Abstract
The mortality of colorectal carcinoma often results from the progression of metastatic disease, which is predominantly hepatic. Though recent advances in surgical, locoregional, and systemic therapies have yielded modest survival improvements, treatment of these aggressive lesions is limited to palliation for the vast majority of patients. Oncolytic viral therapy represents a promising novel therapeutic modality that has achieved tumor regression in several preclinical and clinical models. Evidence further suggests that locoregional viral administration may improve viral efficacy while minimizing toxicity. This study will review the theories behind hepatic arterial infusion of oncolytic virus, as well as herpes viral design, preclinical data, and clinical progress in regional liver therapy using oncolytic virus to treat hepatic colorectal carcinoma metastases.
INTRODUCTION
Both primary and secondary hepatic malignancies are notoriously aggressive, and have long evaded traditional therapies (1;2). While recent advancements in surgical and ablative technologies hold promise, especially for patients with few or isolated lesions, hepatic metastases remain the most frequent cause of mortality resulting from colorectal carcinoma, and treatment of colorectal metastases remains primarily palliative (2–5). Additionally, despite recent chemotherapeutic improvements, many patients receiving systemic chemotherapy still suffer from the debilitating toxicities that result from low tumor specificity achieved by the vast majority of common chemotherapeutic agents (6). Regionally directed chemotherapy via hepatic arterial infusion (HAI) offers a more tumor-specific treatment modality and has been investigated in combination with systemic therapy to achieve disease responses that can facilitate tumor resection in properly selected patients, but the need for novel therapies is ever-present (7).
In recent years, oncolytic viral therapy has emerged as a promising alternative therapy for a wide range of cancers. Currently, genetically engineered attenuated viruses are the most commonly used gene delivery vehicles in clinical trials (8;9). With high tumor specificity and minimal host toxicity, viral agents hold great clinical promise. While a variety of engineered DNA and wild-type RNA viruses have been used, herpes simplex virus type 1 (HSV-1) possesses several unique characteristics that lend it superiority in both genetic manipulation and tumor selectivity (9;10). At least 12 currently published clinical trials and several more trials in progress have studied engineered HSV-1 subtypes with promising results against a wide range of cancers, including high-grade malignant gliomas, melanoma, squamous cell carcinomas of the head and neck, recurrent breast cancer, pancreatic cancer, and hepatic colorectal metastases (11–14). Furthermore, several authors examining engineered HSV-1 subtypes in preclinical models have found sustained viral function in both hypoxic and necrotic microenvironments typical of metastases, which are known to promote resistance to standard adjuvant therapies (15;16). These characteristics make HSV-1 an ideal weapon in the fight against hepatic colorectal metastases.
This article will review the principles of locoregional oncolytic virotherapy, the structure and function of oncolytic herpes viral constructs and their efficacy against hepatic colorectal metastases, and results of recent preclinical experiments and clinical trials using HAI of oncolytic virus.
CONCEPT OF LOCOREGIONAL ONCOLYTIC VIROTHERAPY USING HSV-1
While survival remains dismal for patients with hepatic colorectal metastases who fail first-line systemic chemotherapy, recent studies have shown survival advantages with decreased toxicities following salvage chemotherapy administered via HAI in combination with systemic infusions (17). Furthermore, this success was achieved despite the use of doses far smaller than those used in systemic therapy. Applying these findings to viral therapy, several authors have postulated that HAI can facilitate decreased viral doses, thereby minimizing systemic toxicity and minimizing host immune system interference. Replication-competent oncolytic HSV-1 (oHSV) offers the added benefit of producing a large number of progeny from relatively few infected cells. Clinically, this translates to efficacy without the need for large systemic viral loads. HAI amplifies the already significant tumor specificity of viral therapy by allowing for the delivery of vector to the liver at concentrations far exceeding those reached systemically (9). Because these vectors are inherently oncotropic themselves, virus can be delivered at extremely high local concentrations with minimal toxicity to noncancerous tissue.
The dual hepatic blood supply also provides an anatomic advantage for HAI. Other researchers have established that whereas normal hepatocytes receive blood primarily from portal circulation, hepatic metastases receive blood supply predominantly from the hepatic artery (18). Building on this principle, experiments using HAI of chemotherapy have demonstrated that HAI delivers high drug concentrations to hepatic metastases with enhanced antitumoral effects and minimal damage to normal liver tissue (19;20). Investigators of oncolytic viral therapy took a cue from these theories and outcomes to examine viral HAI.
While viral oncolysis is often perceived as a single treatment modality, in truth it refers to a wide array of therapies based on individual virus families, each with their own unique advantages. oHSV vectors possess several unique characteristics that set them apart from other viruses currently being explored. Perhaps most importantly, HSV encodes a thymidine kinase (TK) gene that renders it susceptible to antiherpetic medications like acyclovir, which stands as a safeguard should excessive extratumoral viral replication occur (21). Furthermore, many characteristics of tumor microbiology yield a cellular microenvironment that naturally facilitates preferential viral replication compared with noncancerous tissues. For instance, rapidly proliferating tumors often outgrow their own blood supply, resulting in regions of hypoxia and tumor necrosis. Low tumor oxygen tension has been correlated with increased metastases and recurrence in a variety of cancers (22–24). Moreover, tumor hypoxia is known to facilitate resistance to standard adjuvant therapies (25;26). However, preclinical studies have demonstrated that viral replication is tolerant of hypoxia and that oHSV can even be engineered for enhanced viral replication in a hypoxic environment (16). Still more preclinical data indicate that cancer cell death, like that found in a necrotic tumor microenvironment, can enhance HSV-1 replication in neighboring cells (15). With these principles in mind, our group hypothesized that oHSV vectors would be ideal for therapy of hepatic colorectal metastases, and tested this hypothesis in both preclinical and clinical settings (17).
EVOLUTION OF ONCOLYTIC HERPES SIMPLEX VIRUS
Over the past two decades, breakthroughs of genetic engineering have allowed investigators to design new generations of oHSV vectors that are constantly improving in their ability to selectively infect and treat a wide range of cancers. The foundations of viral oncolysis trace back over a century, with the earliest reports of what are now known to be viral diseases inducing cancer regression prior to the discovery of viruses (27). Subsequently, interest in viral therapy ebbed and flowed, with interest in the use of natural viruses peaking in the 1950s and 1960s, marked by formal clinical trials and attempts to generate distinctly oncotropic viral strains through selective breeding (28–30). At that time, interest in viruses as potential antineoplastic therapies was abandoned due to unacceptable side effects that eventually ended the trials (31). It would not be until the emergence of modern genetic engineering in the 1990s that viral oncolysis would resurface in earnest with renewed potential as a cancer therapy. What separates the modern approach to viral cancer therapeutics from earlier experimentation is the advent of genetic engineering: the ability to manipulate the vector genome in order to augment the specificity and efficacy of treatment.
