.5–0.9; <25: RR=1.1, 95% CI 0.6–1.9; p-interaction=.08). Excluding CIN2 did not meaningfully change RRs for cofactors (not shown).
Discusson
In this study of colsposcopy clinic patients, women in the two age strata defined by clinical guidelines for use of HR-HPV testing in cervical cancer screening differed in their distribution of cervical cancer risk factors, type-specific HPV associations with CIN3+, and cofactors for CIN3+. Subgroup variation by age in the risk of CIN3+ indicates that adjusting for age as a confounder may not be adequate to control for the relations among age, HPV types, cofactors, and CIN3+.
Consistent with the hypothesis that HR-HPV types other than HPV-16 require more time to progress to clinically significant disease, we found that non-vaccine HR-HPV types and alpha-7 (HPV-18 related) types were only associated with CIN3+ in women aged ≥30. However, the RR for CIN3+ by HPV16 status were very high in all age groups, suggesting that among women with abnormal screening, a finding of HPV16 is important regardless of age; a sensitivity analysis excluding women with multiple type infections suggested that the association with HPV16 might be stronger for younger women. We also noted that 59% of CIN3+ cases were HPV-16 positive (i.e. 105/178, see Table 2), and HPV16-positive CIN3+ had a slightly younger age at diagnosis and shorter interval since first intercourse than HPV16-negative CIN3+ cases, consistent with results from a large U.S. screening trial(17) and supporting the hypothesis that HPV-16 infections can lead to significant abnormalities more quickly than other HR-HPV types. Previous studies have suggested an overrepresentation of HPV-16 or HPV-16 and HPV-18 in neoplasia affecting younger women in case series of CIN3+, CIN2, or CIN2+(10, 17–20). HPV-16 is the most oncogenic type and clearly an important contributor to high-grade cervical neoplasia at all ages. Our findings suggest that other HR-HPV types may become more important to risk of CIN3+ as women age, although the magnitude of their risk remains very low compared to that of HPV-16.
Our study partially supports the existing literature on cofactors for cervical neoplasia among HR-HPV positive women, although the racial make-up of the study population is quite different from most previous studies. Among primarily white women referred to one colposcopy center, cofactors for CIN3 (vs. <CIN2) included increasing parity and current smoking, in agreement with our study, and also included higher income (i.e. ≥40,000 vs. <10,000), and extremes of body mass index (i.e. ≥30 and <20), after adjustment for age quintiles(21). Another US study investigated reproductive cofactors for CIN3 among women with LSIL or ASCUS cytology (ALTS)(22). In contrast with our findings, the authors identified increased risk of CIN3 (vs. CIN<2) with current injectable contraceptive use, but not OC, Norplant, parity, gravidity, or age at first pregnancy, after adjusting for HPV-16 DNA, education, age, and smoking status. A large data pooling study identified a graded increase in CIN3/carcinoma in situ with increasing parity and age at first full-term pregnancy, although when these risk factors were combined in a model, age at first full-term pregnancy was the stronger risk factor(23). The study did not report on whether the association remained after restricting the analysis to HR-HPV positive women (23). None of these previous studies reported on whether the observed associations were uniform across age groups(21, 22). A pooled analysis of international case-control studies found that smoking was associated with similar 2-fold greater odds of both carcinoma in situ and invasive cervical cancer among HPV positive women, and found no evidence of age-group differences in the effects of smoking with invasive and in situ cases combined(24). Our selection of candidate cofactors for CIN3+ was informed by the larger body of literature on invasive cervical cancer, which has identified associations with age at first full-term pregnancy and number of full-term pregnancies(23), lifetime number of sexual partners(25), hormonal contraceptive use(26), and smoking (24, 27). It is important to consider cofactors for CIN3+ because precancerous lesions are the target of screening activities, and cofactors for this endpoint might differ from those for invasive cancers because of the timing of exposures, the portion of the pathophysiologic process affected by the risk factor, the age of cases, or differences in control selection.
