Abstract
Introduction
Occupational exposure to silica may be associated with chronic kidney disease (CKD). Most studies have been conducted in occupational cohorts with high levels of exposure but small numbers of cases. We analyzed data from a population-based case-control study of occupational silica exposure and CKD.
Methods
Cases were hospital patients with newly diagnosed CKD and community controls were selected using random digit dialing and frequency matched by age, gender, race and proximity to the hospital. Silica exposure estimates were assigned by industrial hygiene review of lifetime job history data and weighted for certainty and intensity. Conditional logistic regression was used to estimate the odds ratios (ORs) for CKD conditioned on demographic, lifestyle and clinical variables.
Results
The mean age of participants was 62 years (range, 30-83 years), 56% were male and 54% were white. Any silica exposure (compared to none) was associated with a 40% increased risk of CKD (OR=1.40, 95% confidence interval [CI]: 1.04, 1.89) in a multivariable adjusted model. The mean cumulative duration of silica exposure was significantly higher in exposed cases than in exposed controls (33.4 vs. 24.8 years, respectively). Overall, compared to non-exposed participants, the ORs (95% CI) for those below and above the median duration of silica exposure were 1.20 (95% CI: 0.77, 1.86) and 1.76 (95% CI: 1.14, 2.71), respectively.
Conclusions
We found a positive relationship between occupational silica exposure and CKD. A dose-response trend of increasing CKD risk with increasing duration of silica exposure was observed and was particularly strong among non-whites.
INTRODUCTION
Silica, a chemical compound found in abundance in nature, is comprised of quartz, a constituent of rock, and makes up 90-95% of sand. Most silica in commercial use is obtained by processing (such as crushing or milling) from naturally occurring sources and can be found in abrasive cleaners, toothpaste, scouring powder, and metal polish. Also, polished vitreous silica has excellent ultraviolet-visible-infrared transparency and heat resistance and is thus used for lenses, prisms, laser parts, and optical fibers. In an occupational setting, silica can be harmful when inhaled as a dust, particularly when such exposure is prolonged. Occupational industries in which workers may be exposed to silica include (but are not limited to) agriculture, agricultural chemicals, asphalt and roofing, automobile repair, ceramics and pottery, construction, dentistry, and mining1. Recent reports suggest that between 1-3 million people in the United States work in occupational environments with potential exposure to silica and at least 10% of these workers may have dangerously high exposures (at least 2-10 times the recommended exposure limit)2.
Prior research suggests that silica exposure is associated not only with silicosis, lung disease3, rheumatoid arthritis4, small vessel vascultitis5 and other autoimmune diseases6, but also with kidney damage 6-12. For example, in one analysis of data from 3 existing cohort studies, Steenland and colleagues observed that individuals who were exposed to silica had a 5.1% excess risk of end-stage renal disease (ESRD) and a 1.8% excess risk of death from kidney disease11. The mechanisms by which silica may damage the renal system can be either through direct (silica particles in the kidney) or indirect toxicity13. This indirect toxicity likely occurs when the lungs, after being exposed to silica particles, begin to produce macrophages to attack the particles. This process, in addition to lymph node stimulation, activates the immune system and can lead to glomerulonephritis14.
Other studies, by contrast, have not reported an association between silica and kidney function 15-16. However, many of these studies are limited by small sample sizes and/or data that were not collected to characterize occupational exposure, health outcomes, or covariate information. Therefore, the purpose of the current study was to examine, in a well-characterized case-control study, the association between occupational silica exposure and various subtypes (including earlier stages) of physician-diagnosed chronic kidney disease (CKD).
MATERIALS AND METHODS
We used data from a case-control study examining several risk factors for CKD 17-19. The study population consisted of hospital patients and community controls, age 30-79 years residing in North Carolina between 1980 and 1982.