Modern oHSV Development—The First Three Generations
While a variety of oncolytic viral vectors have yielded promising findings in experimental and clinical models, oHSV possesses several qualities that make it ideal for oncolytic therapy. It efficiently infects a broad range of cells and species, and has a large well-characterized genome, less than half of which is required for viral replication, making the virus a prime target for genetic manipulation (32). In fact, oHSV vectors can accept the largest genetic inserts (up to 30 kb) of any oncolytic virus under investigation, with the next largest adenovirus accepting only 10 kb inserts (32). Furthermore, as a replication-competent virus, oHSV can achieve replication even in quiescent cells, making it ideal for the infection of putative cancer stem cells. In terms of safeguards for the host, the virus remains inherently episomal, which lends protection from insertional mutagenesis (33), and as previously mentioned HSV-1 is the only virus under clinical investigation for which consistently effective, FDA-approved antiviral therapy is readily available (34).
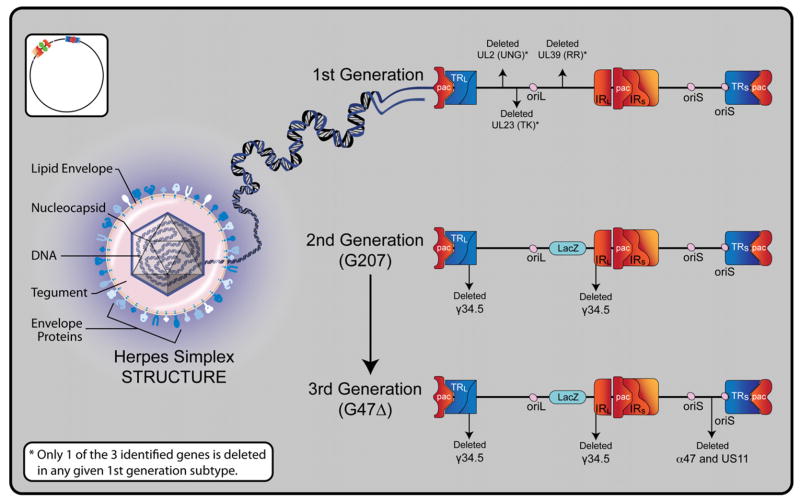
The development of herpes oncolysis as a viable experimental therapy began with a finite series of strains featuring key shifts in basic vector design. These initial strains are frequently grouped into three classes or “generations” of vectors. While the field of oHSV vector design has since exploded into a wide range of diverse vectors, a brief review of these first three generations provides a convenient starting point for understanding the underpinnings of current and future oHSV design (Figure 1).
Figure 1. Overview of Oncolytic HSV-1 Structure and Generations.
Herpes simplex virus type 1 is a double-stranded DNA virus with icosahedral symmetry. A central core contains viral DNA within a nucleocapsid. Tegument between the nucleocapsid and outer envelope contains various proteins that are transferred to a host cell upon fusion. The lipid envelope carries viral glycoproteins that facilitate cellular fusion and specificity. The 152 kb DNA genome is comprised of unique long and short segments, each flanked by inverted (IRL and IRS) and terminal repeats (TRL and TRS). The genome further contains three DNA packaging (pac) signals, which enable construction of virions. There are two different origins of replication, one in the unique long segment (oriL), and one in the unique short segment (oriS). Several genes are duplicated as a result of the inverted repeats. These include oriS, γ34.5, α0, and α4. Representative constructs are shown of each of the three generations of oncolytic HSV-1. In the first generation, potential single deletions of uracil deglycosylase (UNG), ribonucleotide reductase (RR), and thymidine kinase (TK) are represented. In the second generation, the G207 construct reflects double deletions of γ34.5 and the addition of a LacZ gene at the site of UL39 (RR), which effectively inactivates RR and enables histochemical identification of viral replication via β-galactosidase detection. In the third generation, the G47Δ construct reflects its derivation from the G207 backbone with the deletion of the α47 gene and the overlapping US11 promoter region. Since α47 encodes an inhibitor of antigen presentation, its deletion prevents the down-regulation of MHC class I peptides on the surface of virally infected cells, thereby diminishing host immune responses and enhancing viral efficacy.
First Generation
The first obvious requirement in the construction of a therapeutic vector is to minimize toxicity that would result from infection of noncancerous tissues. Thus, the first generation of genetically engineered oHSV vectors was created by simply deleting genes thought to be essential for infection of normal tissues but redundant for the infection of cancer cells. First generation oHSV vectors all featured mutations of genes encoding for either ribonucleotide reductase (RR), tyrosine kinase (TK), or γ134.5. The common thread among these genes is that their gene products are all crucial to viral replication in relatively quiescent normal cells, but nonessential in rapidly dividing cancer cells harboring a ready supply of viable genetic machinery. For example, the first recombinant oHSV, dlsptk, was generated through the deletion of the UL23 gene encoding TK—an enzyme that processes nucleotides to facilitate replication of DNA. This mutated HSV-1 could infect individual normal cells but failed to replicate at a rate sufficient to sustain infection. Conversely, in rapidly dividing cancer cells, where a surplus of ready nucleotides is provided by an overactive native cellular replication machinery, viral replication would proceed unhindered to allow for progressive lytic infection (35).
Second Generation
While the first generation of oHSVs offered key proofs of principle in terms of tumor specificity and decreased host toxicities, ultimately concern regarding viral resistance to first-line antiherpes medications (in the case of TK deletions) and reversion to wild type resulted in development of a new generation of multimutated vectors.
The prototypical second generation oHSV vector, G207, added a deletion of the U39 gene (encoding RR) to the first generation 1716 strain, creating a vector that lacked both U39 and γ134.5. This G207 vector has been studied in both preclinical and clinical models, and remains in use by many labs both in its original form and as a backbone for additional manipulations. In a historical sense, the second generation of vectors is notable for first addressing all basic safety concerns required in vector design. All further developments in vector design since have focused on increasing vector efficacy rather than safety.
