Abstract
Maternal endothelial dysfunction in preeclampsia is associated with increased soluble fms-like tyrosine kinase-1 (sFlt-1), a circulating antagonist of vascular endothelial growth factor and placental growth factor. Angiotensin II (Ang II) is a potent vasoconstrictor that increases concomitant with sFlt-1 during pregnancy. Therefore, we speculated that Ang II may promote the expression of sFlt-1 in pregnancy. Here we report that infusion of Ang II significantly increases circulating levels of sFlt-1 in pregnant mice, thereby demonstrating that Ang II is a regulator of sFlt-1 secretion in vivo. Furthermore, Ang II stimulated sFlt-1 production in a dose- and time-dependent manner from human villous explants and cultured trophoblasts but not from endothelial cells, suggesting that trophoblasts are the primary source of sFlt-1 during pregnancy. As expected, Ang II–induced sFlt-1 secretion resulted in the inhibition of endothelial cell migration and in vitro tube formation. In vitro and in vivo studies with losartan, small interfering RNA specific for calcineurin and FK506 demonstrated that Ang II–mediated sFlt-1 release was via Ang II type 1 receptor activation and calcineurin signaling, respectively. These findings reveal a previously unrecognized regulatory role for Ang II on sFlt-1 expression in murine and human pregnancy and suggest that elevated sFlt-1 levels in preeclampsia may be caused by a dysregulation of the local renin/angiotensin system.
Keywords: sFlt-1, angiotensin II, AT1 receptor, pregnancy, placenta, calcineurin
The human placenta is principally a vascular organ that functions to achieve a physiological union of the maternal and fetal blood supplies. A major physiological role of the placenta is to develop an extensive vascular network allowing for nutrient, waste, and gas exchange between the maternal and fetal circulations. To accomplish this, the placenta produces a variety of angiogenic factors including vascular endothelial growth factor (VEGF) and placental growth factor.1 Cytotrophoblasts invade the maternal spiral arterioles and acquire properties of endothelial cells in a process termed pseudovasculogenesis.2 The balance between proangiogenic and antiangiogenic factors modulates this vascular remodeling. VEGF promotes angiogenesis through interaction with 2 high-affinity receptors, VEGF receptor-1 (VEGFR-1, also known as Flt-1) and VEGF receptor-2 (VEGFR-2, also known as KDR/Flk-1). The production of antiangiogenic factors increases toward the end of gestation, when continued placental growth is no longer needed. One such antiangiogenic factor is a soluble form of VEGFR-1 (sFlt-1) that binds to circulating VEGF and placental growth factor, thereby inhibiting their angiogenic activities.
It was first proposed in 1997 that low levels of VEGF in maternal decidua may be a contributory factor in preeclampsia because of increased levels of endogenous sFlt-1, which may antagonize the beneficial effects of VEGF in this disorder.3 Subsequently, a number of groups demonstrated the production of sFlt-1 from human placental explants1,4 and cultured cytotrophoblasts.5,6 Circulating levels of sFlt-1 increase significantly during the last 2 months of pregnancy7–9 but are present only in trace quantities in sera from nonpregnant women. Soluble Flt-1 is increased in the maternal circulation in preeclampsia before onset of clinical manifestations9,10 and correlates with severity of the disease.11 Over-expression of sFlt-1 in pregnant rats results in a preeclampsia-like phenotype.12 Furthermore, conditioned medium (CM) from human placental explants obtained from uncomplicated pregnancies induces in vitro angiogenesis, whereas CM from preeclamptic placentas inhibits angiogenesis.4,12 More importantly, removal of sFlt-1 from preeclamptic placenta CM promotes angiogenesis, suggesting that placental vascular growth may be restricted in preeclampsia because of an imbalance of pro- and antiangiogenic factors.1,4 However, the factors regulating the synthesis and secretion of sFlt-1 during pregnancy are largely unknown.
The presence of a local renin/angiotensin system has been detected in many tissues, including the brain, kidney, adrenal gland, and heart.13 In the placenta, the local generation of Ang II is responsible for the activation of Ang II type 1 (AT1) receptors present on trophoblasts.14,15 In mice, the renin/angiotensin system undergoes major changes in response to pregnancy.15 One of the most striking changes is a 5- to 10-fold rise in the levels of circulating prorenin and renin,15 which is associated with increased renin gene expression in placenta. As the increase in placental renin gene expression is concomitant with the increase in sFlt-1, we speculated that the production of Ang II in the placenta could regulate sFlt-1 expression. In this study, we report that in pregnant mice, infusion of Ang II induces sFlt-1 secretion, demonstrating that Ang II is a regulator of sFlt-1 secretion in vivo via AT1 receptor activation and calcineurin signaling.
