Abstract
Introduction
Gastroesophageal cancer remains a leading cause of cancer deaths and is uniformly fatal in patients presenting with metastases and recurrence. This study sets out to determine the effect of a third-generation, replication-competent, oncolytic herpes simplex type 1 virus containing transgenes encoding for a fusogenic membrane glycoprotein and Fcy::Fur, against gastroesophageal cancer.
Methods
The cytotoxic effect of the virus was tested on human gastroesophageal cancer cell lines OCUM-2MD3, MKN-45, AGS, MKN-1, MKN-74, and BE-3 at sequential multiplicities of infection (MOI). Cytotoxicity was measured using a lactate dehydrogenase assay. Viral replication was tested by serially diluting supernatants from viral infections and titering on VERO cells via standard plaque assay. Correlations of cytotoxicity and viral replication were also investigated.
Results
All cell lines were susceptible to viral infection and demonstrated a dose-dependent effect, with greater and faster cytotoxicity at higher MOIs. Viral replication was supported in the cell lines tested, with peak titers by day 5, some supporting as high as >40,000× amplification. Cell lines with longer doubling times (>30 hours) also achieved higher viral titers at an MOI of 0.1. Cell lines with shorter doubling times achieved 50% cell kill in fewer days, with an average of 2.3 days for cell lines with doubling times under 30 hours compared with 4.4 days for cell lines with doubling times over 30 hours.
Conclusion
These results suggest that this third-generation oncolytic herpesvirus can effectively infect and lyse gastroesophageal cancer cells and may provide a novel therapy against gastroesophageal cancer.
Keywords: gastroesophageal cancer, herpes virus, oncolytic
INTRODUCTION
Gastroesophageal cancer remains one of the most common malignancies worldwide and is the second-leading cause of cancer-related death. A significant proportion of patients will also either present with metastasis or develop recurrent disease despite treatment. Five-year survival rates for surgically resectable disease is 45%–47% [1]. However, for patients who present with unresectable disease or who recur after resection, the disease is nearly uniformly fatal. It is evident that novel therapies are needed.
Oncolytic herpesviruses have been proven effective against a wide variety of malignancies, both in vitro and in vivo. Several viruses have been brought to clinical trials for patients with metastatic colorectal, head and neck, breast, and prostate cancer, melanoma, and glioma [2]. These modified viruses have been engineered to be tumor specific with less systemic toxicity. The third-generation virus vector studied here (OncovexGALV/CD, Biovex Inc., Woburn, MA) was designed to more specifically target cancer cells via double deletions of γ134.5, a neurovirulence gene, as well as a single deletion of the US12 gene, which codes for the ICP 47 protein. Also added is gibbon ape leukemia virus (GALV) fusogenic membrane glycoprotein and the Fcy::Fur gene. The resulting virus is more lytic to tumor cells with a lessened toxic effect on normal cells, and presents almost no chance for reversion to wild type.
In this study, we report preclinical data supporting this third-generation, attenuated oncolytic herpesvirus as an effective therapeutic agent against gastroesophageal cancer.
MATERIALS AND METHODS
Cell lines
Human gastric cancer OCUM-2MD3 cells were a gift from Dr. Masakazu Yashiro (Osaka City University Medical School, Japan) and were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with high glucose, 2 mmol/L L-glutamine, 0.5 mmol/L sodium pyruvate, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal calf serum (FCS). The human gastric cancer cell line MKN-45, a poorly differentiated adenocarcinoma, was obtained as a gift from Dr. Yutaka Yoneumura (Kanazawa University, Japan) and was maintained in Roswell Park Memorial Institute (RPMI) medium supplemented with 10% FCS, 1% penicillin, and 1% streptomycin. Human AGS cells (a gastric adenocarcinoma epithelial cell line) were obtained from American Type Culture Collection (ATCC; Manassas, VA) and were routinely cultured in Ham’s F-12K medium with 10% fetal bovine serum (FBS). MKN-1, an adenosquamous cell carcinoma, and MKN-74, a well-differentiated adenocarcinoma cell line, were kindly provided by Dr. T. Suzuki (Fukushima Medical College, Japan) and were cultured in RPMI supplemented with 10% FBS, 1% penicillin, and 1% streptomycin. BE-3 is a human esophageal adenocarcinoma cell line and was provided by Dr Nasser Altorki (New York Presbyterian Hospital, New York). BE-3 was cultured in RPMI supplemented with 10% FBS, 1% penicillin, and 1% streptomycin.