Cases were patients from one of four North Carolina medical centers (Duke University Medical Center, North Carolina Memorial Hospital, Charlotte Memorial Hospital and North Carolina Baptist Hospital) with newly diagnosed CKD, identified by review of kidney-related ICD-9 discharge diagnoses. Potential cases had at least one of the following ICD-9 codes given as part of their discharge diagnosis: 250.4 (diabetes with renal manifestations), 403 (hypertensive renal disease), 404 (hypertensive heart and renal disease), 582 (chronic glomerulonephritis, including interstitial nephritis), 583 (nephritis and nephropathy, including diabetic nephropathy), 585 (chronic renal failure), 586 (renal failure, unspecified), 587 (renal sclerosis, unspecified), 590.0 (chronic pyelonephritis), 590.8 (other pyelonephritis, not specified as chronic or acute), and 593.9 (unspecified disorder of kidney and ureter). Inclusion criteria required that patients, in addition to having newly diagnosed CKD, also had 2 or more measurements of serum creatinine, all of which must have been greater than 1.5 mg/dL. Patients were excluded on the basis of age under 30 years, residence outside North Carolina, preexisting kidney disease or evidence of prior creatinine measurements greater than 1.5 mg/dL (ascertained from medical histories and chart review), and evidence of normal kidney function (despite the diagnosis of CKD). In addition, patients with systemic lupus erythematosus, polycystic kidney disease, or missing silica exposure data were excluded.
Controls were selected using random digit dialing and Medicare recipient listings and were frequency matched to case patients by age (within 5 years), gender, race and proximity to the hospital (residence in or adjacent to counties containing study hospitals). Medical histories and exposure data were obtained for both cases and controls with telephone interviews conducted by trained study personnel.
Six hundred and seven of 709 case patients could be contacted for interview, 554 of whom (91%) participated in the study (78% overall response rate for cases). Among the control participants, 608 of 717 could be contacted and 520 (86%) were interviewed (73% overall response rate for controls). Interviews revealed 4 controls with a history of probable kidney disease and these participants were excluded from the study. Job histories were provided by proxy for 302 cases. (Proxies included, for example, a spouse or caregiver). After also excluding those with missing silica exposure data, a total of 504 cases and 457 controls were included in the analysis. The sample demographics were similar before and after exclusions.
A detailed account of participants’ work history was collected. In addition to general questions on exposure to sand or silica and employment in industries or occupations with potential for exposure (e.g. stone, clay, glass manufacturing), data were collected on all jobs that were held for two or more consecutive years. Along with open-ended descriptions of job-specific activities and what the employer specialized in, participants were also asked (1) if, for each reported job, they were exposed to sand or silica at least 5 times; or (2) if they were exposed to other dusty conditions at least 5 times.
Based on complete information from lifetime job histories, silica exposure was assessed independently (and blinded to case-status) by authors (CGP and LNF) from specific occupational data. For each job held, qualitative dose estimates were assessed (high, moderate, and low) and differences resolved by consensus review. Examples of exposure ratings are available in the Appendix. A certainty rating was applied to each exposure estimate based on the extent and quality of data provided by respondents. Jobs for which exposure was possible, but unlikely, were also identified to permit sensitivity analysis. For each individual, lifetime exposure ratings were calculated based on the assigned exposure dose and the length of time at each job. Exposure scores were weighted by a factor of 1.0 for high certainty, 0.75 for moderate certainty, and 0.25 for low certainty. Weighting for exposure intensity ranged from 0 (unexposed) to 3 (high), for increasing levels of exposure. The product of intensity by certainty was summed across years spent at each job with silica exposure for analysis of cumulative exposure. In addition to the job-based exposure estimates, self-reported work in specific industries or with specific materials collected by checklist was grouped as probable (i.e., ever have a job in stone, clay or glass manufacture; and work with sand or silica, clay, ceramics or pottery products, and ceramic glazes) and possible (i.e., ever have a job in: chemical manufacture, auto mechanics or repair, plumbing, heating or air conditioning, smelting, lead or other metal industry, paint manufacture, commercial painting or spray painting; work with paint such as restoring homes, removing house paint, ship repair or repainting).
During the interview, data on demographics, clinical measures, medications and medical conditions (including hypertension, diabetes, gout, urinary tract infections, pyelonephritis and kidney stones) were collected. Annual income, years of education, and height and weight were also ascertained. Body mass index (BMI) was calculated using weight in kilograms divided by height in meters squared.