Materials and Methods
Cell Culture
HTR-8/SVneo cells, an immortalized line of human trophoblasts,16 were cultured in RPMI medium 1640 (Invitrogen, Grand Island, NY) supplemented with 5% FBS and 1% antibiotic–antimycotic solution. Human umbilical vein endothelial cells (HUVECs) were grown in endothelial growth medium (EGM)-2 medium (Cambrex). All cells were cultured at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Ang II Treatment and Collection of Conditioned Medium
For sFlt-1 induction, HTR-8/SVneo cells were plated in 12-well plates at 1×105 cells per well overnight. The next day, cells were changed to serum-free medium and treated with Ang II for 4 days. To ensure that an adequate concentration of Ang II was maintained throughout the experiments, fresh Ang II was added each day. The cells and their CM were collected and centrifuged at room temperature for 15 minutes at 1000g. Cell pellets were collected for Western blotting. In some cases, cells were preincubated with 100 nmol/L losartan (Merck & Co Inc) or FK506 (Fujisawa Pharmaceutical, Osaka, Japan) for 30 minutes before addition of Ang II. To deplete sFlt-1 from CM, CM was first incubated with monoclonal anti-Flt-1 antibody (1:250 dilution) for 2 hours at 4°C before adding Protein-A beads overnight as previously described.4
Tissue Collection and Villous Culture With Ang II Treatment
Human placental tissue was collected as previously described.17,18 After dissection, 5 small fragments (15 to 20 mg wet weight) of placental villi were dissected from the placenta, teased apart, and placed on 24-well plates. Placental villous explants were cultured in phenol red–free DMEM medium (Sigma Ltd, Poole, UK) supplemented with 100 μg/mL streptomycin, 100 U/mL penicillin, and 0.2% BSA, in 5% CO2 at 37°C. Ang II was incubated with villous fragments for up to 72 hours at 37°C. For the inhibitor studies, losartan was added at 100 nmol/L for 30 minutes before stimulation with Ang II. After 24 hours, the conditioned media were collected and stored at −20°C for further analysis.
sFlt-1 ELISA
Soluble Flt-1 levels in sera and CM were measured using either a commercial kit (R&D Systems, Minneapolis, Minn) or an in-house ELISA.
Semiquantitative RT-PCR
Total RNA was isolated by TRIzol reagent as described previously.16 Superscript 1-step RT-PCR with Platinum Taq kit (Invitrogen) was used for reverse transcriptase-polymerase chain reaction (RT-PCR) according the protocol provided by the vendor. Flt-1 primer sequences for RT-PCR were as described19: sense primer, 5′-TTTGCATAGCTTCCAATAAAGTTG-3′; antisense primer, 5′-CATGACAGTCTAAAGTGGTGGAAC-3′. β-Actin was used as internal control and primer sequences as following: sense primer, 5′-GCTCTGGCTCCTAGCACCAT-3′; antisense primer, 5′-CCACCGATCCACACAGAGTAC-3′. RT-PCR products were run on 2% agarose gels and quantified using Bio-Rad Quantity One software. Flt-1 mRNA expression was represented by the ratio Flt-1/β-actin mRNA.
Quantitative Real-Time RT-PCR
Following stimulation villous explant, tissue samples were homogenized using a PreCellys 24 homogenizer in 1 mL of TRIzol, and the RNA was extracted, further purified, and subjected to DNase-1 digestion on RNeasy mini columns (Qiagen). Total RNA (1 μg) was reverse transcribed with oligo-dT18 primers using the cDNA Synthesis Kit (Bioline) for 70 minutes at 48°C. Real-time PCR was performed using SYBR green PCR SensiMix (Quantace) on a Corbett Research Rotagene RG-3000 under the following conditions: 95°C, 10 seconds; 58°C, 15 seconds; 72°C, 15 seconds; and fluorescence was quantified after 85°C, 15 seconds, for 40 cycles. Triplicate cDNA samples and standards were amplified with primers specific for sFlt-1 (sense, 5′-AGGAGATGCTCCTCCCAAA-3′; antisense, 5′- GTGCAGGGATCCTCCAAAT-3′; 147-bp product) or β-actin, as described previously.20 The mean threshold cycle (CT) for each sample was compared against standard curves produced using 6 serial dilutions of a plasmid containing a C-terminal fragment of sFlt-1 or β-actin cDNA, and relative sFlt-1 expression was calculated following normalization to β-actin.