Virus
This third-generation herpesvirus is an attenuated, replication-competent, oncolytic herpes simplex type 1 virus, derived from the JS-1 strain. It contains double deletions of the γ134.5 gene and deletion of the US12 gene. The GALV env gene, which codes for the membrane glycoprotein of the gibbon ape leukemia virus, was cloned into the ICP34.5 locus with truncation of 16 amino acids in the transmembrane R peptide, thereby rendering the GALV glycoprotein constitutively active. The Fcy::Fur gene was also cloned into an ICP34.5 locus. The GALV env R- gene and Fcy::Fur gene are under CMV and RSV promoters, respectively.
Cytotoxicity assay
All gastroesophageal cell lines were plated at a density of 2×104 cells per well in 12-well plates in 1 mL of medium. The plates were incubated for 4 hours, after which the medium was carefully suctioned off. Virus was diluted in 100 μL of cold medium and then added in 3 sequential multiplicities of infection (MOI, number of viral particles per cancer cell), ranging from 0.001 to 1.0. Medium was added back to each well to a total volume of 1 mL after 20 minutes. An additional 1 mL of medium was added to all wells on day 4. At daily intervals, the supernatants were collected from each well and frozen. The cells were then washed with phosphate-buffered saline (PBS) and lysed with 1.35% Triton-X solution to release intracellular lactate dehydrogenase (LDH). The amount of LDH present directly correlated to the number of lysed cells. This was quantified using a Cytotox 96 colorimetric assay (Promega, Madison, WI), which measured the conversion of a tetrazolium salt into a red formazan product in the presence of LDH, using a microplate spectrophotometer at 490 nm. Results are demonstrated as percentages of surviving treated cells to control, untreated cells. All conditions were tested in triplicate.
Viral replication
Five gastroesophageal cell lines, AGS, BE3, MKN-1, MKN-45, and MKN-74, were plated at a density of 2×104 cells per well in 12-well plates in 1 mL of medium. After incubation for 4 hours, the medium was suctioned off, and virus was added to each well in 100 μL of medium at an MOI of 0.1. After 20 minutes, medium was added back to each well to a total volume of 1 mL. On days 1, 3, 5, and 7, the supernatant was collected and frozen at −80°C. Serial dilutions of supernatant were added to confluent VERO cells and incubated for 4 hours. The wells were then washed with medium and 1% agarose with medium was added. After 48 hours of incubation, 2 mL of 2% neutral red was added to each well. Viral plaques were counted at 24 hours. Supernatants from each cell line were counted in triplicate.
RESULTS
Viral cytotoxicity
All 6 human gastroesophageal cancer cell lines were susceptible to oncolysis by this third generation oncolytic herpesvirus. The OCUM-2MD3, MKN-45, and AGS cell lines overall were more sensitive to viral lysis compared with MKN-74, MKN-1 and BE-3. All cell lines demonstrated a dose-dependent effect, with greater and faster cytotoxicity at higher MOI concentrations. In OCUM-2MD3, MKN-45, and AGS cell lines, virus killed all cells within 24 hours at an MOI of 1.0 (data not shown). Comparing cytotoxicity with an MOI of 0.1 across the 6 cell lines, the least sensitive cell line was MKN-1, followed by BE-3, MKN-74, AGS, MKN-45, and OCUM-2MD3. Cytotoxicity ranged from 56.1% to 98.1%, respectively (Figure 1).
Figure 1.