Third Generation
The third generation oHSV vectors reflected recognition of the need for increased efficacy. G47Δ was created by deleting the α47 gene and the overlapping US11 promoter region from the G207 backbone. This α47 gene encodes for a protein that protects the virus from host immune responses by down-regulating host cell expression of major histocompatibility complex class 1 (MHC-1). Deleting α47 thereby restores MHC-1, allowing tumor cells to present antigen to circulating T-cells in response to infection. Todo et al. showed that this deletion had the intended response of increasing tumor reduction by enhancing antitumor immune response (36). Another third generation viral construct, NV1066, deleted single copies of α0, α4, and γ34.5, which altogether resulted in decreased host neurovirulence with increased tumor specificity (37). NV1066 further contains an enhanced green fluorescent protein insert, which causes infected cells to fluoresce green under the proper fluorescent microscopy or laparoscopy (37).
Arming Vectors: Insertion of Genes Encoding for Anticancer Proteins
While deletion of viral genes results in high viral tumor selectivity, incomplete tumor responses in preclinical and clinical models using these initial vectors prompted the development of oHSV strains that could augment tumor cell kill. These new vectors built on the constructs outlined above by strategically inserting genes intended to increase the vectors’ anticancer efficacy without compromising the selective attenuation seen in noncancer cells. A wide variety of transgenes have been incorporated, with many different purposes. Intended functions range from activating chemotherapy prodrugs (38–43), to augmenting host immune response to tumor cells (44–49), to inducing anti-angiogenesis proteins (50–54). Still other vectors have been constructed with tumor-specific promoters, designed to respond either to certain elements of a microenvironment, like hypoxia (typical of tumor cells), or to an antigen or protein expressed predominantly by cancer cells (16;55–58). The progress of these armed vectors marks a promising future for targeted oncolytic virotherapies.
PRECLINICAL SUCCESS
Locoregional oncolytic viral therapy has been extensively tested in a variety of cancers, with several murine models demonstrating excellent tumor response to local therapy. For example, carotid perfusion of oHSV cured experimental oral cancer in a hamster model (59), while single doses of oHSV via peritoneal and pleural perfusion in murine models have shown both tumor regression and cure in experimental animals with disseminated xenografts of human gastric cancer, colorectal cancer, esophageal cancer, and mesothelioma (60–64). Accordingly, a wealth of preclinical data have been reported by several groups showing efficacy of oncolytic viral therapy against colorectal cancer and hepatic colorectal metastases in models of both local and systemic therapy (Table 1). Kooby and colleagues were among the first to demonstrate oHSV efficacy against colorectal cancer and hepatic metastases, using G207 administered by intratumoral injection of subcutaneous tumors as well as portal infusion directed at hepatic metastases in a murine model. They noted significantly fewer nodules in the treated livers, as detailed in Table 1 (65).
Table 1.
Oncolytic Herpes Simplex Virus vs. Colorectal Carcinoma and Hepatic Metastases in Preclinical Models
| Senior Author, Year | Virus | In vivo Model and Relevant Findings | Route/Maximum Dose |
|---|---|---|---|
| Fong, 1999 (65) | HSV-1 (G207) | Athymic rats: Flank tumors from 3 CRC cell lines—complete response, partial response, or reduced growth rate noted with viral treatment Morris hepatoma model—splenic tumor challenge with portal infusion of virus 7 days later 11 days postinfusion, treated livers with 13 +/− 10 nodules vs. 80 +/− 30 nodules in untreated (P<0.05) |
Flank tumors—IT 1 × 107 PFU RH7777 hepatic micrometastases—portal vein 1 × 108 PFU |
| Tanabe, 2000 (69) | HSV-1 (hrR3) | Immunocompetent and incompetent mice with diffuse liver metastases: Diffuse CRC liver metastases model—splenic tumor challenge with splenic viral injection 8 days later 14 days postinjection, virally treated mice with 1–5 nodules vs. untreated mice with nodules “too numerous to count” |
Hepatic metastases—splenic injection 5 × 107 PFU |
| Fong, 2007 (70) | HSV-1 (NV1023 or NV1034 – GM-CSF) | Immunocompetent mice: CRC and hepatoma flank tumors—viral treatment 2 weeks after tumor cell injection 14 days posttreatment, significantly diminished tumor volumes in virally treated groups. Enhanced efficacy of NV1034 vs. NV1023 |
CT26 flank tumors—IT 5 × 107 PFU Hepa 1–6 Tumors—IT, 5 × 106 |
| Fong, 2007 (85) | HSV-1 (NV1023, NV1034-GM-CSF or NV1042-IL-12) | Immune-deficient mice: CRC splenic injections—viral treatment 24 hours postinjection. 14 days posttreatment, all viral groups with significant reduction in mean surface liver nodules vs. control. Enhanced efficacy of NV1042>NV1034>NV1023 |
CT26 splenic and hepatic micrometastases—splenic injections 1 × 107 PFU |
| Tanabe, 2009 (67) | HSV-1 (hrR3) | Immunocompetent mice: CRC peritoneal metastases—viral treatment began 4 days post CRC dissemination. Virus administered QOD for 3 doses. 48 hours after last viral dose, abdominal organs removed en bloc, tumor weight significantly lower in IP group. |
MC26 peritoneal metastases—IV or IP 1 × 108 PFU |
h = hours, IT = intratumoral, PFU = plaque-forming units, CRC = murine colorectal carcinoma, IV = intravenous, IP = intraperitoneal, QOD = every other day, RH7777 = murine hepatoma cell line, CT26 = human colorectal carcinoma cell line, Hepa 1–6 = mouse hepatoma cell line, MC26 = murine colorectal carcinoma cell line.
While it should be mentioned that systemic therapy has also shown great success preclinically with low rates of adverse effects (66), recent evidence suggests that local viral delivery confers tumoricidal effects superior to those resulting from systemic delivery, while exposing the host organs to minimal levels of virus. For example, Kulu et al. compared intraperitoneal to intravenous administration of oHSV to treat disseminated peritoneal colorectal carcinomatosis in mice (67). After three doses, these researchers found that intraperitoneal administration resulted in more restricted biodistribution, less host toxicity, and greater efficacy against peritoneal metastases compared with intravenous administration (67). In terms of dosing frequency, preclinical studies have shown that multiple doses of both systemic and local virotherapy result in significantly enhanced tumoricidal effects, an important consideration in the development of clinical models (20;66;68).