Western Blot Analysis
Proteins were analyzed by Western blots as described.16
In Vitro Migration
The in vitro cell migration assay was preformed as described.21 Briefly, HUVECs (2.5×104 per well) were prepared with 500 μL of EGM-2 basal medium and added to the inserts of the 24-well plates. CM was used as a chemoattractant and added to the outside of the insert chamber in triplicate (BD Biosciences). After 24 hours of incubation at 37°C, migrated cells were stained with Quick-diff and counted. Four to 10 fields were counted and the mean recorded.
In Vitro Tube Formation
The endothelial cells in vitro tube formation assay was performed as described12 using 96-well plates coated with growth factor-reduced Matrigel (BD Biosciences) and incubated at 37°C for 30 minutes. HUVECs, at a density of 2×104 cells per well, were incubated with CM and plated on precoated 96-well plates and incubated at 37°C in a CO2 incubator. Images were taken after 4 hours of incubation, and the total tube length per field was measured using Metaphore software. Five to 10 fields were used to determine the mean tube length per field.
Transfection of Small Interfering RNA
HTR cells (1.0×105) cells were transfected with 3 μg of small interfering RNA (siRNA) specific for calcineurin catalytic subunit α (CN) or nonspecific target (DHARMACON) using RNAiFect transfection reagent (Qiagen). After 48 hours posttransfection, cells were cultured in serum-free medium and treated with Ang II for 72 hours. Secreted sFlt-1 levels in cell culture were measured by ELISA.
Animals
C57BL/6J pregnant or nonpregnant mice (18 to 22 g; Harlan, Indianapolis, Ind) were anesthetized with sodium pentobarbital (50 mg/kg IP), and osmotic minipumps (Alzet model 2001; Alza, Palo Alto, Calif) were implanted subcutaneously in the nape of the neck. All animal studies were reviewed and approved by the Animal Welfare Committee, University of Texas Houston Health Science Center. Ang II was delivered at a rate of 1.5 mg/kg body weight per day into 10-day pregnant mice or nonpregnant female mice for 7 days.22–25 Control mice were infused with saline. For calcineurin pathway experiments, the calcineurin inhibitor FK506 (2 mg/kg body weight per day) was given daily by intraperitoneal injection 24 hours after the minipump was implanted until mice were euthanized. Mouse systolic blood pressure was measured using mouse tail-cuff (AD instruments, Colorado Springs, Colo).
Statistical Analysis
All values are expressed as the mean±SEM. Statistical significance was determined by ANOVA test using GraphPad Prism software, and significance set at a probability value of P<0.05.
Results
sFlt-1 Secretion Is Induced in Pregnant Mice Infused With Ang II
Circulating levels of sFlt-1 rise during the second half of pregnancy.6,8,19,26 To determine whether Ang II can stimulate sFlt-1 production in vivo, Ang II was infused into pregnant (gestation day 10) and nonpregnant mice and the levels of sFlt-1 measured in the maternal circulation at different time points. Ang II infusion resulted in significantly increased circulating levels of sFlt-1 in the pregnant mice by day 5 (gestation day 15) and sFlt-1 levels remained significantly above the saline-treated pregnant animals (Figure 1). In contrast, Ang II infusion in nonpregnant mice did not result in increased levels of sFlt-1 in the circulation or adverse outcome. These results provide the first direct evidence for the ability of Ang II to stimulate an increase in sFlt-1 secretion in the maternal circulation during normal pregnancy, a finding consistent with the view that during pregnancy sFlt-1 is primarily derived from trophoblasts.4
Figure 1.

Ang II infusion induces sFlt-1 secretion in pregnant mice. Ang II (1.5 mg/kg body weight per day) was infused via subcutaneous osmotic minipump into 10-day pregnant mice (n=5) or nonpregnant female mice (n=7) for 7 days. Control mice (n=5) were infused with saline. Sera were collected at different time points, as indicated, and the concentration of sFlt-1 was determined by ELISA. *P<0.01 vs saline-infused nonpregnant mice (infusion days 5 and 7). **P<0.05 vs saline-infused pregnant mice (infusion days 5 and 7).
