Abstract
Chemically functionalized probes are required for tunneling measurements made via chemical contacts (“Recognition Tunneling”). Here, we describe the etching of gold STM probes suitable for chemical functionalization with moieties bearing thiol groups. Insulated with high density polyethylene, these probes may be used in aqueous electrolytes with sub pA leakage currents. The area of the exposed probe surface was characterized via the saturation current in an electroactive solution (0.1 M K3Fe(CN)6). Twenty five percent of the probes had an exposed region of 10 nm radius or less.
INTRODUCTION
The scanning tunneling microscope was applied to imaging surfaces in aqueous electrolyte not long after its invention.1 The key to doing this was to insulate the probe almost completely, leaving only a small amount of metal exposed at its apex. Probes have been etched from Pt or a Pt/Ir alloy and insulated with wax, paint or glass.2, 3, 4, 5, 6, 7 In 2006, Ohshiro and Umezawa demonstrated selective chemical contrast in an STM image using a gold probe fucntionalized with a DNA base.8 We showed that contrast in this mode is not only dominated by adhesion9 but also found that single molecule reads of chemical identity were possible by monitoring stochastic tunneling noise.10, 11, 12, 13, 14 The key to these measurements lies in making sharp gold probes and insulating them with a coating that is resistant to organic solvents. Even if the measurements are carried out in buffered aqueous electrolyte, functionalization is usually carried out in an organic solvent because of the limited solubility of many organic thiols in water. We have described aspects of our experimental techniques in the supplements to several other papers10, 11, 12, 13, 14 but a more detailed description is called for. Here, we describe the etching process that produces gold probes suitable for insulation and functionalization, how these probes are coated with high density polyethylene (HDPE), and finally we describe how they are characterized by electrochemical measurements in a highly electroactive electrolyte that greatly magnifies the leakage currents that would be found in the less active solutions normally used for tunneling measurements.
METHODS
Probe etching
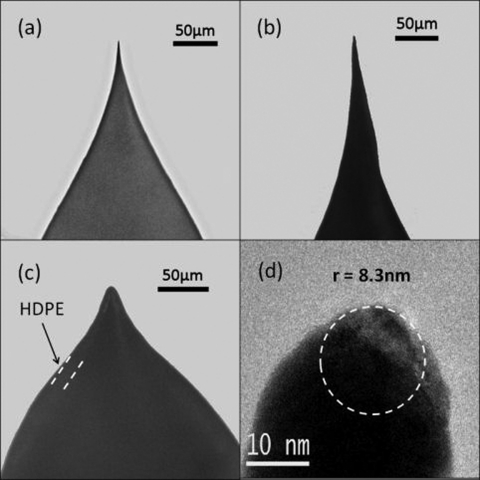
A 0.25 mm diameter gold wire (Aesar, 99.999% pure) is lowered using a precision mechanical translation stage into an etching solution consisting of 1:1 HCl and EtOH.15 A 5 kHz square wave of 40 V peak to peak (generated with a Krohn-Hite 5800 a signal generator and a Yamaha HTR 5920 audio amplifier) is applied between the gold wire and a platinum foil counter electrode. The wire is lowered into the etching solution until the RMS current reaches 400 mA, corresponding to 2 mm–3 mm immersion depth. Etching is continued at constant peak to peak voltage until the current falls to zero. In the second step, the tip is then withdrawn from the solution and the voltage reduced to ∼12 V peak to peak. The gold wire is then quickly immersed in the same solution until it reaches a peak current of 40–80 mA (total time ∼0.25 s) and then immediately pulled out of the etching solution. This two-step process produces a probe shape like that shown in the optical micrograph in Fig. 1a. A sharp apex sits on top of a cone of ∼25° half-angle. The broad cone is required to support the high density polyethylene (HDPE) coating while the sharp apex penetrates it. It is important that the probe surface be smooth and the apex sharp (i.e., no obvious end resolvable under 250× magnification). An example of a sharp probe that cannot be insulated properly is shown in Fig. 1b. Etches are repeated until the desired shape is achieved, either from the first (40 V) or the second (12 V) step depending on whether the overall shape is bad (Fig. 1b) or the end just needs further sharpening. Satisfactory probes are rinsed with EtOH and blown dry with nitrogen gas.