As oncolytic viruses gained more evidence-based ground, concerns regarding host immune response were raised. Investigators wondered whether a host immune system would attack therapeutic viruses, whether viruses would prove effective against a pre-immunized host, and whether the tumor responses seen in vivo occurred as a result of tumor lysis, or secondary to host immune activity. Yoon et al. investigated several of these aspects by performing extensive in vivo testing in immune-competent and immune-deficient mice to find that oHSV-mediated tumor inhibition was equivalent, and concluded that tumor destruction is mitigated primarily by viral oncolysis rather than host immune response (69). Malhotra and colleagues further investigated the role of the immune system in viral oncolysis by comparing the effects of modified first generation oHSV subtype NV1023 to its derivative, NV1034, a subtype capable of granulocyte-macrophage colony stimulating factor (GM-CSF) secretion. Mouse flank tumors decreased in size after injection with both types of virus; however, enhanced antitumor efficacy was seen with NV1034 injection, suggesting that local cytokine production could add to the already potent antitumor effects of oHSV (70). Interestingly, Malhotra et al. also showed that intratumoral administration of both subtypes of HSV-1 protected against future tumor rechallenges, and that this protection was tumor specific (70). Armed with these and other promising preclinical conclusions, investigators proceeded to clinical trials.
CLINICAL TRIALS
HSV-1 subtypes G207 and NV1020 have been clinically evaluated in several phase I and II trials for treatment of a variety of malignancies including malignant glioma (12;13;71;72), melanoma <12003, 18936≫, squamous cell carcinomas of the head and neck (73), recurrent breast cancer, and pancreatic cancer (74). Previous studies utilized intratumoral or intravenous delivery of HSV-1 subtypes. Our group has utilized oHSV via an intra-arterial delivery method that demonstrates preferential viral selection for tumor tissue over normal liver tissues, as well as excellent long-term safety data (17;21).
Others have utilized alternate local treatment modalities against primary and secondary hepatic tumors, such as intratumoral virus administration. Park et al. administered a total of four doses of up to 3 × 109 plaque-forming units (PFU) of oncolytic poxvirus, JX-594, every three weeks via intratumoral injection to 14 patients with an array of primary and metastatic liver tumors (75). The authors noted radiographic disease regression in three patients and stable disease in six with side effects including fever, chills, and direct hyperbilirubinemia (75;76). With optimistic tumor responses and a similar adverse effect profile as is seen with HAI of other viral therapies, intratumoral administration affirms the safety and efficacy of locoregional oncolytic virotherapy.
Though Kemeny and colleagues were the first to use oHSV via HAI in a clinical setting, others had shown safety and efficacy of HAI using adenovirus (Table 2) (77;78). In 2001, Habib et al. described results of dl1520 administered five times to three hepatocellular carcinoma patients intratumorally, three patients with hepatic colorectal metastases via the hepatic artery, and three additional patients with hepatic colorectal metastases intravenously in a phase I trial (78). The trial used an escalating dose design, with doses peaking at 3 × 1011 PFU (78). Habib and colleagues further conducted a phase II trial utilizing two to four viral doses via HAI with concurrent continuous 5-fluorouracil (5-FU) in 7 patients with hepatic colorectal metastases, noting stable disease in six of seven patients and 50% carcinoembryonic antigen (CEA) reductions in three of six patients with initially elevated CEA (78). Similarly, Reid et al. established safety of HAI of Onyx-015 in nine patients with hepatic colorectal metastases (77). Toxicities were mild, but the exact nature of tumor response to virus was difficult to assess in this study, which utilized combination therapy with virus and HAI of 5-FU and leucovorin after 2 doses of Onyx-015 alone, all after varied chemotherapeutic regimens (77). In a phase II continuation of this study, with long-term follow-up, Reid et al. observed a median survival of 12 months and noted that 11 of the 24 patients (46%) examined in the phase II trial experienced either tumor reduction or tumor enlargement followed by regression of greater than or equal to 10% (79).
Table 2.
Hepatic Arterial Infusion of Oncolytic Virus vs. Hepatic Colorectal Metastases in Clinical Trials
| Senior Investigator, Year, Phase | Virus | # Pts | Dosage Range (Particles) and Schema per Patient | Toxicity Seen in >50% Patients | Tumor Response |
|---|---|---|---|---|---|
| Reid 2001, I (77) | Adenovirus (Onyx-015) | 11* | 2 × 108 – 2 × 1012 2 virus-only doses then 3 virus + 5-FU/leucovorin |
Virus alone: pyrexia, chills, elevated Alk Phos & AST | Dose-response tumor reduction seen with combination therapy at > 6 × 1011 2 partial response 2 stable disease |
| Reid 2002, II (86) | Adenovirus (Onyx-015) | 27** | 2 × 109 – 2 × 1012 2 virus-only doses (25) then 3 virus + 5-FU/leucovorin (18) |
Virus alone: pyrexia, chills, increased Alk Phos and AST, nausea | 3 ≥ 50% regression 4 MR? 9 +/− 25% change 11 disease progression ≥25% growth |
| Fong 2006, I (17) | HSV-1 (NV1020) | 12 | 3 × 106 – 1 × 108 Single dose—3 patients per dose cohort 28 days after viral dose, HAI chemo |
Pyrexia, pain, diarrhea, fatigue, atelectasis, headache | After virus only: Dose-response tumor reduction 1 39%, 1 20% reduction 7 stable disease 3 disease progression After virus + chemo: 12 partial response (range 39%–81% reduction) |
| Habib 2001, I/II (78) | Adenovirus (dl1520) | 7*** | 3 × 1011 PFU One cycle = 3 consecutive days receiving above dose. Patients received 2–4 cycles. Concurrent daily HAI of 5-FU for 3 months, beginning 1 week prior to viral therapy |
None (2 patients with reported “shivers,” 1 with pyrexia) | 50% CEA decrease in 3 of 6 patients with pretreatment elevated CEA 6 stable disease 1 disease progression |
| Tawfik 2008, II (84) | HSV-1 (NV1020) | 22 | 1 × 108 PFU Up to 4 doses, 1 per week 2 additional cycles of chemo |
Transient (<24 h) viral syndrome | Virus alone: 1 complete response 9 stable disease Virus and chemo: 1 complete response, 1 partial response, 11 stable disease |
Hepatic metastases of gastrointestinal carcinomas, 9 colorectal + 2 pancreatic.