Ang II Stimulates the Release of sFlt-1 From Human Placenta via AT1 Receptor Activation
As infusion of Ang II induced the release of sFlt-1 in pregnant mice, we investigated the ability of Ang II to stimulate the release of sFlt-1 from normal human placenta. Placental villous explants were treated with increasing concentrations of Ang II (10 to 100 nmol/L) for 48 and 72 hours, and the CM was analyzed by ELISA. Ang II induced a concentration- and time-dependent increase in the levels of sFlt-1 (Figure 2A). Pretreatment of villous explants with 100 nmol/L losartan, an AT1 receptor specific antagonist, blocked Ang II–induced release of sFlt-1 observed at 72 hours (Figure 2B). Consistent with these findings, real-time PCR revealed a ≈3-fold increase in sFlt-1 transcripts after 24 hours of Ang II stimulation, and this was attenuated by pretreatment with losartan (Figure 2C), indicating that Ang II via AT1 receptor stimulates an increase of sFlt-1 mRNA abundance in human placenta.
Figure 2.
Ang II induces the release of sFlt-1 from human placental explants via AT1 receptor activation. A, Normal placental villous explants were incubated in medium containing 0.2% BSA and treated with Ang II (10 to 1000 nmol/L). The CM was collected at 48 and 72 hours, and levels of sFlt-1 were determined by ELISA. B, Normal villous explants were pretreated with 100 nmol/L losartan, before treatment with Ang II (100 nmol/L) for 72 hours, and CM was assayed for levels of sFlt-1. C, Real-time PCR analysis for sFlt-1 mRNA on villous explants pretreated with 100 nmol/L losartan, before stimulation with Ang II (100 and 1000 nmol/L) for 24 hours. Values represent expression of sFlt-1 mRNA normalized to β-actin mRNA and are expressed as relative threshold cycle number (CT) value. *P<0.05 vs control, **P<0.05 vs 10 nmol/L Ang II treatment, ***P<0.05 vs 48 hours of Ang II treatment.
Ang II Induces sFlt-1 Release via AT1 Receptor Activation in Human Trophoblasts
Previous reports have shown that sFlt1 is detected in the trophoblasts and endothelium of placenta.4,27,28 To identify the cell types responsible for Ang II–mediated sFlt-1 release in the placenta, levels of sFlt-1 were measured in endothelial cells and trophoblasts CM following Ang II stimulation. Interestingly, Ang II induced a time- and concentration-dependent increase in sFlt-1 in an immortalized human trophoblast line (HTR-8/SVneo cells) but not in HUVECs (Figure 3A and 3B), demonstrating that Ang II only induces sFlt-1 expression in trophoblast, which is inhibited by losartan (Figure 3C through 3F). The increase in the level of secreted sFlt-1 was accompanied by an increase in the abundance of sFlt-1 protein and mRNA levels (Figure 3D through 3G). These results indicate that Ang II mediated AT1 receptor activation results in an increase in the synthesis of sFlt-1 by human trophoblasts but not in endothelial cells.
Figure 3.
Ang II induces the synthesis and secretion of sFlt-1 by human trophoblasts but not endothelial cells via AT1 receptor activation. A, HTR-8/SVneo trophoblasts and HUVECs were treated for 72 hours with increasing concentrations of Ang II (10 to 1000 nmol/L), and CM was assayed for levels of sFlt-1. Data are expressed as mean±SEM of 3 independent experiments performed in triplicate. **P<0.01 vs control. B, HTR-8/SVneo trophoblasts were treated with 100 nmol/L Ang II for various times, and the sFlt-1 concentration in CM was determined by ELISA. Each sample was analyzed in triplicate, and each experiment was repeated at least 3 times. *P<0.05 vs control, **P<0.05 vs 2-day Ang II treatment, ***P<0.05 vs 3-day Ang II treatment. C through G, HTR-8/SVneo cells were treated with 100 nmol/L Ang II for 4 days in the presence or absence of 100 nmol/L losartan. *P<0.02 vs control, **P<0.01 vs Ang II treatment. C, ELISA was used to quantify the sFlt-1 concentration in CM. D, Cellular extracts were analyzed by Western blotting to determine the level of sFlt-1 protein using monoclonal mouse anti–Flt-1 (1:1000 dilution) antibody. E, sFlt-1 protein levels were normalized to β-actin and represented as a ratio of sFlt-1/β-actin from densitometric analysis of multiple experiments. Data are expressed as mean±SEM. *P<0.05 vs control. F and G, Total RNA was isolated from HTR-8/SVneo cells treated for 4 days with 100 nmol/L Ang II. RT-PCR products were visualized by electrophoresis on 2% agarose gels, and semiquantitative RT-PCR was used to quantify Flt-1 mRNA normalized to that of β-actin mRNA abundance. Ratios of Flt-1 mRNA/β-actin were obtained by densitometric analysis. *P<0.05 vs control, **P<0.05 vs Ang II treatment.