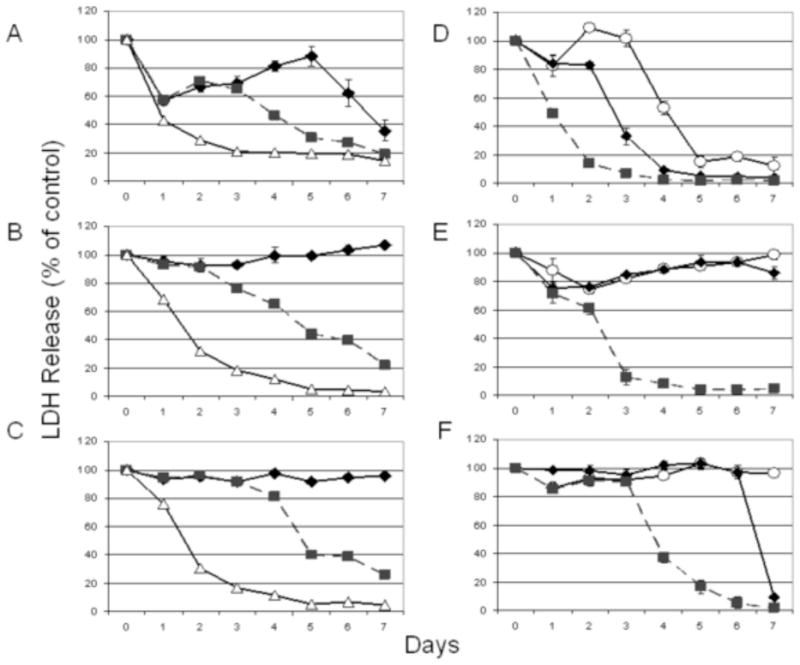
Cytotoxicity of virus against 6 human gastroesophageal carcinoma cell lines in vitro. All cell lines were susceptible to viral oncolysis. The (A) MKN-74, (B) MKN-1, and (C) BE-3 cell lines required higher multiplicities of infection (MOI) to achieve cytotoxicity. MOI refers to the ratio of viral plaque-forming units relative to number of tumor cells. Open triangle, MOI = 1.0; square, MOI = 0.1; and diamond, MOI = 0.01. The (D) OCUM-2MD3, (E) MKN-45, and (F) AGS cell lines were susceptible to viral oncolysis at low MOI levels. Square, MOI = 0.1; diamond, MOI = 0.01; open circle, MOI = 0.001.
The OCUM-2MD3 cell line proved to be particularly susceptible to viral oncolysis, with near 100% cell kill by day 5, at an MOI of 0.001. With an MOI of 0.1, >90% cell kill was achieved by day 3. The AGS cell line also proved quite susceptible with an MOI of 0.1, with 95% cell kill by day 6. At an MOI of 0.01, 90% cytotoxicity was seen by day 7. The MKN-74 cell line demonstrated 80% cytotoxicity by day 5 at an MOI of 1.0 and 80% cytotoxicity by day 7 at an MOI of 0.1 (Figure 1).
The MKN-1, BE-3, and MKN-45 cell lines required higher viral doses to achieve cytotoxicity. 50% cell kill was achieved in both BE-3 and MKN-1 by day 2 at an MOI of 1.0 and by day 5 at an MOI of 0.1. The MKN-45 cell line achieved 50% cell kill by day 3 and 100% by day 5 at an MOI of 0.1. MKN-1 showed 95% cytotoxicity by day 5 at an MOI of 1.0 and 78% cell kill by day 7 at an MOI of 0.1. BE-3 similarly showed 95% cytotoxicity by day 5 and 74% cytotoxicity by day 7 with an MOI of 0.1.
Viral replication
Viral replication was supported in the 5 cell lines tested, and all demonstrated peak viral titers on day 5 (Figure 2). BE-3 demonstrated the greatest viral titer, with a peak of 8.125×107 plaque-forming units (pfu) on day 5 with a virus MOI of 0.1. This represents a >40,000× amplification. While the other cell lines did not achieve titers of this magnitude, they also demonstrated viral amplification with an MOI of 0.1. The MKN-74 cell line displayed a >12,000× amplification with a peak titer of 2.44×107 pfu on day 5. MKN-45 achieved a >9600× amplification with a peak titer of 1.925×107 pfu. The AGS and MKN-1 cell lines allowed for 468× and 123× amplification with peak titers of 9.375×105 and 2.46×105 pfu, respectively.
Figure 2.