Hepatic metastases of gastrointestinal carcinomas, 24 colorectal + 3 pancreatic.
Phase I portion of study examined patients with hepatocellular carcinoma, 7 represents patients studied in phase II, all with multiple bilateral colorectal liver metastases.
Pts = patients; HSV-1 = herpes simplex virus type 1; HAI = hepatic artery infusion; Alk Phos = alkaline phosphatase; AST = aspartate transaminase, chemo = chemotherapy; CEA = carcinoembryonic antigen; 5-FU = 5-fluorouracil; MR = magnetic resonance; PFU = plaque-forming units.
Similarly, our group observed favorable results using just a single dose of HSV-1 (NV1020) via HAI followed by HAI of chemotherapy in 12 patients. We noted partial responses (defined as greater than or equal to 25% tumor reduction) in all patients and a 25 month median survival, with one patient who remained alive 65 months after dosing, and 72 months after initial diagnosis (17;21). Of note, this patient was in the highest dose (1 × 108 PFU) cohort and exhibited the most dramatic response to therapy seen in the trial (Figure 2) (17;21). In all patients, to assess the effects of virotherapy alone, NV1020 was administered without any concomitant therapy (17;21). Two of the three patients in the highest dose cohort noted respective 39% and 20% reductions in radiographic tumor volume with virus alone (17;21). With preclinical data showing significantly enhanced tumor responses with multiple doses, we expect in future trials to establish the maximum tolerated dose as a function of multiple doses in order to achieve enhanced tumor responses.
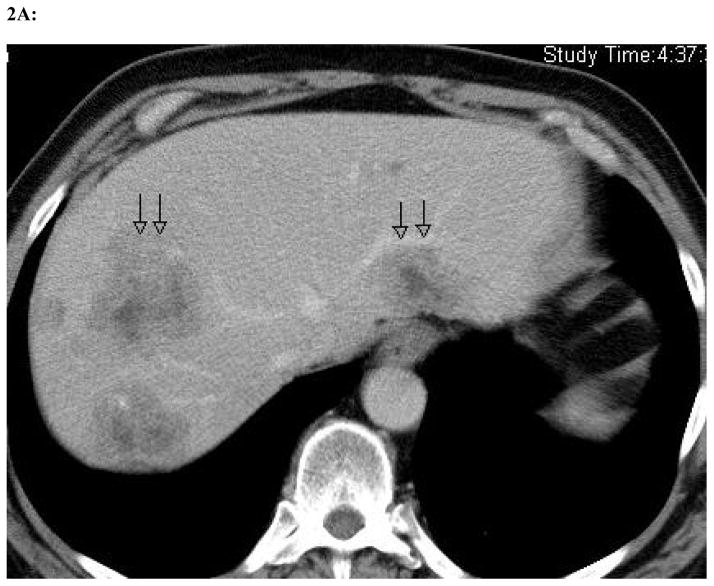
Figure 2. Radiographic change in a single patient at maximum dose of 1 × 108 plaque-forming units.
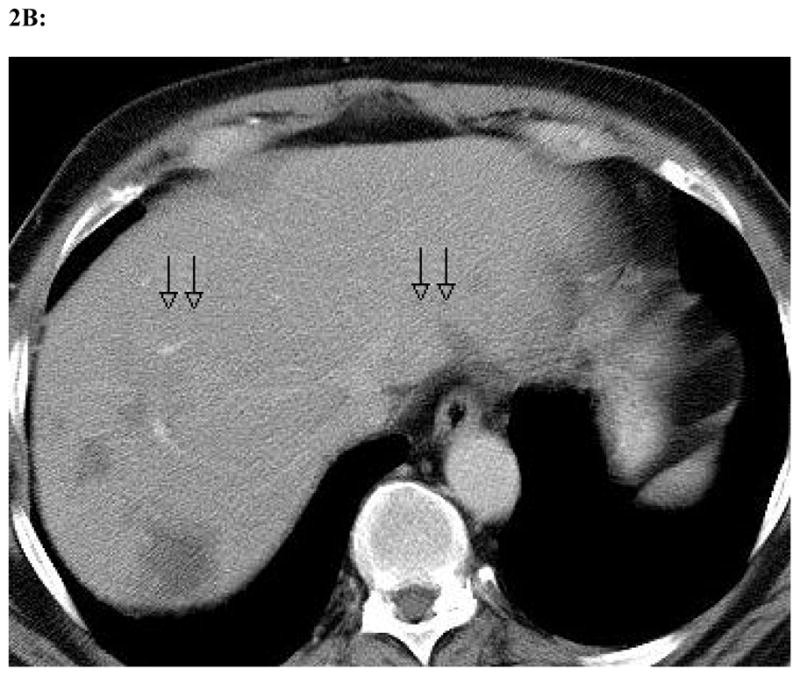
Representative slices from computed tomography scans performed before (a) and one month after (b) oHSV treatment with NV1020 via hepatic arterial infusion. As delineated by the black arrows, the tumors have clearly decreased significantly in size with viral therapy as the only treatment modality employed between the two scans.
Nevertheless, the responses achieved in this study are quite remarkable when one considers that all of the patients included had previously failed 5-FU and leucovorin treatment as well as subsequent salvage regimens, consisting predominantly of irinotecan, and were exhibiting rising CEA levels at the time of viral administration (17). Furthermore, 12 patients were treated in four cohorts with each subsequent cohort receiving an incrementally increased dose. Thus, only three patients received maximum dosage of 1 × 108 PFU allowed in this study. All told, all 12 patients achieved partial responses following HAI of floxuridine with dexamethasone one month after viral therapy, with average tumor dimensions showing sustained decreases of 35%–37% at 6–12 months, and maximum responses varying from 39%–81% tumor reductions at various time points (21). In addition to radiographic tumor diminution, all patients experienced a decrease in CEA levels. No major complications were suffered and while all patients were HSV-1 seropositive prior to the study, no patient demonstrated signs of virus reactivation (17;21). Finally, no blood, urine, vaginal swabs, or rectal swabs cultured positive for HSV (17;21). Thus, oHSV warrants further investigation as a tool against hepatic colorectal metastases.