AT1 Receptor Activation Induces sFlt-1 Release via the Calcineurin Pathway
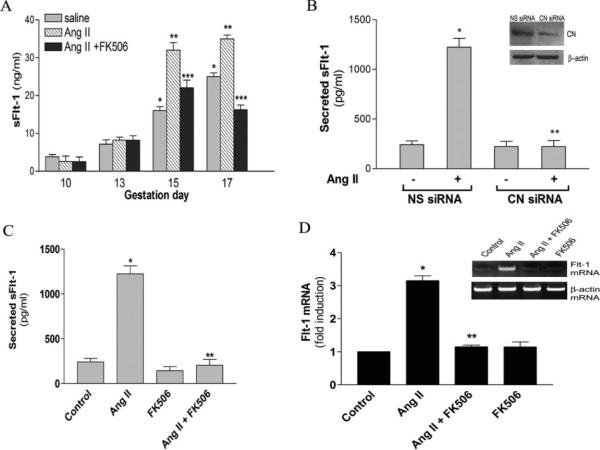
Calcineurin is among many signaling molecules that may function downstream of AT1 receptor activation. To test the involvement of calcineurin in sFlt-1 production, pregnant mice were infused with Ang II with or without daily subcutaneous injection of FK506, a well-known selective calcineurin inhibitor.29,30 As shown in Figure 4A, the increased sFlt-1 secretion induced by Ang II at gestation days 15 and 17 was completely inhibited by FK506, indicating that the induction of sFlt-1 by Ang II is mediated through calcineurin signaling in vivo. We also found that FK506 is able to attenuate the elevated systolic blood pressure and reduced fetal growth resulting from Ang II infusion (Table). Consistently, we showed that sFlt-1 induction by Ang II in human trophoblasts was inhibited by siRNA specific for calcineurin catalytic subunit α (Figure 4B). As shown in the inset of Figure 4B, siRNA specific for calcineurin catalytic subunit α successfully reduced calcineurin α protein levels (Figure 4B, inset) in human trophoblasts. Similarly, we found that Ang II–mediated induction of sFlt-1 secretion and sFlt-1 protein and mRNA expression in cultured human trophoblasts was inhibited by FK506 (Figure 4C and 4D). These results indicate that calcineurin signaling functions downstream of AT1 receptor activation and is essential for Ang II–mediated stimulation of sFlt-1 synthesis and secretion from human trophoblasts.
Figure 4.
Calcineurin signaling functions downstream of the AT1 receptor to mediate Ang II-induced sFlt-1 production by from pregnant mice (A) and human trophoblasts (B through D). A, Subcutaneous osmotic minipump was used to deliver saline or Ang II (1.5 mg/kg body weight per day) to pregnant mice beginning at gestation day 10. FK506 (2 mg/kg body weight per day) was injected intraperitone-ally daily into pregnant mice (n=3 to 5) from gestation days 10 to 17. *P<0.05 vs control saline-infused pregnant mice (10 days of gestation). **P<0.05 vs saline-infused mice at each gestational time point. ***P<0.05 vs Ang II–infused pregnant mice (days 15 and 17 of gestation). B, HTR-8/SVneo cells were transfected with nonspecific siRNA (NS siRNA) and specific siRNA for calcineurin catalytic subunit α (CN siRNA). The cellular level of calcineurin catalytic subunit α were evaluated by Western blot analysis using mouse monoclonal anti-calcineurin catalytic subunit α antibody (1:5000 dilution; Sigma) after 48 hours of transfection. A typical result is shown in the inset. The transfected cells (48 hours of posttransfection) were treated with Ang II for 4 days, and the sFlt-1 secretion was measured by ELISA. C, HTR-8/SVneo cells were treated with 100 nmol/L Ang II for 4 days in the presence or absence of FK506. Secreted sFlt-1 in CM was determined by ELISA. D, inset, Semiquantitative RT-PCR was used to measure Flt-1 mRNA. Flt-1 mRNA abundance was normalized to β-actin mRNA abundance. Ratio of Flt-1 mRNA/β-actin was obtained by densitometric analysis. All data are expressed as mean±SEM. *P<0.02 vs control, **P<0.01 vs Ang II treatment.