Means and standard deviations for continuous variables and percents for categorical variables were calculated for various traits of the study population by case and control status. The distribution of baseline characteristics among cases and controls were compared using Student’s t-tests and chi-square tests.
In order to examine the association between occupational silica exposure and CKD, odds ratios (OR) were calculated using conditional logistic regression models. The models were conditioned on race, gender, and proximity to hospital. Since cases were matched within five years of age to controls (as opposed to matched in five-year age groups), age was included in the models as continuous variable. Initial regression models adjusted for age and education. Potential confounders also controlled for in fully adjusted models included age, respondent status (proxy vs. self), education level, body mass index, and history of hypertension and diabetes. We added individual covariates to a base model that included age and education and demonstrated only slight differences in ORs. The OR was unchanged after adjusting for all covariates in a single model compared to the base model. Thus, to improve the precision of estimates for analyses of CKD subtype we report estimates from the reduced models. Multiplicative interaction terms of silica exposure with diabetes and with hypertension status were evaluated using product terms in fully adjusted regression models. All statistical analyses were conducted using SAS 8.0 (SAS Institute, Cary, NC).
RESULTS
Characteristics of study participants are given in Table 1. The mean age was 62 years (range, 30-83 years), 56% were male and 54% were white. The age, race, and gender distributions of cases and controls were almost identical due to matching. Cases, however, were more likely than controls to have fewer years of education, have higher BMI, have histories of hypertension and diabetes, use analgesic medications daily, and have proxy respondents. Table 2 shows assigned levels of silica exposure in cases and controls. The prevalence of occupational exposure to silica was high and more frequent among cases than controls (48.8% vs. 40.3%, respectively). Among those with silica exposure, the median duration of silica exposure after weighting for both certainty and intensity was higher in cases than in controls (22.5 vs. 13.0 years).
Table 1.
Characteristics* of Patients with Chronic Kidney Disease and Community Controls in North Carolina
| CKD Cases N=504 |
Controls N=457 |
p-value | |||
|---|---|---|---|---|---|
| Mean age, years (SD) | 62.8 | (12.0) | 61.6 | (12.1) | 0.11 |
| White race | 275 | (54.6%) | 244 | (53.4%) | 0.72 |
| Male gender | 298 | (59.1%) | 271 | (59.3%) | 0.96 |
| Education level | |||||
| < 8 years | 193 | (38.8%) | 120 | (26.4%) | |
| 8-11 years | 150 | (30.2%) | 134 | (29.5%) | <0.0001 |
| 12+ years | 154 | (31.0%) | 200 | (44.1%) | |
| Alcoholic drinks/day | |||||
| <1 | 381 | (75.6%) | 343 | (75.15) | |
| 1-2 | 71 | (14.1%) | 75 | (16.4%) | 0.44 |
| 3+ | 52 | (10.3%) | 39 | (8.5%) | |
| Moonshine consumption | 57 | (12.1%) | 36 | (8.0%) | 0.04 |
| Ever smoked | 274 | (55.0%) | 275 | (61.3%) | 0.05 |
| Mean PYs smoked, years (SD) | 20.1 | (29.6) | 19.2 | (27.9) | 0.65 |
| Mean BMI, kg/m2 (SD) | 26.9 | (5.6) | 25.4 | (4.4) | <0.0001 |
| History of diabetes | 142 | (28.8%) | 28 | (6.1%) | <0.0001 |
| History of hypertension | 305 | (61.2%) | 135 | (29.5%) | <0.001 |
| History of analgesic use | |||||
| Never/occasionally | 247 | (54.2%) | 304 | (70.2%) | |
| Weekly | 129 | (28.4%) | 106 | (24.5%) | <0.0001 |
| Daily | 79 | (17.4%) | 23 | (5.3%) | |
| Proxy respondents | 273 | (54.2%) | 41 | (9.0%) | <0.0001 |
| CKD case subtypes | |||||
| Nephrosclerosis | 98 | (19.4%) | -- | -- | |
| Diabetic nephropathy | 95 | (18.9%) | -- | -- | |
| Glomerular nephritis | 68 | (13.5%) | -- | -- | |
| Interstitial nephritis | 100 | (19.8%) | -- | -- | |
| End-stage renal disease (ESRD)+ | 34 | (6.8%) | -- | -- | |
| Renal insufficiency+ | 109 | (21.6%) | -- | -- | |
N(%) unless noted differently
Not otherwise specified
CKD= chronic kidney disease; SD=standard deviation; PY=pack years
Table 2.