Several mechanisms for cofactors for cervical cancer and CIN3 have been suggested. Smoking by-products are found in cervical mucus(28), and could induce immune suppression against active HPV infection or direct oncogenic effects(29). Reproductive factors, including parity, are related to sexual activity, raising concern that such variables are proxies for HPV exposure(23). However, associations with parity and other reproductive history and sexual behavior variables have remained after restricting cases and controls to HR-HPV positive women in this and many other studies. Multiparity might promote neoplastic changes through hormonal influences or local tissue changes during the vaginal birth process that expose the transformation zone to carcinogenic agents(23, 30). Our finding of a protective effect of current condom use echoes previous findings of protective effects of barrier contraceptives for invasive cervical cancer among HPV-positive women(31, 32). While this association might be attributable to residual confounding by HPV exposure, condoms also protect against other sexually transmitted infections, such as Chlamydia trachomatis, which could act as cofactors for CIN3+, perhaps by contributing to HPV persistence(31).
Age group differences in cofactors suggest that the timing of exposures or elapsed time between exposure and detection of precancerous lesions are important. We speculate that the lack of association between multiparity and CIN3+ among women <30 in this study could be attributable to relatively low parity in this age group and insufficient follow-up time between multiple pregnancies and future development of disease. The distributions of nearly all the classical cervical cancer risk factors we examined differed between the two age groups. Several of these characteristics, such as education, income, BMI, gravidity, parity, and menopausal status are truly age-related—that is, with the passage of time, women have more opportunities to acquire these risk factors. Other age group variation, especially in behaviors like smoking, alcohol, condom or other contraceptive use, may instead represent cohort differences. For example, the prevalence of smoking declined from 1978 to 2002(33), thus the lower rates of current smoking noted among younger women in this study might be attributable to birth cohort differences rather than true age differences. While population trends suggest declining age at onset of sexual activity(34), the younger onset of sexual activity in women under 30 in this colposcopy cohort might be a proxy for age at first exposure to HPV, and reflect a selection effect rather than a cohort effect. As screening practices and prevention strategies change (e.g. introduction of HR-HPV co-testing and HPV vaccine), the descriptive epidemiology of colposcopy patients will also change. When researchers adjust for age in regression models of cervical cancer outcomes, part of the intent is to adjust for the time needed for cervical cancer to develop; however, adjusting for age also adjusts for other behavioral and clinical characteristics representing a mixture of age and cohort effects.
Several limitations should be considered when interpreting this study; most of these are related to the referral population. The study design assumed that all women entering care at the colposcopy clinic had a legitimate reason for referral. Consequently, relatively few exclusion criteria were applied, and information on referral indications were not collected. Based on the study years (2000–2004), most women would have been referred following an abnormal Pap test (HR-HPV testing had not yet been approved for screening); however, some women may have been referred for additional evaluation and treatment following a biopsy in another setting. Thus, some women may have had their most severe lesion removed during a prior biopsy, which would lead to outcome misclassification (e.g. classified as CIN0 when a CIN3 lesion had been removed). We would expect this to have the effect of reducing differences between outcome groups. Women were recruited from urban public hospital-affiliated clinics which serve large inner city minority populations of relatively disadvantaged women who depend on public health services. While this has the benefit of providing more information on this population which has been relatively underrepresented in other similar studies(21, 22), generalizability to non-urban or relatively advantaged populations is unknown. All associations are conditional on women having abnormal screening tests, and cannot be generalized to women with normal cytology. Finally, despite a substantial number of CIN3+ cases, statistical power to detect interactions was limited.