Table.
Ang II Infusion Into Pregnant Mice Leads to Increased Systolic Blood Pressure and Restricted Fetal Growth
| Pregnant Mouse | n | Systolic Blood Pressure (mm Hg) | Fetal Size (g) |
|---|---|---|---|
| Saline | 5 | 91±5 | 1.08±0.02 |
| Ang II | 5 | 145±8* | 0.61±0.05* |
| Ang II+FK506 | 4 | 121±7† | 0.81±0.06† |
| FK506 | 3 | 93±5 | 1.06±0.05 |
Systolic blood pressure and fetal size were measured at gestation day 17. Data are expressed as mean±SEM.
P<0.05 vs control,
P<0.05 vs Ang II treatment.
Fetal weight is the average of the litter for each group.
Ang II–Induced Trophoblast-Derived sFlt-1 Inhibits Endothelial Cell Migration and Tube Formation
It has been shown that placental-derived sFlt-1 is responsible for reduced angiogenesis.4 To determine whether the sFlt-1 secreted by trophoblasts is biologically active, human trophoblast cells were treated with Ang II, and the effect of CM on endothelial cell migration and tube formation was assessed. Trophoblast CM induced endothelial cell migration and tube formation; however, CM from trophoblast treated with Ang II produced 50% less migration (Figure 5B) and reduced tube formation (Figure 5C). CM from trophoblasts treated with Ang II in the presence of losartan or FK506 induced similar levels of endothelial cell migration and tube formation to that seen with CM of untreated trophoblasts. These results indicate that blockade of AT1 receptor or calcineurin signaling overrode the inhibitory properties of the CM from Ang II–treated trophoblasts (Figure 5). Moreover, removal of sFlt-1 from Ang II–treated trophoblast CM using anti–sFlt-1 anti-body (Figure 5A) eliminated the antimigratory (Figure 5B) and antiangiogenic activity (Figure 5C), proving that sFlt-1 accounted for the reduced angiogenic activity seen in CM from Ang II–treated trophoblasts.
Figure 5.
Increased sFlt-1 secreted by Ang II–treated human trophoblasts inhibits in vitro endothelial cell migration and tube formation. HTR-8/SVneo cells were cultured for 4 days with various treatments. CM was collected and added to cultured HUVECs. A, Western blot analysis showed that the secreted sFlt-1 from HTR-8/SVneo cells in CM was removed by immuno-precipitation with anti-Flt-1. B, HUVECs were grown in the chamber containing serum-free medium, and CM was used as chemoattractant. Migrated cells were counted after 24 hours of incubation. Each sample was analyzed in triplicate and repeated 3 times. C, Representative photomicrographs of HUVECs plated on growth factor–reduced Matrigel and stimulated with different CM. D, Images were taken by light microscopy, and tube lengths were measured using metamorphic software. Four to 10 fields were used to obtain average tube length. All data are expressed as mean±SEM. *P<0.05 vs control, **P<0.05 vs Ang II treatment.
Discussion
The regulation of angiogenesis in pregnancy is tightly controlled; however, the molecular mechanism of sFlt-1 induction in pregnancy is unknown. Here we report that Ang II stimulates an increase in circulating sFlt-1 in pregnant mice, human placental villous explants, and trophoblasts but not from endothelial cells. Ang II induces sFlt-1 release via the AT1 receptor and calcineurin pathway. Furthermore, we demonstrate that Ang II–induced sFlt-1 reduces the ability of trophoblast-derived CM to promote angiogenesis. Taken together, our findings identify a previously unrecognized regulatory role for Ang II in the regulation of sFlt-1 expression during pregnancy.