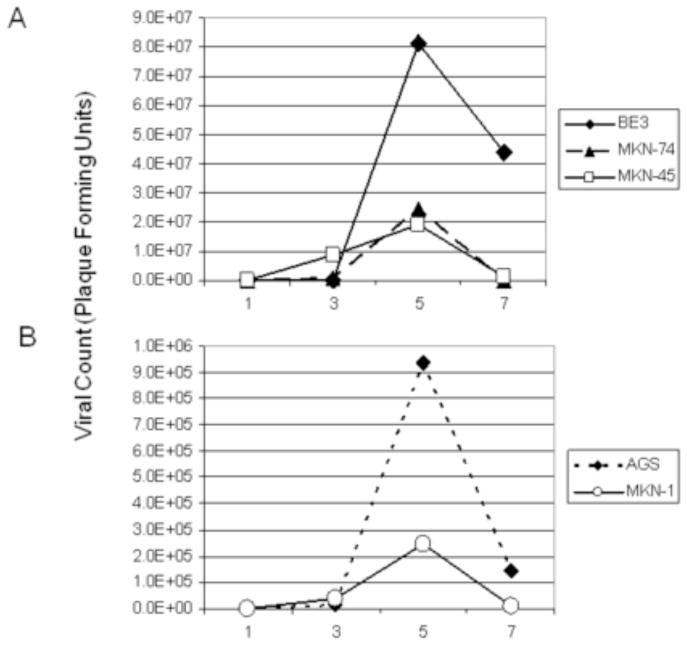
Viral replication was supported by 5 gastroesophageal cancer cell lines. The viral dose used had an MOI of 0.1. (A) Viral plaque assays demonstrated high viral yield by day 5 in all cell lines, with BE-3, MKN-74, and MKN-45 achieving the highest titers. (B) The AGS and MKN-1 cell lines also supported viral proliferation, although not to similar levels of amplification. The best viral proliferation was seen in the BE-3 cell line, with a peak viral titer of 8.125×107 on day 5 after infection with virus (2×103 pfu), which represented a >40,000-fold amplification.
Correlation of Doubling Time to Viral Titer and Cytotoxicity
The OCUM-2MD3, MKN-45, and AGS cell lines had doubling times of 12, 22.9, and 20 hours, respectively. The MKN-1, MKN-74, and BE-3 cell lines had slower doubling times at 31, 36, and 39 hours, respectively. Cell lines that had longer doubling times (>30 hours) achieved higher viral titers at an MOI of 0.1, compared with those cell lines with shorter doubling times (Figure 3A). An exponential curve was fitted using nonlinear least squares and demonstrated an R2 value of 0.667. Spearman’s rank correlation of doubling time relative to viral titer demonstrated a coefficient of 0.7. Cell lines with shorter doubling times (OCUM-2MD3, MKN-45, and AGS) also achieved 50% cell kill in fewer days at an MOI of 0.1 compared with those cell lines with longer doubling times (Figure 3B). The average number of days for cell lines with doubling times under 30 hours to achieve 50% cell kill was 2.3 days compared with 4.4 days for those lines with doubling times over 30 hours. The R2 value for the correlation between doubling time and days to 50% cytotoxicity was 0.72.
Figure 3.
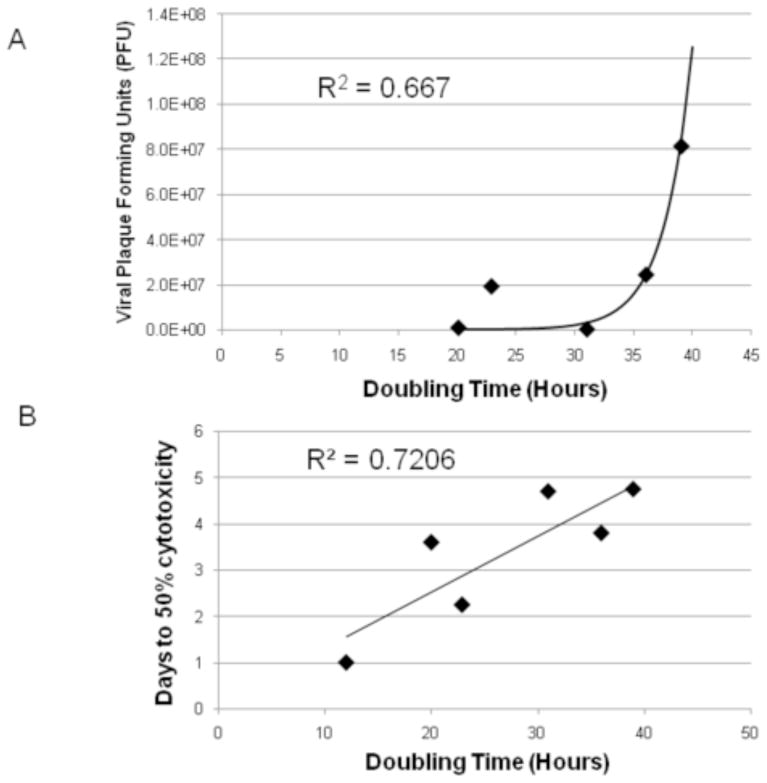
Correlation of the doubling time of 5 gastroesophageal cancer cell lines respective to their viral titer at an MOI of 0.1 on day 5 (A). An exponential line of fit had an R2 value of 0.667. Correlation of days to 50% cell kill of 6 gastroesophageal cancer cell lines at an MOI of 0.1 respective to the cell lines’ doubling time (B). Linear correlation had a R2 value of 0.72.