Occupational Silica Exposure among Patients with Chronic Kidney Disease and Community Controls in North Carolin
| CKD Cases N=504 |
Controls N=457 |
|
|---|---|---|
| Any silica exposure*, N (%) | 246 (48.8) | 184 (40.3) |
| Level of silica exposure, N (%) | ||
| None (not exposed) | 177 (35.1) | 194 (42.5) |
| Low (maybe exposed) | 81 (16.1) | 79 (17.3) |
| Medium (low/possible) | 199 (39.5) | 149 (32.6) |
| High (high/probable) | 47 ( 9.33) | 35 ( 7.7) |
| Median duration of silica exposure, years | ||
| weighted for certainty and intensity | 22.5 | 13.0 |
| weighted for intensity only | 30.0 | 18.0 |
Defined as medium or high level of silica exposure
CKD=chronic kidney disease
In conditional logistic models adjusted for age and education, silica exposure was associated with a 37% increased risk of CKD (OR=1.37, 95% CI: 1.02, 1.85). Limiting models to only self-respondents resulted in a higher OR of 1.53 [95% CI: 1.01, 2.30] (not shown). In analyzing subtypes of CKD (Table 3), silica exposure was significantly associated with a greater risk of renal insufficiency (OR=1.74, 95% CI: 1.05, 2.87). While silica exposure was not significantly associated with ESRD (OR=1.75, 95% CI: 0.76, 4.01) or other subtypes, consistently positive estimates for subtypes were observed (albeit lacking precision).
Table 3.
Adjusted* Conditional Odds Ratios and 95% Confidence Intervals for Chronic Kidney Disease Subtypes Associated With Occupational Silica Exposure**
| OR (95% CI) | |
|---|---|
| CKD (all) | 1.37 (1.02, 1.85) |
| Nephrosclerosis | 1.21 (0.72, 2.04) |
| Diabetic Nephropathy | 1.40 (0.82, 2.39) |
| Glomerulonephritis | 1.13 (0.63, 2.03) |
| Interstitial Nephritis | 1.30 (0.76, 2.23) |
| ESRD | 1.74 (0.76, 4.01) |
| Renal Insufficiency | 1.74 (1.05, 2.87) |
Adjusted for age and education.
Defined as medium or high level of silica exposure versus not exposed or low silica exposure
CKD=chronic kidney disease; OR=odds ratio; CI=confidence interval
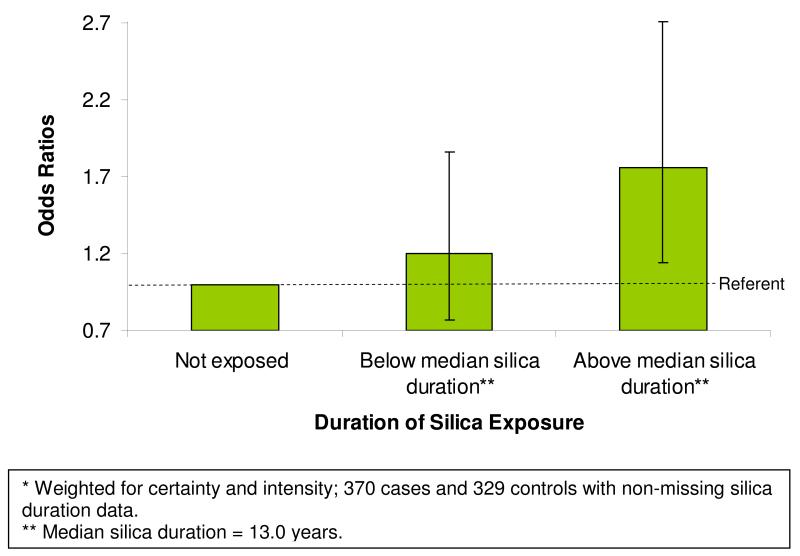
Duration of occupational silica exposure, weighted for certainty and intensity, was examined by comparing non-exposed study participants to those below and above the median of exposure duration in years (median duration=13.0 years) based on the job history. The odds ratios of CKD associated with duration of occupational silica exposure demonstrated a positive linear relationship (Figure 1). Compared to those not exposed, those below the median duration of silica exposure had an OR of 1.20 (95% CI: 0.77, 1.86) and those above the median duration of silica exposure had an OR of 1.76 (95% CI: 1.14, 2.71). The ORs for CKD associated with duration of silica exposure were in general higher for non-whites than whites but did not demonstrate a dose-response trend (not shown). No differences were observed in the relationship between silica duration and CKD between males and females (not shown).