In addition to establishment of optimum dosing and infusion schedules, future studies of HAI of oncolytic virotherapy may include evaluation of viral efficacy via correlation with CEA level. In our group’s NV1020 trial, all patients experienced partial response and CEA reduction following viral and chemotherapy, and the patient with the greatest radiographic tumor reduction in response to one dose of virus alone (39%) also had the greatest reduction of CEA level (75%) (17;21). While CEA levels vary greatly between patients, relative change in CEA in a single patient has been correlated with tumor recurrence and tumor burden (80–83). Accordingly, CEA could serve as a marker for oHSV efficacy during treatment, perhaps guiding future dosing strategies in individual patients. Correlation of CEA level and tumor regression has also been confirmed in trials examining HAI of adenovirus, which further showed that radiographic response often lagged behind clinical responses like decreased CEA levels (79). With physiologic and radiologic improvements, oHSV holds substantial hope for the continued clinical success of HSV-1 subtypes against hepatic and other malignancies.
CONCLUSION
Finally, with some authors reporting favorable extrahepatic responses with HAI in the clinical setting, it is clear that the full potential of locoregional virotherapy has yet to be realized (84). With side effects that are subjectively more tolerable than those of chemotherapy, HAI of oncolytic viruses clearly holds great promise as a successful treatment modality for patients with highly aggressive metastatic disease. This therapy warrants optimization and further testing to achieve future incorporation into first-line regimens against hepatic colorectal disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008 May;134(6):1752–63. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 2.Saad ED, Hoff PM. Chemotherapy of Metastatic Colorectal Cancer. Curr Treat Options Gastroenterol. 2005 Jun;8(3):239–47. doi: 10.1007/s11938-005-0016-x. [DOI] [PubMed] [Google Scholar]
- 3.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, et al. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008 Sep;248(3):475–86. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 4.Kornprat P, Jarnagin WR, DeMatteo RP, Fong Y, Blumgart LH, D’Angelica M. Role of intraoperative thermoablation combined with resection in the treatment of hepatic metastasis from colorectal cancer. Arch Surg. 2007 Nov;142(11):1087–92. doi: 10.1001/archsurg.142.11.1087. [DOI] [PubMed] [Google Scholar]
- 5.Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, III, Dorfman GS, et al. American society of clinical oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010 Jan 20;28(3):493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 6.Lee JJ, Chu E. An update on treatment advances for the first-line therapy of metastatic colorectal cancer. Cancer J. 2007 Sep;13(5):276–81. doi: 10.1097/PPO.0b013e3181570062. [DOI] [PubMed] [Google Scholar]
- 7.Kemeny NE, Huitzil Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to Resectability Using Hepatic Artery Infusion Plus Systemic Chemotherapy for the Treatment of Unresectable Liver Metastases From Colorectal Carcinoma. J Clin Oncol. 2009 May 26; doi: 10.1200/JCO.2008.20.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varghese S, Rabkin SD. Oncolytic herpes simplex virus vectors for cancer virotherapy. Cancer Gene Ther. 2002;9(12):967–78. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 9.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005 Nov 21;24(52):7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 10.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007 Feb;4(2):101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 11.Fong Y, Kemeny N, Jarnagin W, Stanziale S, Guilfoyle B, Gusani N, et al. Phase 1 study of a replication-competent herpes simplex oncolytic virus for treatment of hepatic colorectal metastases. Proc Am Soc Clin Oncol. 2002;21:8a. [Google Scholar]
- 12.Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7(10):867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 13.Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000 May;7(10):859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 14.Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009 Dec 1;27(34):5763–71. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 15.Nagano S, Perentes JY, Jain RK, Boucher Y. Cancer cell death enhances the penetration and efficacy of oncolytic herpes simplex virus in tumors. Cancer Res. 2008 May 15;68(10):3795–802. doi: 10.1158/0008-5472.CAN-07-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinblatt M, Pin RH, Federoff HJ, Fong Y. Utilizing tumor hypoxia to enhance oncolytic viral therapy in colorectal metastases. Ann Surg. 2004;239(6):892–9. doi: 10.1097/01.sla.0000128308.36393.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemeny NE, Jarnagin W, Gonen M, Haviland D, D’Angelica M, Blumgart L, et al. Phase I trial of hepatic arterial infusion (HAI) with floxuridine (FUDR) and demamethasone (DEX) in combination with systemic oxaliplatin (OXAL), fluorouracil (FU) + leucovorin (LV) after resection of hepatic metastases from colorectal cancer. J Clin Oncol. 2005;23(16S Pt I of II):3579. [Google Scholar]
- 18.Ackerman NB. The blood supply of experimental metastases. IV. Changes in vascularity with tumor growth. Surgery. 1974;75:589–96. [PubMed] [Google Scholar]
- 19.Ensminger WD, Gyves JW. Clinical pharmacology of hepatic arterial chemotherapy. Sem Oncol. 1983;10:176–82. [PubMed] [Google Scholar]
- 20.Shinozaki K, Ebert O, Woo SL. Eradication of advanced hepatocellular carcinoma in rats via repeated hepatic arterial infusions of recombinant VSV. Hepatology. 2005 Jan;41(1):196–203. doi: 10.1002/hep.20536. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Kim T, Bhargava A, Schwartz L, Brown K, Brody L, et al. A Herpes Oncolytic Virus Can Be Delivered Via the Vasculature to Produce Biologic Changes in Human Colorectal Cancer. Mol Ther. 2008 Nov 18; doi: 10.1038/mt.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56(19):4509–15. [PubMed] [Google Scholar]
- 23.Brizel DM, Sibley GS, Prosnitz LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38(2):285–9. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56(5):941–3. [PubMed] [Google Scholar]