DISCUSSION
Gastroesophageal cancer remains the fourth most common malignancy worldwide, with estimates of over 1 million new cases in 2007 [3]. It is the second leading cause of cancer-related deaths in males worldwide and the fourth leading cause in females, with estimates of over 800,000 total deaths per year [3]. A high incidence still occurs in Asian countries such as China and Japan.
Surgery remains the only potential cure. Surgical therapy, however, is not indicated for recurrent or metastatic disease. Peritoneal carcinomatosis is discovered synchronously in up to 20% of patients [4,5]. Median survival ranges from 7.6 to 9.9 months in the presence of peritoneal metastases, despite surgical or medical intervention [5]. Recurrent disease after therapy for gastroesophageal cancer is still high and the prognosis remains poor. Locoregional recurrence occurred in 45%–58% of patients having undergone surgery with either D2 or D1 lymphadenectomies, respectively [6, 7]. Surgery, therefore, while providing an excellent means of therapy for early gastroesophageal cancer, does not benefit the majority of advanced gastroeophageal cancer patients.
Chemotherapy is a useful adjuvant therapy, improving overall and disease-free survival, compared to surgical therapy alone. Several large trials have been conducted, including the North American Intergroup 0116 trial and the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. These trials demonstrated improved median survival in the chemotherapy group compared with the surgery alone group. The overall median survival, however, was improved to only 36 months in the adjuvant treatment setting [8]. For patients with advanced disease, survival even with chemotherapy is only 7.5–12 months. While chemotherapy offers some benefit, the therapeutic potential is limited. It is possible gastroesophageal cancers are too resistant to currently available chemotherapy regimens. Those patients with advanced disease who are not candidates, or who fail surgical or medical treatment, all die within 3–5 months [1].
Because the overall median survival in patients with locally advanced or metastatic gastroesophageal cancer has remained poor despite advancements in medical and surgical therapies, novel therapies are being investigated in preclinical studies. Adenovirus vectors have been used for gene transfer in mediating apoptosis in gastric cancer and are a hopeful therapeutic agent but are not yet clinically available [9]. Attenuated oncolytic herpesviruses have been widely investigated for various types of cancer, including glioma, melanoma, mesothelioma, breast, bladder, colorectal, hepatocellular, and salivary gland tumors [10,11]. Several clinical trials are being conducted to determine efficacy and applicability of various herpesviruses toward different types of cancer [12,13].
Oncolytic herpesvirus therapy has great promise as cancer therapy from many respects. The genome has been sequenced and characterized so that nonessential genes may be deleted [14]. As much as 30 kb of the genome may be deleted for insertion of genes tailored for cancer therapy [15]. Oncolytic herpesviruses have been genetically attenuated to be tumor specific and non-neurovirulent. Double deletion of the γ134.5 gene attenuates the virus to make it less neurotoxic, and deletion of ICP47 increases antigen presentation within infected tumor cells [16]. Importantly, antiherpes treatment such as acyclovir and ganciclovir make oncolytic herpes a clinically useful and safe vector [17].
Previous preclinical studies have been conducted evaluating the use of 2 oncolytic herpesviruses, G207 and NV1020, in relation to gastric cancer and carcinomatosis. The G207 virus contains a double deletion of the γ134.5 gene and an inactivation of the UL39 gene, which encodes ICP 6, a large subunit of ribonucleotide reductase. NV1020 contains only 1 deletion of the γ134.5 gene. These studies found that both G207 and NV1020 were tumor specific with a significant therapeutic benefit [18–20].