Figure 1.
Age and Education Adjusted Conditional Odds Ratios (and 95% Confidance Intervals) for Chronic Kidney Disease Associated with Increasing Duration of Occupational Silica Exposure*.
DISCUSSION
In this well-characterized case-control study of occupational exposures and CKD in North Carolina, a positive and consistent association was observed between silica exposure and CKD. Occupational exposure to silica was associated with a 37% increased risk of CKD and appeared to be specifically related to unclassified renal insufficiency, representing earlier stages of diagnosed CKD. Assessment of duration of occupational exposures showed trends of increasing CKD risk with increased duration of silica exposure.
Approximately 3 million workers in USA and Europe are exposed to crystalline silica20. Despite awareness of the dangers of silica exposure and improving safety measures in occupational settings, recent evidence of higher than the NIOSH recommended exposure limit of 0.05 mg/m3 for occupational exposure to silica has been reported across multiple industries (Yassin et al., 2005). In addition to workers in the traditional dusty trades industries associated with risk of silicosis, farmers and other workers in the agricultural sector may be also exposed to silica. In North Carolina, Archer and colleagues reported very high exposure levels of respirable silica in the agricultural industry (e.g., 3.91 mg/m3 (standard deviation [SD]: 2.07 mg/m3) for sweet potato transplanting)21, although another population survey in the same region indicated that exposures are likely to vary by crops grown and location22.
Evidence suggests that there are a myriad of occupational and environmental exposures that may contribute to the development of and progression of CKD23-27. Recent reports have highlighted silicon-containing compounds as being particularly damaging to the renal system, particularly for individuals who experience intense and prolonged exposure (e.g., miners, sandblasters, glassmakers, brick and grain workers)23-24. For example, Steenland et al. 27 examined the association between silica and kidney disease, comparing 325 male cases with ESRD to 325 matched population-based controls. They obtained information on participants’ occupational histories (whether, in each job held for greater than 6 months since age 18, participants were exposed to a variety of occupational exposures). Results suggested that men who were exposed to silica, particularly in foundries, brick making or sandblasting, were more likely to have ESRD compared to those who were not exposed and that individuals had increase risk of ESRD with increasing levels of silica exposure. Steenland and colleagues26 also followed a cohort of approximately 4,000 workers in the industrial sand industry with quantitative silica exposure measurements, showing excess mortality from acute and chronic kidney disease and increased incidence of ESRD with increasing cumulative silica exposure.
In another cohort study of approximately 3,000 male Italian ceramic workers, Rapiti and colleagues25 collected information about study participants’ employment history, smoking status and results from x-ray film readings. When the authors compared the rate of ESRD among industry workers with the rate of ESRD among the regional population, they observed that the ceramic workers had over a 3-fold risk of ESRD and that this risk was particularly high for those with a longer latency since their first exposure to silica. An excess risk was also observed among workers without silicosis.
Our study is among the few population-based studies to explore the relationship between occupational silica exposure, using detailed occupational histories, and CKD including CKD subtypes. We included a large, well-defined population of CKD cases and carefully selected community controls as well as comprehensive collection and assessment of occupational exposure histories. In contrast to some of the prior research conducted in this field, which primarily involved white males, our study extended generalizability by the inclusion of a diverse sample (approximately half non-white and half female). Further, we were able to control for a variety of potential confounding variables frequently not available in other occupational studies. Finally, in previously published studies, the association between specific subtypes of CKD and silica exposure has not been fully investigated, as was done in our study.