This study’s internal validity is supported by efforts to reduce misclassification of both the exposures and outcomes. The HPV typing was conducted in an experienced laboratory with extensive quality control procedures. The highly sensitive genotyping assay mitigates concern that some cofactors are markers for undetected HR-HPV. The pathology findings were subject to an expert review process, and follow-up information was available on 2/3 of participants to enhance detection of CIN3 missed at baseline, including clarifying a portion of previously indeterminate outcomes. CIN3 cases identified during follow-up were interpreted as prevalent cases having previous false negative results or lack of biopsy at baseline colposcopy rather than as cases that developed during the follow-up period(35). Although these follow-up cases did not undergo the same rigorous review process as the baseline outcomes, we feel confident that the CIN3 cases ascertained at follow-up are valid, since this diagnosis is not subject to as much interpretation as CIN2(36, 37). In addition, the observed associations were similar when performed using only the baseline outcomes (data not shown). Our decision to include CIN2 with CIN0-1 in the reference category in our models was based on the goal of avoiding spectrum bias and the related potential for generating overly optimistic estimates of effects when intermediate outcome categories are excluded from analyses.
The age distribution of the study population may impact study findings regarding relations among HPV types, cofactors, and CIN3+ because of age group differences in risk factor prevalence, in the time since exposure to HR-HPV or other risk factors, or to differential impact of specific HPV types. Additional research on type-specific risk may eventually support a clinical application of HPV genotyping in certain situations. Because HR-HPV cofactors may inform disease risk without invasive procedures or additional expense, future efforts to incorporate clinical and demographic data into screening algorithms might improve the efficiency of existing markers. As cervical cancer screening strategies for vaccinated cohorts of women are developed and clinical HR-HPV testing is incorporated, it will be important to consider the natural history of HPV and cervical neoplasia and secular trends in cofactors in the pursuit of appropriately sensitive and specific screening tools applied to the appropriate age groups or other sub-populations.
Supplementary Material
Figure 2.
Prevalence of the most common HPV types in colposcopy referral study population (N=1658) overall (black bars), among women aged <30 (grey bars) and among women ≥30 (white bars). Figure includes the 26 types found in at least 1% of the study population (i.e. at least as common as HPV-11, the least common vaccine type). Age-group comparisons for all types at least as common as HPV-42 were statistically significant (p-value from chi-square test <.05).
NOTE: HR, high-risk (i.e. detected by clinical HR-HPV tests); LR, low-risk. Other types are possibly high-risk but not detected by clinical HR-HPV tests. Not shown in figure: HPV-70 (0.5%), HPV-67 (0.1%), HPV-69 (0.1%), HPV-IS39 (0.1%), and HPV-85 (0%).
Acknowledgments
We would like to thank Drs. Jin-Mann Lin, Roumiana Boneva and Mona Saraiya for their comments and suggestions on this manuscript.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
The views expressed in this publication are the views of the authors and do not necessarily reflect the views of the Ontario Ministry of Health and Long-Term Care.
Grant Support
This work was supported in part by the National Cancer Institute’s Early Detection Research Network (EDRN), Interagency Agreements Y1-CN-0101-01 and Y1-CN-5005-01. Rosane Nisenbaum gratefully acknowledges the support of the Ontario Ministry of Health and Long-Term Care.