- 25.Bush RS, Jenkin RD, Allt WE, Beale FA, Bean H, Dembo AJ, et al. Definitive evidence for hypoxic cells influencing cure in cancer therapy. Br J Cancer Suppl. 1978;37(3):302–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Tannock I, Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981;43(2):245–8. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinkovics JG, Horvath JC. Natural and genetically engineered viral agents for oncolysis and gene therapy of human cancers. Arch Immunol Ther Exp (Warsz ) 2008 Dec;56( Suppl 1):3s–59s. doi: 10.1007/s00005-008-0047-9. [DOI] [PubMed] [Google Scholar]
- 28.Southam CM, Moore AE. Clinical studies of viruses as antineoplastic agents, with particular reference to Egypt 101 virus. Cancer. 1952;5:1025–34. doi: 10.1002/1097-0142(195209)5:5<1025::aid-cncr2820050518>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.HOSTER HA, ZANES RP, Jr, VON HE. Studies in Hodgkin’s syndrome; the association of viral hepatitis and Hodgkin’s disease; a preliminary report. Cancer Res. 1949 Aug;9(8):473–80. [PubMed] [Google Scholar]
- 30.Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9:1211–8. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007 Sep 8;254(2):178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(21):11307–12. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987 Jun;158(2):265–75. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 34.Balfour HH., Jr Antiviral drugs. N Engl J Med. 1999 Apr 22;340(16):1255–68. doi: 10.1056/NEJM199904223401608. [DOI] [PubMed] [Google Scholar]
- 35.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252(5007):854–6. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 36.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98(11):6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong R, Joe J, Kim S, Shah J, Horsburg B, Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Human Gene Therapy. 2002;13(10):1213–23. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 38.Tyminski E, Leroy S, Terada K, Finkelstein DM, Hyatt JL, Danks MK, et al. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005 Aug 1;65(15):6850–7. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 39.Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008 May;16(5):879–85. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braidwood L, Dunn PD, Hardy S, Evans TR, Brown SM. Antitumor activity of a selectively replication competent herpes simplex virus (HSV) with enzyme prodrug therapy. Anticancer Res. 2009 Jun;29(6):2159–66. [PubMed] [Google Scholar]
- 41.Ishida D, Nawa A, Tanino T, Goshima F, Luo CH, Iwaki M, et al. Enhanced cytotoxicity with a novel system combining the paclitaxel-2′-ethylcarbonate prodrug and an HSV amplicon with an attenuated replication-competent virus, HF10 as a helper virus. Cancer Lett. 2009 Jul 13; doi: 10.1016/j.canlet.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Simpson GR, Han Z, Liu B, Wang Y, Campbell G, Coffin RS. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006 May 1;66(9):4835–42. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 43.Nawa A, Nozawa N, Goshima F, Nagasaka T, Kikkawa F, Niwa Y, et al. Oncolytic viral therapy for human ovarian cancer using a novel replication-competent herpes simplex virus type I mutant in a mouse model. Gynecol Oncol. 2003;91(1):81–8. doi: 10.1016/s0090-8258(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 44.Bennett JJ, Malhotra S, Wong RJ, Delman K, Zager J, St Louis M, et al. Interleukin 12 secretion enhances antitumor efficacy of oncolytic herpes simplex viral therapy for colorectal cancer. Annals Of Surgery. 2001 Jun;233(6):819–26. doi: 10.1097/00000658-200106000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarnagin WR, Delman K, Kooby D, Mastorides S, Zager J, Brennan MF, et al. Neoadjuvant interleukin-12 immunogene therapy protects against cancer recurrence after liver resection in an animal model. Ann Surg. 2000 May;231(5):762–71. doi: 10.1097/00000658-200005000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varghese S, Rabkin SD, Liu R, Nielsen PG, Ipe T, Martuza RL. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 2006 Mar;13(3):253–65. doi: 10.1038/sj.cgt.7700900. [DOI] [PubMed] [Google Scholar]
- 47.Han ZQ, Assenberg M, Liu BL, Wang YB, Simpson G, Thomas S, et al. Development of a second-generation oncolytic Herpes simplex virus expressing TNFalpha for cancer therapy. J Gene Med. 2007 Feb;9(2):99–106. doi: 10.1002/jgm.999. [DOI] [PubMed] [Google Scholar]
- 48.Wong RJ, Chan MK, Yu Z, Kim TH, Bhargava A, Stiles BM, et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12. Clinical Cancer Research. 2004;10(1 Pt 1):251–9. doi: 10.1158/1078-0432.CCR-0197-3. [DOI] [PubMed] [Google Scholar]
- 49.Wong RJ, Patel SG, Kim S, DeMatteo RP, Malhotra S, Bennett JJ, et al. Cytokine gene transfer enhances herpes oncolytic therapy in murine squamous cell carcinoma. Human Gene Therapy. 2001 Feb 10;12(3):253–65. doi: 10.1089/10430340150218396. [DOI] [PubMed] [Google Scholar]
- 50.Aghi M, Rabkin S, Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98(1):38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- 51.Wakimoto H, Kesari S, Farrell CJ, Curry WT, Jr, Zaupa C, Aghi M, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009 Apr 15;69(8):3472–81. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullen JT, Donahue JM, Chandrasekhar S, Yoon SS, Liu W, Ellis LM, et al. Oncolysis by viral replication and inhibition of angiogenesis by a replication-conditional herpes simplex virus that expresses mouse endostatin. Cancer. 2004 Aug 15;101(4):869–77. doi: 10.1002/cncr.20434. [DOI] [PubMed] [Google Scholar]
- 53.Yang CT, Lin YC, Lin CL, Lu J, Bu X, Tsai YH, et al. Oncolytic herpesvirus with secretable angiostatic proteins in the treatment of human lung cancer cells. Anticancer Res. 2005 May;25(3B):2049–54. [PubMed] [Google Scholar]
- 54.Liu TC, Zhang T, Fukuhara H, Kuroda T, Todo T, Canron X, et al. Dominant-negative fibroblast growth factor receptor expression enhances antitumoral potency of oncolytic herpes simplex virus in neural tumors. Clin Cancer Res. 2006 Nov 15;12(22):6791–9. doi: 10.1158/1078-0432.CCR-06-0263. [DOI] [PubMed] [Google Scholar]
- 55.Reinblatt M, Pin RH, Fong Y. Carcinoembryonic antigen directed herpes viral oncolysis improves selectivity and activity in colorectal cancer. Surgery. 2004;136(3):579–84. doi: 10.1016/j.surg.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 56.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005 Apr 1;65(7):2832–9. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 57.Kasuya H, Pawlik TM, Mullen JT, Donahue JM, Nakamura H, Chandrasekhar S, et al. Selectivity of an oncolytic herpes simplex virus for cells expressing the DF3/MUC1 antigen. Cancer Research. 2004;64(7):2561–7. doi: 10.1158/0008-5472.can-03-3431. [DOI] [PubMed] [Google Scholar]