In a related analysis, data from this study were used to explore associations between CKD and a range of occupations and occupational exposures (Sponholtz, submitted), with analyses suggesting an association between self-reported exposure to “sand and silica” or dusty conditions, especially within industries such as agricultural production and construction. This study extends that work by incorporating a more comprehensive industrial hygiene review and assessment of the potential for silica exposure based on self-reported exposures and prior knowledge of materials used and tasks associated with specific occupations.
There are several potential limitations of the current study that warrant mention. First, the self-reported nature of the occupational exposure variables may have introduced possible bias. However, exposure review was blinded to case status, and cases were not likely to be aware of the silica hypothesis. Cases did not appear to indiscriminately over-report other types of exposures, for example metals as assessed based on self-report to metals and metal fumes, and work across a variety of industries (e.g., smelting, lead or metal industries) (results not shown). Non-differential exposure misclassification can also be a concern, especially when the exposure is rare. However, in the present assessment, silica exposure was higher than in many other studies, in part, due to the inclusion of some types of agricultural occupations (e.g., farm worker). The accuracy of retrospective exposure assessment is limited by the extent of recall and information available for categorizing jobs into exposed and unexposed categories. Our method of expert assessment is considered the gold standard in retrospective exposure assessment, and is similar to that used in another recent population-based study of silica, nephritis and autoimmune diseases5. Silica exposure variables were constructed based on the level as well as the duration of silica exposure, and took into account the intensity and certainty of the exposure. Our findings were consistent using variables adjusting for certainty and taking into account exposure duration. Finally, we utilized proxy respondents, which may have resulted in some misreporting of exposure variables. However, several studies have confirmed that the use of proxy respondents provide adequate data for most exposures 28-30. After excluding proxy respondents from our analysis, estimates from the logistic regression models did not change dramatically and tended to produce estimates that were further from the null. For example in fully adjusted logistic regression models limited to self-respondents, the odds ratio for CKD associated with any silica exposure was 1.56 (95% CI: 1.06, 2.29) vs. 1.40 (95% CI: 1.04, 1.89) for the full sample.
In conclusion, we observed a positive relationship between occupational silica exposure and CKD, with strong specific association with renal insufficiency with a dose-response trend. These results provide confirmatory evidence of the relationship between occupational silica exposure and CKD and, additionally, suggest that exposure to silica may be associated with earlier stages of the kidney disease. Thus, future studies are warranted to examine the association of silica exposure and the level of kidney function.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01-ES-049028).
APPENDIX
Silica exposure assessment
1. Examples of output from blinded industrial hygiene review of occupation and industry data from lifetime job history are shown in the table below. Reviews were based on prior knowledge regarding occupation and industry. Self-reported exposure to sand or silica or dusty condition for each job was also evaluated and compared with expected exposures based on prior knowledge.
| Occupation | Industry | Industrial Hygiene Review: Silica exposure rating based on prior knowledge of jobs and tasks |
Self-report, On this job, exposed to: |
||
|---|---|---|---|---|---|
| Intensity | Certainty | Sand or silica |
Dusty conditions |
||
| 804: Truck drivers, heavy |
060: Construction | High | High | yes | yes |
| 613-617: Extractive occup |
040-050: Mining | High | High | no | yes |
| 563: Brickmasons & stonemasons |
060: Construction | High | High | yes | yes |
| 869: Construction laborers |
060: Construction | High | Moderate | yes | yes |
| 453: Janitors & cleaners |
130: Tobacco mfg | Mod | High | no | yes |
| 585: Plumbers,pipefitters & steamfitters |
060: Construction | Mod | Low | no | yes |
| 567: Carpenters | 060: Construction | Low | Moderate | no | yes |
| 479: Farm workers | 010-031: Agriculture,etc. |
Low | Moderate | no | yes |
| 453: Janitors & cleaners |
132-152: Textile mfg |
Low | Low | no | yes |
Other self-reported jobs or tasks data associated with high or moderate silica exposure were grouped as probable or possible exposure. Tasks were rated as either probable or possible exposure as follows:
Probable exposure: work in stone, clay or glass manufacture, or used silica-containing on materials on the job (sand or silica, clay, ceramics, or other pottery products, ceramic glazes)
Possible exposure: work in chemical manufacture, plumbing, smelting, lead industry, other metal industry, paint manufacture, commercial painting, auto mechanics, or used paint restoring homes, ship repair or repainting.