Footnotes
Conflicts of Interest:
None
References
- 1.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007 Jun 1;195(11):1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010 Mar 3;102(5):315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S, et al. Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev. 2010 Jun;19(6):1585–94. doi: 10.1158/1055-9965.EPI-09-1235. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Wentzensen N. From Human Papillomavirus to Cervical Cancer. Obstet Gynecol [Review] 2010 Jul;116(1):177–85. doi: 10.1097/AOG.0b013e3181e4629f. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC, Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of humanpapillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol. 2004 Feb;103(2):304–9. doi: 10.1097/01.AOG.0000109426.82624.f8. [DOI] [PubMed] [Google Scholar]
- 6.Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, Terry G, et al. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3033–42. doi: 10.1158/1055-9965.EPI-08-0508. [DOI] [PubMed] [Google Scholar]
- 7.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 463: Cervical cancer in adolescents: screening, evaluation, and management. Obstet Gynecol. 2007 Aug;116(2 Pt 1):469–72. doi: 10.1097/AOG.0b013e3181eeb30f. [DOI] [PubMed] [Google Scholar]
- 8.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007 Oct;197(4):340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 9.McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008 May;9(5):425–34. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol. 2009 Jan;113(1):18–25. doi: 10.1097/AOG.0b013e31818f5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakobsson M, Gissler M, Paavonen J, Tapper AM. Loop electrosurgical excision procedure and the risk forpreterm birth. Obstet Gynecol. 2009 Sep;114(3):504–10. doi: 10.1097/AOG.0b013e3181b052de. [DOI] [PubMed] [Google Scholar]
- 12.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006 Feb 11;367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 13.Winget MD, Baron JA, Spitz MR, Brenner DE, Warzel D, Kincaid H, et al. Development of common data elements: the experience of and recommendations from the early detection research network. Int J Med Inform. 2003 Apr;70(1):41–8. doi: 10.1016/s1386-5056(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 14.Rajeevan MS, Swan DC, Nisenbaum R, Lee DR, Vernon SD, Ruffin MT, et al. Epidemiologic and viral factors associated with cervical neoplasia in HPV-16-positive women. Int J Cancer. 2005 May 20;115(1):114–20. doi: 10.1002/ijc.20894. [DOI] [PubMed] [Google Scholar]
- 15.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003 May 15;157(10):940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 16.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology ofhuman cancer. Vaccine. 2006 Aug 31;24(Suppl 3):S3/1–10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 17.Castle PE, Schiffman M, Wheeler CM, Wentzensen N, Gravitt PE. Human papillomavirus genotypes in cervical intraepithelial neoplasia grade 3. Cancer Epidemiol Biomarkers Prev. Jul;19(7):1675–81. doi: 10.1158/1055-9965.EPI-10-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carozzi FM, Tornesello ML, Burroni E, Loquercio G, Carillo G, Angeloni C, et al. Prevalence of human papillomavirus types in high-grade cervical intraepithelial neoplasia and cancer in Italy. Cancer Epidemiol Biomarkers Prev. Sep;19(9):2389–400. doi: 10.1158/1055-9965.EPI-10-0131. [DOI] [PubMed] [Google Scholar]
- 19.Coupe VM, Berkhof J, Bulkmans NW, Snijders PJ, Meijer CJ. Age-dependent prevalence of 14 high-risk HPV types in the Netherlands: implications for prophylactic vaccination and screening. Br J Cancer. 2008 Feb 12;98(3):646–51. doi: 10.1038/sj.bjc.6604162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porras C, Rodriguez AC, Hildesheim A, Herrero R, Gonzalez P, Wacholder S, et al. Human papillomavirus types by age in cervical cancer precursors: predominance of human papillomavirus 16 in young women. Cancer Epidemiol Biomarkers Prev. 2009 Mar;18(3):863–5. doi: 10.1158/1055-9965.EPI-08-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SS, Zuna RE, Wentzensen N, Dunn ST, Sherman ME, Gold MA, et al. Human papillomavirus cofactors by disease progression and human papillomavirus types in the study to understand cervical cancer early endpoints and determinants. Cancer Epidemiol Biomarkers Prev. 2009 Jan;18(1):113–20. doi: 10.1158/1055-9965.EPI-08-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castle PE, Walker JL, Schiffman M, Wheeler CM. Hormonal contraceptive use, pregnancy and parity, and the risk of cervical intraepithelial neoplasia 3 among oncogenic HPV DNA-positive women with equivocal or mildly abnormal cytology. Int J Cancer. 2005 Dec 20;117(6):1007–12. doi: 10.1002/ijc.21279. [DOI] [PubMed] [Google Scholar]