A number of groups have shown that a complete renin/angiotensin system is present in the placenta of humans and other mammals.15,31 Analysis of pregnant mice revealed that renin gene expression is developmentally regulated during normal pregnancy, achieving maximal values near the end of gestation.15 Ang II is also increased in the maternal circulation during normal human pregnancy.32–34 Using pregnant mice, villous explants, and human trophoblasts, we provide in vivo and in vitro evidence for Ang II as a positive modulator of sFlt-1 synthesis and secretion via AT1 receptor activation. These results imply that the local renin angiotensin system is intact in placental villous explants and is capable of generating endogenous Ang II and activating AT1 receptors to induce sFlt-1 synthesis and secretion. Furthermore, we showed that FK506, a calcineurin specific inhibitor,24–25 inhibited Ang II–mediated sFlt-1 production in pregnant mice and by cultured human trophoblasts. Moreover, siRNA specific for calcineurin subunit α mRNA completely abolished the Ang II–induced sFlt-1 secretion. Thus, the results obtained from both in vivo and in vitro experiments using FK506 or siRNA specific for calcineurin subunit α suggest that calcineurin signaling is required for Ang II–mediated sFlt-1 induction in trophoblasts during pregnancy. In other experiments, we have shown that Ang II treatment of human trophoblasts results in the activation of nuclear factor of activated T cells (NFAT), a well-known transcription factor and substrate of calcineurin. Ang II–mediated activation of NFAT is blocked by both FK506 and cyclosporin A (see the online data supplement, available at http://circres.ahajournals.org). Our earlier studies have shown that Ang II is capable of inducing NFAT nuclear translocalization and activation in human trophoblasts.16 Altogether, our results suggest that Ang II–induced synthesis and secretion of sFlt-1 involve AT1 receptor activation, calcineurin signaling, NFAT nuclear translocation, and the transcriptional activation of the Flt-1 gene.
Toward the end of gestation, continued placental vascular development is terminated, a process accomplished in part through the production of antiangiogenic factors. Multiple groups have demonstrated that sFlt-1, an antagonist to the action of VEGF and placental growth factor, is secreted from cytotrophoblasts in late pregnancy.6,8,19,26 The circulating levels of sFlt-1 are relatively low during the first trimester, begin to rise during the second trimester, and achieve relatively high levels during the third trimester. Thus, we propose that increased production of Ang II in the placenta late in the pregnancy stimulates production of antiangiogenic factors such as sFlt-1 to reduce continued placenta vascular development. We have provided the direct evidence that increased trophoblast sFlt-1 secretion by Ang II functions to antagonize angiogenesis as was earlier reported for preeclamptic CM.4 However, the biological significance of Ang II–mediated sFlt-1 secretion during pregnancy requires further investigation.
It is well known that serum concentrations of sFlt-1 are significantly higher in women with preeclampsia.35–39 However, Ang II levels are not elevated in women with preeclampsia. Thus, the following question remains: what factors contribute to the increased sFlt-1associated with preeclampsia? We40–42 and others43–47 have shown that autoantibodies present in the serum of women with preeclampsia are capable of activating AT1 receptors. In addition, these autoantibodies are able to induce the synthesis and secretion of plasminogen activator inhibitor-1, a feature that may also contribute to the shallow trophoblast invasion associated with preeclampsia.40 In this study, infusion of Ang II resulted in an increased arterial blood pressure and induced restricted fetal growth in pregnant mice (Table), which are 2 hallmark features of preeclampsia. We speculate that the AT1 receptor activating autoantibodies associated with preeclampsia may be responsible for the increased sFlt-1 observed in this disorder.
Supplementary Material
Acknowledgments
Sources of Funding This work was supported by NIH grants HL076558 (to Y.X.) and HD34130 (to R.E.K.). This work was also supported by grants from the British Heart Foundation, European Union, and Medical Research Council (to A.A.). S. Ahmad, P.W.H., and A.A. belong to the European Vascular Genomics Network, a Network of Excellence supported by the European Community's Sixth Framework Programme for Research Priority 1 Life Sciences, Genomics and Biotechnology for Health (contract LSHM-CT-2003-503254).
Footnotes
Disclosures None.