Several phase I and II clinical trials have evaluated the clinical efficacy of second generation oncolytic herpesviruses against melanoma and metastatic colon cancer, among others [13, 31]. Both intratumoral and intraarterial injections of virus were performed, with low toxicity effects observed. Local inflammation, erythema, and a transient febrile response were the most commonly observed effects from intratumoral injection [13]. Intraarterial injection produced more severe effects, including transient rise in liver enzymes, diarrhea, and leukocytosis, although these symptoms all cleared by one month after administration. Additionally, no patients demonstrated evidence of viral reactivation in blood, saliva, rectal or vaginal swabs in close follow-up. Furthermore, the patients with high response to virus administration had clinical response and survived well past expected for disease prognosis [32].
These clinical trials were all performed with second generation oncolytic herpesvirus and clearly demonstrated safety and clinical benefits in human administration, with low toxicity to healthy human cells. Successive generations of oncolytic herpesviruses have been engineered to further increase antitumor efficacy as well as the safety and immunogenic profile of these viruses [17].
We studied a third-generation, attenuated, replication-competent oncolytic herpesvirus containing transgenes encoding GALV and Fcy::Fur to determine if it could play a therapeutic role in gastroesophageal cancer. It has already been shown to be cytotoxic in vitro to human fibrosarcoma, colorectal and pancreatic carcinoma, glioblastoma, astrocytoma, and lung epidermoid carcinoma cell lines [21]. The virus construct is unique in that it contains a double deletion of the γ134.5 gene as well as a single deletion of the US12 gene, making it tumor specific and immune response stimulating, respectively. In place of these genes, the gene coding for the GALV fusogenic protein and gene Fcy::Fur were inserted. Expression of the GALV protein caused the infected cells to form large syncytia and has been shown to potentiate spread of virus and efficacy of viral therapy [22].
Table 1 illustrates characteristics of the 6 gastroesophageal cancer cell lines studied. All cell lines were derived from human gastroesophageal tumors or metastases from primary gastric cancer. The OCUM-2MD3, MKN-45, and MKN-74 cell lines, especially, are used frequently in animal models of peritoneal carcinomatosis, having a predilection for peritoneal dissemination. Adhesion characteristics have been studied, and loss of expression of E-cadherin has been implicated in tumor progression and metastasis. All of the cell lines except for BE-3 and MKN-74 have a low level or downregulation of E-cadherin. Further genetic characterizations are listed for each cell line [23–26].
Table 1.
Characteristics of gastroesophageal cancer cell lines.
| Cell Name | Type and Derivation | Tum or formation | Mouse model | Adhesion status | Genetic Characterizations |
|---|---|---|---|---|---|
|
| |||||
| AGS | moderately differentiated adenocarcinoma EBV- | flank tum or | nude mice | low level of E-cadherin expresses LI-cadherin | Expresses Villin 1 LGALS4 |
| OCUM-2MD3 | scirrhous poorly differentiated carcinoma derived from OCUM-2M | peritoneal dissemination | nude mice | downregulation of E-cadherin and integrin β4 | down regulation of squamous cell differentiation marker gene cluster |
| BE3 | Barrett’s esophagus associated esophageal adenocarcinoma from distal esophagus | flank tum or | nude mice | unknown | Expresses pIκκβ(S181) pTSC1(S513) pS6Kl(T389) members of the inflammation associated signaling pathway |
| MKN-1 | adenosquamous carcinoma established from lymph node metastases | flank tum or | nude mice | E-cadherin silenced by promoter hyperm ethylation | missense mutation of p53 |
| MKM-45 | poorly differentiated adenocarcinoma diffuse type | peritoneal dissemination | nude SCID mice | wild type allele of E-cedherin lost | CEA + amplification of c-m et proto-oncogene wild type p53 expresses low levels bcl-2 |
| MKN-74 | intestinal type moderately differentiated tubular adenocarcinoma derived from liver metastases | peritoneal dissemination | nude SCID mice | no genetic alteration to E-cadherin | CEA − wild typep53 does not express bcl-2 |
All of the gastroesophageal cancer cell lines studied were susceptible to viral oncolysis. While the OCUM-2MD3, MKN-74, and AGS cell lines proved to be particularly susceptible with an MOI as low as 0.01, an MOI of 1.0 was cytotoxic in the less sensitive cell lines BE3, MKN-1, and MKN-45. It is evident then that this third-generation, modified, oncolytic herpesvirus would be a useful therapy as a single agent against gastroesophageal cancer, as all cell lines are susceptible to viral oncolysis.