Figure 1.
Profiles of the etched and insulated gold probes. Optical images under 250× magnification of (a) a good etched gold STM probe which is smooth, straight, and sharp such that it reaches an apex of radius less than 1 μm, (b) a poor etched gold STM probe which is not smooth or sharp, such that the apex of this tip is visibly rounded, and (c) a good coated STM probe with a smooth and continuous coating which comes to a point at the apex, and has no visible protrusions there. An TEM image (d) of typical good STM probe with radius of curvature equal to 8.3 nm in this case.
Polymer coating
Etched probes are immersed in 1:3 H2O2 and H2SO4 (“piranha”) solution for 1–2 min (caution, piranha etch can react explosively with organics) then rinsed with H2O, EtOH, and air dried with N2 gas. Alfa Aesar high density polyethylene granules (with no traceable amounts of plasticizers) are melted onto the platform of tip coating instrument,2 previously heated to 270–280 °C. When the polymer melt is clear, the gold tip is pushed up through the molten blob at a rate of 30 μm/s–100 μm/s. Once the HDPE begins to harden on the apex of the probe (now above the reservoir of melted polymer) the gold tip is withdrawn to below the level of the melted HDPE and the first step repeated. A good quality STM probe has a HDPE coating that comes to a point at the apex, but has no visible protrusions there. The coating should be smooth and continuous along the length of the probe, and the gold probe within the HDPE must be straight to the top of the coating. The coating should be at least 5 μm thick near the apex of the tip, and thicker about the remainder of the gold probe (Fig. 1a). A poor quality tip may have clearly visible sharp protrusions from the apex of the coating. If still sharp, this tip may be re-coated in order to reduce the amount of visible protrusion. A poor quality tip may also be bent such that the exposure of the tip is not at the top of the coating (or it is leaving it completely insulated). Such probes are discarded.
Leakage measurements
An aqueous solution of 100 mM K3Fe(CN)6 in 1 M KCl was prepared using 18.2 MΩ water. All solutions were deoxygenated with argon gas for 1–2 min before use. The high concentration of K3Fe(CN)6 ensures that the Fe(CN)6 is the most significant electroactive species. This minimizes contamination from trace oxygen or other possible contaminants, and it allows us to measure small exposed surface areas. Repeated cycling of the probes in this solution shows that the HDPE is stable. We prepared Ag/AgCl wires as reference electrodes by immersing clean silver wire in concentrated HClO4 solution for 1 h followed by overnight immersion in 4 M saturated KCl solution. Before use, the Ag/AgCl wire is rinsed with water and dried with N2 gas (http://www.warneronline.com/pdf/whitepapers/chloriding_wire.pdf). Electrodes formed in this way exhibit some small drift in potential, but this is unimportant for the present application. Cyclic voltammograms were obtained using the IV spectroscopy function of a scanning tunneling microscope (Agilent PicoSPM) in a two electrode configuration in which the gold probe was biased relative to the Ag/AgCl reference and the current through the probe recorded using the built-in 1 nA/V current to voltage converter. The two electrode configuration is stable at the small (nA) currents recorded here. We wrote a LabVIEW program to drive the spectroscopy sweeps in order to achieve greater control over the sweep direction, starting bias, sweep speed, etc. The CVs were found to be independent of scan rate at high concentrations of electrolyte. The data we show here are taken at 100 mV/s.