References
- 1.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta. 2000;21(suppl A):S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A. Heparin-binding angiogenic growth factors in pregnancy. Trophoblast Res. 1997;10:215–258. [Google Scholar]
- 4.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 7.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 10.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 11.Levine RJ, Thadhani R, Qian C, Lam C, Lim KH, Yu KF, Blink AL, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Urinary placental growth factor and risk of preeclampsia. JAMA. 2005;293:77–85. doi: 10.1001/jama.293.1.77. [DOI] [PubMed] [Google Scholar]
- 12.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, Barnes N, Ahmed A. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J Clin Invest. 1998;101:442–454. doi: 10.1172/JCI119881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y, Wen H, Prashner HR, Chen R, Inagami T, Catanzaro DF, Kellems RE. Pregnancy-induced changes in renin gene expression in mice. Biol Reprod. 2002;66:135–143. doi: 10.1095/biolreprod66.1.135. [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem. 2002;277:24601–24608. doi: 10.1074/jbc.M201369200. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed A, Li XF, Dunk C, Whittle MJ, Rushton DI, Rollason T. Colocalisation of vascular endothelial growth factor and its Flt-1 receptor in human placenta. Growth Factors. 1995;12:235–243. doi: 10.3109/08977199509036883. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A, Li XF, Shams M, Gregory J, Rollason T, Barnes NM, Newton JR. Localization of the angiotensin II and its receptor subtype expression in human endometrium and identification of a novel high-affinity angiotensin II binding site. J Clin Invest. 1995;96:848–857. doi: 10.1172/JCI118131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210–217. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Malarstig A, Tenno T, Jossan S, Aberg M, Siegbahn A. A quantitative real-time PCR method for tissue factor mRNA. Thromb Res. 2003;112:175–183. doi: 10.1016/j.thromres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duka A, Schwartz F, Duka I, Johns C, Melista E, Gavras I, Gavras H. A novel gene (Cmya3) induced in the heart by angiotensin II-dependent but not salt-dependent hypertension in mice. Am J Hypertens. 2006;19:275–281. doi: 10.1016/j.amjhyper.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz F, Duka A, Triantafyllidi E, Johns C, Duka I, Cui J, Gavras H. Serial analysis of gene expression in mouse kidney following angiotensin II administration. Physiol Genomics. 2003;16:90–98. doi: 10.1152/physiolgenomics.00108.2003. [DOI] [PubMed] [Google Scholar]
- 24.Keen HL, Ryan MJ, Beyer A, Mathur S, Scheetz TE, Gackle BD, Faraci FM, Casavant TL, Sigmund CD. Gene expression profiling of potential PPARgamma target genes in mouse aorta. Physiol Genomics. 2004;18:33–42. doi: 10.1152/physiolgenomics.00027.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lavoie JL, Bianco RA, Sakai K, Keen HL, Ryan MJ, Sigmund CD. Transgenic mice for studies of the renin-angiotensin system in hypertension. Acta Physiol Scand. 2004;181:571–577. doi: 10.1111/j.1365-201X.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 26.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 27.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirashima M, Lu Y, Byers L, Rossant J. Trophoblast expression of fms-like tyrosine kinase 1 is not required for the establishment of the maternal-fetal interface in the mouse placenta. Proc Natl Acad Sci U S A. 2003;100:15637–15642. doi: 10.1073/pnas.2635424100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu DY, Luo J, Bu F, Zhang W, Wei Q. Effects of cyclosporin A, FK506 and rapamycin on calcineurin phosphatase activity in mouse brain. IUBMB Life. 2006;58:429–433. doi: 10.1080/15216540600791555. [DOI] [PubMed] [Google Scholar]
- 30.Schwaninger M, Blume R, Oetjen E, Knepel W. The immunosuppressive drugs cyclosporin A and FK506 inhibit calcineurin phosphatase activity and gene transcription mediated through the cAMP-responsive element in a nonimmune cell line. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:541–545. doi: 10.1007/BF00173216. [DOI] [PubMed] [Google Scholar]
- 31.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol. 1998;19:232–252. doi: 10.1006/frne.1998.0166. [DOI] [PubMed] [Google Scholar]
- 32.Alhenc-Gelas F, Tache A, Saint-Andre JP, Milliez J, Sureau C, Corvol P, Menard J. The renin-angiotensin system in pregnancy and parturition. Adv Nephrol Necker Hosp. 1986;15:25–33. [PubMed] [Google Scholar]
- 33.Skinner SL, Lumbers ER, Symonds EM. Analysis of changes in the renin-angiotensin system during pregnancy. Clin Sci. 1972;42:479–488. doi: 10.1042/cs0420479. [DOI] [PubMed] [Google Scholar]
- 34.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 36.Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393–399. doi: 10.1385/1-59259-989-3:393. [DOI] [PubMed] [Google Scholar]
- 37.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 39.Dimitrakova ED, Dimitrakov JD, Karumanchi SA, Pehlivanov BK, Milchev NP, Dimitrakov DI. Placental soluble fms-like tyrosine-kinase-1 (sFlt-1) in pregnant women with preeclampsia. Folia Med (Plovdiv) 2004;46:19–21. [PubMed] [Google Scholar]
- 40.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 41.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 42.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stepan H, Faber R, Dornhofer N, Huppertz B, Robitzki A, Walther T. New insights into the biology of preeclampsia. Biol Reprod. 2006;74:772–776. doi: 10.1095/biolreprod.105.045997. [DOI] [PubMed] [Google Scholar]
- 45.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. see comment. [DOI] [PubMed] [Google Scholar]
- 46.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 47.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.