Cell lines with shorter doubling times were more susceptible to viral oncolysis and demonstrated faster cytotoxicity. OCUM-2MD3, MKN-45, and AGS cell lines, the most sensitive of the gastroesophageal cancer cell lines, had doubling times under 30 hours, while the other three cell lines, MKN-74, MKN-1, and BE3, had doubling times over 30 hours [27–30]. This oncolytic herpesvirus would therefore be particularly useful against gastric cancers with fast growth rates, as there is enhanced in vitro sensitivity of cell lines with doubling times under 30 hours. Paradoxically, higher viral titers were achieved in those cell lines with longer doubling times, indicating that immediate cytotoxicity may be detrimental to ultimate viral replication. It also indicates that while some replication is important for viral spread and further infection, a very high level of replication is not necessary for complete killing of cancer.
Additionally, for cell lines with a predisposition for peritoneal dissemination, in particular the OCUM-2MD3 and MKN74 cell lines, this virus appears to be particularly effective in killing. This may prove to be important clinically, as those patients with peritoneal seeding have a high likelihood of recurrence.
CONCLUSION
This study demonstrates that a third-generation, modified, oncolytic herpesvirus can effectively infect, replicate within, and lyse gastroesophageal cancer cells. Cell lines that are fast growing with short doubling times, and cell lines that are particularly tumorigenic in the peritoneum, proved to be the most susceptible. For those unfortunate patients who have metastatic disease or recurrence from gastric cancer, this virus may offer a new therapeutic approach. These data would encourage future clinical trials of this agent as palliative and adjuvant therapies for gastroesophageal cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alberts SR, Cervantes A, van de Velde CJH. Gastric cancer: epidemiology, pathology and treatment. Annals of Oncology. 2003;14:ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 2.Kasuya H, Takeda S, Nomoto S, Nakao A. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Therapy. 2005;12:725–736. doi: 10.1038/sj.cgt.7700830. [DOI] [PubMed] [Google Scholar]
- 3.Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 4.Yonemura Y, Bandou E, Kawamura T. Quantitative prognostic indicators of peritoneal dissemination of gastric cancer. European Journal of Surgical Oncology. 2006;32:602–606. doi: 10.1016/j.ejso.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Gretschel S, Siegel R, Estevez-Schwarz L, et al. Surgical Strategies for gastric cancer with synchronous peritoneal carcinomatosis. British Journal of Surgery. 2006;93:1530–1535. doi: 10.1002/bjs.5513. [DOI] [PubMed] [Google Scholar]
- 6.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended Lymph-Node Dissection for Gastric Cancer. The New England Journal of Medicine. 1999;340:908–14. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 7.Hartgrink HH, van de Velde CJH, Putter H, et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. Journal of Clinical Oncology. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Khan FA, Shukla AN. Pathogenesis and treatment of gastric carcinoma: “An up-date with brief review”. Journal of Cancer Research and Therapy. 2006;2(4):196–199. doi: 10.4103/0973-1482.29830. [DOI] [PubMed] [Google Scholar]
- 9.Ohashi M, Ueno H, Tanaka T, et al. Adenovirus mediated p53 tumour suppressor gene therapy for human gastric cells in vitro and in vivo. Gut. 1999;44:366–371. doi: 10.1136/gut.44.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song TJ, Eisenberg DP, Adusumilli PS, et al. Oncolytic Herpes Viral Therapy is Effective in the Treatment of Hepatocellular Carcinoma Cell Lines. Journal of Gastrointestinal Surgery. 2006;10:532–542. doi: 10.1016/j.gassur.2005.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid V, Yu Z, Schuman T, et al. Herpes oncolytic therapy of salivary gland tumors. International Journal of Cancer. 2007;122:202–208. doi: 10.1002/ijc.23030. [DOI] [PubMed] [Google Scholar]
- 12.Kasuya H, Takeda S, Nomoto S, Nakao A. The potential of oncolytic virus therapy for pancreatic cancer. Cancer Gene Therapy. 2005;12:725–736. doi: 10.1038/sj.cgt.7700830. [DOI] [PubMed] [Google Scholar]