RESULTS AND DISCUSSION
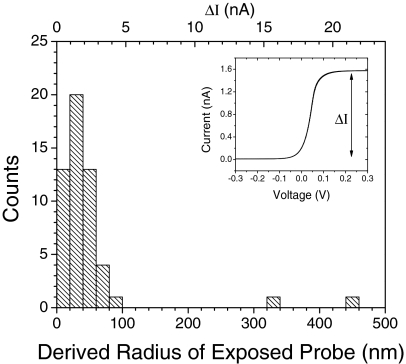
Most importantly, probes must have a small exposed metal surface area in order to minimize leakage current.11, 16 We have also noticed that pick-up of ac noise correlates with dc leakage.17 Presumably, thin layers of insulation lead to greater interfacial capacitance and lower impedance path for ac coupling (see for example, the 120 Hz spikes in Fig. 2b of Huang et al.13). The radius of the exposed surface area of the tip, r, can be obtained from the saturation current, ΔI, in an ultramicroelectrode voltammogram (see the inset, Fig. 2) using ΔI = 4πnFDCr where n = 1 for a 1 electron transfer (FeIII ⇔ FeII), F is the Faraday constant, D is the ion diffusion constant (taken to be 7.6 × 10−6 cm2 s−1) (Ref. 18), and C is the concentration of the redox active species in moles/cm3. The formula assumes a hemispherical surface.18 Figure 2 shows a distribution of the values of the radii for the exposed areas determined from these measurements. Most commonly we obtain coated STM probes of 20 nm–40 nm exposed-radius, although ∼25% of the probes have a measured radius of 10 nm or less. This small exposed surface area ensures that the dominant phenomenon in a tunneling measurement is that of electron tunneling.
Figure 2.
Distribution of leakage currents and corresponding exposed radii. The saturation current (ΔI) of coated STM probes was measured by cyclic voltammetry in 100 mM K3Fe(CN)6 in 1 M KCl (see inset).
Leakage in electrolytes used for tunneling measurements (typically 1 mM phosphate buffer) is much smaller than the nA leakages measured in 0.1 M potassium ferricyanide—typically 1 pA–20 pA at – 0.5 tip bias. While it is reduced by roughly the ratio of the concentrations of the phosphate to ferricyanide, there is no correlation between the measured leakage in K3Fe(CN)6 and the leakage measured in phosphate buffer. This is because the leakage is almost certainly dominated by small amounts of contamination (such as dissolved oxygen) that is more electroactive than the phosphate buffer. We tested the durability of the probes by measuring leakage in K3Fe(CN)6 before and after prolonged immersion in phosphate buffer (>9 h) under bias (0.5 V) and also before and after potential sweeps over ± 0.5 V at rates between 1 and 1000 mV/s. No change in the probes was detected.
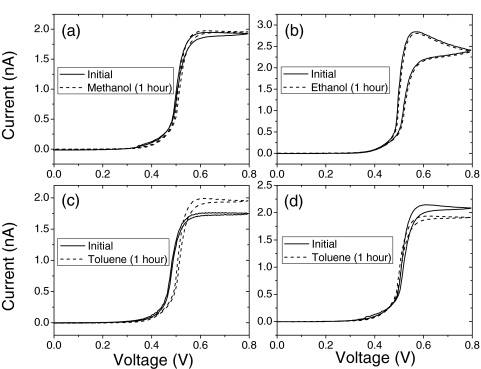
We turn finally to functionalization of the probes. As mentioned earlier, this usually requires the use of an organic solvent to dissolve a thiolated organic reagent. We placed the coated STM probes in methanol, ethanol, and toluene for 1 h at room temperature. The STM probes measured exposed surface area did not change appreciably after exposure to the alcohols (Figs. 3a, 3b) In addition, the coatings are stable in air and H2O for long periods of time, as long as organic contamination build up can be safely removed from the surface of the probe. In toluene, we found both small increases and decreases in exposed surface area (Figs 3c, 3d). Toluene dissolves bulk polyethylene at elevated temperatures (88–110 °C) (Ref. 19) so it seems likely that there will be some solvent penetration at much lower temperatures around a small opening in the film where more chain ends are exposed. The solvent could swell the film to lower leakage, or cause it to part from the metal, thereby increasing it.
Figure 3.
Stability of the coated probes in solvents. Cyclic voltammagrams of high density polyethylene coated gold STM probes exposed to various solvents (a - methanol, b - ethanol, c and d - toluene) for 1 h. Measurements taken in 100 mM K3Fe(CN)6 in 1 M KCl.