- 58.Pan W, Bodempudi V, Esfandyari T, Farassati F. Utilizing ras signaling pathway to direct selective replication of herpes simplex virus-1. PLoS ONE. 2009;4(8):e6514. doi: 10.1371/journal.pone.0006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carew JF, Kooby D, Halterman MW, Federoff HJ, Fong Y. Selective infection and cytolysis of human head and neck squamous cell carcinoma with sparing of normal mucosa by a cytolytic herpes simplex virus type 1 (G207) Proceedings of the American Head and Neck Society. 1999 doi: 10.1089/10430349950017608. Ref Type: Abstract. [DOI] [PubMed] [Google Scholar]
- 60.Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Therapy. 2002;9(11):935–45. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 61.Stanziale SF, Stiles BM, Bhargava A, Kerns SA, Kalakonda N, Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum Gene Ther. 2004;15(6):609–18. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- 62.Kucharczuk JC, Randazzo B, Chang MY, Amin KM, Elshami AA, Sterman DH, et al. Use of a “replication-restricted” herpes virus to treat experimental human malignant mesothelioma. Cancer Research. 1997;57(3):466–71. [PubMed] [Google Scholar]
- 63.Stiles BM, Bhargava A, Adusumilli PS, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134(2):357–64. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 64.Stiles B, Adusumilli P, Bhargava A, Stanziale S, Kim T, Chan M, et al. Minimally-invasive localization of oncolytic herpes simplex viral therapy of micrometastatic pleural cancer. Cancer Gene Therapy. 2006;13(1):53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB J. 1999 Aug;13(11):1325–34. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 66.Delman KA, Bennett JJ, Zager JS, Burt BM, McAuliffe PF, Petrowsky H, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Human Gene Therapy. 2000;11(18):2465–72. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- 67.Kulu Y, Dorfman JD, Kuruppu D, Fuchs BC, Goodwin JM, Fujii T, et al. Comparison of intravenous versus intraperitoneal administration of oncolytic herpes simplex virus 1 for peritoneal carcinomatosis in mice. Cancer Gene Ther. 2009 Apr;16(4):291–7. doi: 10.1038/cgt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA, Fong Y. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. Journal of the American College of Surgeons. 2001;193(1):12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- 69.Yoon SS, Nakamura H, Carroll NM, Bode BP, Chiocca EA, Tanabe KK. An oncolytic herpes simplex virus type 1 selectively destroys diffuse liver metastases from colon carcinoma. FASEB J. 2000;14(2):301–11. [PubMed] [Google Scholar]
- 70.Malhotra S, Kim T, Zager J, Bennett J, Ebright M, D’Angelica M, et al. Use of an oncolytic virus secreting GM-CSF as combined oncolytic and immunotherapy for treatment of colorectal and hepatic adenocarcinomas. Surgery. 2007 Apr;141(4):520–9. doi: 10.1016/j.surg.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Therapy. 2002;9(6):398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 72.Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Therapy. 2004;11(22):1648–58. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 73.Mace AT, Ganly I, Soutar DS, Brown SM. Potential for efficacy of the oncolytic Herpes simplex virus 1716 in patients with oral squamous cell carcinoma. Head Neck. 2008 Aug;30(8):1045–51. doi: 10.1002/hed.20840. [DOI] [PubMed] [Google Scholar]
- 74.Nakao A, Takeda S, Shimoyama S, Kasuya H, Kimata H, Teshigahara O, et al. Clinical experiment of mutant herpes simplex virus HF10 therapy for cancer. Curr Cancer Drug Targets. 2007 Mar;7(2):169–74. doi: 10.2174/156800907780058808. [DOI] [PubMed] [Google Scholar]
- 75.Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008 Jun;9(6):533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 76.Liu TC, Hwang T, Park BH, Bell J, Kirn DH. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol Ther. 2008 Sep;16(9):1637–42. doi: 10.1038/mt.2008.143. [DOI] [PubMed] [Google Scholar]
- 77.Reid T, Galanis E, Abbruzzese J, Sze D, Andrews J, Romel L, et al. Intra-arterial administration of a replication-selective adenovirus (dl1520) in patients with colorectal carcinoma metastatic to the liver: a phase I trial. Gene Ther. 2001;8(21):1618–26. doi: 10.1038/sj.gt.3301512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Habib NA, Sarraf CE, Mitry RR, Havlik R, Nicholls J, Kelly M, et al. E1B-deleted adenovirus (dl1520) gene therapy for patients with primary and secondary liver tumors. Human Gene Therapy. 2001;12(3):219–26. doi: 10.1089/10430340150218369. [DOI] [PubMed] [Google Scholar]
- 79.Reid TR, Freeman S, Post L, McCormick F, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Therapy. 2005;12(8):673–81. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 80.Wong IH, Yeo W, Chan AT, Johnson PJ. Quantitative relationship of the circulating tumor burden assessed by reverse transcription-polymerase chain reaction for cytokeratin 19 mRNA in peripheral blood of colorectal cancer patients with Dukes’ stage, serum carcinoembryonic antigen level and tumor progression. Cancer Lett. 2001;162(1):65–73. doi: 10.1016/s0304-3835(00)00630-3. [DOI] [PubMed] [Google Scholar]
- 81.Choi MY, Lee KM, Chung JK, Lee DS, Jeong JM, Park JG, et al. Correlation between serum CEA level and metabolic volume as determined by FDG PET in postoperative patients with recurrent colorectal cancer. Ann Nucl Med. 2005;19(2):123–9. doi: 10.1007/BF03027391. [DOI] [PubMed] [Google Scholar]
- 82.Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O’Brien M, et al. The prognostic value of CEA, beta HCG, AFP, CA125, CA19–9 and C-erb B-2, beta HCG immunohistochemistry in advanced colorectal cancer. Ann Oncol. 1995;6(6):581–7. doi: 10.1093/oxfordjournals.annonc.a059248. [DOI] [PubMed] [Google Scholar]
- 83.Novis BH, Gluck E, Thomas P, Steele GD, Zurawski VR, Jr, Stewart R, et al. Serial levels of Ca 19–9 and CEA in colonic cancer. J Clin Oncol. 1986;4:987. doi: 10.1200/JCO.1986.4.6.987. [DOI] [PubMed] [Google Scholar]
- 84.Reid TR, Geller DA, Tawfik H. Phase II study of an oncolytic herpes simplex virus (NV1020) in patients with colorectal cancer metastatic to the liver (mCRC). Presented at the 2009 GI cancer symposium of ASCO; San Francisco, CA. 2010. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeRubertis BG, Stiles BM, Bhargava A, Gusani NJ, Hezel M, D’Angelica M, et al. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 2007 Jun;14(6):590–7. doi: 10.1038/sj.cgt.7701053. [DOI] [PubMed] [Google Scholar]
- 86.Reid T, Galanis E, Abbruzzese J, Sze D, Wein LM, Andrews J, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Research. 2002;62(21):6070–9. [PubMed] [Google Scholar]