- 13.Kemeny N, Brown K, Covey A, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Human Gene Therapy. 2006;17(12):1214–24. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- 14.Roizman B. The function of herpes simplex virus genes: A primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everts B, Van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Therapy. 2005;12:141–161. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 16.Liu BL, Robinson M, Han Z-Q, et al. ICP34.5 deleted herpes simplex virus with enhanced incolytic, immune stimulating, and anti-tumour properties. Gene Therapy. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 17.Varghese S, Rabkin S. Oncolytic herpes simplex virus vectors for cancer vibotherapy. Cancer Gene Therapy. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 18.Bennett JJ, Kooby DA, Delman K, et al. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. Journal of Molecular Medicine. 2000;78:166–74. doi: 10.1007/s001090000092. [DOI] [PubMed] [Google Scholar]
- 19.Bennett JJ, Delman KA, Burt B, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Therapy. 2002;9:935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 20.Stanziale SF, Stiles BM, Bhargava A, et al. Oncolytic Herpes Simplex Virus-1 Mutant Expressing Green Fluorescent Protein Can Detect and Treat Peritoneal Cancer. Human Gene Therapy. 2004;15:609–618. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- 21.Simpson GR, Han Z, Wang Y, Campbell G, Coffin RS. Combination of a Fusogenic Glycoprotein, Prodrug Activation, and Oncolytic Herpes Simplex Virus for Enhanced Local Tumor Control. Cancer Research. 2006;66(9):4835–42. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 22.Fu X, Tao L, Jin A, et al. Expression of a Fusogenic Membrane Glycoprotein by an Oncolytic Herpes Simplex Virus Potentiates the Viral Antitumor Effect. Molecular Therapy. 2003;7(6):748–54. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 23.Hippo Y, Yashiro M, Ishii M, et al. Differential Gene Expression Profiles of Scirrhous Gastric Cancer Cells with High Metastatic Potential to Peritoneum or Lymph Nodes. Cancer Research. 2001;61:889–895. [PubMed] [Google Scholar]
- 24.Ji J, Chen X, Leung SY, et al. Comprehensive Analysis of the Gene Expression Profiles in Human Gastric Cancer Cell Lines. Oncogene. 2002;21:6549–6556. doi: 10.1038/sj.onc.1205829. [DOI] [PubMed] [Google Scholar]
- 25.Yen CJ, Izzo JG, Lee DF, et al. Bile Acid Exposure Up-regulates Tuberous Sclerosis Complex 1/Mammalian Target of Rapamycin Pathway in Barrett’s-Associated Esophageal Adenocarcinoma. Cancer Research. 2008;68(8):2632–2640. doi: 10.1158/0008-5472.CAN-07-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokozaki H. Molecular Characteristics of Eight Gastric Cancer Cell Lines Established in Japan. Pathology International. 2000;50:767–777. doi: 10.1046/j.1440-1827.2000.01117.x. [DOI] [PubMed] [Google Scholar]
- 27.Mitsuhashi Y, Inaba M, Sugiyama Y, et al. In Vitro Measurement of Chemosensitivity of Human Small Cell Lung and Gastric Cancer Cell Lines toward Cell Cycle Phase-Nonspecific Agents under the Clinically Equivalent Area under the Curve. Cancer. 2006;70(10):2540–2546. doi: 10.1002/1097-0142(19921115)70:10<2540::aid-cncr2820701024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Altorki N, Schwartz GK, Blundell M, et al. Characterization of Cell Lines Established from Human Gastric-Esophageal Adenocarcionmas Biologic Phenotype and Invasion Potential. Cancer. 1993;72(3):649–657. doi: 10.1002/1097-0142(19930801)72:3<649::aid-cncr2820720305>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Feng RH, Zhu ZG, Li JF, et al. Inhibition of human telomerase in MKN-45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World Journal of Gastroenterology. 2002;8(3):436–440. doi: 10.3748/wjg.v8.i3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura K, Yashiro M, Matsuoka T, et al. A Novel Molecular Targeting Compound as K-samII/FGF-R2 Phosphorylation Inhibitor, Ki23057, for Scirrhous Gastric Cancer. Gastroenterology. 2006;131:1530–1541. doi: 10.1053/j.gastro.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman HL, Kim DW, Deraffele G, et al. Local and Distant Immunity Induced by Intralesional Vaccination with an Oncolytic Herpes Virus Encoding GM-CSF in Patients with Stage IIIc and IV Melanoma. Ann Surg Oncol. 2009 Nov 14; doi: 10.1245/s10434-009-0809-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Fong Y, Kim T, Bhargava A, et al. A herpes oncolytic virus can be delivered via the vasculature to produce biologic changes in human colorectal cancer. Mol Ther. 2009;17:389–394. doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]