We functionalized the probes with three thiol-terminated reagents: 1 mM 1-octanethiol20 in toluene, 1 mM 4-mercaptobenzamide13 in ethanol, and 1 mM 4(5)-(2-thioethyl)imidazole-2-carboxamide21 in methanol. Insulated probes were cleaned prior to functionalization by rinsing with ethanol and H2O, blown dry with nitrogen gas, and then immersed in solutions of the thiolated reagents for 1 h. We were able to test the efficiency of the functionalization process by making recognition tunneling measurements on a functionalized gold surface, and comparing the tunneling data to controls in which the probe was functionalized, but the substrate was left bare. In all cases one functionalized electrode produced a different tunneling signal from two functionalized electrodes (see, for example, the data in Chang et al.22). Thus, we are confident that the procedure described above results in functionalization of the probes with almost 100% yield provided that the probes are satisfactorily etched and coated in the first place.
Table 1 lists the relative change in leakage current of the probes as measured in 0.1 M in K3Fe(CN)6 before and after functionalization. Interestingly, all probes functionalized with 1-octanethiol in toluene show an increase in leakage, while toluene alone caused both increases and decreases. Thus, although the monolayer should block current, any blocking is overwhelmed by damage to the coating caused by the combined effect of the thiol and the solvent. Note, however, that the leakage of these probes is much less in a less electroactive solution, so they are suitable for tunneling measurements down to few pA. Depositions done in the two alcohols (Table 1) resulted in both increase and decrease in leakage, and the two groups were separated for analysis. Most of the probes showed an increase in leakage suggesting that the thiols destabilize the adhesion of the HDPE layer. Nonetheless, leakage is still relatively small and almost nonexistent in 1 mM phosphate buffer solutions.
Table 1.
Relative change in leakage current measured in 0.1 M K3Fe(CN)6 after functionalization of the probes. Numbers <1 correspond to a decrease in leakage on functionalization.
| Change in | ||
|---|---|---|
| Reagent | leakage (nA) | N |
| 1-Octanethiol in toluene | 4.8 ± 4 | 7 |
| 4-Mercaptobenzamide in methanol | 0.1 ± 0.1 | 2 |
| 4-Mercaptobenzamide in methanol | 1.6 ± 1.6 | 6 |
| 4(5)-(2-Thioethyl)imidazole-2-carboxamide in ethanol | 1.0 | 1 |
| 4(5)-(2-Thioethyl)imidazole-2-carboxamide in ethanol | 0.6 ± 0.7 | 7 |
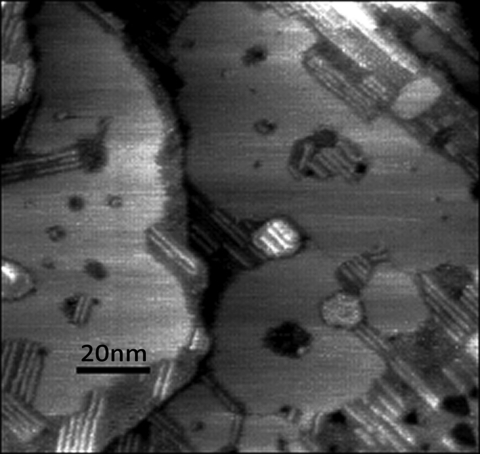
Finally, we note that, despite the softness of these gold probes, they are capable of molecular resolution in aqueous electrolyte, as shown in Fig. 4.
Figure 4.
STM image of a mixed monolayer showing stacks of 4-mercaptobenzamide molecules (rod-like features) embedded in an octanethiol monolayer. The image was taken in 10 mM phosphate buffer solution (pH 7.0) at a tip bias of −0.1 V and a tunneling current of 6 pA using one of the insulated, unfunctionalized gold probes.
In conclusion, we have shown that HDPE-coated gold STM probes are stable in a variety of working conditions, giving low leakage currents in buffered aqueous electrolytes, even after functionalization with thiolated reagents, provided that the correct etching and coating procedures are followed. The yield of low-leakage (∼1 pA in 1 mM buffer) stable, functionalized probes is high enough (>95%) to permit routine measurement of tunneling signals mediated by molecules stably attached to the probe.
ACKNOWLEDGMENTS
This work was supported by grants from the Sequencing Technology Program of the National Human Genome Research Institute (HG004378 and HG006323). We acknowledge useful discussions with Dr. Peiming Zhang and Shuo Huang.