REFERENCES
- 1.Stratta P, Canavese C, Messuerotti A, Fenoglio I, Fubini B. Silica and renal diseases: no longer a problem in the 21st century? J Nephrol. 2001 Jul-Aug;14(4):228–247. [PubMed] [Google Scholar]
- 2.Parks CG, Conrad K, Cooper GS. Occupational exposure to crystalline silica and autoimmune disease. Environ Health Perspect. 1999 Oct;107(Suppl 5):793–802. doi: 10.1289/ehp.99107s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attfield MD, Costello J. Quantitative exposure-response for silica dust and lung cancer in Vermont granite workers. Am J Ind Med. 2004 Feb;45(2):129–138. doi: 10.1002/ajim.10348. [DOI] [PubMed] [Google Scholar]
- 4.Klockars M, Koskela RS, Jarvinen E, Kolari PJ, Rossi A. Silica exposure and rheumatoid arthritis: a follow up study of granite workers 1940-81. Br Med J (Clin Res Ed) 1987 Apr 18;294(6578):997–1000. doi: 10.1136/bmj.294.6578.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogan SL, Cooper GS, Savitz DA, et al. Association of Silica Exposure with Anti-Neutrophil Cytoplasmic Autoantibody Small-Vessel Vasculitis: A Population-Based, Case-Control Study. Clin J Am Soc Nephrol. 2007;2:290–299. doi: 10.2215/CJN.03501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan SL, Satterly KK, Dooley MA, Nachman PH, Jennette JC, Falk RJ. Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol. 2001 Jan;12(1):134–142. doi: 10.1681/ASN.V121134. [DOI] [PubMed] [Google Scholar]
- 7.Fenwick S, Main J. Increased prevalence of renal disease in silica-exposed workers. Lancet. 2000 Sep 9;356(9233):913–914. doi: 10.1016/S0140-6736(00)02686-6. [DOI] [PubMed] [Google Scholar]
- 8.IA EL-S, Gadallah M, Shouman AE, Nessim DE. Subclinical nephrotoxicity caused by smoking and occupational silica exposure among Egyptian industrial workers. Arch Med Res. 2003 Sep-Oct;34(5):415–421. doi: 10.1016/S0188-4409(03)00077-8. [DOI] [PubMed] [Google Scholar]
- 9.Rosenman KD, Moore-Fuller M, Reilly MJ. Kidney disease and silicosis. Nephron. 2000 May;85(1):14–19. doi: 10.1159/000045624. [DOI] [PubMed] [Google Scholar]
- 10.Saita G, Zavaglia O. Renal function in silicotics.] Med Lav. 1951 Feb;42(2):41–48. [PubMed] [Google Scholar]
- 11.Steenland K. One agent, many diseases: exposure-response data and comparative risks of different outcomes following silica exposure. Am J Ind Med. 2005 Jul;48(1):16–23. doi: 10.1002/ajim.20181. [DOI] [PubMed] [Google Scholar]
- 12.Gregorini G, Ferioli A, Donato F, et al. Association between silica exposure and necrotizing crescentic glomerulonephritis with p-ANCA and anti-MPO antibodies: a hospital-based case-control study. Adv Exp Med Biol. 1993;336:435–440. doi: 10.1007/978-1-4757-9182-2_77. [DOI] [PubMed] [Google Scholar]
- 13.Steenland K, Rosenman K, Socie E, Valiante D. Silicosis and end-stage renal disease. Scand J Work Environ Health. 2002 Dec;28(6):439–442. doi: 10.5271/sjweh.696. [DOI] [PubMed] [Google Scholar]
- 14.Steenland K, Goldsmith DF. Silica exposure and autoimmune diseases. Am J Ind Med. 1995 Nov;28(5):603–608. doi: 10.1002/ajim.4700280505. [DOI] [PubMed] [Google Scholar]