- 23.International Collaboration of Epidemiologic Studies of Cervical Cancer. Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33,542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006 Sep 1;119(5):1108–24. doi: 10.1002/ijc.21953. [DOI] [PubMed] [Google Scholar]
- 24.Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case--control study. Cancer Causes Control. 2003 Nov;14(9):805–14. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 25.Cervicalcarcinoma and sexual behavior: collaborative reanalysis of individual data on 15, 461 women with cervical carcinoma and 29, 164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2009 Apr;18(4):1060–9. doi: 10.1158/1055-9965.EPI-08-1186. [DOI] [PubMed] [Google Scholar]
- 26.Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, Plummer M, et al. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003 Apr 5;361(9364):1159–67. doi: 10.1016/s0140-6736(03)12949-2. [DOI] [PubMed] [Google Scholar]
- 27.Haverkos HW, Soon G, Steckley SL, Pickworth W. Cigarette smoking and cervical cancer: Part I: a meta-analysis. Biomed Pharmacother. 2003 Mar;57(2):67–77. doi: 10.1016/s0753-3322(03)00196-3. [DOI] [PubMed] [Google Scholar]
- 28.Prokopczyk B, Cox JE, Hoffmann D, Waggoner SE. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J Natl Cancer Inst. 1997 Jun 18;89(12):868–73. doi: 10.1093/jnci/89.12.868. [DOI] [PubMed] [Google Scholar]
- 29.Gadducci A, Barsotti C, Cosio S, Domenici L, Riccardo Genazzani A. Smoking habit, immune suppression, oral contraceptive use, and hormone replacement therapy use and cervical carcinogenesis: a review of the literature. Gynecol Endocrinol. 2011 Mar 25;27(8):597–604. doi: 10.3109/09513590.2011.558953. [DOI] [PubMed] [Google Scholar]
- 30.Autier P, Coibion M, Huet F, Grivegnee AR. Transformation zone location and intraepithelial neoplasia of the cervix uteri. Br J Cancer. 1996 Aug;74(3):488–90. doi: 10.1038/bjc.1996.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silins I, Ryd W, Strand A, Wadell G, Tornberg S, Hansson BG, et al. Chlamydia trachomatis infection and persistence of human papillomavirus. Int J Cancer. 2005 Aug 10;116(1):110–5. doi: 10.1002/ijc.20970. [DOI] [PubMed] [Google Scholar]
- 32.Hildesheim A, Herrero R, Castle PE, Wacholder S, Bratti MC, Sherman ME, et al. HPV co-factors related to the development of cervical cancer: results from a population-based study in Costa Rica. Br J Cancer. 2001 May 4;84(9):1219–26. doi: 10.1054/bjoc.2001.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Steffen LM, Jacobs DR, Zhou X, Blackburn H, Berger AK, et al. Trends in cardiovascular risk factor levels in the Minnesota Heart Survey (1980–2002) as compared with the National Health and Nutrition Examination Survey (1976–2002): A partial explanation for Minnesota’s low cardiovascular disease mortality? Am J Epidemiol. Mar 1;173(5):526–38. doi: 10.1093/aje/kwq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finer LB. Trends in premarital sex in the United States, 1954–2003. Public Health Rep. 2007 Jan-Feb;122(1):73–8. doi: 10.1177/003335490712200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gage JC, Hanson VW, Abbey K, Dippery S, Gardner S, Kubota J, et al. International Collaboration of Epidemiologic Studies of Cervical Cancer. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006 Aug;108(2):264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 36.Dalla Palma P, Giorgi Rossi P, Collina G, Buccoliero AM, Ghiringhello B, Gilioli E, et al. The reproducibility of CIN diagnoses among different pathologists: data from histology reviews from a multicenter randomized study. Am J Clin Pathol. 2009 Jul;132(1):125–32. doi: 10.1309/AJCPBRK7D1YIUWFP. [DOI] [PubMed] [Google Scholar]
- 37.Ismail SM, Colclough AB, Dinnen JS, Eakins D, Evans DM, Gradwell E, et al. Observer variation in histopathological diagnosis and grading of cervical intraepithelial neoplasia. BMJ. 1989 Mar 18;298(6675):707–10. doi: 10.1136/bmj.298.6675.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.