References
- Sonnenfeld R. and Hansma P. K., Science 232, 211 (1986). 10.1126/science.232.4747.211 [DOI] [PubMed] [Google Scholar]
- Nagahara L. A., Thundat T., and Lindsay S. M., Rev. Sci. Instrum. 60, 3128 (1989). 10.1063/1.1140590 [DOI] [Google Scholar]
- Zhang B. and Wang E., Electrochim. Acta 39, 103 (1994). 10.1016/0013-4686(94)85015-1 [DOI] [Google Scholar]
- Mao B. W., Ye J. H., Zhuo X. D., Mu J. Q., Fen Z. D., and Tian Z. W., Ultramicroscopy 42–44, 464 (1992). 10.1016/0304-3991(92)90308-7 [DOI] [Google Scholar]
- Schulte A. and Chow R., Anal. Chem. 68, 3054 (1996). 10.1021/ac960210n [DOI] [PubMed] [Google Scholar]
- Bach C., Nichols R., Meyer H., and Besenhard J., Surf. Coat. Technol. 67, 139 (1994). 10.1016/0257-8972(94)90112-0 [DOI] [Google Scholar]
- Slevin C., Gray N., Macpherson J., Webb M., and Unwin P., Electrochem. Commun. 1, 282 (1999). 10.1016/S1388-2481(99)00059-4 [DOI] [Google Scholar]
- Ohshiro T. and Umezawa Y., Proc. Natl. Acad. Sci. U.S.A. 103, 10 (2006). 10.1073/pnas.0506130103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., He J., Kibel A., Lee M., Sankey O. F., Zhang P., and Lindsay S. M., Nat. Nanotechnol. 4, 297 (2009). 10.1038/nnano.2009.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., He J., Lin L., Zhang P., Liang F., Young M., Huang S., and Lindsay S., Nanotechnology 20, 185102 (2009). 10.1088/0957-4484/20/18/185102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Huang S., He J., Liang F., Zhang P., Li S., Chen X., Sankey O. F., and Lindsay S. M., Nano Lett. 10, 1070 (2010). 10.1021/nl1001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Chang S., He J., Zhang P., Liang F., Tuchband M., Li S., and Lindsay S., J. Phys. Chem. C 114, 20443 (2010). 10.1021/jp104792s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., He J., Chang S., Zhang P., Liang F., Li S., Tuchband M., Fuhrman A., Ros R., and Lindsay S. M., Nat. Nanotechnology 5, 868 (2010). 10.1038/nnano.2010.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S., He J., Sankey O., Hapala P., Jelinek P., Zhang P., Chang S., and Huang S., Nanotechnology 21, 262001 (2010). 10.1088/0957-4484/21/26/262001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B., Picardi G., and Pettinger B., Rev. Sci. Instrum. 75, 837 (2004). 10.1063/1.1688442 [DOI] [Google Scholar]
- Conyers J. and White H. S., Anal. Chem. 72, 4441 (2000). 10.1021/ac000399+ [DOI] [PubMed] [Google Scholar]
- Abadal G., Perez-Murano F., Barniol N., and Aymerich X., IEEE Trans. Instrum. Meas. 52, 859 (2003). 10.1109/TIM.2003.814683 [DOI] [Google Scholar]
- Bard A. J. and Faulkner L. R., Electrochemical Methods: Fundamentals and Applications (Wiley, New York, 1980). [Google Scholar]
- Boutevin B., Lusinchi M., Pietrasanta Y., and Robin J. J., Polym. Eng. Sci. 36, 879 (1996). 10.1002/pen.10475 [DOI] [Google Scholar]
- Bain C. D., Troughton E. B., Tao Y. T., Evall J., Whitesides G. M., and Nuzzo R. G., J. Am. Chem. Soc. 111, 321 (1989). 10.1021/ja00183a049 [DOI] [Google Scholar]
- Liang F., Li S., Lindsay S., and Zhang P., “Studies on Chemical and Hydrogen Bonding Properties of Imidazole-2-carboxamide for Use in DNA Sequencing by Recognition Tunnelling,” Chemistry (submitted). [DOI] [PMC free article] [PubMed]
- Chang S., He J., Zhang P., Gyarfas B., and Lindsay S., J. Am. Chem. Soc. 133, 14267 (2011). 10.1021/ja2067737 [DOI] [PMC free article] [PubMed] [Google Scholar]