- 15.Calvert GM, Rice FL, Boiano JM, Sheehy JW, Sanderson WT. Occupational silica exposure and risk of various diseases: an analysis using death certificates from 27 states of the United States. Occup Environ Med. 2003 Feb;60(2):122–129. doi: 10.1136/oem.60.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fored CM, Nise G, Ejerblad E, et al. Absence of association between organic solvent exposure and risk of chronic renal failure: a nationwide population-based case-control study. J Am Soc Nephrol. 2004 Jan;15(1):180–186. doi: 10.1097/01.asn.0000103872.60993.06. [DOI] [PubMed] [Google Scholar]
- 17.Sandler DP, Smith JC, Weinberg CR, et al. Analgesic use and chronic renal disease. N Engl J Med. 1989 May 11;320(19):1238–1243. doi: 10.1056/NEJM198905113201903. [DOI] [PubMed] [Google Scholar]
- 18.Vupputuri S, Sandler DP. Lifestyle risk factors and chronic kidney disease. Ann Epidemiol. 2003 Nov;13(10):712–720. doi: 10.1016/s1047-2797(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 19.Vupputuri S, Soucie JM, McClellan W, Sandler DP. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004 Mar;14(3):222–228. doi: 10.1016/S1047-2797(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 20.Calvert GM, Steenland K, Palu S. End-stage renal disease among silica-exposed gold miners. A new method for assessing incidence among epidemiologic cohorts. Jama. 1997 Apr 16;277(15):1219–1223. [PubMed] [Google Scholar]
- 21.Archer JD, Cooper GS, Reist PC, Storm JF, Nylander-French LA. Exposure to respirable crystalline silica in eastern North Carolina farm workers. AIHA J (Fairfax, Va) 2002 Nov-Dec;63(6):750–755. doi: 10.1080/15428110208984765. [DOI] [PubMed] [Google Scholar]
- 22.Parks CG, Cooper GS, Nylander-French LA, Storm JF, Archer JD. Assessing exposure to crystalline silica from farm work: a population-based study in the Southeastern United States. Ann Epidemiol. 2003 May;13(5):385–392. doi: 10.1016/s1047-2797(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 23.Brewster UC. Chronic kidney disease from environmental and occupational toxins. Conn Med. 2006 Apr;70(4):229–237. [PubMed] [Google Scholar]
- 24.Nuyts GD, Van Vlem E, Thys J, et al. New occupational risk factors for chronic renal failure. Lancet. 1995 Jul 1;346(8966):7–11. doi: 10.1016/s0140-6736(95)92648-8. [DOI] [PubMed] [Google Scholar]
- 25.Rapiti E, Sperati A, Miceli M, et al. End stage renal disease among ceramic workers exposed to silica. Occup Environ Med. 1999;56:559–561. doi: 10.1136/oem.56.8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steenland K, Sanderson W, Calvert GM. Kidney disease and arthritis in a cohort study of workers exposed to silica. Epidemiology. Jul 2001;12(4):405–412. doi: 10.1097/00001648-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Steenland NK, Thun MJ, Ferguson CW, Port FK. Occupational and other exposures associated with male end-stage renal disease: a case/control study. Am J Public Health. 1990 Feb;80(2):153–157. doi: 10.2105/ajph.80.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle CA, Brann EA, The Selected Cancers Cooperative Study Group Proxy respondents and the validity of occupational and other exposure data. Am J Epidemiol. 1992 Sep 15;136(6):712–721. doi: 10.1093/oxfordjournals.aje.a116550. [DOI] [PubMed] [Google Scholar]
- 29.Campbell PT, Sloan M, Kreiger N. Utility of Proxy versus Index Respondent Information in a Population-Based Case-Control Study of Rapidly Fatal Cancers. Ann Epidemiol. 2006 Dec 16; doi: 10.1016/j.annepidem.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke. 2002 Nov;33(11):2593–2599. doi: 10.1161/01.str.0000034395.06874.3e. [DOI] [PubMed] [Google Scholar]